Sol-Gel Processed Cobalt-Doped Methylated Silica Membranes Calcined under N2 Atmosphere: Microstructure and Hydrogen Perm-Selectivity
Abstract
:1. Introduction
2. Experimental Part
2.1. Fabrication of Methyl-Modified Co/SiO2 Sols
2.2. Fabrication of Unsupported Co/MSiO2 Materials
2.3. Fabrication of Supported Co/MSiO2 Membranes
2.4. Characterization
3. Results
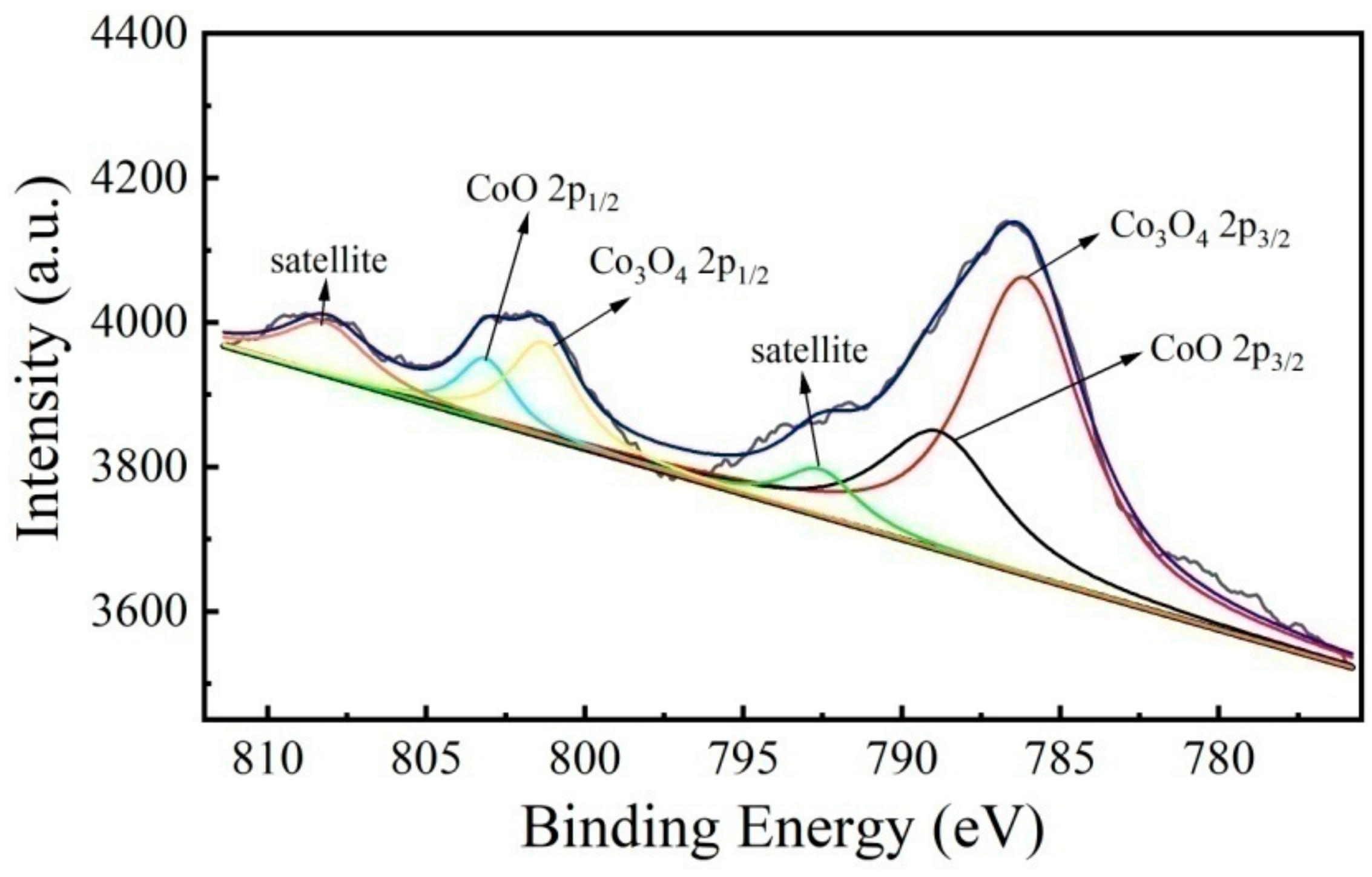
3.1. Phase Structure Analysis
3.2. FTIR Analysis
3.3. Pore-Structure Analysis
3.4. TEM Analysis
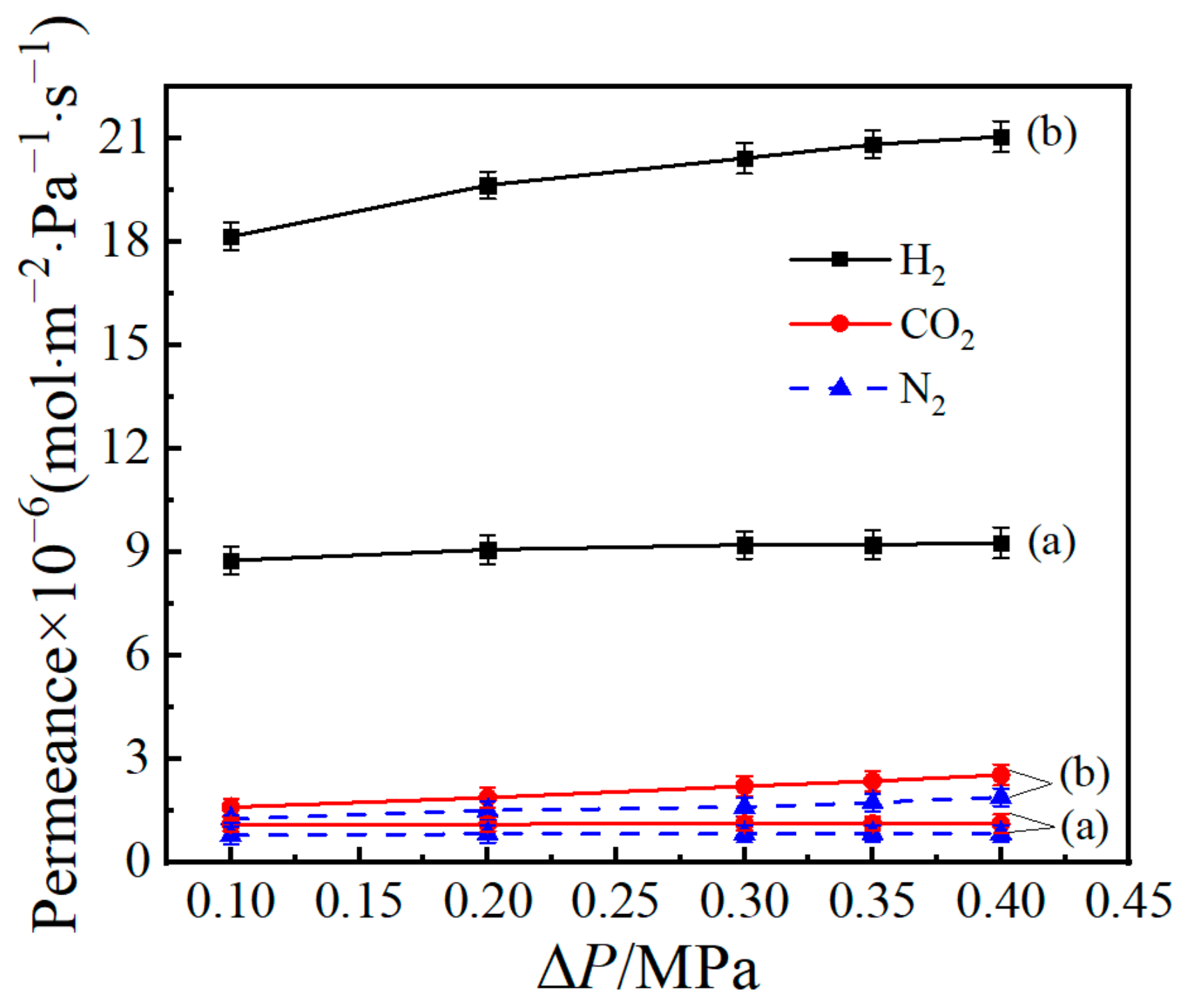
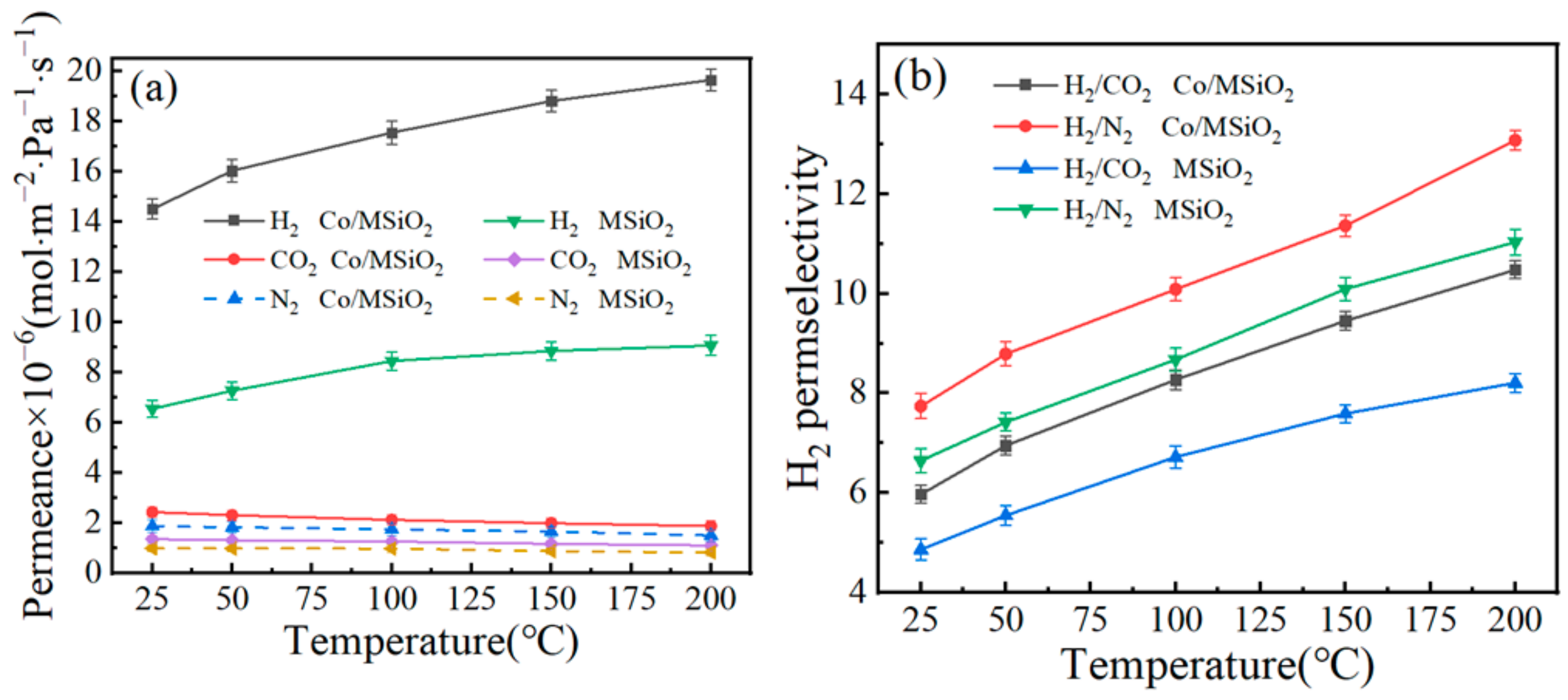
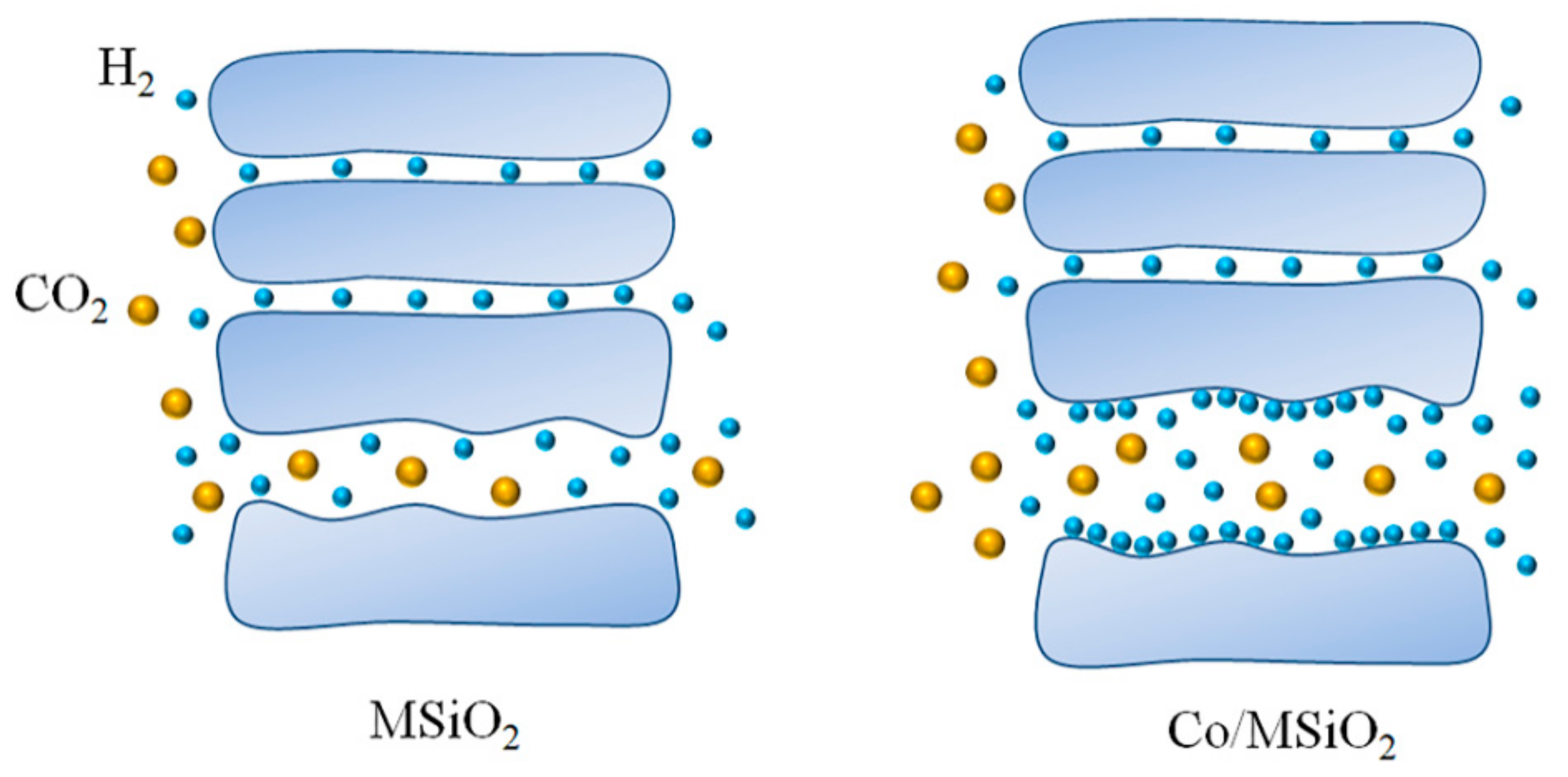
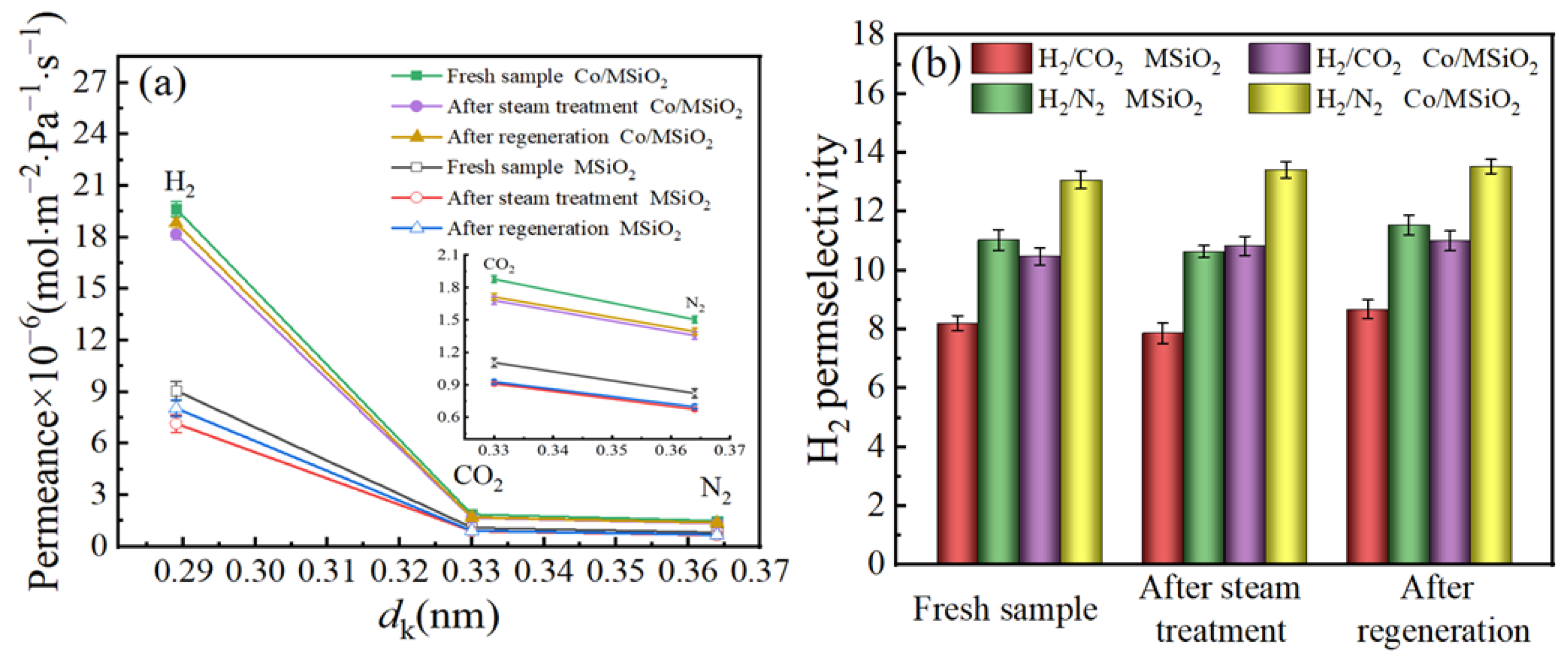
3.5. Gas-Permeance Analysis
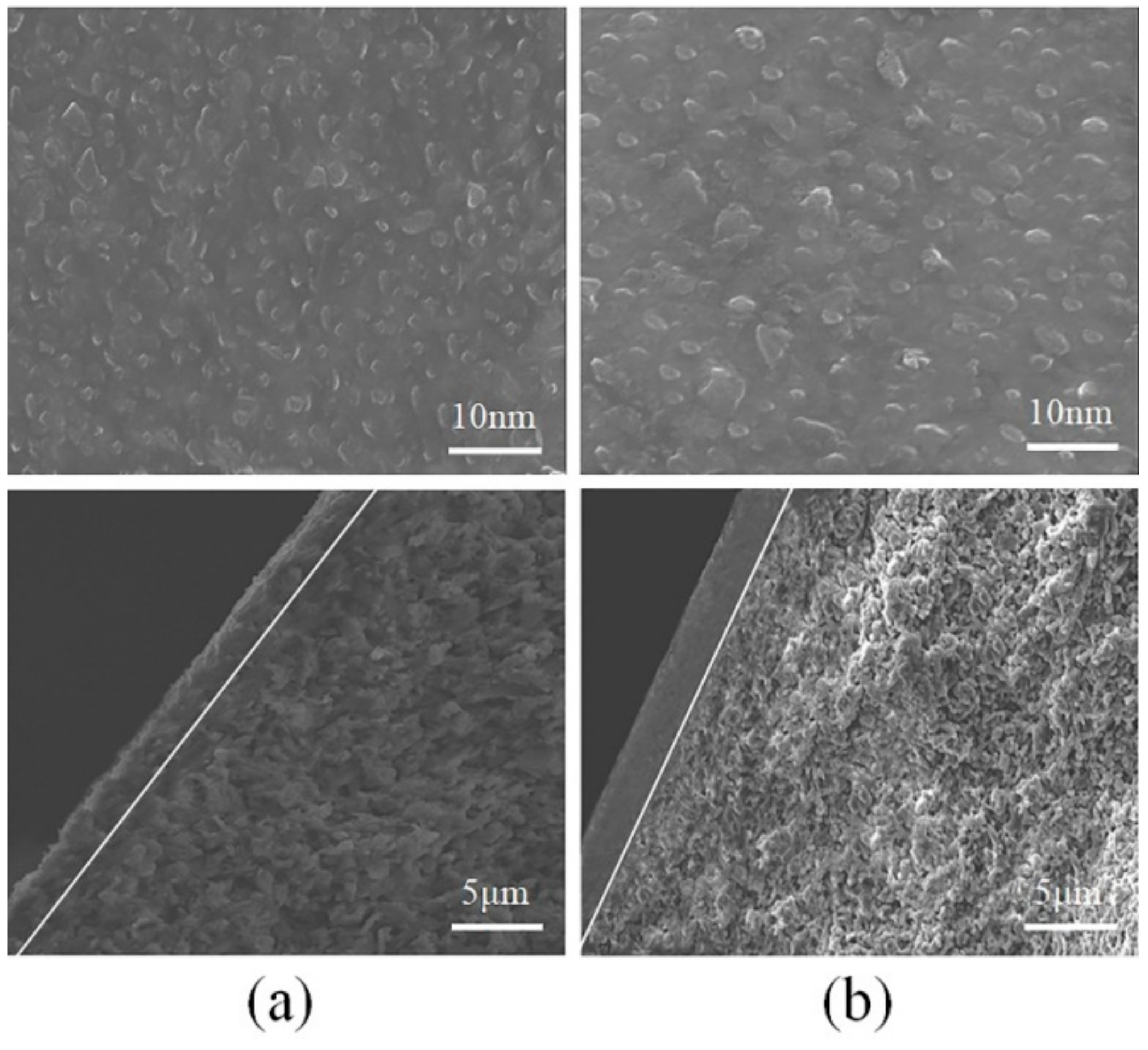
3.6. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wang, H.; Fu, W.; Yang, X.; Zhang, H.; Huang, Z.; Li, J. Recent Advancement in Heterostructured Catalysts for Hydrogen Evolution Reaction. J. Mater. Chem. A 2020, 8, 6926–6956. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, C.-Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2019, 258, 114078. [Google Scholar] [CrossRef]
- Chen, S.; Pei, C.; Gong, J. Insights into Interfacial Catalysis of Steam Reforming Reactions for Hydrogen Production. Energy Environ. Sci. 2019, 12, 3473–3495. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. Development and multi-objective optimization of a newly proposed industrial heat recovery based cascaded hydrogen and ammonia synthesis system. Sci. Total Environ. 2020, 743, 140671. [Google Scholar] [CrossRef]
- Edwards, P.; Kuznetsov, V.; David, W.I. Hydrogen energy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 365, 1043–1056. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, H.; Lei, J.; Song, H.; Qi, H. Controlling pore structures of Pd-doped organosilica membranes by calcination atmosphere for gas separation. Chin. J. Chem. Eng. 2019, 27, 3036–3042. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, L.; Xu, D.; Ciora, R.; Liu PK, T.; Manousiouthakis, V.I.; Tsotsis, T.T. A carbon molecular sieve membrane-based reactive separation process for pre-combustion CO2 capture. J. Membr. Sci. 2020, 605, 118028. [Google Scholar] [CrossRef]
- Tahmasbi, D.; Hossainpour, S.; Babaluo, A.A.; Rezakazemi, M.; Mousavi Nejad Souq, S.S.; Younas, M. Hydrogen separation from synthesis gas using silica membrane: CFD simulation. Int. J. Hydrogen Energy 2020, 45, 19381–19390. [Google Scholar] [CrossRef]
- Ameh, A.E.; Eze, C.P.; Antunes, E.; Cornelius, M.-L.U.; Musyoka, N.M.; Petrik, L.F. Stability of fly ash-based BEA-zeolite in hot liquid phase. Catal. Today 2019, 357, 416–424. [Google Scholar] [CrossRef]
- Prodinger, S.; Derewinski, M.A. Recent Progress to Understand and Improve Zeolite Stability in the Aqueous Medium. Pet. Chem. 2020, 60, 420–436. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, J.-H.; Hong, Y.; Lee, C.-Y. Removal of BTX using granular octyl-functionalized mesoporous silica nanoparticle. Int. Biodeterior. Biodegrad. 2014, 95, 219–224. [Google Scholar] [CrossRef]
- Araki, S.; Imasaka, S.; Tanaka, S.; Miyake, Y. Pervaporation of organic/water mixtures with hydrophobic silica membranes functionalized by phenyl groups. J. Membr. Sci. 2011, 380, 41–47. [Google Scholar] [CrossRef]
- Messaoud, S.B.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Alkylamine-silica hybrid membranes for carbon dioxide/methane separation. J. Membr. Sci. 2015, 477, 161–171. [Google Scholar] [CrossRef]
- Kato, H.; Lundin, S.-T.B.; Ahn, S.-J.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Gas Separation Silica Membranes Prepared by Chemical Vapor Deposition of Methyl-Substituted Silanes. Membranes 2019, 9, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.-J.; Ko, K.-J.; Choi, H.-K.; Moon, J.-H.; Lee, C.-H. Kinetic effects of methane on binary mixture separation on methyltriethoxysilane templated silica membranes. Sep. Purif. Technol. 2017, 182, 151–159. [Google Scholar] [CrossRef]
- Wei, Q.; Ding, Y.-L.; Nie, Z.-R.; Liu, X.-G.; Li, Q.-Y. Wettability, pore structure and performance of perfluorodecyl-modified silica membranes. J. Membr. Sci. 2014, 466, 114–122. [Google Scholar] [CrossRef]
- Ma, X.; Janowska, K.; Boffa, V.; Fabbri, D.; Magnacca, G.; Calza, P.; Yue, Y. Surfactant-Assisted Fabrication of Alumina-Doped Amorphous Silica Nanofiltration Membranes with Enhanced Water Purification Performances. Nanomaterials 2019, 9, 1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawal, S.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Development of an acetylacetonate-modified silica-zirconia composite membrane applicable to gas separation. J. Membr. Sci. 2020, 599, 117844. [Google Scholar] [CrossRef]
- Darmawan, A.; Karlina, L.; Astuti, Y.; Sriatun; Motuzas, J.; Wang, D.; da Costa, J.C.D. Structural evolution of nickel oxide silica sol-gel for the preparation of interlayer-free membranes. J. Non-Cryst. Solids 2016, 447, 9–15. [Google Scholar] [CrossRef]
- Yang, J.; Tian, L. Preparation, Characterization and Surface Free Energy of Nickel-Doped Silica Organic–Inorganic Hybrid Membrane for H2/CO2 Separation. J. Nanosci. Nanotechnol. 2019, 19, 3180–3186. [Google Scholar] [CrossRef]
- Lee, J.; Ha, J.-H.; Song, I.-H.; Park, J.-W. Facile surface modification of ceramic membranes using binary TiO2/SiO2 for achieving fouling resistance and photocatalytic degradation. J. Sol. Gel. Sci. Technol. 2019, 91, 198–207. [Google Scholar] [CrossRef]
- Yang, X.; Sheridan, S.; Ding, L.; Wang, D.K.; Smart, S.; Diniz da Costa, J.C.; Liubinas, A.; Duke, M. Inter-layer free cobalt-doped silica membranes for pervaporation of ammonia solutions. J. Membr. Sci. 2018, 553, 111–116. [Google Scholar] [CrossRef]
- Pakdehi, S.G.; Rahimi, E.; Shafiei, K. Effect of Co-SiO2 mesoporous layer coating step on liquid fuel dimethyl amino ethyl azide (DMAZ) dehydration performance. Chem. Eng. Technol. 2019, 42, 996–1001. [Google Scholar] [CrossRef]
- Yang, J.; Fan, W.; Bell, C.-M. Effect of calcination atmosphere on microstructure and H2/CO2 separation of palladium-doped silica membranes. Sep. Purif. Technol. 2019, 210, 659–669. [Google Scholar] [CrossRef]
- Song, H.; Zhao, S.; Lei, J.; Wang, C.; Qi, H. Pd-doped organosilica membrane with enhanced gas permeability and hydrothermal stability for gas separation. J. Mater. Sci. 2016, 51, 6275–6286. [Google Scholar] [CrossRef]
- Karakiliç, P.; Huiskes, C.; Luiten-Olieman, M.W.J.; Nijmeijer, A.; Winnubst, L. Sol-gel processed magnesium-doped silica membranes with improved H2/CO2 separation. J. Membr. Sci. 2017, 543, 195–201. [Google Scholar] [CrossRef]
- Boffa, V.; Blank, D.H.A.; Elshof, J.E. Hydrothermal stability of microporous silica and niobia-silica membranes. J. Membr. Sci. 2008, 319, 256–263. [Google Scholar] [CrossRef]
- Ballinger, B.; Motuzas, J.; Smart, S.; da Costa, J.C.D. Palladium cobalt binary doping of molecular sieving silica membranes. J. Membr. Sci. 2014, 451, 185–191. [Google Scholar] [CrossRef]
- Darmawan, A.; Motuzas, J.; Smart, S.; Julbe, A.; da Costa, J.C.D. Binary iron cobalt oxide silica membrane for gas separation. J. Membr. Sci. 2015, 474, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Díez, B.; Roldán, N.; Martín, A.; Sotto, A.; Perdigón-Melón, J.A.; Arsuaga, J.; Rosal, R. Fouling and biofouling resistance of metal-doped mesostructured silica/polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2017, 526, 252–263. [Google Scholar] [CrossRef]
- Battersby, S.; Smart, S.; Ladewig, B.; Liu, S.; Duke, M.C.; Rudolph, V.; da Costa, J.C.D. Hydrothermal stability of cobalt silica membranes in a water gas shift membrane reactor. Sep. Purif. Technol. 2009, 66, 299–305. [Google Scholar] [CrossRef]
- Smart, S.; Vente, J.F.; da Costa, J.C.D. High temperature H2/CO2 separation using cobalt oxide silica membranes. Int. J. Hydrog. Energy 2012, 37, 12700–12707. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, D.K.; Martens, D.L.; Smart, S.; Strounina, E.; da Costa, J.C.D. Physicochemical characterisation and hydrothermal stability investigation of cobalt-incorporated silica xerogels. RSC Adv. 2014, 4, 18862–18870. [Google Scholar] [CrossRef]
- Esposito, S.; Turco, M.; Ramis, G.; Bagnasco, G.; Pernice, P.; Pagliuca, C.; Bevilacqua, M.; Aronne, A. Cobalt-silicon mixed oxide nanocomposites by modified sol-gel method. J. Solid State Chem. 2007, 180, 3341–3350. [Google Scholar] [CrossRef]
- Uhlmann, D.; Liu, S.; Ladewig, B.P.; da Costa, J.C.D. Cobalt-doped silica membranes for gas separation. J. Membr. Sci. 2009, 326, 316–321. [Google Scholar] [CrossRef]
- Ma, H.; Xu, J.; Chen, C.; Zhang, Q.; Ning, J.; Miao, H.; Zhou, L.; Li, X. Catalytic aerobic oxidation of ethylbenzene over Co/SBA-15. Catal. Lett. 2007, 113, 104–108. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.; Luo, H.; Jin, F.; Ruan, T.; Wang, D. MoO2 nanobelts modified with an MOF-derived carbon layer for high performance lithium-ion battery anodes. J. Alloys Compd. 2019, 803, 664–670. [Google Scholar] [CrossRef]
- Igi, R.; Yoshioka, T.; Ikuhara, Y.H.; Iwamoto, Y.; Tsuru, T. Characterization of Co-Doped Silica for Improved Hydrothermal Stability and Application to Hydrogen Separation Membranes at High Temperatures. J. Am. Ceram. Soc. 2008, 91, 2975–2981. [Google Scholar] [CrossRef]
- Morad, I.; Liu, X.; Qiu, J. Crystallization-induced valence state change of Mn2+→Mn4+ in LiNaGe4O9 glass-ceramics. J. Am. Ceram. Soc. 2020, 103, 3051–3059. [Google Scholar] [CrossRef]
- Riva, R.; Miessner, H.; Vitali, R.; Del Piero, G. Metal–support interaction in Co/SiO2 and Co/TiO2. Appl. Catal. A Gen. 2000, 196, 111–123. [Google Scholar] [CrossRef]
- Lukashuk, L.; Yigit, N.; Li, H.; Bernardi, J.; Föttinger, K.; Rupprechter, G. Operando XAS and NAP-XPS investigation of CO oxidation on meso- and nanoscale CoO catalysts. Catal. Today 2019, 336, 139–147. [Google Scholar] [CrossRef]
- Okoye-Chine, C.G.; Mbuya, C.O.L.; Ntelane, T.S.; Moyo, M.; Hildebrandt, D. The effect of silanol groups on the metal-support interactions in silica-supported cobalt Fischer-Tropsch catalysts. A temperature programmed surface reaction. J. Catal. 2020, 381, 121–129. [Google Scholar] [CrossRef]
- Azmiyawati, C.; Niami, S.S.; Darmawan, A. Synthesis of silica gel from glass waste for adsorption of Mg2+, Cu2+, and Ag+ metal ions. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 1–6. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Shao, Z.; Wang, H.; Sun, Y. Direct CO2 hydrogenation to ethanol over supported Co2C catalysts: Studies on support effects and mechanism. J. Catal. 2020, 382, 86–96. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Ye, Y.; Tang, Y.; Li, X.; Gu, F.; Li, L. Composite Si-O-Metal network catalysts with uneven electron distribution: Enhanced activity and electron transfer for catalytic ozonation of carbamazepine. Appl. Catal. B 2020, 263, 118311. [Google Scholar] [CrossRef]
- Khalil, A.; Ali, N.; Khan, A.; Asiri, A.M.; Kamal, T. Catalytic potential of cobalt oxide and agar nanocomposite hydrogel for the chemical reduction of organic pollutants. Int. J. Biol. Macromol. 2020, 164, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chen, S.; Bezrukov, A.A.; Mostrom, M.; Terskikh, V.V.; Franz, D.; Wang, S.-Q.; Kumar, A.; Chen, M.; Space, B.; et al. Ultramicropore engineering by dehydration to enable molecular sieving of H2 by calcium trimesate. Angew. Chem. Int. Ed. 2020, 59, 16188–16194. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hagg, M.-B. Optimization of Carbonization Process for Preparation of High Performance Hollow Fiber Carbon Membranes. Ind. Eng. Chem. Res. 2011, 50, 8065–8072. [Google Scholar] [CrossRef]
- De Vos, R.M.; Verweij, H. Improved performance of silica membranes for gas separation. J. Membr. Sci. 1998, 143, 37–51. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.K.; Martens, D.L.; Smart, S.; da Costa, J.C.D. Binary gas mixture and hydrothermal stability investigation of cobalt silica membranes. J. Membr. Sci. 2015, 493, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Hacarlioglu, P.; Lee, D.; Gibbs, G.V.; Oyama, S.T. Activation energies for permeation of He and H2 through silica membranes: An ab initio calculation study. J. Membr. Sci. 2008, 313, 277–283. [Google Scholar] [CrossRef]
- Qureshi, H.F.; Nijmeijer, A.; Winnubst, L. Influence of sol–gel process parameters on the micro-structure and performance of hybrid silica membranes. J. Membr. Sci. 2013, 446, 19–25. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirano, Y.; Fujii, H.; Tsuru, T.; Asaeda, M. Hydrothermal Stability and Performance of Silica-Zirconia Membranes for Hydrogen Separation in Hydrothermal Conditions. J. Chem. Eng. Jpn. 2001, 34, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, H.F.; Besselink, R.; ten Elshof, J.E.; Nijmeijer, A.; Winnubst, L. Doped microporous hybrid silica membranes for gas separation. J. Sol Gel Sci. Technol. 2015, 75, 180–188. [Google Scholar] [CrossRef]



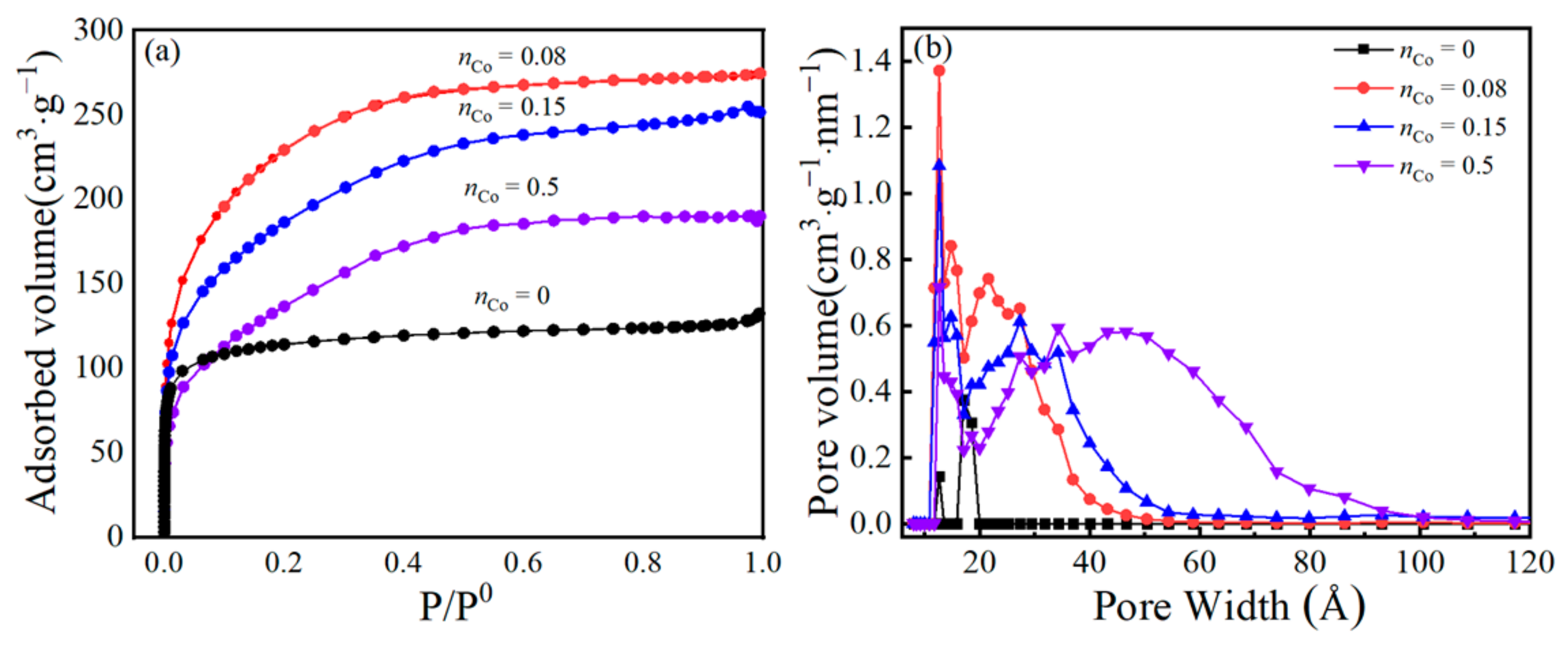
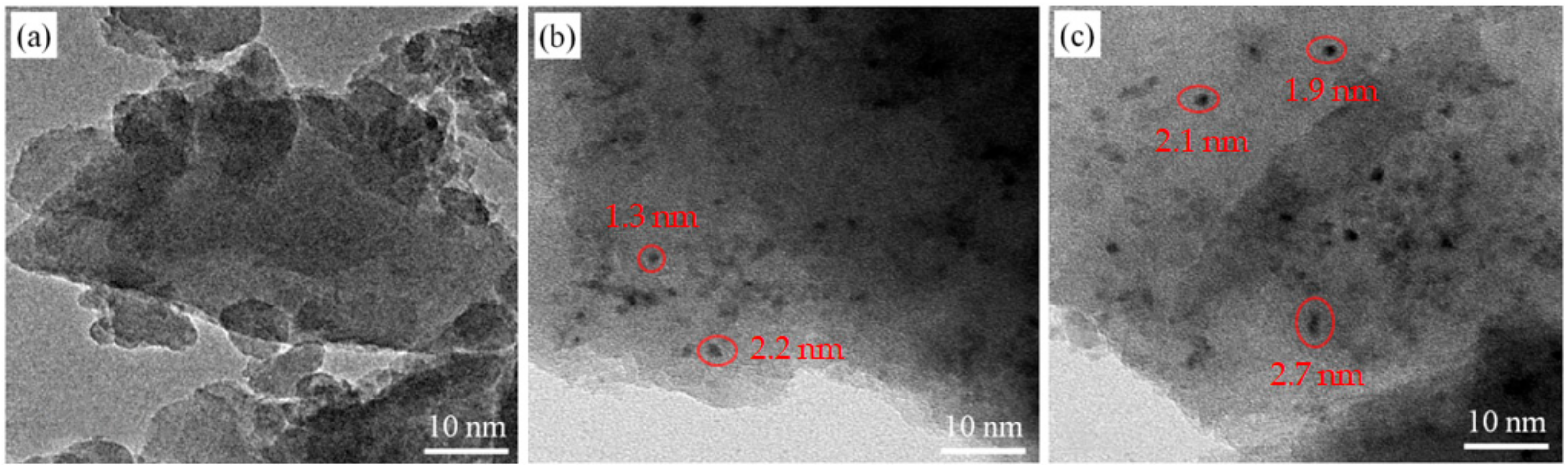
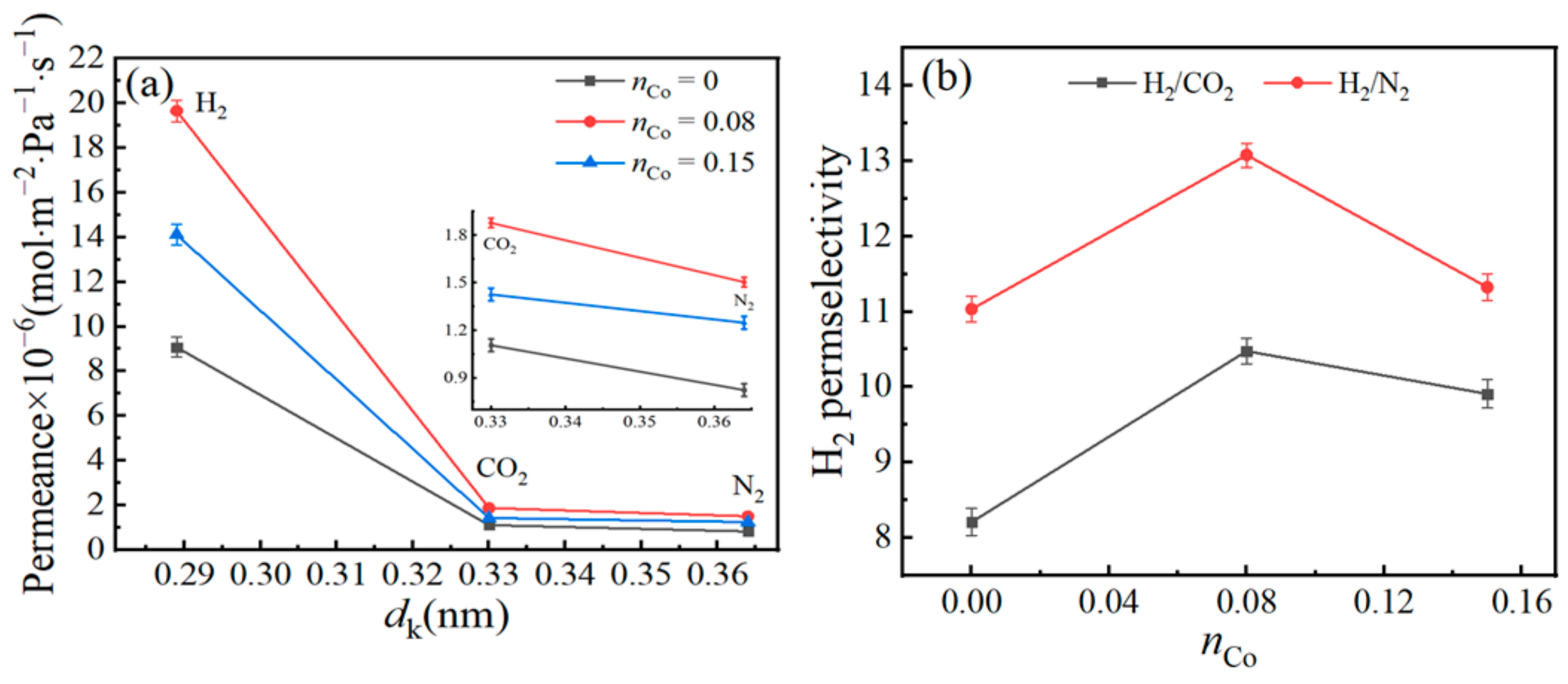
| Membrane Materials | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | VM/Vt | Mean Pore Size (nm) |
|---|---|---|---|---|---|
| nCo = 0 | 389.38 | 0.230 | 0.150 | 0.652 | 1.75 |
| nCo = 0.08 | 775.34 | 0.424 | 0.045 | 0.106 | 2.34 |
| nCo = 0.15 | 644.10 | 0.402 | 0.035 | 0.087 | 2.73 |
| nCo = 0.5 | 494.54 | 0.399 | 0.034 | 0.085 | 3.14 |
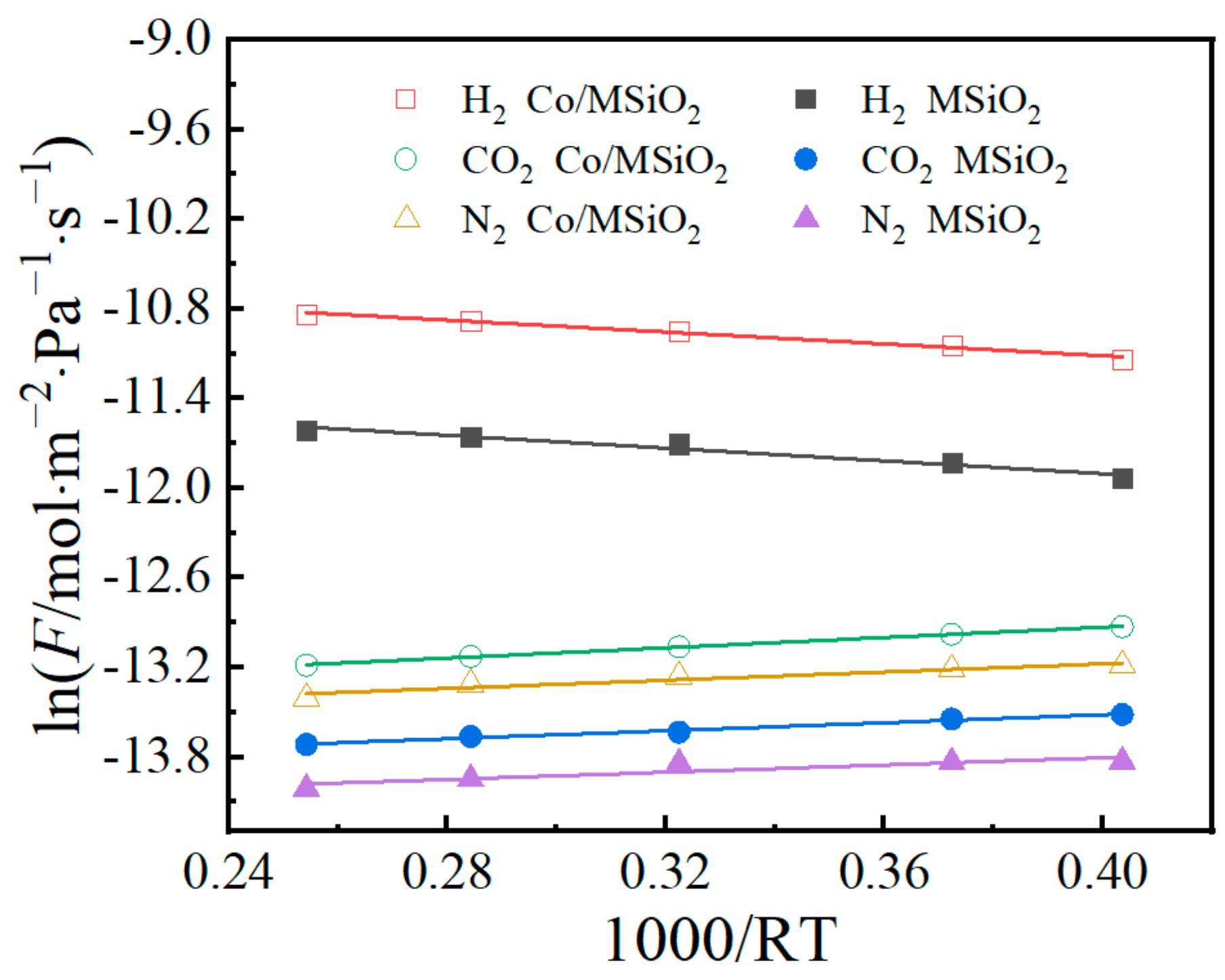
| Gases | Ea/KJ·mol−1 | |
|---|---|---|
| MSiO2 | Co/MSiO2 | |
| H2 | 2.14 | 1.98 |
| CO2 | −1.34 | −1.72 |
| N2 | −1.21 | −1.37 |
| Membrane Type | Temperature and Pressure | Permeance of H2 (mol·m−2·Pa−1·s−1) | Perm-Selectivities of H2 | Calcination Atmosphere | Ea Value of H2 (KJ·mol−1) | Pore Diameter (nm) | |
|---|---|---|---|---|---|---|---|
| H2/CO2 | H2/N2 | ||||||
| Si(400) [49] | 200 °C, 1 bar | 1.74 × 10−6 | 7.5 | 64.4 | Air | 8 | 0.38–0.55 |
| SiO2 [52] | 200 °C, 2 bar | 4.62 × 10−7 | 3.7 | 10.5 | N2 | — | 0.30–0.54 |
| SiO2–ZrO2 [53] | 500 °C, 100 KPa | 2 × 10−6 | 15 | 190 | Air | 13 | — |
| Pd/SiO2 [25] | 200 °C, 0.3 MPa | 7.26 × 10−7 | 4.3 | 14 | H2, N2 | — | ~0.57 |
| Nb/SiO2 [54] | 200 °C, 2 bar | 5.03 × 10−7 | 3.5 | 6.5 | N2 | — | ~0.55 |
| Co/SiO2 [50] | 200 °C, 500 KPa | 5 × 10−8 | 31.6 | — | Air | 13.8 | — |
| Co/SiO2 * | 200 °C, 0.2 MPa | 1.97 × 10−5 | 10.48 | 13.08 | N2 | 1.98 | 0.3–2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yang, J.; Mu, R.; Guo, Y.; Hou, H. Sol-Gel Processed Cobalt-Doped Methylated Silica Membranes Calcined under N2 Atmosphere: Microstructure and Hydrogen Perm-Selectivity. Materials 2021, 14, 4188. https://doi.org/10.3390/ma14154188
Wang L, Yang J, Mu R, Guo Y, Hou H. Sol-Gel Processed Cobalt-Doped Methylated Silica Membranes Calcined under N2 Atmosphere: Microstructure and Hydrogen Perm-Selectivity. Materials. 2021; 14(15):4188. https://doi.org/10.3390/ma14154188
Chicago/Turabian StyleWang, Lunwei, Jing Yang, Ruihua Mu, Yingming Guo, and Haiyun Hou. 2021. "Sol-Gel Processed Cobalt-Doped Methylated Silica Membranes Calcined under N2 Atmosphere: Microstructure and Hydrogen Perm-Selectivity" Materials 14, no. 15: 4188. https://doi.org/10.3390/ma14154188
APA StyleWang, L., Yang, J., Mu, R., Guo, Y., & Hou, H. (2021). Sol-Gel Processed Cobalt-Doped Methylated Silica Membranes Calcined under N2 Atmosphere: Microstructure and Hydrogen Perm-Selectivity. Materials, 14(15), 4188. https://doi.org/10.3390/ma14154188






