Research Progress of Transparent Electrode Materials with Sandwich Structure
Abstract
:1. Introduction
2. Sandwich Structure Transparent Electrodes of Different Materials
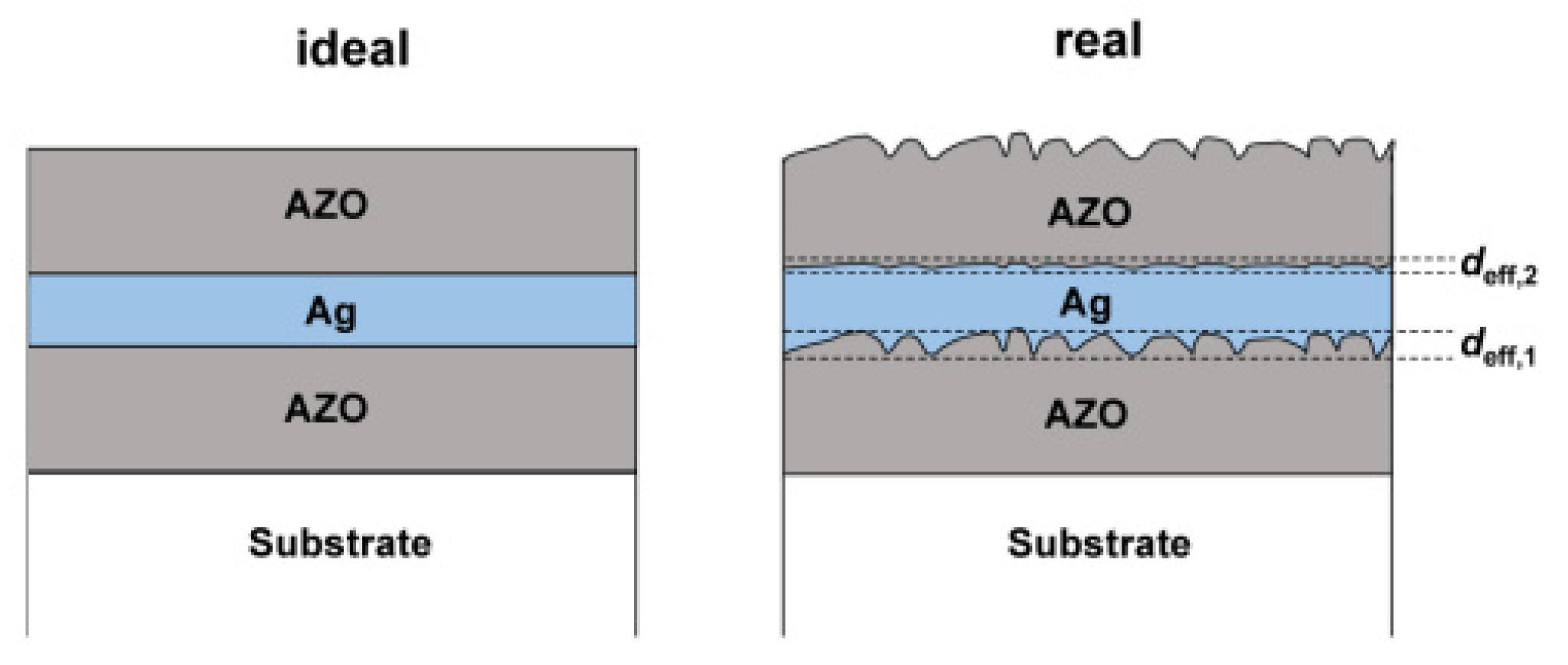
2.1. Metal-Based Composite Electrodes
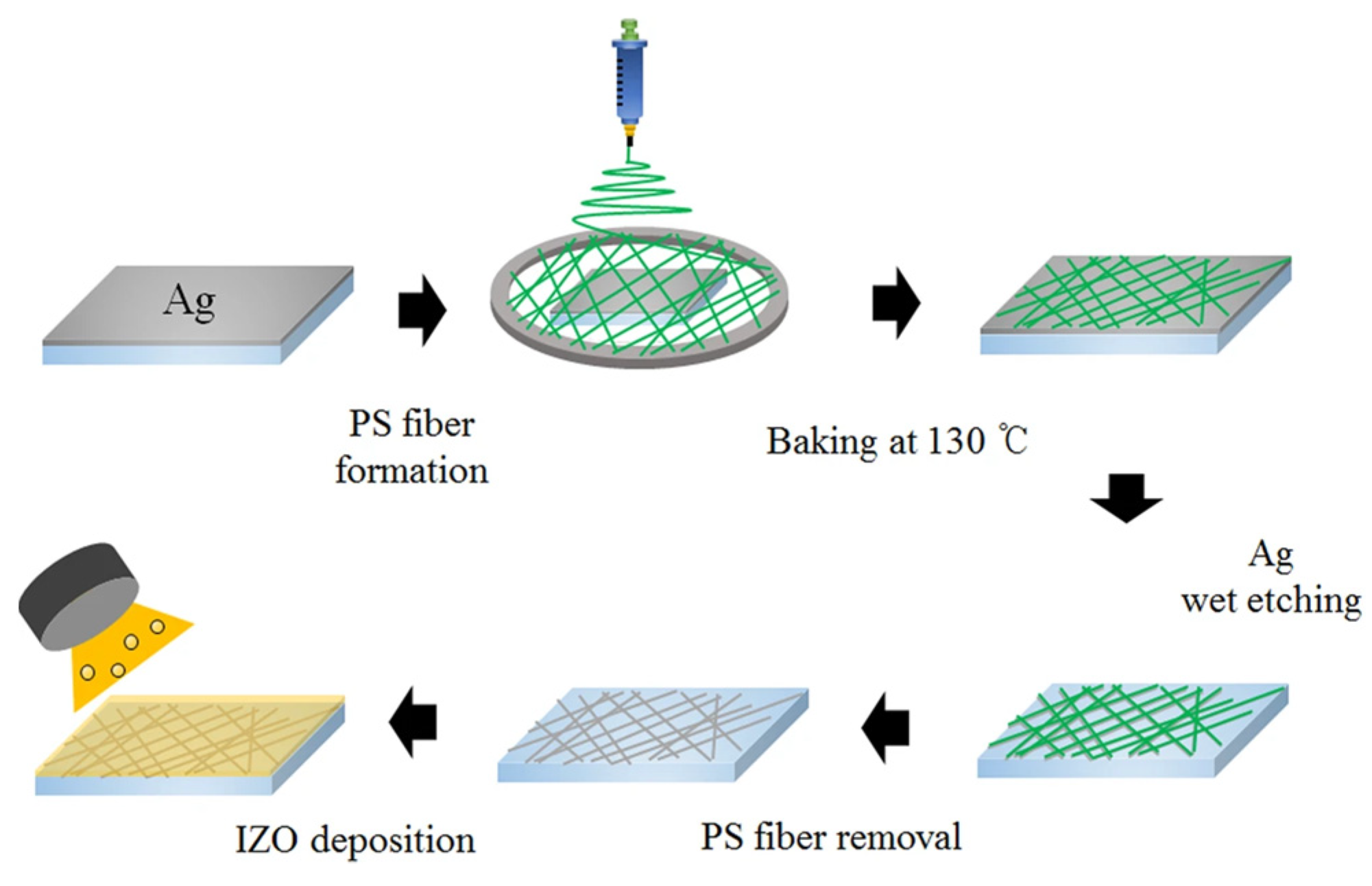
2.2. Composite Electrodes with Alternating Metal/Compound Materials
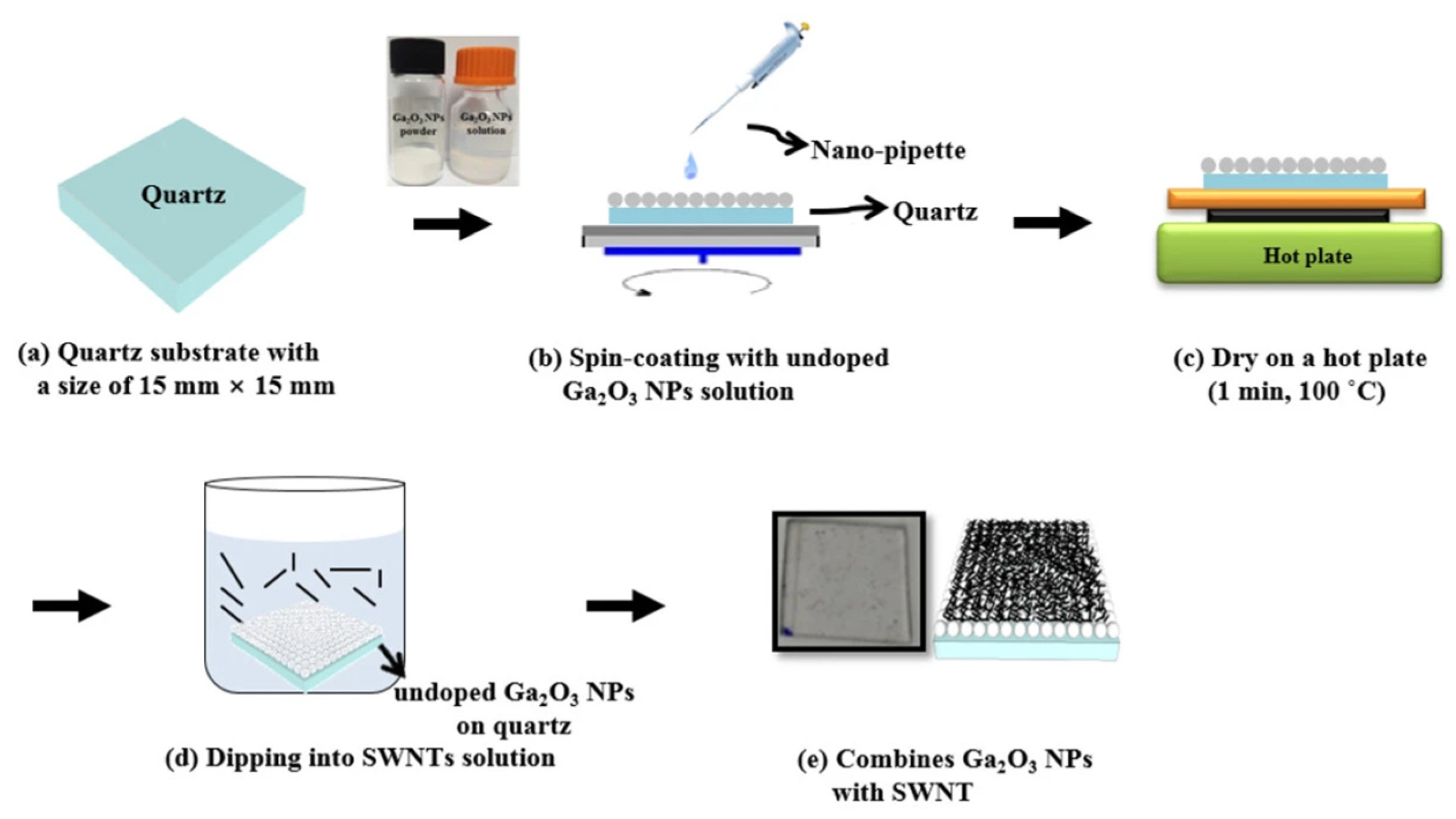
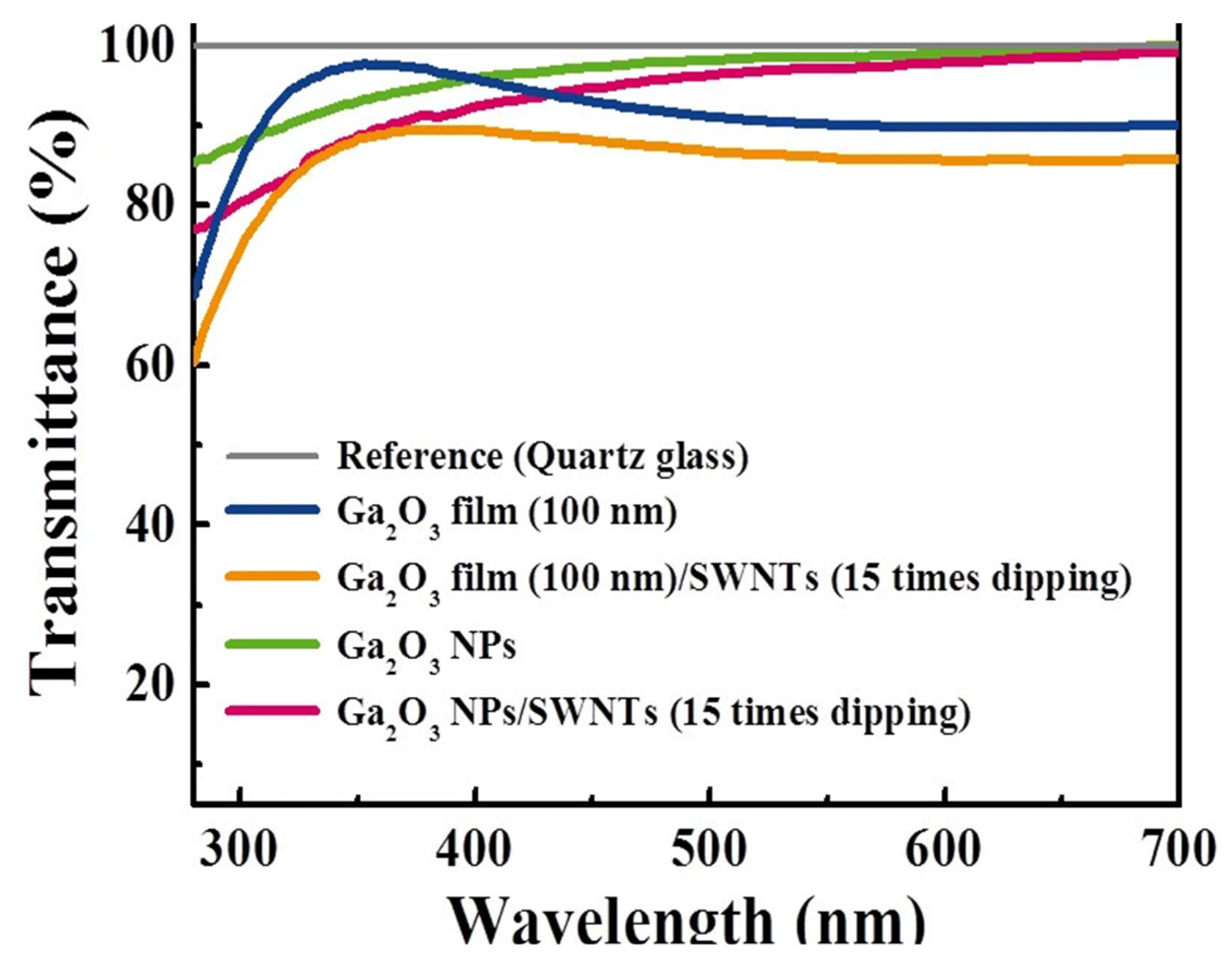
2.3. Carbon-Based Composite Electrodes
2.4. Organic-Dominated Composite Electrodes
3. Comparison and Outlook
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2007, 104, 20142. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Wu, H.C.; Chen, S.C.; Ma Lee, C.W.; Wang, X. Absorption amelioration of amorphous Si film by introducing metal silicide nanoparticles. Nanoscale Res. Lett. 2017, 12, 224. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Hrong, R.H.; Zeng, Y.Y.; Wang, W.K.; Tsai, C.L.; Fu, Y.K.; Kuo, W.H. Transparent electrode design for AlGaN deep-ultraviolet light-emitting diodes. Optics Express 2017, 25, 32206–32213. [Google Scholar] [CrossRef]
- Sun, H.; Liao, M.H.; Chen, S.C.; Li, Z.Y.; Lin, P.C.; Song, S.M. Synthesis and characterization of n-type NiO:Al thin films for fabrication of p-n NiO homojunctions. J. Phys. D 2018, 51, 105109. [Google Scholar] [CrossRef]
- Yu, F.; Liu, W.B.; Ke, S.W.; Kurmoo, M.; Zuo, J.L.; Zhang, Q.C. Electrochromic two-dimensional covalent organic framework with a reversible dark-to-transparent switch. Nat. Commun. 2020, 11, 6. [Google Scholar] [CrossRef]
- Chen, S.C.; Kuo, T.Y.; Lin, H.C.; Chen, R.Z.; Sun, H. Optoelectronic properties of p-type NiO films deposited by direct current magnetron sputtering versus high power impulse magnetron sputtering. Appl. Surf. Sci. 2020, 508, 145106. [Google Scholar] [CrossRef]
- Badeker, K. Concerning the electricity conductibility and the thermoelectric energy of several heavy metal bonds. Ann. Phys. 1907, 22, 749–766. [Google Scholar]
- Grundmann, M. Karl Badeker (1877–1914) and the discovery of transparent conductive materials. Phys. Status Solidi Appl. Mater. Sci. 2015, 212, 1409–1426. [Google Scholar] [CrossRef]
- Kim, C.L.; Jung, C.W.; Oh, Y.J.; Kim, D.E. A highly flexible transparent conductive electrode based on nanomaterials. NPG Asia Mater. 2017, 9, 9. [Google Scholar] [CrossRef]
- Li, X.H.; Song, K.Q.; Cong, D.L.; Zhang, M.; Sun, C.Y.; Wu, H.L.; Li, Z.S. Preparation and photoelectric properties of ITO thi films with high transparency and low emissivity. Surf. Technol. 2020, 49, 126–132. [Google Scholar]
- Boehme, M.; Charton, C. Properties of ITO on PET film in dependence on the coating conditions and thermal processing. Surf. Coat. Technol. 2005, 200, 932–935. [Google Scholar] [CrossRef]
- Peng, H.L.; Dang, W.H.; Cao, J.; Chen, Y.L.; Wu, W.; Zheng, W.S.; Li, H.; Shen, Z.X.; Liu, Z.F. Topological insulator nanostructures for near-infrared transparent flexible electrodes. Nat. Chem. 2012, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.Q.; Shi, H.Z.; Wang, S.Y.; Xu, J.P.; Ma, J.S.; Ma, B.; Wen, M.Y.; Li, J.Q.; Zhao, J.L.; et al. Large-Area Tunable Red/Green/Blue Tri-Stacked Quantum Dot Light-Emitting Diode Using Sandwich-Structured Transparent Silver Nanowires Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 48820–48827. [Google Scholar] [CrossRef]
- Wang, K.L.; Xin, Y.Q.; Zhao, J.F.; Song, S.M.; Chen, S.C.; Lu, Y.B.; Sun, H. High transmittance in IR region of conductive ITO/AZO multilayers deposited by RF magnetron sputtering. Ceram. Inter. 2018, 44, 6769–6774. [Google Scholar] [CrossRef]
- Liu, F.; Dai, M.J.; Lin, S.S.; Shi, Q.; Sun, H. Electrical and magnetic properties of (In, Co) co-doped ZnO films deposited using radio frequency magnetron sputtering. Chin. J. Eng. 2021, 43, 1–7. [Google Scholar]
- Bingel, A.; Stenzel, O.; Naujok, P.; Muller, R.; Shestaeva, S.; Steglich, M.; Schulz, U.; Kaiser, N.; Tunnermann, A. AZO/Ag/AZO transparent conductive films: Correlation between the structural, electrical, and optical properties and development of an optical model. Opt. Mater. Express 2016, 6, 3217–3232. [Google Scholar] [CrossRef]
- Minami, T.; Sato, H.; Imamoto, H.; Takata, S. Substrate-temperature dependence of transparent conducting al-doped ZnO thin-films prepared by magnetron sputtering. Jpn. J. Appl. Phys. Part 2 Lett. 1992, 31, L257–L260. [Google Scholar] [CrossRef]
- Park, J.; Heo, S.; Park, K.; Song, M.H.; Kim, J.Y.; Kyung, G.; Ruoff, R.S.; Park, J.U.; Bien, F. Research on flexible display at Ulsan National Institute of Science and Technology. NPJ Flex. Electron. 2017, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Ameri, S.K.; Ho, R.; Jang, H.W.; Tao, L.; Wang, Y.H.; Wang, L.; Schnyer, D.M.; Akinwande, D.; Lu, N.S. Graphene Electronic Tattoo Sensors. ACS Nano 2017, 11, 7634–7641. [Google Scholar] [CrossRef]
- Liu, X.N.; Gao, J.; Gong, J.H.; Wang, W.X.; Chen, S.C.; Dai, M.J.; Lin, S.S.; Shi, Q.; Sun, H. Optoelectronic properties of an AZO/Ag multilayer employed as a flexible electode. Ceram. Inter. 2021, 47, 5671–5676. [Google Scholar] [CrossRef]
- Lee, J.; An, K.; Won, P.; Ka, Y.; Hwang, H.; Moon, H.; Kwon, Y.; Hong, S.; Kim, C.; Lee, C.; et al. A dual-scale metal nanowire network transparent conductor for highly efficient and flexible organic light emitting diodes. Nanoscale 2017, 9, 1978–1985. [Google Scholar] [CrossRef]
- Han, Y.C.; Lim, M.S.; Park, J.H.; Choi, K.C. ITO-free flexible organic light-emitting diode using ZnS/Ag/MoO3 anode incorporating a quasi-perfect Ag thin film. Org. Electron. 2013, 14, 3437–3443. [Google Scholar] [CrossRef]
- Zhang, Q.C. Shooting flexible electronics. Front. Phys. 2020, 16, 3. [Google Scholar]
- Ji, C.G.; Liu, D.; Zhang, C.; Guo, L.J. Ultrathin-metal-film-based transparent electrodes with relative transmittance surpassing 100%. Nat. Commun. 2020, 11, 8. [Google Scholar] [CrossRef]
- Leem, D.S.; Edwards, A.; Faist, M.; Nelson, J.; Bradley, D.D.C.; De Mello, J.C. Efficient Organic Solar Cells with Solution-Processed Silver Nanowire Electrodes. Adv. Mater. 2011, 23, 4371–4375. [Google Scholar] [CrossRef]
- Kim, A.; Won, Y.; Woo, K.; Kim, C.H.; Moon, J. Highly Transparent Low Resistance ZnO/Ag Nanowire/ZnO Composite Electrode for Thin Film Solar Cells. ACS Nano 2013, 7, 1081–1091. [Google Scholar] [CrossRef]
- Seo, T.H.; Kim, B.K.; Shin, G.; Lee, C.; Kim, M.J.; Kim, H.; Suh, E.K. Graphene-silver nanowire hybrid structure as a transparent and current spreading electrode in ultraviolet light emitting diodes. Appl. Phys. Lett. 2013, 103, 5. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovel, H.J. Transparency of thin metal-films on semiconductor substrates. J. Appl. Phys. 1976, 47, 4968–4970. [Google Scholar] [CrossRef]
- Bingel, A.; Steglich, M.; Naujok, P.; Muller, R.; Schulz, U.; Kaiser, N.; Tunnermann, A. Influence of the ZnO:Al dispersion on the performance of ZnO:Al/Ag/ZnO:Al transparent electrodes. Thin Solid Films 2016, 616, 594–600. [Google Scholar] [CrossRef]
- Luo, E.Z.; Heun, S.; Kennedy, M.; Wollschlager, J.; Henzler, M. Surface-roughness and conductivity of thin Ag films. Phys. Rev. B 1994, 49, 4858–4865. [Google Scholar] [CrossRef]
- Liu, C.H.; Yu, X. Silver nanowire-based transparent, flexible, and conductive thin film. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Park, C.H.; Kwack, J.H.; Lee, D.J.; Kim, J.G.; Choi, J.; Bae, B.H.; Park, S.J.; Kim, E.; Park, Y.W.; et al. Ag fiber/IZO Composite Electrodes: Improved Chemical and Thermal Stability and Uniform Light Emission in Flexible Organic Light-Emitting Diodes. Sci. Rep. 2019, 9, 7. [Google Scholar] [CrossRef]
- You, J.; Lee, S.M.; Eom, H.S.; Chang, S.T. Highly Transparent Conducting Electrodes Based on a Grid Structure of Silver Nanowires. Coatings 2021, 11, 11. [Google Scholar]
- Sepulveda-Mora, S.B.; Cloutier, S.G. Figures of Merit for High-Performance Transparent Electrodes Using Dip-Coated Silver Nanowire Networks. J. Nanomater. 2012, 2012, 7. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yang, H.Z.; Chen, S.C.; Lu, Y.B.; Xin, Y.Q.; Yang, T.L.; Sun, H. Impact of active layer thickness of nitrogen-doped In-Sn-Zn-O films on materials and thin film transistor performances. J. Phys. D 2018, 51, 175101. [Google Scholar] [CrossRef]
- Yu, X.M.; Yu, X.; Zhang, J.J.; Chen, L.Q.; Zhou, Y.T.; Wei, J. Fully solution processed Ag NWs/ZnO TF/ZnO NR composite electrodes with tunable light scattering properties for thin-film solar cells. J. Alloy. Compd. 2019, 791, 1231–1240. [Google Scholar] [CrossRef]
- Morita, Y.; Ohtani, N. Fabrication of aluminum and gallium codoped ZnO multilayer transparent conductive films by spin coating method and discussion about improving their performance. Jpn. J. Appl. Phys. 2018, 57, 02CB03. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; An, H.M.; Kim, H.D.; Kim, T.G. Transparent conductive oxide films mixed with gallium oxide nanoparticle/single-walled carbon nanotube layer for deep ultraviolet light-emitting diodes. Nanoscale Res. Lett. 2013, 8, 507. [Google Scholar] [CrossRef] [Green Version]
- Pang, S.P.; Hernandez, Y.; Feng, X.L.; Mullen, K. Graphene as Transparent Electrode Material for Organic Electronics. Adv. Mater. 2011, 23, 2779–2795. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photon. 2010, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, Y.; Zhu, D. Chemical doping of graphene. J. Mater. Chem. 2011, 21, 3335–3345. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Gu, Y.T. Mechanical properties of graphene: Effects of layer number, temperature and isotope. Comput. Mater. Sci. 2013, 71, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Chortos, A.; Lei, T.; Jin, L.H.; Kim, T.R.; Bae, W.G.; Zhu, C.X.; Wang, S.H.; Pfattner, R.; Chen, X.Y.; et al. Ultratransparent and stretchable graphene electrodes. Sci. Adv. 2017, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, E.; Cha, S.; Kim, Y.; Kim, C. Transparent and flexible electrode composed of a graphene multilayer interlayer-doped with MoO3. Org. Electron. 2020, 77, 6. [Google Scholar] [CrossRef]
- D’Arsie, L.; Esconjauregui, S.; Weatherup, R.; Guo, Y.Z.; Bhardwaj, S.; Centeno, A.; Zurutuza, A.; Cepek, C.; Robertson, J. Stability of graphene doping with MoO3 and I-2. Appl. Phys. Lett. 2014, 105, 5. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.; Kidambi, P.R.; Bayer, B.C.; Weijtens, C.; Kuhn, A.; Centeno, A.; Pesquera, A.; Zurutuza, A.; Robertson, J.; Hofmann, S. Metal Oxide Induced Charge Transfer Doping and Band Alignment of Graphene Electrodes for Efficient Organic Light Emitting Diodes. Sci. Rep. 2014, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.T.; Liu, Y.Y.; Zhang, W.J.; Guo, X.L.; Yin, L.L.; Wang, Y.X.; Li, L.; Zhang, Y.; Wang, Z.M.; Zhang, T. Growth of graphene/Ag nanowire/graphene sandwich films for transparent touch-sensitive electrodes. Mater. Chem. Phys. 2019, 221, 78–88. [Google Scholar] [CrossRef]
- Ronova, I. Structural aspects in polymers: Interconnections between conformational parameters of the polymers with their physical properties. Struct. Chem. 2010, 21, 541–553. [Google Scholar] [CrossRef]
- Vosgueritchian, M.; Lipomi, D.J.; Bao, Z.A. Highly Conductive and Transparent PEDOT:PSS Films with a Fluorosurfactant for Stretchable and Flexible Transparent Electrodes. Adv. Funct. Mater. 2012, 22, 421–428. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.P.; Li, P.C.; Xia, Y.J.; Zhang, X.; Du, D.H.; Isikgor, F.H.; Ouyang, J.Y. Review on application of PEDOTs and PEDOT: PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Luo, L.X.; Huang, W.N.; Yang, C.L.; Zhang, J.; Zhang, Q.C. Recent advances on π-conjugated polymers as active elements in high performance organic field-effect transistors. Front. Phys. 2021, 16, 33500. [Google Scholar] [CrossRef]
- Kim, I.C.; Kim, T.H.; Lee, S.H.; Kim, B.S. Extremely Foldable and Highly Transparent Nanofiber-Based Electrodes for Liquid Crystal Smart Devices. Sci. Rep. 2018, 8, 8. [Google Scholar] [CrossRef]
- Singh, D.; Tao, R.; Lubineau, G. A synergetic layered inorganic-organic hybrid film for conductive, flexible, and transparent electrodes. NPJ Flex. Electron. 2019, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- You, J.B.; Meng, L.; Song, T.B.; Guo, T.F.; Yang, Y.; Chang, W.H.; Hong, Z.R.; Chen, H.J.; Zhou, H.P.; Chen, Q.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef]
- Chauvin, A.; Heu, W.T.C.; Buh, J.; Tessier, P.Y.; El Mel, A.A. Vapor dealloying of ultra-thin films: A promising concept for the fabrication of highly flexible transparent conductive metal nanomesh electrodes. NPJ Flex. Electron. 2019, 3, 5. [Google Scholar] [CrossRef]





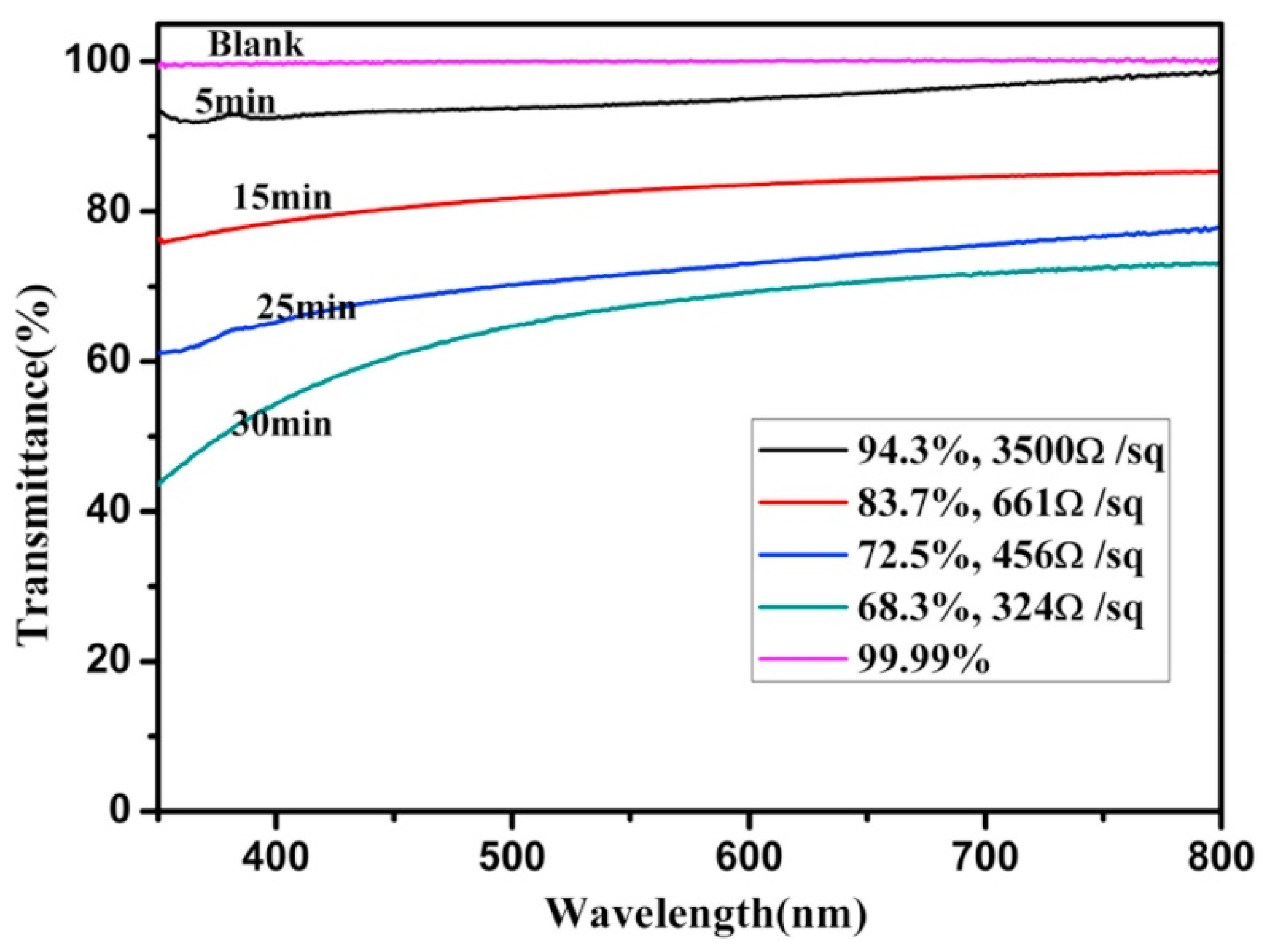
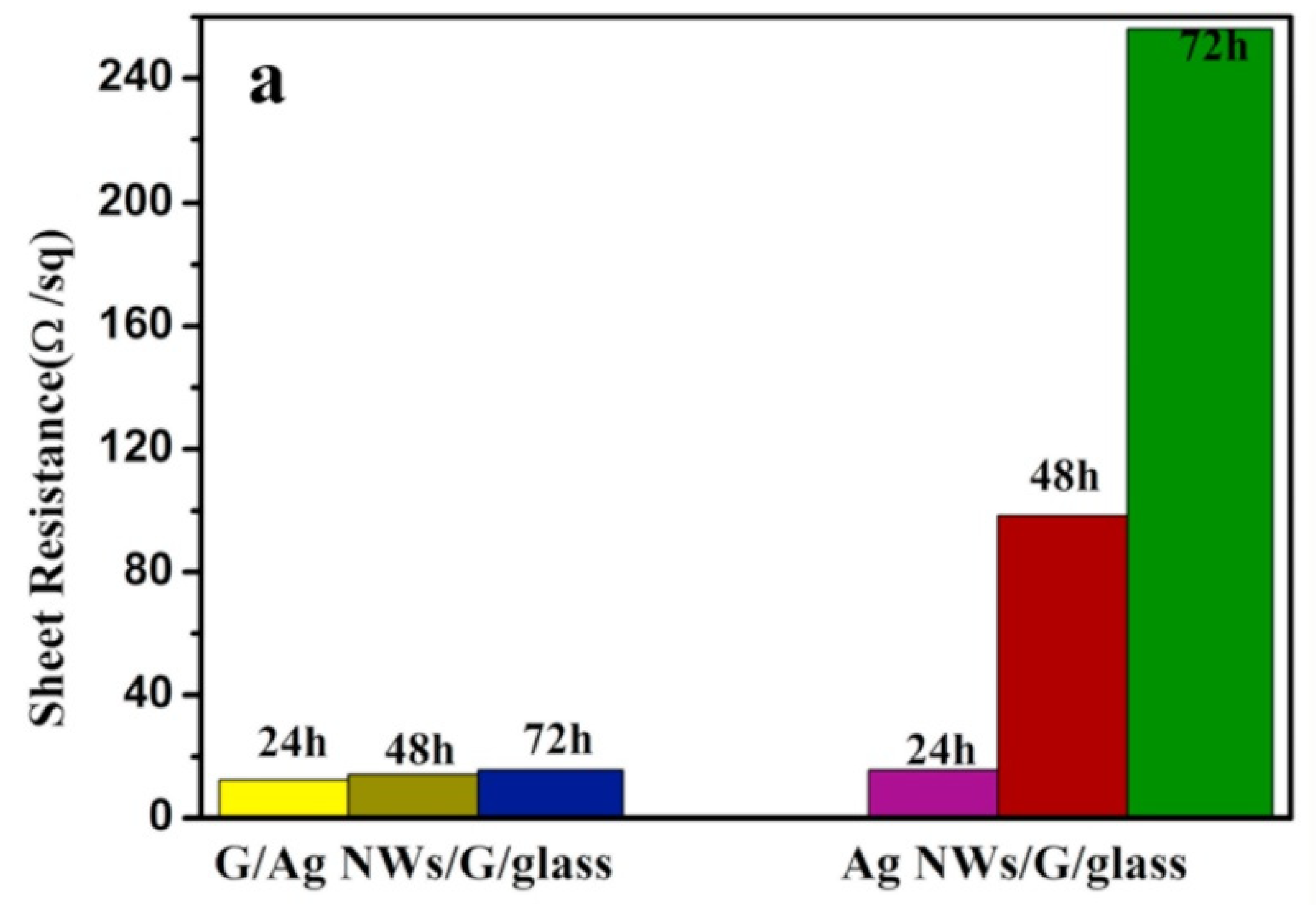
| Material | Thickness/nm | Sheet Resistance/Ω·sq−1 | Transmittance | References |
|---|---|---|---|---|
| AZO/Ag/AZO | 37/10/37 | 6.2 | 79.9% | [18] |
| IZO/Ag Fiber/PS | 240 | 10 | 80% | [35] |
| Glass/AgNWs/OTS | 130.5 | 20–250 | 88% | [36] |
| AgNWs/ZnO-TF/ZnO-NR | 77.658 | >100 | 80% | [39] |
| AGZO/AGZO/AGZO | 180–240 | 2.5M | 90% | [40] |
| Mutilayer Graphene/Graphene Scrolls | 10–100 | ≈100 | 87–97% | [46] |
| MoO3/Graphene/MoO3 | >15 | 200 | 85% | [47] |
| Graphene/AgNWs/Graphene | —— | 10.8 | 77.6% | [50] |
| ITO/NF-r-CA/AgNWs | —— | 24 | 90% | [54] |
| ITO/PEDOT:PSS/PET | 150 | 40–60 | 60–90% | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.-H.; Yan, Y.-Q.; Yu, G.; Zhang, Z.-Y.; Zhama, T.; Sun, H. Research Progress of Transparent Electrode Materials with Sandwich Structure. Materials 2021, 14, 4097. https://doi.org/10.3390/ma14154097
Qin L-H, Yan Y-Q, Yu G, Zhang Z-Y, Zhama T, Sun H. Research Progress of Transparent Electrode Materials with Sandwich Structure. Materials. 2021; 14(15):4097. https://doi.org/10.3390/ma14154097
Chicago/Turabian StyleQin, Li-Hao, Yong-Qi Yan, Gan Yu, Zhao-Yi Zhang, Tuofu Zhama, and Hui Sun. 2021. "Research Progress of Transparent Electrode Materials with Sandwich Structure" Materials 14, no. 15: 4097. https://doi.org/10.3390/ma14154097
APA StyleQin, L.-H., Yan, Y.-Q., Yu, G., Zhang, Z.-Y., Zhama, T., & Sun, H. (2021). Research Progress of Transparent Electrode Materials with Sandwich Structure. Materials, 14(15), 4097. https://doi.org/10.3390/ma14154097






