Reducing Efficiency of Fucoxanthin in Diatom Mediated Biofabrication of Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Fucoxanthin
2.2. Biofabrication of GNP
2.3. Characterizations of GNPs
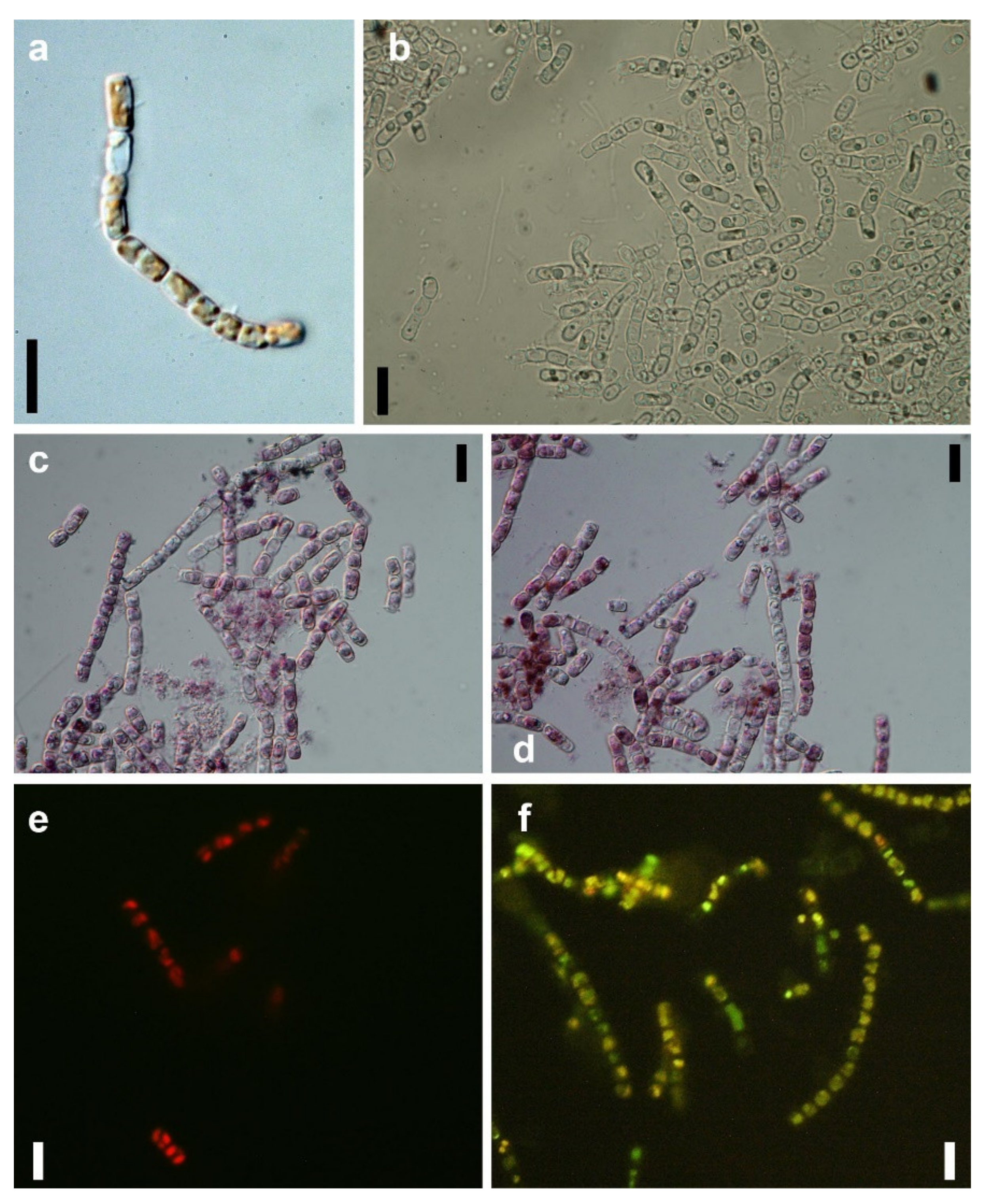
2.4. Microscopic Examination of GNP Loaded N. shiloi
2.5. Quantification of Chlorophyll, Carotenoids and Fucoxanthin
3. Results
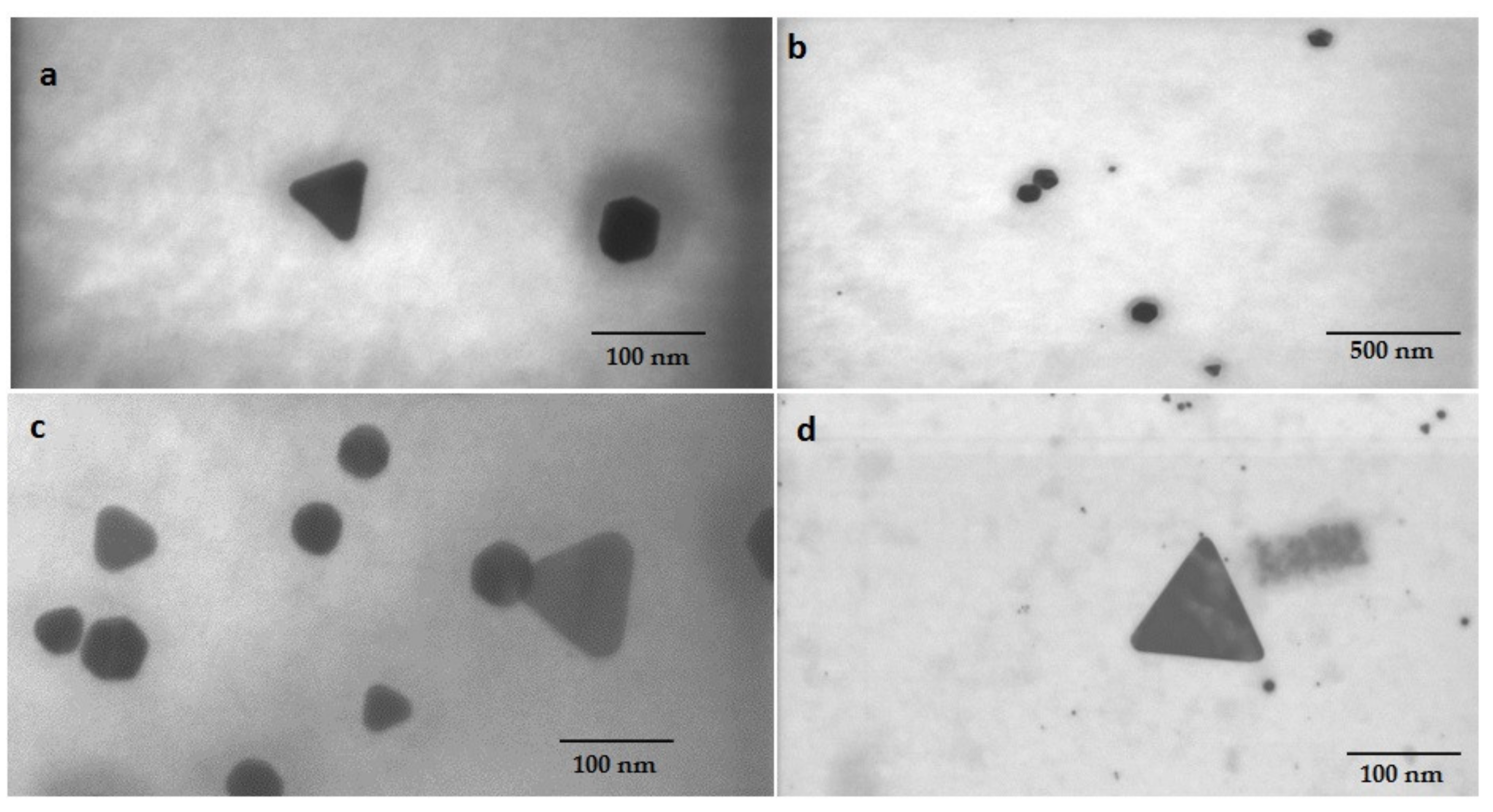
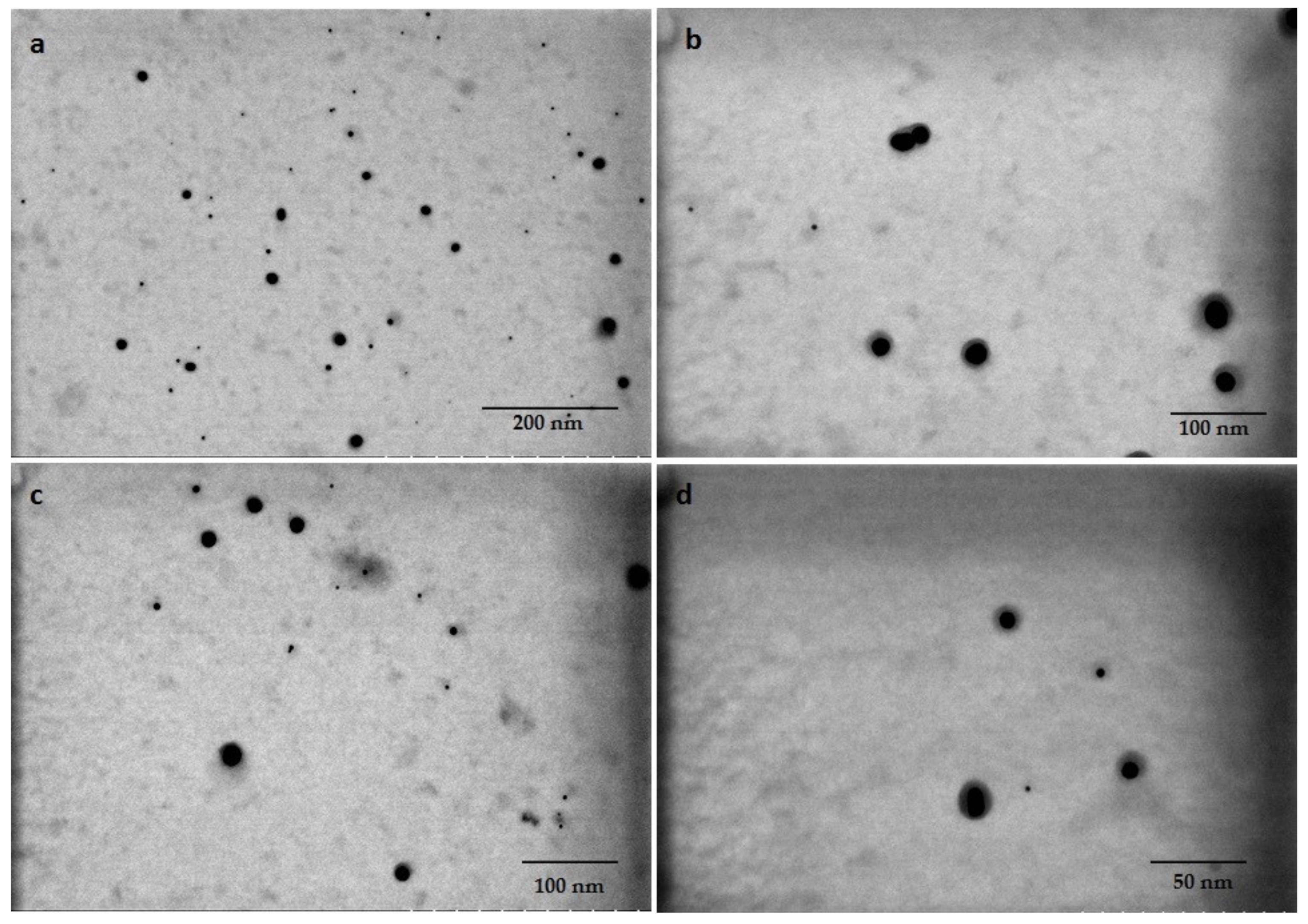
3.1. Diatom Assisted Biogenesis of GNP
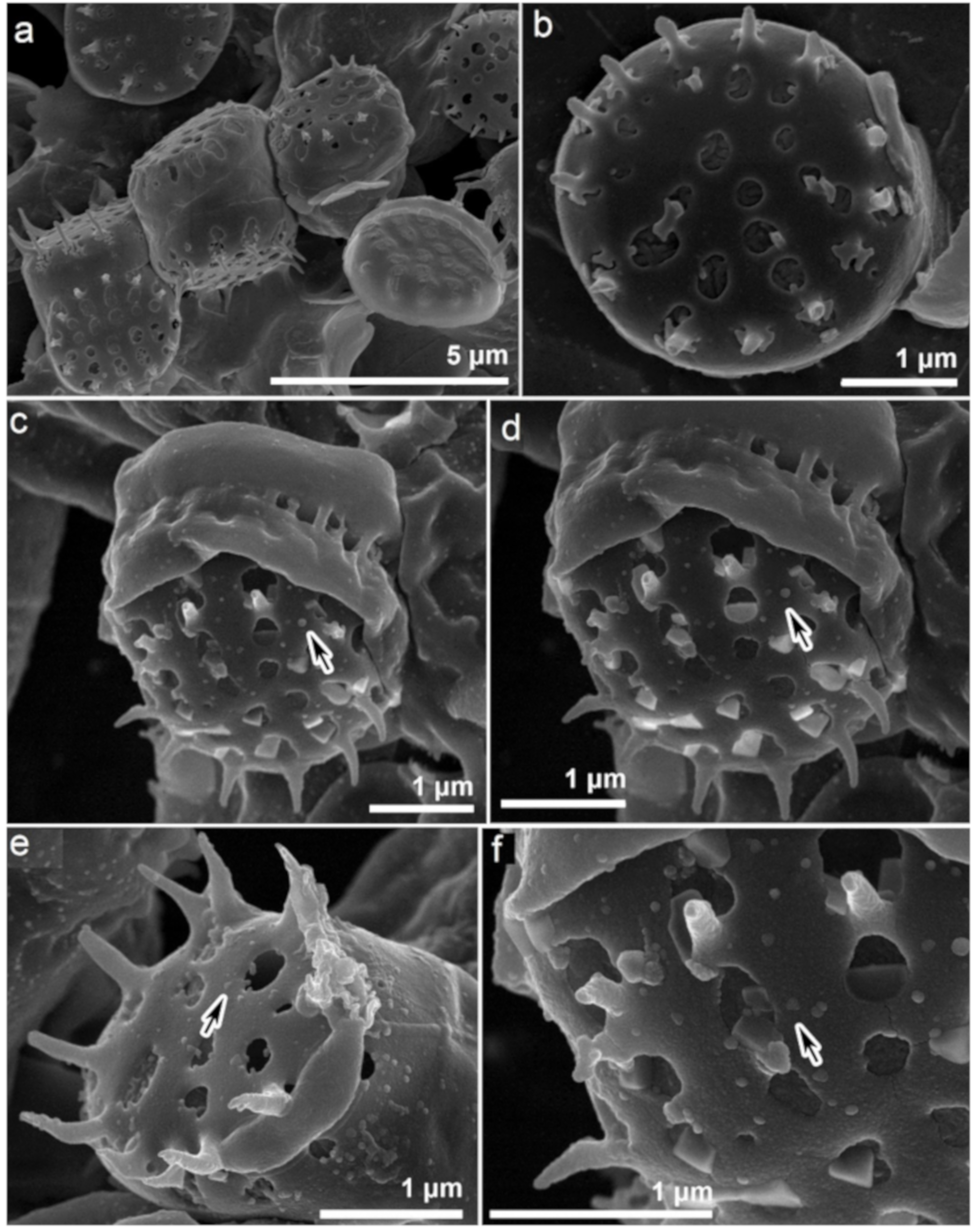
3.2. Morphological Changes in Gold Exposed N. shiloi
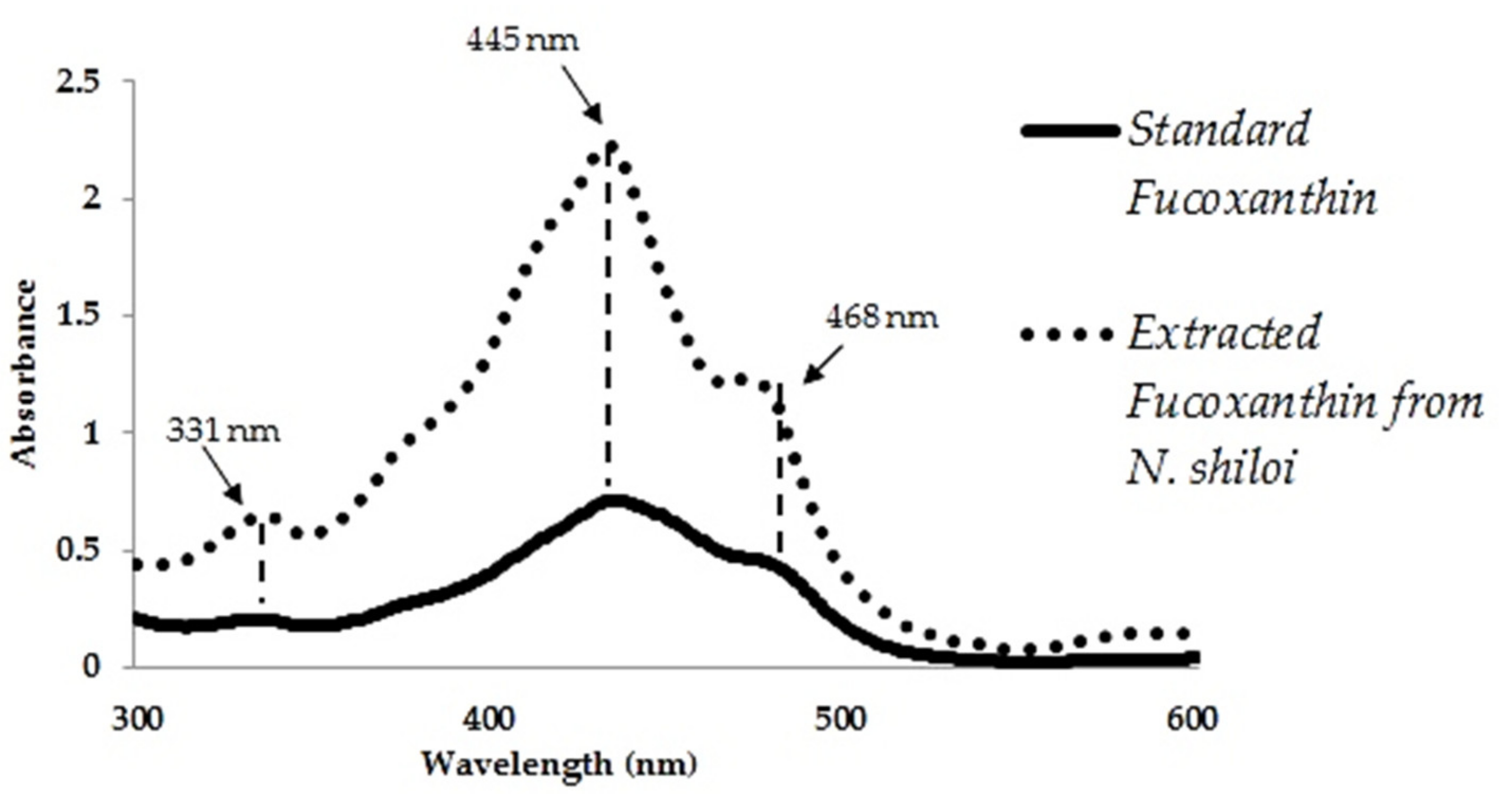
3.3. Confirmation of Au3+ Reduction by Isolated Fucoxanthin
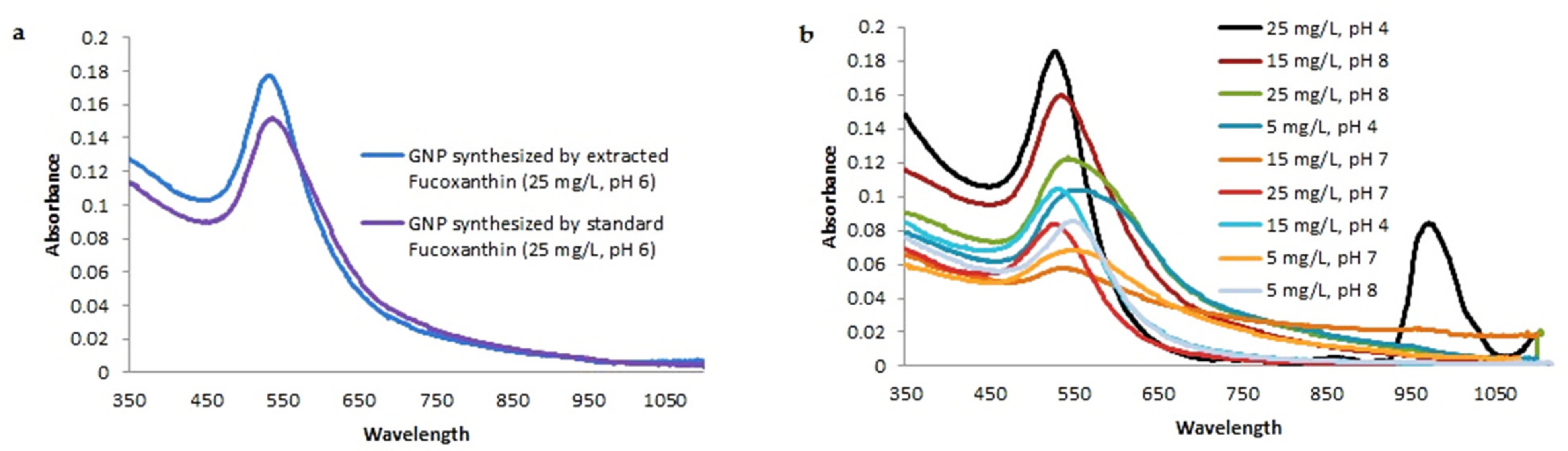
3.4. Characterization of Biogenic GNPs
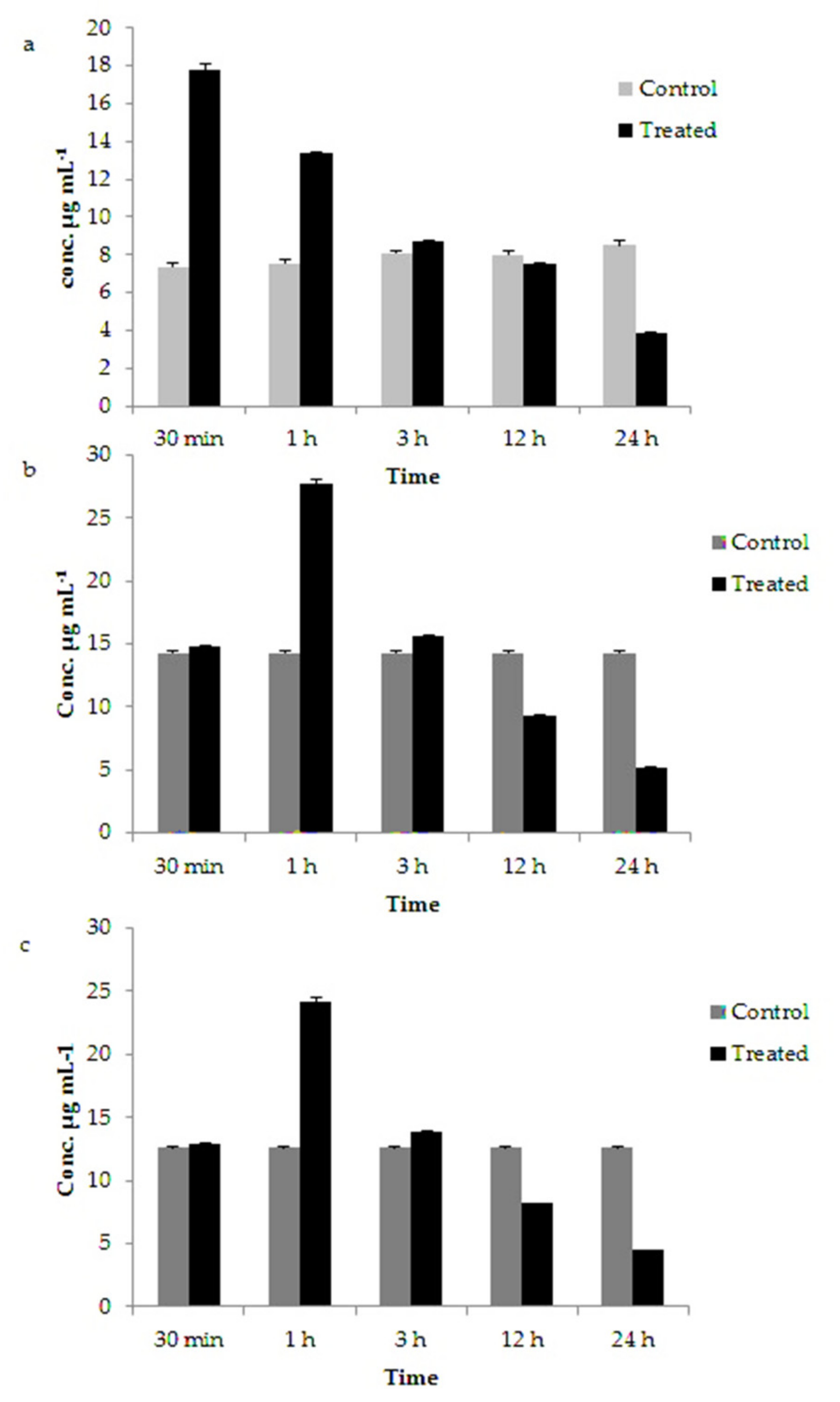
3.5. Variation in Chlorophyll, Carotenoids and Fucoxanthin Content in GNP Loaded N. shiloi
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nair, B.; Pradeep, T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Ahmad, M.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003, 14, 824–828. [Google Scholar] [CrossRef]
- Lengke, M.F.; Southam, G. The effect of thiosulfate-oxidizing bacteria on the stability of the gold-thiosulfate complex. Geochim. Et Cosmochim. Acta 2005, 69, 3759–3772. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold(I)-thiosulfate and gold (III)-chloride complexes. Langmuir 2006, 22, 2780–2787. [Google Scholar] [CrossRef]
- Parial, D.; Patra, H.K.; Roychoudhury, P.; Dasgupta, A.K.; Pal, R. Gold nanorod production by cyanobacteria- a green chemistry approach. J. Appl. Phycol. 2012, 27, 3–10. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.G. Silver based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minaeian, S.; Shahverdi, A.R.; Nohi, A.S.; Shahverdi, H.R. Extracellular biosynthesis of silver nanoparticles by some bacteria. JSIAU 2008, 17, 1–4. [Google Scholar]
- Govindaraju, K.; Kiruthiga, V.; Kumar, V.G.; Singaravelu, G. Extracellular Synthesis of Silver Nanoparticles by a Marine Alga, Sargassum Wightii Grevilli and Their Antibacterial Effects. J. Nanosci. Nanotechnol. 2009, 9, 5497–5501. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium solani: A novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanoparticle Res. 2009, 11, 2079–2085. [Google Scholar] [CrossRef]
- Mohseniazar, M.; Barin, M.; Zarredar, H.; Alizadeh, S.; Shanehbandi, D. Potential of microalgae and Lactobacilli in biosynthesis of silver nanoparticles. BioImpacts 2011, 1, 149–152. [Google Scholar]
- MubarakAli, D.; Sasikala, M.; Gunasekaran, M.; Thajuddin, N. Biosynthesis and characterization of silver nanoparticles using marine cyanobacterium, Oscillatoria willei NTDM01. Dig. J. Nanomater. Biostructures 2011, 6, 385–390. [Google Scholar]
- Sheny, D.S.; Philip, D.; Mathew, J. Synthesis of platinum nanoparticles using dried Anacardium occidentale leaf and its catalytic and thermal applications. Spectrochim. Spectrochim. Acta Part A 2013, 114, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Dauthal, P.; Mukhopadhyay, M. Biosynthesis of Palladium Nanoparticles Using Delonix regia Leaf Extract and Its Catalytic Activity for Nitro-aromatics Hydrogenation. Ind. Eng. Chem. Res. 2013, 52, 18131–18139. [Google Scholar] [CrossRef]
- Roychoudhury, P.; Bhattacharya, A.; Dasgupta, A.; Pal, R. Biogenic synthesis of gold nanoparticle using fractioned cellular components from eukaryotic algae and cyanobacteria. Phycol. Res. 2016, 64, 133–140. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K. Detection limits of DLS and UV–vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Dickinson, C.; Lafir, F.; Brougham, D.F.; Marsili, E. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem. 2012, 14, 1322–1334. [Google Scholar] [CrossRef]
- Chakraborty, N.; Pal, R.; Ramaswami, A.; Nayak, D.; Lahiri, S. Diatom: A potential bioaccumulator of gold. J. Radioanal. Nucl. Chem. 2006, 270, 645–649. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef] [Green Version]
- Roychoudhury, P.; Nandi, C.; Pal, R. Diatom-based biosynthesis of gold-silica nanocomposite and their DNA binding affinity. J. Appl. Phycol. 2016, 28, 2857–2863. [Google Scholar] [CrossRef]
- Budroni, G.; Corma, A. Gold-organic-inorganic high surface-area materials as precursors of highly active catalysts. Angew. Chem. Int. Ed. 2006, 45, 3328–3331. [Google Scholar] [CrossRef]
- Bus, E.; Miller, J.T.; van Bokhoven, J.A. Hydrogen chemisorptions on Al2O3-supported gold catalysts. J. Phys. Chem. B 2005, 109, 14581–14587. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. Supported gold catalysts prepared by in situ reduction technique: Preparation, characterization and catalytic activity measurements. Appl. Catal. A Gen. 2004, 259, 163–168. [Google Scholar] [CrossRef]
- Kim, J.H.; Bryan, W.W.; Lee, T.R. Preparation, characterization, and optical properties of gold, silver, and gold-silver alloy nanoshells having silica cores. Langmuir 2008, 24, 11147–11152. [Google Scholar] [CrossRef] [PubMed]
- Schrofel, A.; Kratosova, G.; Bohunicka, M.; Dobrocka, E.; Vavra, I. Biosynthesis of gold nanoparticles using diatoms—silica-gold and EPS-gold bionanocomposite formation. J. Nanoparticle Res. 2011, 13, 3207–3216. [Google Scholar] [CrossRef] [Green Version]
- Bose, R.; Roychoudhury, P.; Pal, R. In-situ green synthesis of fluorescent silica–silver conjugate nanodendrites using nanoporous frustules of diatoms: An unprecedented approach. Bioprocess Biosyst. Eng. 2021, 44, 1263–1273. [Google Scholar] [CrossRef]
- Pytlik, N.; Kaden, J.; Finger, M.; Naumann, J.; Wanke, S.; Machill, S.; Brunner, E. Biological synthesis of gold nanoparticles by the diatom Stephanopyxis turris and in vivo SERS analyses. Algal Res. 2017, 28, 9–15. [Google Scholar] [CrossRef]
- Feurtet-Mazel, A.; Mornet, S.; Charron, L.; Mesmer-Dudons, N.; Maury-Brachet, R.; Baudrimont, M. Biosynthesis of gold nanoparticles by the living freshwater diatom Eolimna minima, a species developed in river biofilms. Environ. Sci. Pollut. Res. 2016, 23, 4334–4339. [Google Scholar] [CrossRef]
- Vona, D.; Cicco, S.; Ragni, R.; Leone, G.; Lo Presti, M.; Farinola, G. Biosilica/polydopamine/silver nanoparticles composites: New hybrid multifunctional heterostructures obtained by chemical modification of Thalassiosira weissflogii silica shells. MRS Commun. 2018, 8, 911–917. [Google Scholar] [CrossRef]
- Jeffryes, C.; Gutu, T.; Jiao, J.; Rorrer, G.L. Two-stage photobioreactor process for the metabolic insertion of nanostructured germanium into the silica microstructure of the diatom Pinnularia sp. Mater. Sci. Eng. C 2008, 28, 107–118. [Google Scholar] [CrossRef]
- Jeffryes, C.; Gutu, T.; Jiao, J.; Rorrer, G.L. Metabolic insertion of nanostructured TiO2 into the patterned biosilica of the diatom Pinnularia sp. by a two-stage bioreactor cultivation process. ACS Nano 2008, 2, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Kaden, J.; Brückner, S.I.; Machill, S.; Krafft, C.; Pöppl, A.; Brunner, E. Iron incorporation in biosilica of the marine diatom Stephanopyxis turris: Dispersed or clustered? Biometals 2017, 30, 71–82. [Google Scholar] [CrossRef]
- Tomoyo katayama, A.M.; Satoru, T. Responses of pigment composition of the marine diatom Thalassiosira weissflogii to silicate availability during dark survival and recovery. Plankton Benthos Res. 2011, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Papagiannakis, E.; van Stokkum, I.H.; Fey, H.; Büchel, C.; van Grondelle, R. Spectroscopic characterization of the excitation energy transfer in the fucoxanthin-chlorophyll protein of diatoms. Photosynth. Res. 2005, 86, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Flora, S.J.S. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med. Cell. Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Jena, J.; Pradhan, N.; Dash, B.P.; Panda, P.K.; Mishra, B.K. Pigment mediated biogenic synthesis of silver nanoparticles using diatom Amphora sp. and its antimicrobial activity. J. Saudi Chem. Soc. 2015, 19, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Li, C.L.; Witkowski, A.; Ashworth, M.P.; Dąbek, P.; Sato, S.; Zgłobicka, I.; Witak, M.; Khim, J.S.; Kwon, C.-J. The morphology and molecular phylogenetics of some marine diatom taxa within the Fragilariaceae, including twenty undescribed species and their relationship to Nanofrustulum, Opephora and Pseudostaurosira. Phytotaxa 2018, 355, 1. [Google Scholar] [CrossRef]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms.1. Cyclotella nana hustedt, and detonula confervacea (cleve) gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Wang, L.J.; Fan, Y.; Parsons, R.L.; Hu, G.R.; Zhang, P.Y.; Li, F.L. A Rapid Method for the Determination of Fucoxanthin in Diatom. Mar. Drugs 2018, 16, 33. [Google Scholar] [CrossRef] [Green Version]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxides in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Carreto, J.I.; Catoggio, J.A. An indirect method for the rapid estimation of carotenoid contents in Phaeodactylum tricornutum: Possible application to other marine algae. Mar. Biol. 1977, 40, 109–116. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Mohd, B.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Ganesh Babu, M.M.; Gunasekaran, P. Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Colloids Surf. B 2009, 74, 191–195. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Altaf, M.; Jaganyi, D. Characterization of Triangular Gold Nanoparticles Using Aloe arborescens Leaf Extract: A Green Synthesis Approach. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2016, 46, 1332–1335. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L.H. Kinetics and mechanism of the primary steps of degradation of carotenoids by acid in homogeneous solution. J. Agric. Food Chem. 2000, 48, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Gliemmo, M.F.; Latorre, M.E.; Gerschenson, L.N.; Campos, C.A. Color stability of pumpkin (Cucurbita moschata, Duchesne ex Poiret) puree during storage at room temperature: Effect of pH, potassium sorbate, ascorbic acid and packaging material. LWT Food Sci. Technol. 2009, 42, 196–201. [Google Scholar] [CrossRef]
- Arita, S.; Ando, S.; Hosoda, H.; Sakaue, K.; Nagata, T.; Murata, Y.; Shimoishi, Y.; Tada, M. Acceleration effect of sulfides on photodegradation of carotenoids by UVA irradiation. Biosci. Biotechnol. Biochem. 2005, 69, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M. Biological processing of nanostructured silica in diatoms. Prog. Org. Coat. 2003, 47, 256–266. [Google Scholar] [CrossRef]
- De Filippis, L.F.; Pallaghy, C.K. The effects of sublethal concentrations of mercury and zinc on Chlorella I. growth characteristics and uptake of metals. Z. Für Pflanzenphysiol. 1976, 78, 197–207. [Google Scholar] [CrossRef]
- De Filippis, L.F.; Hampp, R.; Ziegler, H. The effects of sublethal concentrations of zinc, cadmium and mercury on Euglena. II. Respiration, photosynthesis and photochemical activities. Arch. Microbiol. 1981, 128, 407–411. [Google Scholar] [CrossRef]
- Sengar, R.S.; Gupta, S.; Gautam, M.; Sharma, A.; Sengar, K. Occurrence, uptake, accumulation and physiological responses of nickel in plants and its effects on environment. Res. J. Phytochem. 2008, 2, 44–60. [Google Scholar] [CrossRef] [Green Version]
- Bartley, G.E.; Scolnlk, P.A.; Glullano, G. Molecular biology of carotenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1994, 45, 287–301. [Google Scholar] [CrossRef]
- Sliwka, H.R.; Melø, T.B.; Foss, B.J. Electron- and energy-transfer properties of hydrophilic carotenoids. Chem. A Eur. J. 2007, 13, 4458–4466. [Google Scholar] [CrossRef]

| Reducing Agents | Concentration of Au3+ Solution (mg L−1) | pH | Reaction Time | Maximum Absorbance (nm) |
|---|---|---|---|---|
| Whole biomass of N. shiloi | 5 | 4 | 20 days | ~550 |
| 7 | 25 days | ~550 | ||
| 8 | 22 days | ~550 | ||
| 15 | 4 | 7 days | ~536 | |
| 7 | 15 days | ~550 | ||
| 8 | 10 days | ~540 | ||
| 25 | 4 | 3 days | ~530 and ~966 | |
| 7 | 7 days | ~535 | ||
| 8 | 5 days | ~539 | ||
| Extracted fucoxanthin from N. shiloi | 25 | 6 | 12 h | ~535 |
| Standard fucoxanthin | 25 | 6 | 12 h | ~537 |
| Parameters | Experimental Condition | Time of Exposure | ||||
|---|---|---|---|---|---|---|
| 30 min | 1 h | 3 h | 12 h | 24 h | ||
| Chlorophyll (µg mL−1) | Control | 7.333 ± 0.202 | 7.543 ± 0.223 | 8.066 ± 0.132 | 8.01 ± 0.223 | 8.48 ± 0.245 |
| 25 mg L−1 Au3+ treated | 17.759 ± 0.329 | 13.354 ± 0.057 | 8.682 ± 0.045 | 7.554 ± 0.040 | 3.847 ± 0.083 | |
| Carotenoids (µg mL−1) | Control | 14.296 ± 0.169 | 14.396 ± 0.078 | 14.55 ± 0.101 | 14.626 ± 0.172 | 14.643 ± 0.189 |
| 25 mg L−1 Au3+ treated | 14.762 ± 0.048 | 27.683 ± 0.386 | 15.66 ± 0.069 | 9.305 ± 0.071 | 5.194 ± 0.042 | |
| Fucoxanthin (µg mL−1) | Control | 12.628 ± 0.022 | 12.680 ± 0.038 | 12.748 ± 0.104 | 12.767 ± 0.123 | 12.771 ± 0.126 |
| 25 mg L−1 Au3+ treated | 12.94 ± 0.039 | 24.099 ± 0.430 | 13.878 ± 0.040 | 8.175 ± 0.043 | 4.481 ± 0.014 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roychoudhury, P.; Dąbek, P.; Gloc, M.; Golubeva, A.; Dobrucka, R.; Kurzydłowski, K.; Witkowski, A. Reducing Efficiency of Fucoxanthin in Diatom Mediated Biofabrication of Gold Nanoparticles. Materials 2021, 14, 4094. https://doi.org/10.3390/ma14154094
Roychoudhury P, Dąbek P, Gloc M, Golubeva A, Dobrucka R, Kurzydłowski K, Witkowski A. Reducing Efficiency of Fucoxanthin in Diatom Mediated Biofabrication of Gold Nanoparticles. Materials. 2021; 14(15):4094. https://doi.org/10.3390/ma14154094
Chicago/Turabian StyleRoychoudhury, Piya, Przemysław Dąbek, Michał Gloc, Aleksandra Golubeva, Renata Dobrucka, Krzysztof Kurzydłowski, and Andrzej Witkowski. 2021. "Reducing Efficiency of Fucoxanthin in Diatom Mediated Biofabrication of Gold Nanoparticles" Materials 14, no. 15: 4094. https://doi.org/10.3390/ma14154094
APA StyleRoychoudhury, P., Dąbek, P., Gloc, M., Golubeva, A., Dobrucka, R., Kurzydłowski, K., & Witkowski, A. (2021). Reducing Efficiency of Fucoxanthin in Diatom Mediated Biofabrication of Gold Nanoparticles. Materials, 14(15), 4094. https://doi.org/10.3390/ma14154094







