Influence of Li2O Incrementation on Mechanical and Gamma-Ray Shielding Characteristics of a TeO2-As2O3-B2O3 Glass System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mechanical Properties
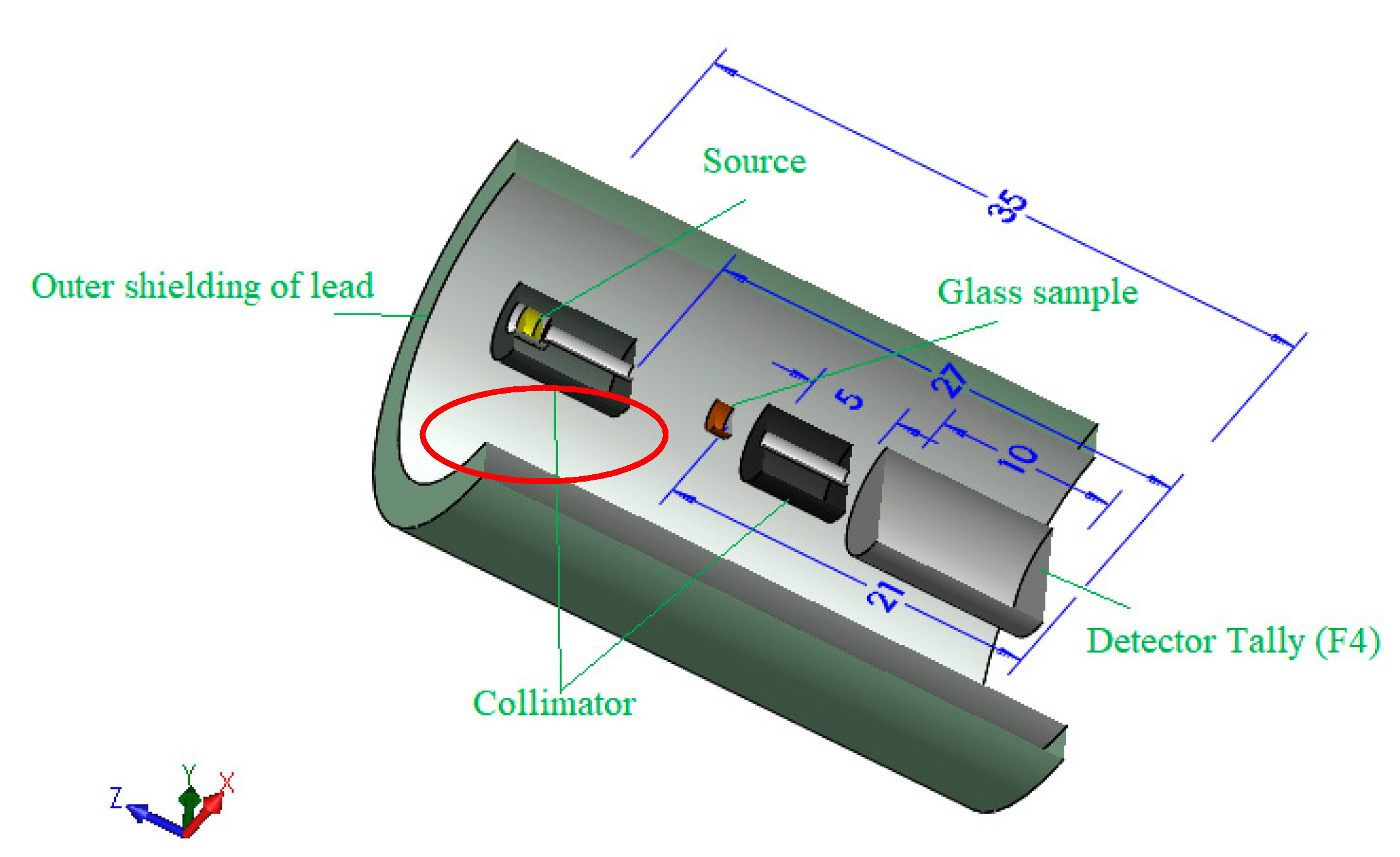
2.2. Gamma-Ray Simulation and Theoretical Calculations
3. Results and Discussion
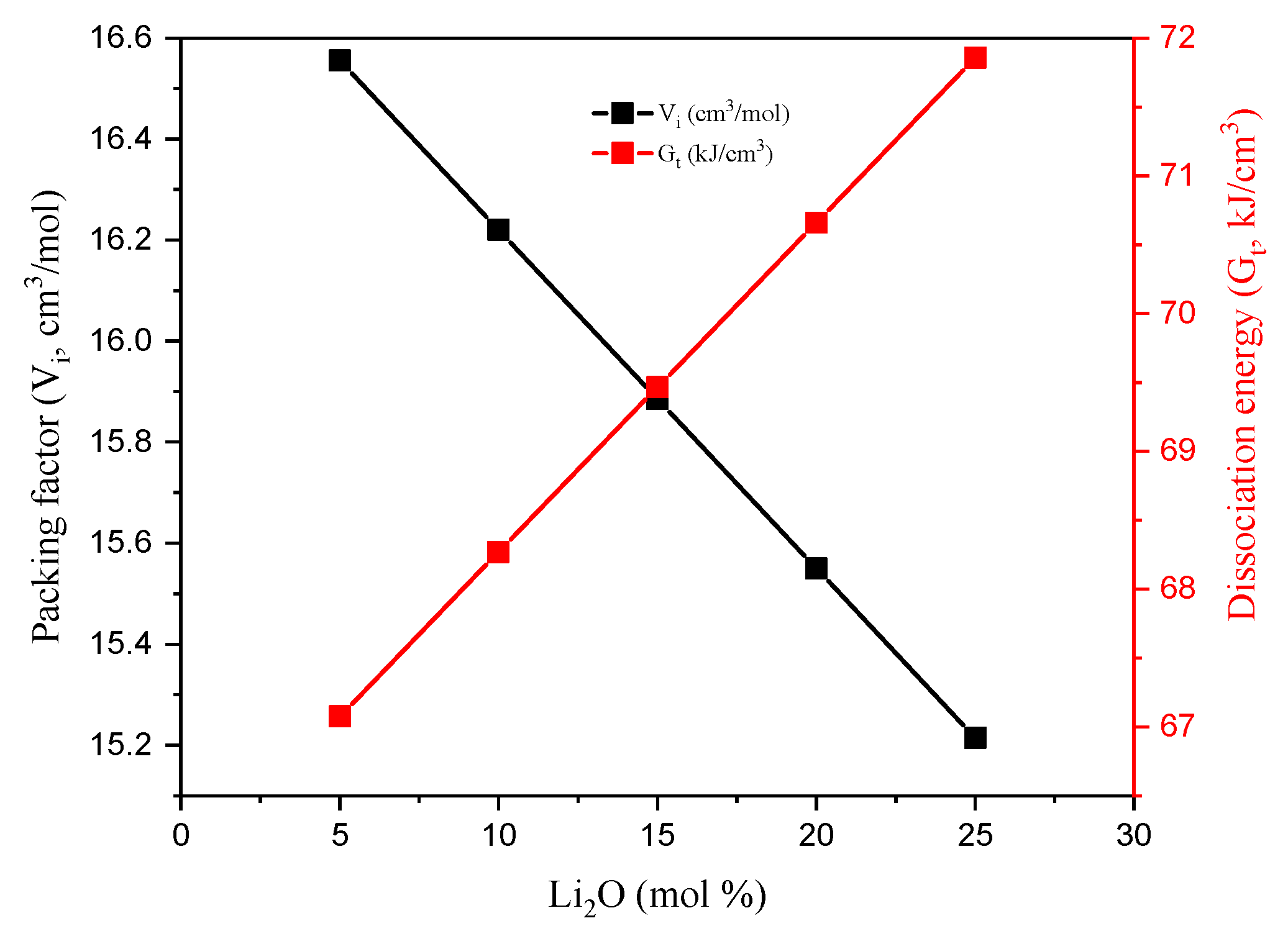
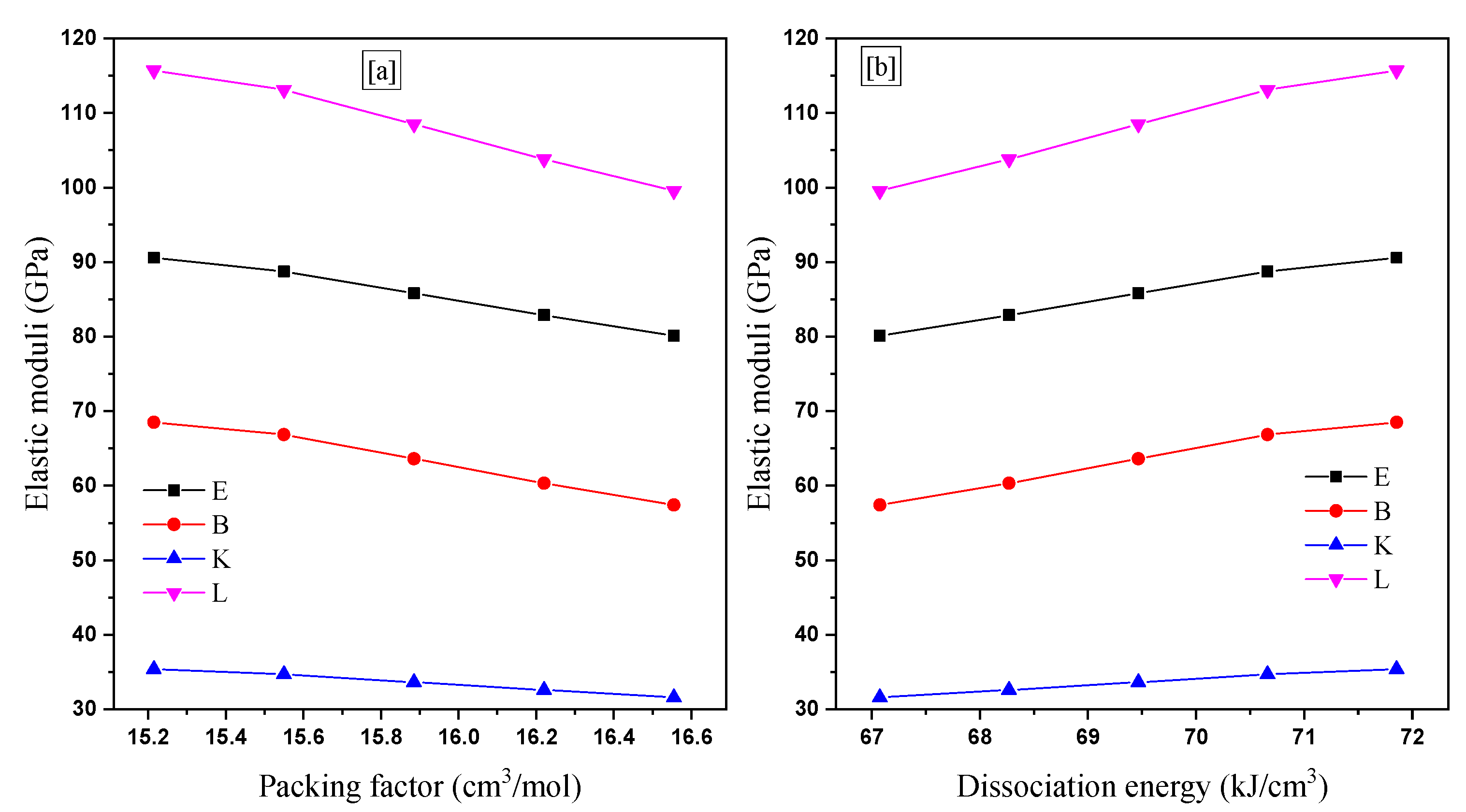
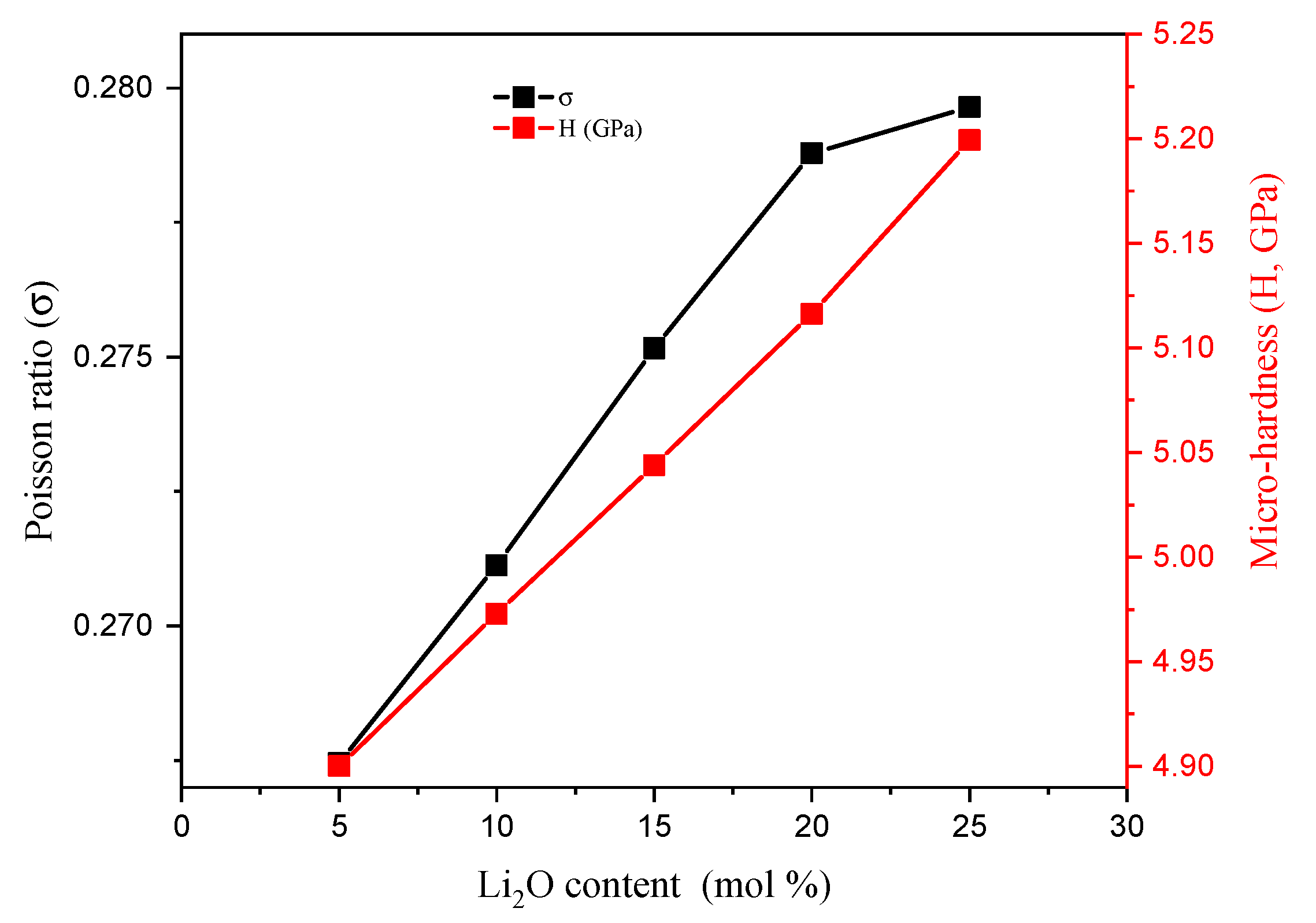
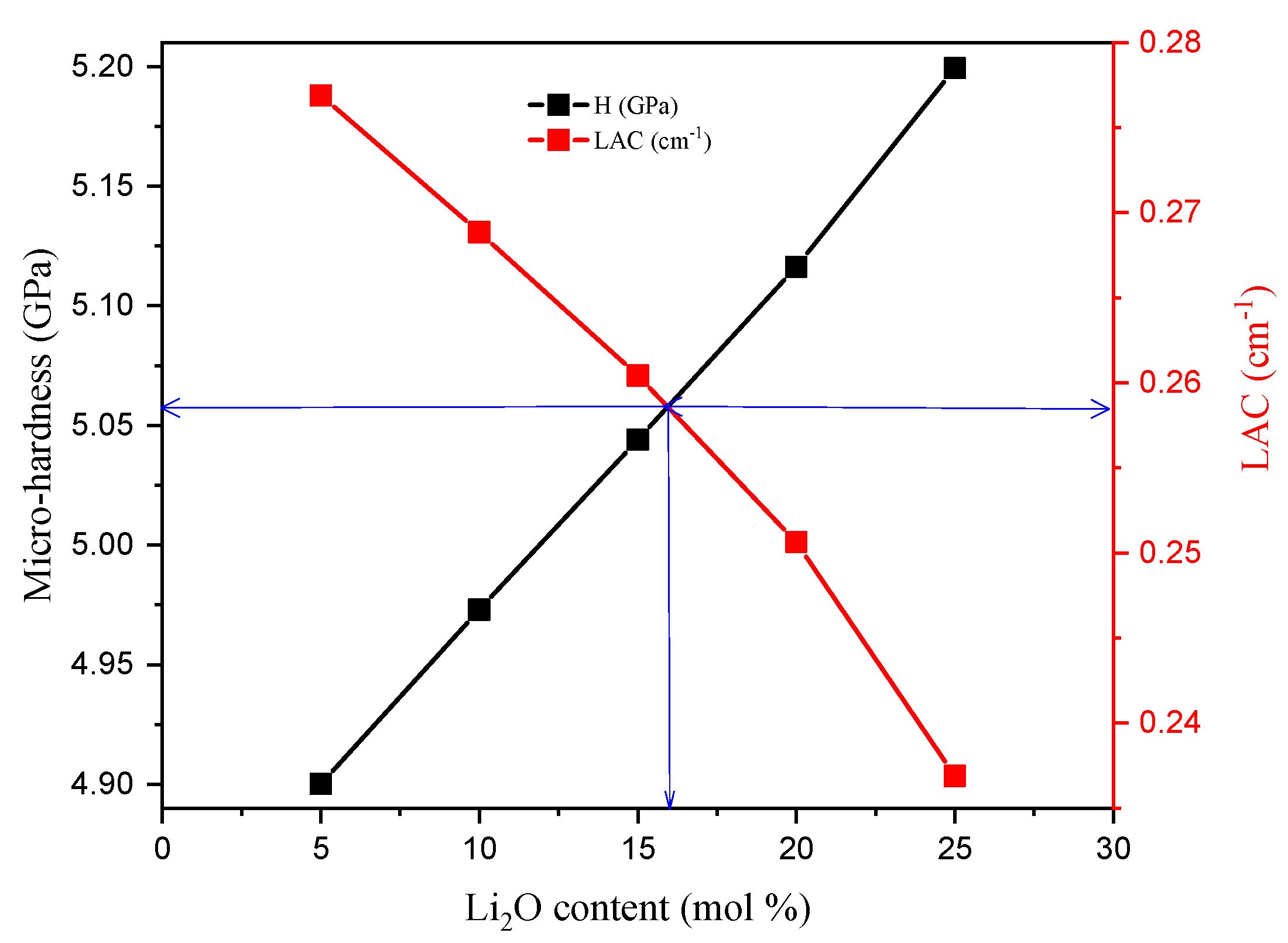
3.1. Mechanical Properties
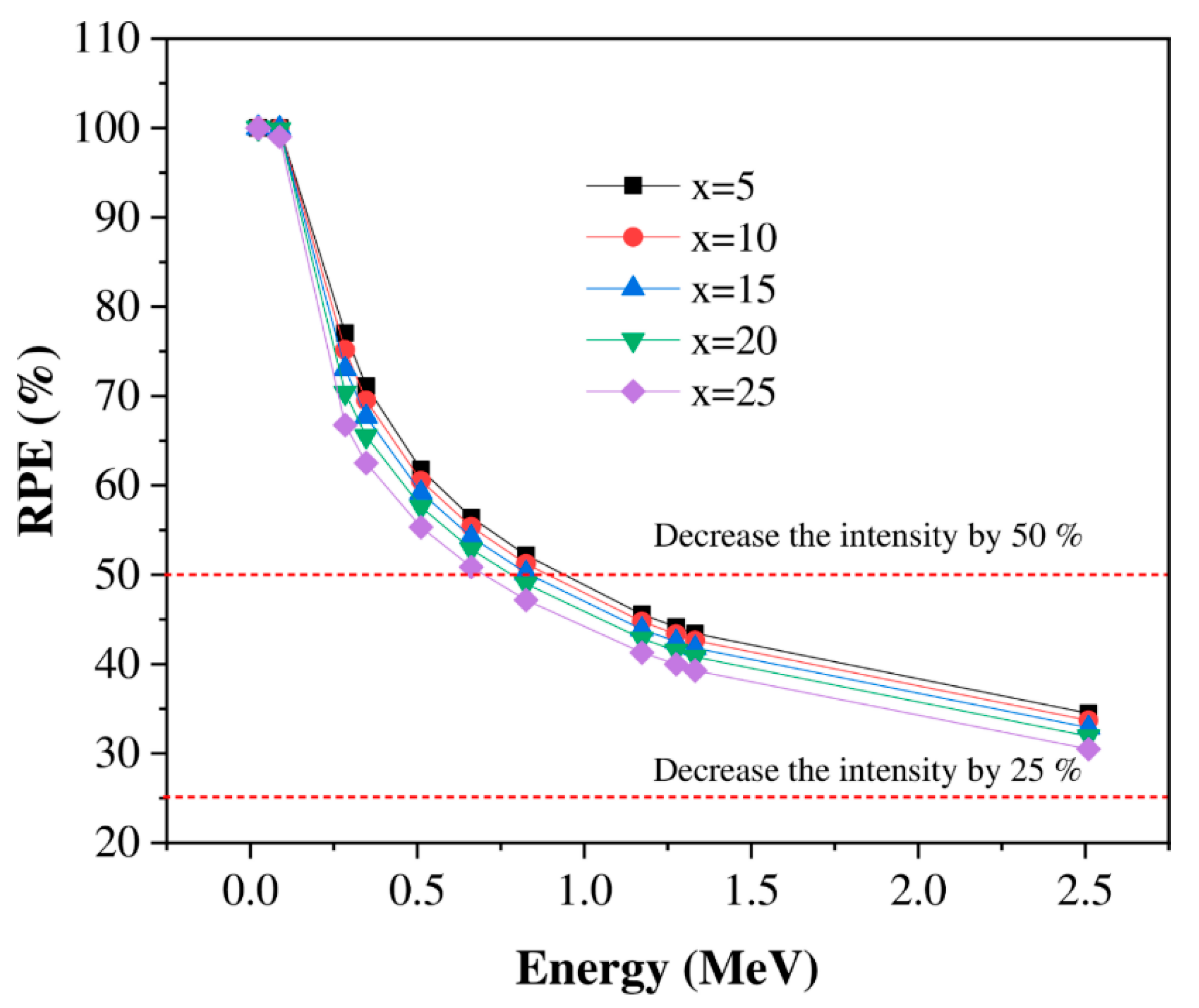
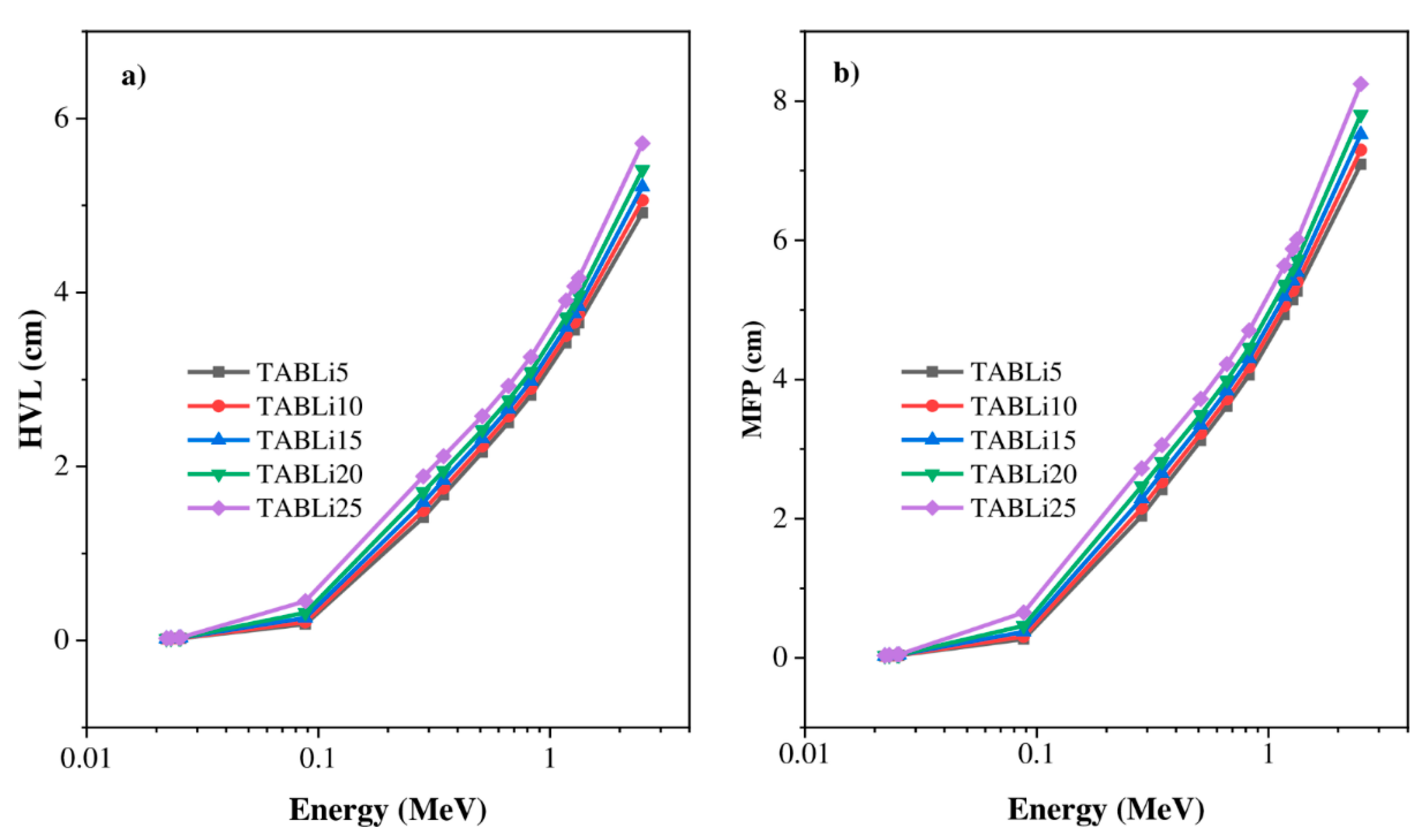
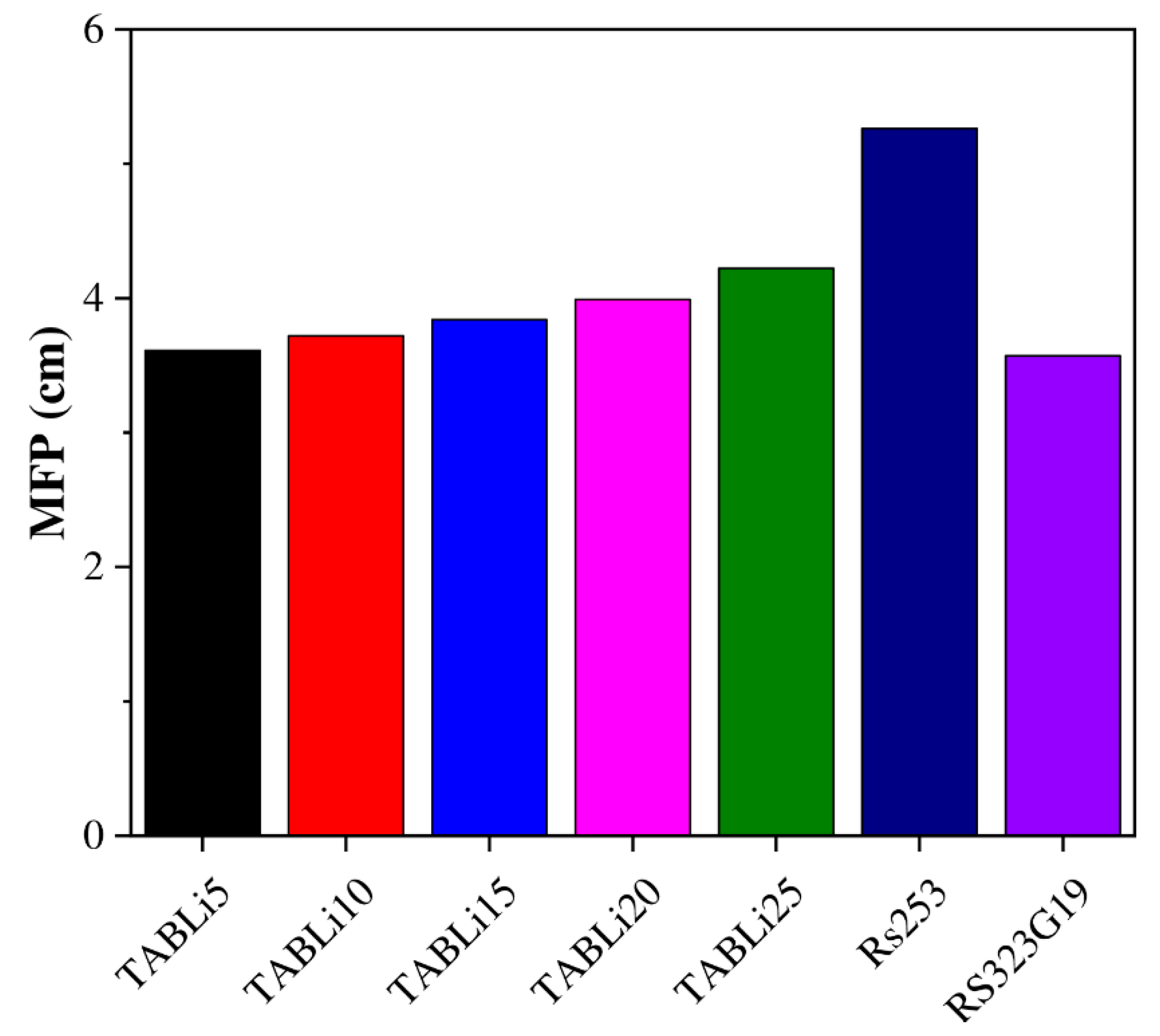
3.2. Shielding Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sayyed, M.I.; Al-Hadeethi, Y.; AlShammari, M.M.; Ahmed, M.; Al-Heniti, S.H.; Rammah, Y.S. Physical, optical and gamma radiation shielding competence of newly boro-tellurite based glasses: TeO2–B2O3–ZnO–Li2O3–Bi2O3. Ceram. Int. 2020, 47, 611–618. [Google Scholar] [CrossRef]
- Abdel Wahab, E.A.; Koubisy, M.S.I.; Sayyed, M.I.; Mahmoud, K.A.; Zatsepin, A.F.; Makhlouf, S.A.; Shaaban, K.S. Novel borosilicate glass system: Na2B4O7-SiO2-MnO2: Synthesis, average electronics polarizability, optical basicity, and gamma-ray shielding features. J. Non-Cryst. Solids 2021, 553, 120509. [Google Scholar] [CrossRef]
- Dong, M.; Xue, X.; Yang, H.; Liu, D.; Wang, C.; Li, Z. A novel comprehensive utilization of vanadium slag: As gamma ray shielding material. J. Hazard. Mater. 2016, 318, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Obaid, S.S.; Sayyed, M.I.; Gaikwad, D.K.; Pawar, P.P. Attenuation coefficients and exposure buildup factor of some rocks for gamma ray shielding applications. Radiat. Phys. Chem. 2018, 148, 86–94. [Google Scholar] [CrossRef]
- Dong, M.; Xue, X.; Yang, H.; Li, Z. Highly cost-effective shielding composite made from vanadium slag and boron-rich slag and its properties. Radiat. Phys. Chem. 2017, 141, 239–244. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mhareb, Y.M.H.A.; Alajerami, S.M.; Mahmoud, K.A.; Imheidat, M.A.; Alshahri, F.; Alqahtani, M.; Al-Abdullah, T. Optical and radiation shielding features for a new series of borate glass samples. Optik 2021, 239, 166790. [Google Scholar] [CrossRef]
- Chanthima, N.; Kaewkhao, J.; Limkitjaroenporn, P.; Tuscharoen, S.; Kothan, S.; Tungjai, M.; Kaewjaeng, S.; Sarachai, S.; Limsuwan, P. Development of BaO–ZnO–B2O3 glasses as a radiation shielding material. Radiat. Phys. Chem. 2017, 137, 72–77. [Google Scholar] [CrossRef]
- Yasmin, S.; Barua, B.S.; Khandaker, M.U.; Chowdhury, F.U.Z.; Rashid, M.A.; Bradley, D.A.; Olatunji, M.A.; Kamal, M. Studies of ionizing radiation shielding effectiveness of silica-based commercial glasses used in Bangladeshi dwellings. Results Phys. 2018, 9, 541–549. [Google Scholar] [CrossRef]
- Kaewjaeng, S.; Kothan, S.; Chaiphaksa, W.; Chanthima, N.; Rajaramakrishna, R.; Kim, H.J.; Kaewkhao, J. High transparency La2O3-CaO-B2O3-SiO2 glass for diagnosis x-rays shielding material application. Radiat. Phys. Chem. 2019, 160, 41–47. [Google Scholar] [CrossRef]
- Gökçe, H.S.; Yalçınkaya, Ç.; Tuyan, M. Optimization of reactive powder concrete by means of barite aggregate for both neutrons and gamma rays. Constr. Build. Mater. 2018, 189, 470–477. [Google Scholar] [CrossRef]
- Şensoy, A.T.; Gökçe, H.S. Simulation and optimization of gamma-ray linear attenuation coefficients of barite concrete shields. Constr. Build. Mater. 2020, 253, 119218. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Jecong, J.F.M.; Hila, F.C.; Balderas, C.V.; Alhuthali, A.M.S.; Guillermo, N.R.D.; Al-Hadeethi, Y. Radiation shielding characteristics of selected ceramics using the EPICS2017 library. Ceram. Int. 2021, 47, 13181–13186. [Google Scholar] [CrossRef]
- Yasmin, S.; Rozaila, Z.S.; Faruque-Uz-Zaman, K.; Barua, B.S.; Rashid, M.A.; Bradley, D.A. The radiation shielding offered by the commercial glass installed in Bangladeshi dwellings. Radiat. Eff. Defects Solids 2018, 173, 657–672. [Google Scholar] [CrossRef]
- Kaky, K.M.; Sayyed, M.I.; Ati, A.A.; Mhareb, M.H.A.; Mahmoud, K.A.; Baki, S.O.; Mahdi, M.A. Germanate oxide impacts on the optical and gamma radiation shielding properties of TeO2-ZnO-Li2O glass system. J. Non-Cryst. Solids 2020, 546, 120272. [Google Scholar] [CrossRef]
- Almuqrin, A.H.; Sayyed, M.I. Radiation shielding characterizations and investigation of TeO2–WO3–Bi2O3 and TeO2–WO3–PbO glasses. Appl. Phys. A 2021, 127, 1–11. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mahmoud, K.A.; Lacomme, E.; AlShammari, M.M.; Dwaikat, N.; Alajerami, Y.S.M.; Alqahtani, M.; El-bashir, B.O.; Mhareb, M.H.A. Development of a novel MoO3-doped borate glass network for gamma-ray shielding applications. Eur. Phys. J. Plus 2021, 136, 1–16. [Google Scholar]
- Mhareb, M.H.A. Physical, optical and shielding features of Li2O–B2O3–MgO–Er2O3 glasses co-doped of Sm2O3. Appl. Phys. A 2020, 126, 1–8. [Google Scholar] [CrossRef]
- Yasaka, P.; Pattanaboonmee, N.; Kim, H.J.; Limkitjaroenporn, P.; Kaewkhao, J. Gamma radiation shielding and optical properties measurements of zinc bismuth borate glasses. Ann. Nucl. Energy 2014, 68, 4–9. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mahmoud, K.A.; Tashlykov, O.L.; Khandaker, M.U.; Faruque, M.R.I. Enhancement of the shielding capability of soda–lime glasses with Sb2O3 dopant: A potential material for radiation safety in nuclear installations. Appl. Sci. 2021, 11, 326. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Sayyed, M.I.; Mahmoud, K.A.; Al-Hadeethi, Y. Direct influence of mercury oxide on structural, optical, and shielding properties of a new borate glass system. Ceram. Int. 2020, 46, 17978–17986. [Google Scholar] [CrossRef]
- Alajerami, Y.S.; Drabold, D.; Mhareb, M.H.A.; Cimatu, K.L.A.; Chen, G.; Kurudirek, M. Radiation shielding properties of bismuth borate glasses doped with different concentrations of cadmium oxides. Ceram. Int. 2020, 46, 12718–12726. [Google Scholar] [CrossRef]
- Sayyed, M.I. Bismuth modified shielding properties of zinc boro-tellurite glasses. J. Alloys Compd. 2016, 688, 111–117. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M.I. A comprehensive study on the effect of TeO2 on the radiation shielding properties of TeO2–B2O3–Bi2O3–LiF–SrCl2 glass system using Phy-X/PSD software. Ceram. Int. 2020, 46, 6136–6140. [Google Scholar] [CrossRef]
- Aygün, B. High alloyed new stainless steel shielding material for gamma and fast neutron radiation. Nucl. Eng. Technol. 2020, 52, 647–653. [Google Scholar] [CrossRef]
- El-Mallawany, R.; El-Agawany, F.I.; Al-Buriahi, M.S.; Muthuwong, C.; Novatski, A.; . Rammah, Y.S. Optical properties and nuclear radiation shielding capacity of TeO2-Li2O-ZnO glasses. Opt. Mater. 2020, 106, 109988. [Google Scholar] [CrossRef]
- Pujari, N.; Birampally, K.; Edukondalu, A.; Vardhani, C.P. Effect of Li2O content on structural and optical properties of Li2O-TeO2-As2O3-B2O3 glasses. J. Phys. Chem. Solids 2021, 148, 109627. [Google Scholar] [CrossRef]
- Makishima, A.; Mackenzie, J.D. Direct calculation of Young’s moidulus of glass. J. Non-Cryst. Solids 1973, 12, 35–45. [Google Scholar] [CrossRef]
- Makishima, A.; Mackenzie, J.D. Calculation of bulk modulus, shear modulus and Poisson’s ratio of glass. J. Non-Cryst. Solids 1975, 17, 147–157. [Google Scholar] [CrossRef]
- Yousef, E.S.; El-Adawy, A.; El-KheshKhan, N. Effect of rare earth (Pr2O3, Nd2O3, Sm2O3, Eu2O3, Gd2O3 and Er2O3 ) on the acoustic properties of glass belonging to bismuth-borate system. Solid State Commun. 2006, 139, 108–113. [Google Scholar] [CrossRef]
- X-5 Monte Carlo Team. MCNP—A General Monte Carlo N-Particle Transport Code, Version 5. La-Ur-03-1987 II; Los Alamos National Laboratory: Los Alamos, NM, USA, 2003. [Google Scholar]
- Erdem, Ş.; Özgür, F.; Bünyamin, A.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar]
- Kilic, G.; Ilik, E.; Mahmoud, K.A.; El-Agawany, F.I.; Alomairy, S.; Rammah, Y.S. The role of B2O3 on the structural, thermal, and radiation protection efficacy of vanadium phosphate glasses. Appl. Phys. A 2021, 127, 265. [Google Scholar] [CrossRef]
- Al-Yousef, H.A.; Alotiby, M.; Hanfi, M.Y.; Alotaibi, B.M.; Mahmoud, K.A.; Sayyed, M.I.; Al-Hadeethi, Y. Effect of the Fe2O3 addition on the elastic and gamma-ray shielding features of bismuth sodium-borate glass system. J. Mater. Sci. Mater. Electron. 2021, 32, 6942–6954. [Google Scholar] [CrossRef]
- Alotaibi, B.M.; Abouhaswa, A.S.; Sayyed, M.I.; Mahmoud, K.A.; Al-Yousef, H.A.; Hila, F.C.; Al-Hadeethi, Y. Structural, optical, and gamma-ray shielding properties of a newly fabricated P2O5–B2O3–Bi2O3–Li2O–ZrO2 glass system. Eur. Phys. J. Plus 2021, 136, 1–22. [Google Scholar]
- Hehn, G. Principles of Radiation Shielding. Nucl. Technol. 1986, 74, 104–105. [Google Scholar] [CrossRef]
- Süsoy Doğan, G. Lithium-boro-tellurite glasses with ZnO additive: Exposure Buildup Factors (EBF) and Nuclear Shielding Properties. Eur. J. Sci. Technol. 2020, 18, 531–544. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Al-Buriahi, M.S.; El-Agawany, F.I.; AbouDeif, Y.M.; Yousef, E.S. Investigation of mechanical features and gamma-ray shielding efficiency of ternary TeO2-based glass systems containing Li2O, Na2O, K2O, or ZnO. Ceram. Int. 2020, 46, 27561–27569. [Google Scholar] [CrossRef]
- Kamislioglu, M.; Altunsoy Guclu, E.E.; Tekin, H.O. Comparative evaluation of nuclear radiation shielding properties of xTeO2 + (100–x)Li2O glass system. Appl. Phys. A 2020, 126, 1–16. [Google Scholar] [CrossRef]
- Singh, K.; Singh, S.; Dhaliwal, A.S.; Singh, G. Gamma radiation shielding analysis of lead-flyash concretes. Appl. Radiat. Isot. 2015, 95, 174–179. [Google Scholar] [CrossRef]
- Singh, K.J.; Singh, N.; Kaundal, R.S.; Singh, K. Gamma-ray shielding and structural properties of PbO-SiO2 glasses. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 944–948. [Google Scholar] [CrossRef]
- Hanfi, M.Y.; Sayyed, M.I.; Lacomme, E.; Akkurt, I.; Mahmoud, K.A. The influence of MgO on the radiation protection and mechanical properties of tellurite glasses. Nucl. Eng. Technol. 2021, 35, 2000–2010. [Google Scholar] [CrossRef]
- Singh, V.P.; Badiger, N.M.; Kaewkhao, J. Radiation shielding competence of silicate and borate heavy metal oxide glasses: Comparative study. J. Non-Cryst. Solids 2014, 404, 167–173. [Google Scholar] [CrossRef]
- Singh, V.P.; Badiger, N.M. Gamma ray and neutron shielding properties of some alloy materials. Ann. Nucl. Energy 2014, 64, 301–310. [Google Scholar] [CrossRef]
- Matori, K.A.; Sayyed, M.I.; Sidek, H.A.A.; Zaid, M.H.M.; Singh, V.P. Comprehensive study on physical, elastic and shielding properties of lead zinc phosphate glasses. J. Non-Cryst. Solids 2017, 457, 97–103. [Google Scholar] [CrossRef]
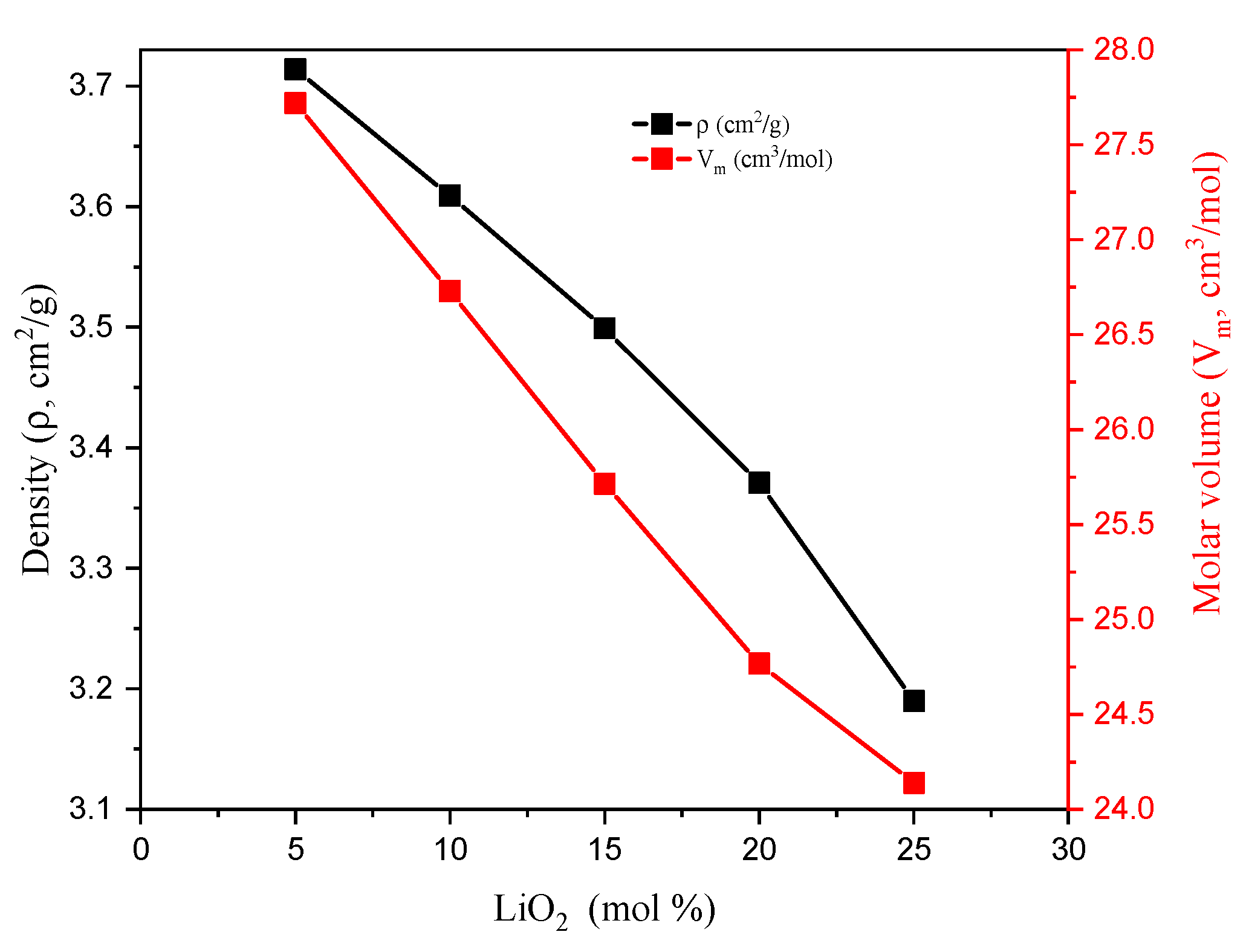


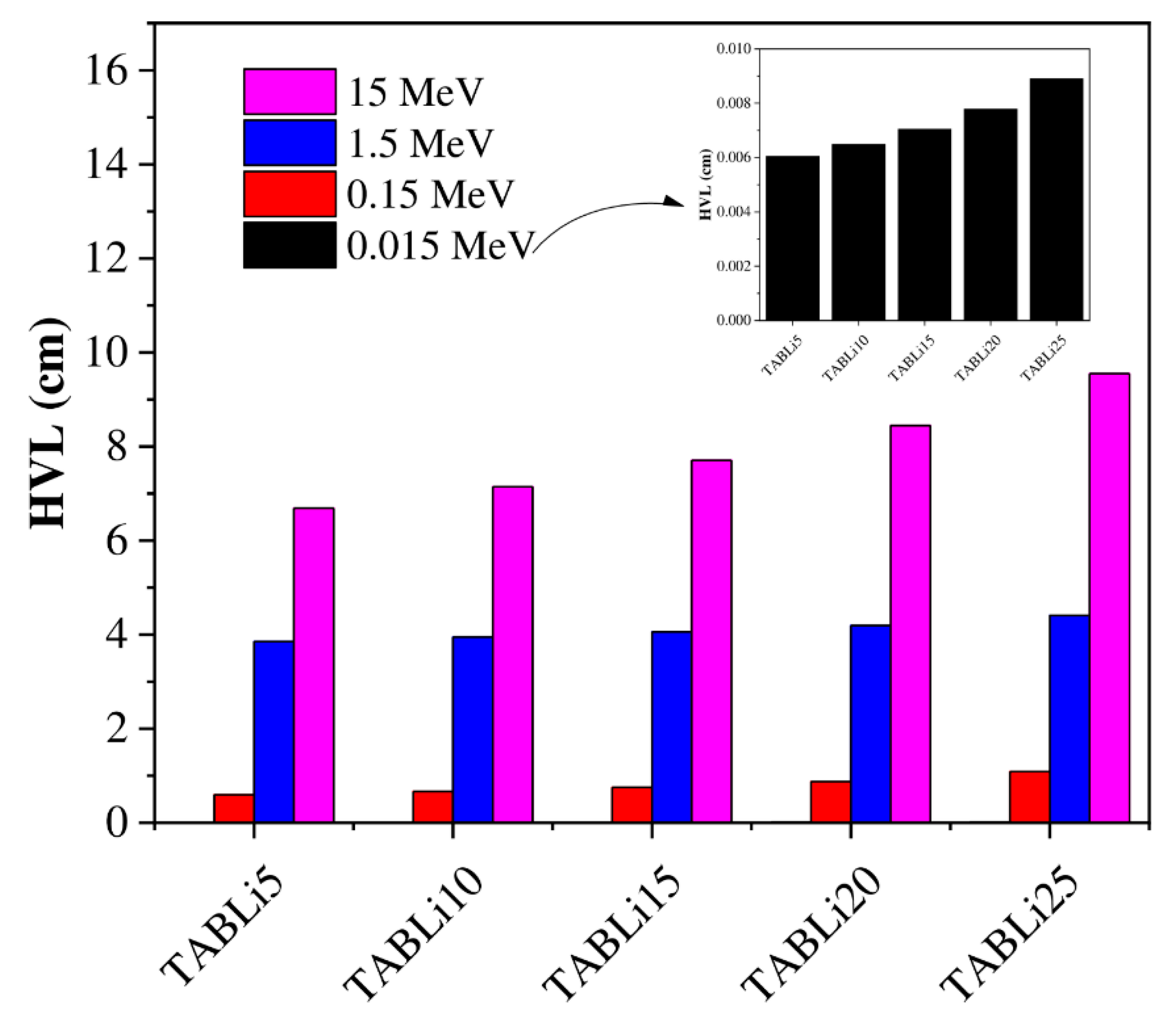
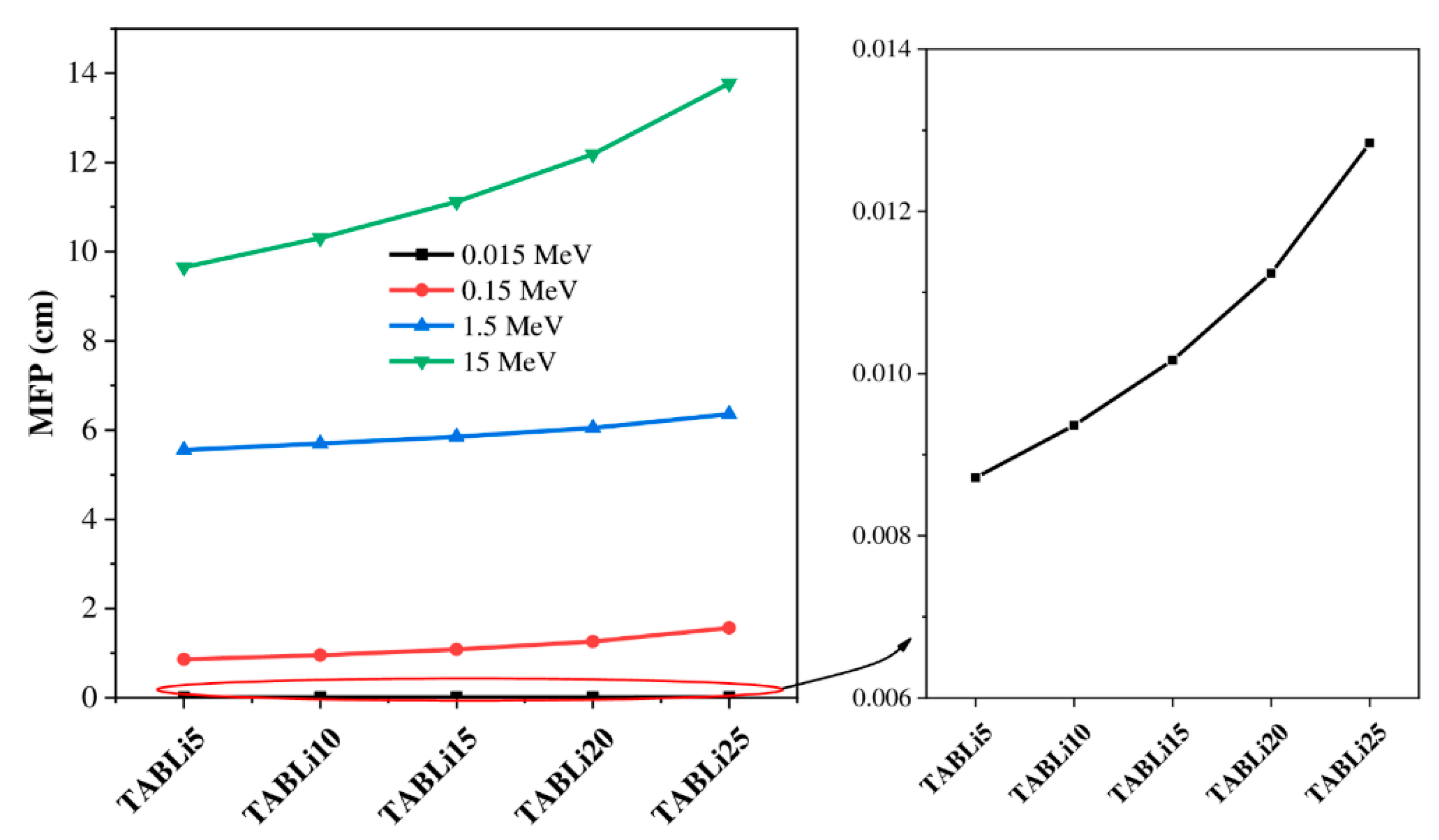
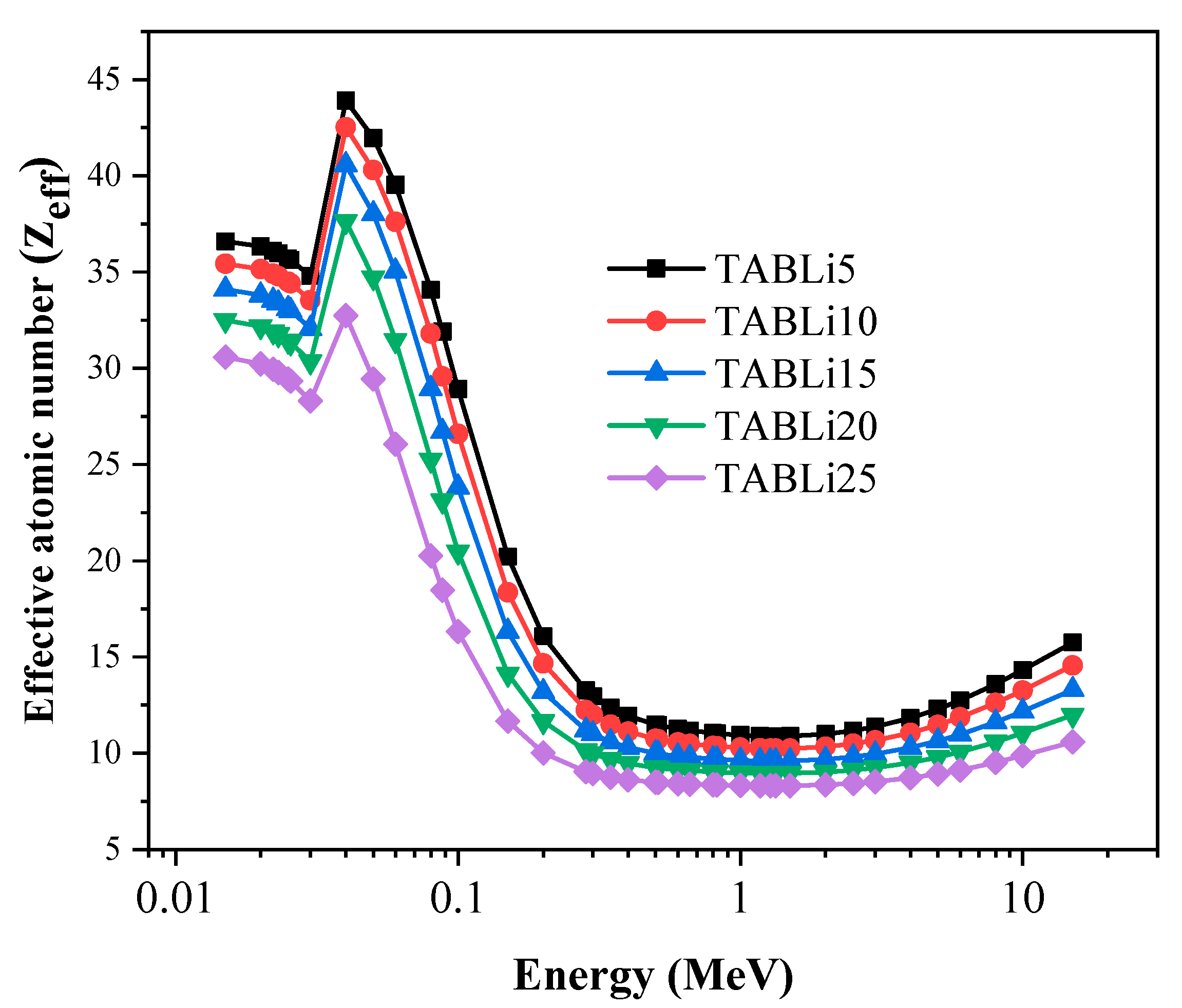


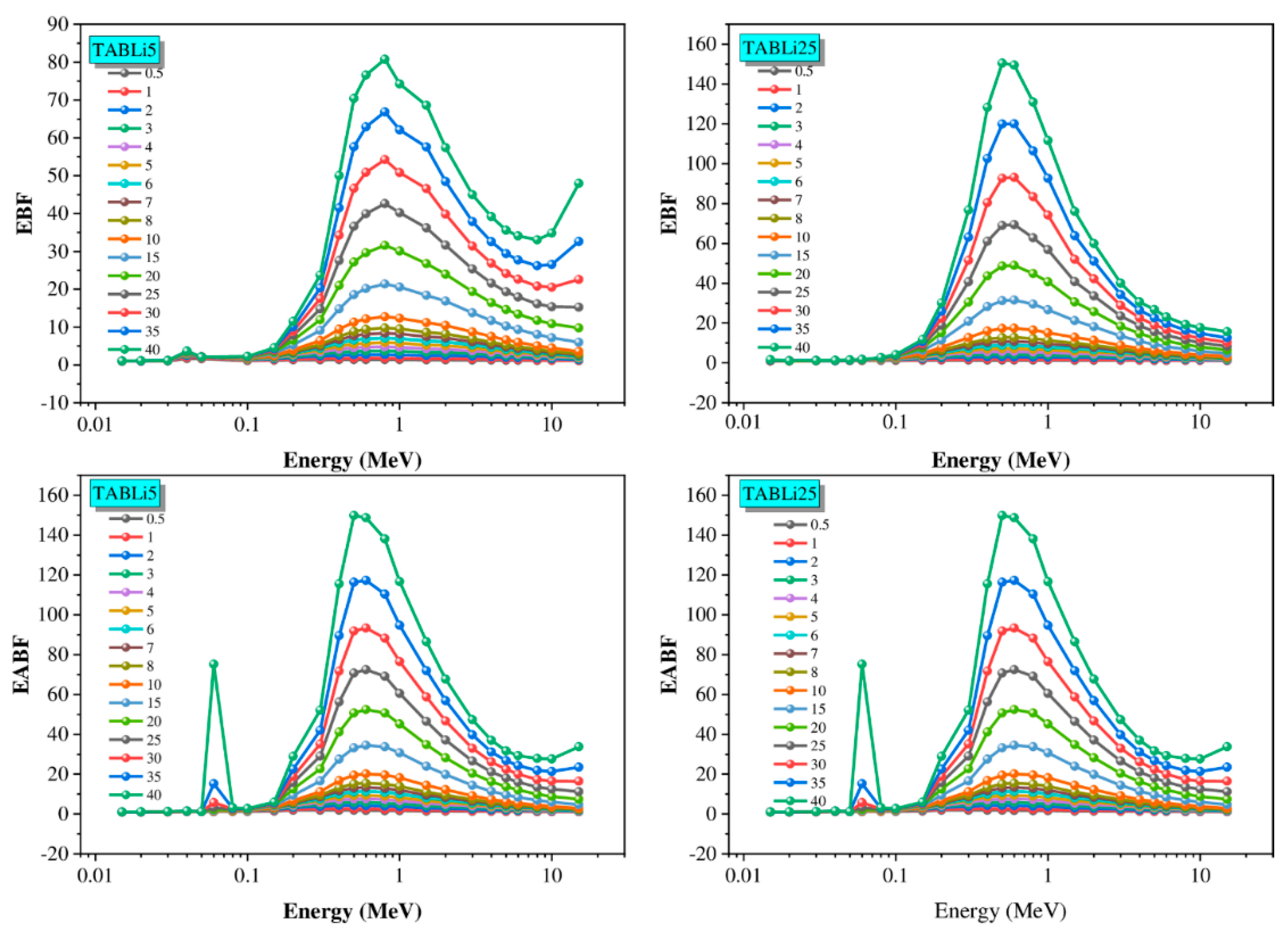

| Composition (wt%) | Density (g/cm3) | MW (g/mol) | Vm (cm3/mol) | ||||
|---|---|---|---|---|---|---|---|
| As2O3 | B2O3 | Li2O | TeO2 | ||||
| TABLi5 | 19.217 | 40.574 | 1.451 | 38.757 | 3.71 | 102.95 | 27.72 |
| TABLi10 | 20.510 | 43.303 | 3.098 | 33.090 | 3.61 | 96.46 | 26.73 |
| TABLi15 | 21.988 | 46.424 | 4.982 | 26.607 | 3.50 | 89.98 | 25.72 |
| TABLi20 | 23.696 | 50.030 | 7.158 | 19.116 | 3.37 | 83.49 | 24.77 |
| TABLi25 | 25.692 | 54.244 | 9.701 | 10.363 | 3.19 | 77.01 | 24.14 |
| TABLi5 | TABLi10 | TABLi15 | TABLi20 | TABLi25 | |
|---|---|---|---|---|---|
| Vt | 0.60 | 0.61 | 0.62 | 0.63 | 0.63 |
| Vl (m/s) | 5177.61 | 5362.85 | 5568.19 | 5792.38 | 6022.41 |
| Vs (m/s) | 2917.22 | 3005.14 | 3101.19 | 3207.99 | 3330.88 |
| Fractal bond conductivity | 2.20 | 2.16 | 2.12 | 2.08 | 2.07 |
| r(B-B), Å | 2.56 | 2.53 | 2.49 | 2.46 | 2.44 |
| r(Li-Li), Å | 2.82 | 2.77 | 2.71 | 2.65 | 2.61 |
| r(Te-Te) Å | 2.79 | 2.77 | 2.73 | 2.70 | 2.67 |
| Energy (MeV) | Mass Attenuation Coefficient (cm2/g) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TABLi5 | TABLi10 | TABLi15 | TABLi20 | TABLi25 | |||||||||||
| MCNP-5 | Phy-X/PSD | ∆ (%) | MCNP-5 | Phy-X/PSD | ∆ (%) | MCNP-5 | Phy-X/PSD | ∆ (%) | MCNP-5 | Phy-X/PSD | ∆ (%) | MCNP-5 | Phy-X/PSD | ∆ (%) | |
| 0.015 | 30.89 | 29.60 | 28.12 | 26.41 | 24.41 | ||||||||||
| 0.022 | 10.91 | 10.92 | −0.07 | 10.46 | 10.46 | −0.05 | 9.95 | 9.95 | −0.02 | 9.61 | 9.35 | 2.67 | 8.89 | 8.65 | 2.71 |
| 0.023 | 9.69 | 9.69 | −0.04 | 9.29 | 9.29 | −0.01 | 8.83 | 8.83 | 0.03 | 8.73 | 8.30 | 4.94 | 8.10 | 7.68 | 5.18 |
| 0.025 | 7.82 | 7.84 | −0.18 | 7.50 | 7.51 | −0.15 | 7.13 | 7.14 | −0.10 | 7.02 | 6.71 | 4.42 | 6.54 | 6.21 | 4.99 |
| 0.026 | 7.41 | 7.43 | −0.23 | 7.11 | 7.12 | −0.20 | 6.76 | 6.77 | −0.15 | 6.65 | 6.37 | 4.26 | 6.19 | 5.89 | 4.88 |
| 0.030 | 4.82 | 4.62 | 4.39 | 4.12 | 3.82 | ||||||||||
| 0.050 | 4.19 | 3.71 | 3.17 | 2.54 | 1.80 | ||||||||||
| 0.080 | 1.25 | 1.12 | 0.96 | 0.79 | 0.59 | ||||||||||
| 0.088 | 0.95 | 0.98 | −4.20 | 0.90 | 0.88 | 1.37 | 0.76 | 0.77 | −0.58 | 0.64 | 0.64 | 1.26 | 0.48 | 0.48 | 0.45 |
| 0.100 | 0.73 | 0.66 | 0.58 | 0.48 | 0.37 | ||||||||||
| 0.150 | 0.31 | 0.29 | 0.26 | 0.23 | 0.20 | ||||||||||
| 0.284 | 0.13 | 0.13 | −0.33 | 0.13 | 0.13 | −0.32 | 0.12 | 0.13 | −0.30 | 0.12 | 0.12 | −0.28 | 0.12 | 0.12 | −0.25 |
| 0.300 | 0.13 | 0.12 | 0.12 | 0.12 | 0.11 | ||||||||||
| 0.347 | 0.11 | 0.11 | −0.18 | 0.11 | 0.11 | −0.17 | 0.11 | 0.11 | −0.16 | 0.11 | 0.11 | −0.15 | 0.10 | 0.10 | −0.13 |
| 0.500 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | ||||||||||
| 0.511 | 0.09 | 0.09 | −0.18 | 0.09 | 0.09 | −0.17 | 0.09 | 0.09 | −0.16 | 0.08 | 0.08 | −0.14 | 0.08 | 0.08 | −0.12 |
| 0.662 | 0.07 | 0.07 | −0.22 | 0.07 | 0.07 | −0.21 | 0.07 | 0.07 | −0.19 | 0.07 | 0.07 | −0.18 | 0.07 | 0.07 | −0.16 |
| 0.800 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | ||||||||||
| 0.826 | 0.07 | 0.07 | −0.19 | 0.07 | 0.07 | −0.18 | 0.07 | 0.07 | −0.17 | 0.07 | 0.07 | −0.16 | 0.07 | 0.07 | −0.14 |
| 1 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | ||||||||||
| 1.173 | 0.05 | 0.06 | −0.90 | 0.05 | 0.06 | −0.83 | 0.06 | 0.06 | −0.75 | 0.06 | 0.06 | −0.66 | 0.06 | 0.06 | −0.55 |
| 1.275 | 0.05 | 0.05 | −0.76 | 0.05 | 0.05 | −0.70 | 0.05 | 0.05 | −0.63 | 0.05 | 0.05 | −0.55 | 0.05 | 0.05 | −0.46 |
| 1.333 | 0.05 | 0.05 | −0.72 | 0.05 | 0.05 | −0.66 | 0.05 | 0.05 | −0.59 | 0.05 | 0.05 | −0.52 | 0.05 | 0.05 | −0.43 |
| 1.5 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | ||||||||||
| 2.506 | 0.04 | 0.04 | −0.45 | 0.04 | 0.04 | −0.43 | 0.04 | 0.04 | −0.40 | 0.04 | 0.04 | −0.37 | 0.04 | 0.04 | −0.34 |
| 3 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | ||||||||||
| 5 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | ||||||||||
| 8 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | ||||||||||
| 10 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 | ||||||||||
| 15 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 | ||||||||||
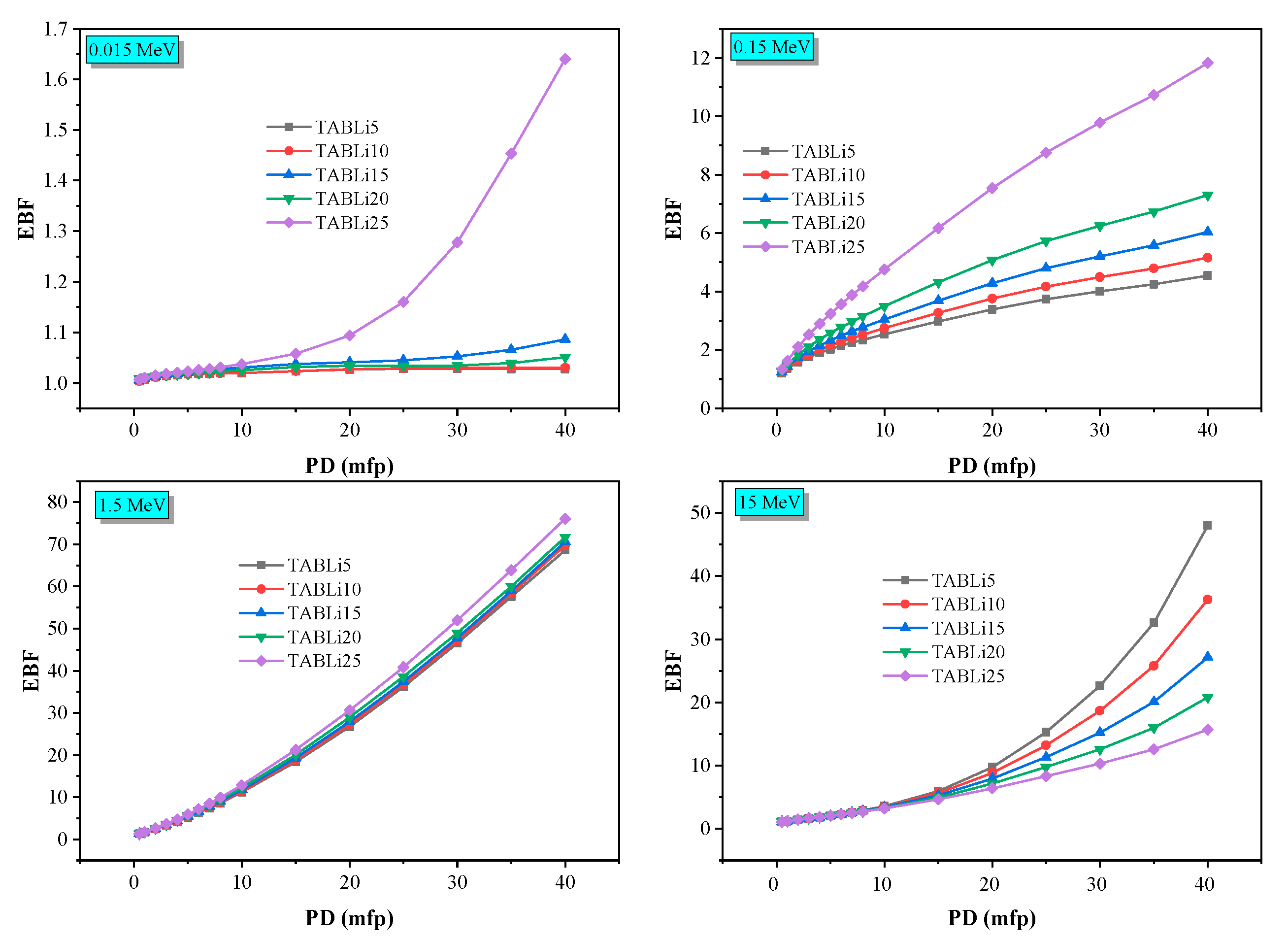
| E (MeV) | Zeq | EBF | EABF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | Xk | a | b | c | d | Xk | ||
| 0.015 | 20.53 | −0.04 | 1.01 | 0.69 | 0.15 | 6.89 | −0.02 | 1.01 | 0.68 | 0.14 | 9.31 |
| 0.02 | 20.93 | 0.26 | 1.02 | 0.39 | −0.18 | 10.98 | 0.26 | 1.02 | 0.35 | −0.20 | 12.47 |
| 0.03 | 21.49 | 0.22 | 1.05 | 0.37 | −0.17 | 16.39 | 0.24 | 1.05 | 0.35 | −0.16 | 14.46 |
| 0.04 | 32.96 | 0.19 | 2.04 | 0.33 | −0.07 | 17.54 | 0.17 | 1.19 | 0.37 | −0.20 | 24.68 |
| 0.05 | 33.61 | 0.02 | 1.88 | 0.23 | −0.06 | 12.52 | 0.08 | 1.20 | 0.23 | −0.04 | 11.06 |
| 0.06 | 34.08 | 0.61 | 1.67 | 0.21 | −0.14 | 15.27 | 0.49 | 1.21 | 0.20 | −0.17 | 14.70 |
| 0.08 | 34.71 | 0.47 | 1.42 | 0.25 | −0.18 | 14.18 | 0.39 | 1.27 | 0.24 | −0.17 | 14.22 |
| 0.1 | 35.13 | 0.23 | 1.22 | 0.40 | −0.13 | 13.82 | 0.23 | 1.28 | 0.39 | −0.13 | 16.50 |
| 0.15 | 35.79 | 0.14 | 1.36 | 0.57 | −0.08 | 14.23 | 0.25 | 1.74 | 0.39 | −0.15 | 13.85 |
| 0.2 | 36.17 | 0.12 | 1.56 | 0.64 | −0.07 | 14.02 | 0.24 | 2.41 | 0.45 | −0.16 | 13.80 |
| 0.3 | 36.61 | 0.05 | 1.69 | 0.84 | −0.03 | 13.76 | 0.11 | 2.59 | 0.69 | −0.08 | 13.59 |
| 0.4 | 36.85 | 0.02 | 1.78 | 0.98 | −0.03 | 13.22 | 0.07 | 2.72 | 0.84 | −0.07 | 13.30 |
| 0.5 | 37.02 | 0.00 | 1.82 | 1.05 | −0.02 | 12.83 | 0.04 | 2.67 | 0.94 | −0.05 | 13.10 |
| 0.6 | 37.11 | −0.01 | 1.83 | 1.09 | −0.02 | 12.13 | 0.02 | 2.59 | 0.99 | −0.04 | 12.75 |
| 0.8 | 37.20 | −0.02 | 1.82 | 1.12 | −0.01 | 11.73 | 0.01 | 2.43 | 1.05 | −0.03 | 12.06 |
| 1 | 37.23 | −0.02 | 1.79 | 1.13 | −0.01 | 11.49 | 0.00 | 2.29 | 1.07 | −0.02 | 11.46 |
| 1.5 | 34.62 | −0.03 | 1.68 | 1.17 | 0.01 | 12.99 | −0.02 | 1.95 | 1.12 | −0.01 | 10.71 |
| 2 | 29.21 | −0.02 | 1.69 | 1.12 | 0.00 | 8.97 | −0.02 | 1.84 | 1.11 | −0.01 | 9.56 |
| 3 | 25.22 | −0.01 | 1.63 | 1.06 | −0.01 | 12.14 | 0.00 | 1.68 | 1.04 | −0.02 | 12.39 |
| 4 | 24.08 | 0.01 | 1.56 | 1.02 | −0.02 | 12.67 | 0.01 | 1.57 | 0.99 | −0.03 | 13.43 |
| 5 | 23.57 | 0.01 | 1.49 | 1.00 | −0.02 | 13.15 | 0.02 | 1.48 | 0.97 | −0.04 | 14.30 |
| 6 | 23.20 | 0.02 | 1.45 | 0.97 | −0.03 | 13.29 | 0.03 | 1.41 | 0.96 | −0.04 | 13.75 |
| 8 | 22.82 | 0.03 | 1.36 | 0.96 | −0.04 | 13.59 | 0.03 | 1.31 | 0.95 | −0.04 | 13.19 |
| 10 | 22.67 | 0.04 | 1.31 | 0.94 | −0.05 | 13.78 | 0.04 | 1.26 | 0.93 | −0.05 | 14.08 |
| 15 | 22.56 | 0.05 | 1.21 | 0.93 | −0.06 | 14.12 | 0.04 | 1.16 | 0.96 | −0.04 | 14.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuqrin, A.H.; Hanfi, M.Y.; Sayyed, M.I.; Mahmoud, K.G.; Al-Ghamdi, H.; Aloraini, D.A. Influence of Li2O Incrementation on Mechanical and Gamma-Ray Shielding Characteristics of a TeO2-As2O3-B2O3 Glass System. Materials 2021, 14, 4060. https://doi.org/10.3390/ma14144060
Almuqrin AH, Hanfi MY, Sayyed MI, Mahmoud KG, Al-Ghamdi H, Aloraini DA. Influence of Li2O Incrementation on Mechanical and Gamma-Ray Shielding Characteristics of a TeO2-As2O3-B2O3 Glass System. Materials. 2021; 14(14):4060. https://doi.org/10.3390/ma14144060
Chicago/Turabian StyleAlmuqrin, Aljawhara H., Mohamed Y. Hanfi, M. I. Sayyed, K. G. Mahmoud, Hanan Al-Ghamdi, and Dalal Abdullah Aloraini. 2021. "Influence of Li2O Incrementation on Mechanical and Gamma-Ray Shielding Characteristics of a TeO2-As2O3-B2O3 Glass System" Materials 14, no. 14: 4060. https://doi.org/10.3390/ma14144060
APA StyleAlmuqrin, A. H., Hanfi, M. Y., Sayyed, M. I., Mahmoud, K. G., Al-Ghamdi, H., & Aloraini, D. A. (2021). Influence of Li2O Incrementation on Mechanical and Gamma-Ray Shielding Characteristics of a TeO2-As2O3-B2O3 Glass System. Materials, 14(14), 4060. https://doi.org/10.3390/ma14144060









