Steel Corrosion Behavior of Reinforced Calcium Aluminate Cement-Mineral Additions Modified Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Specimen Preparation
2.3. Pore Paremeters
2.4. Chloride Corrosion
2.5. Electrochemical Test
2.6. Phase Composition
3. Results and Discussion
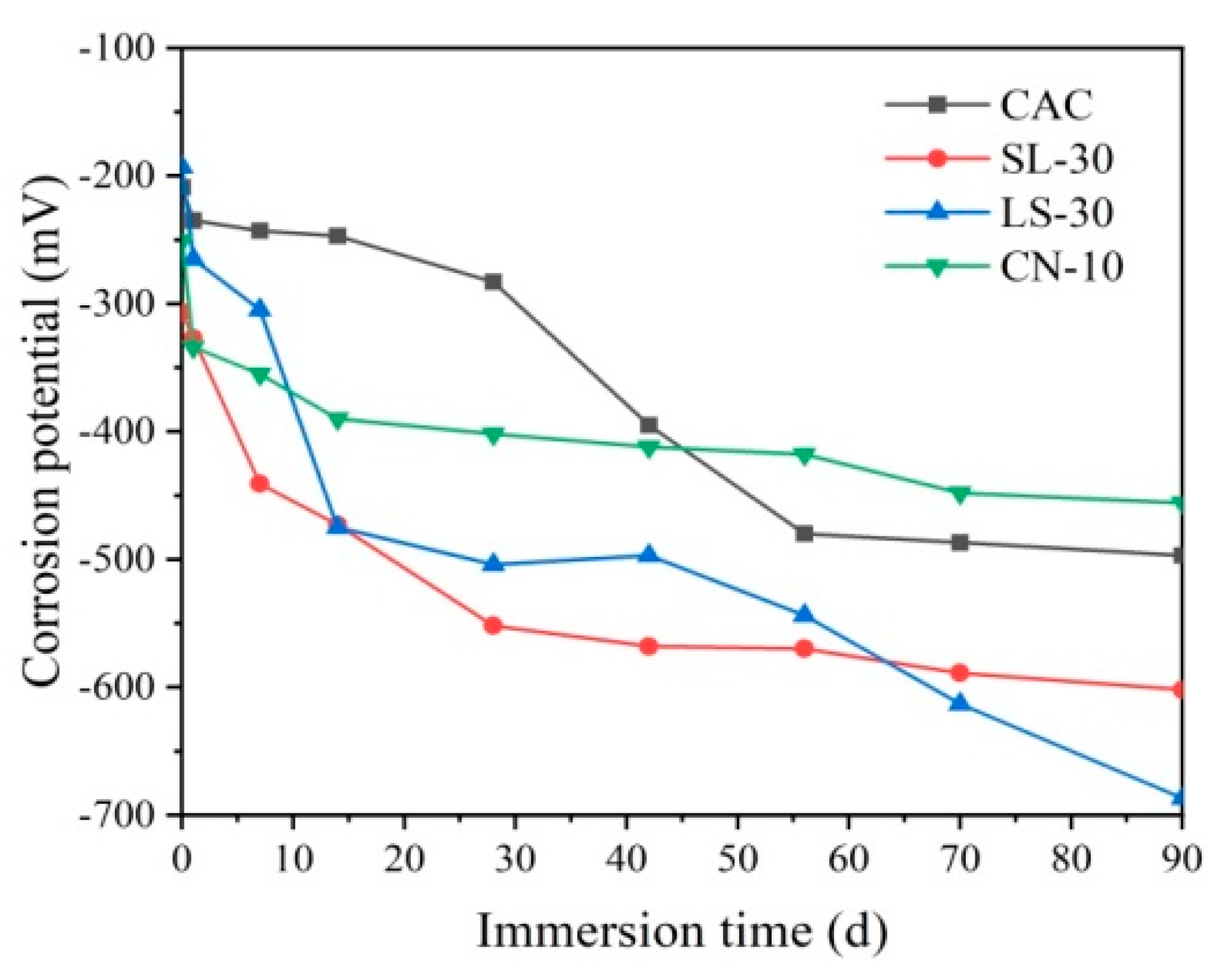
3.1. Corrosion Potential of Steel Reinforcement
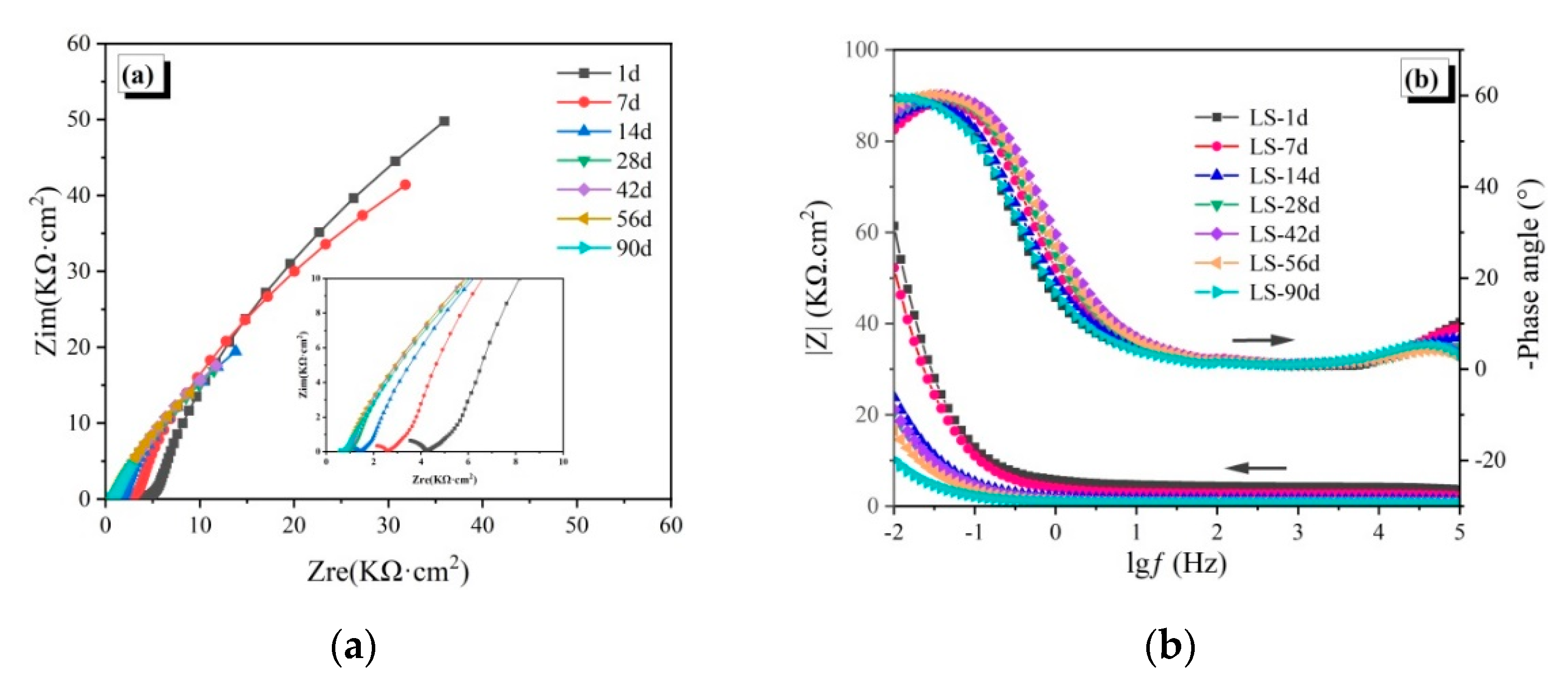
3.2. Electrochemical Impedance Spectroscopy
3.3. Linear Polarization
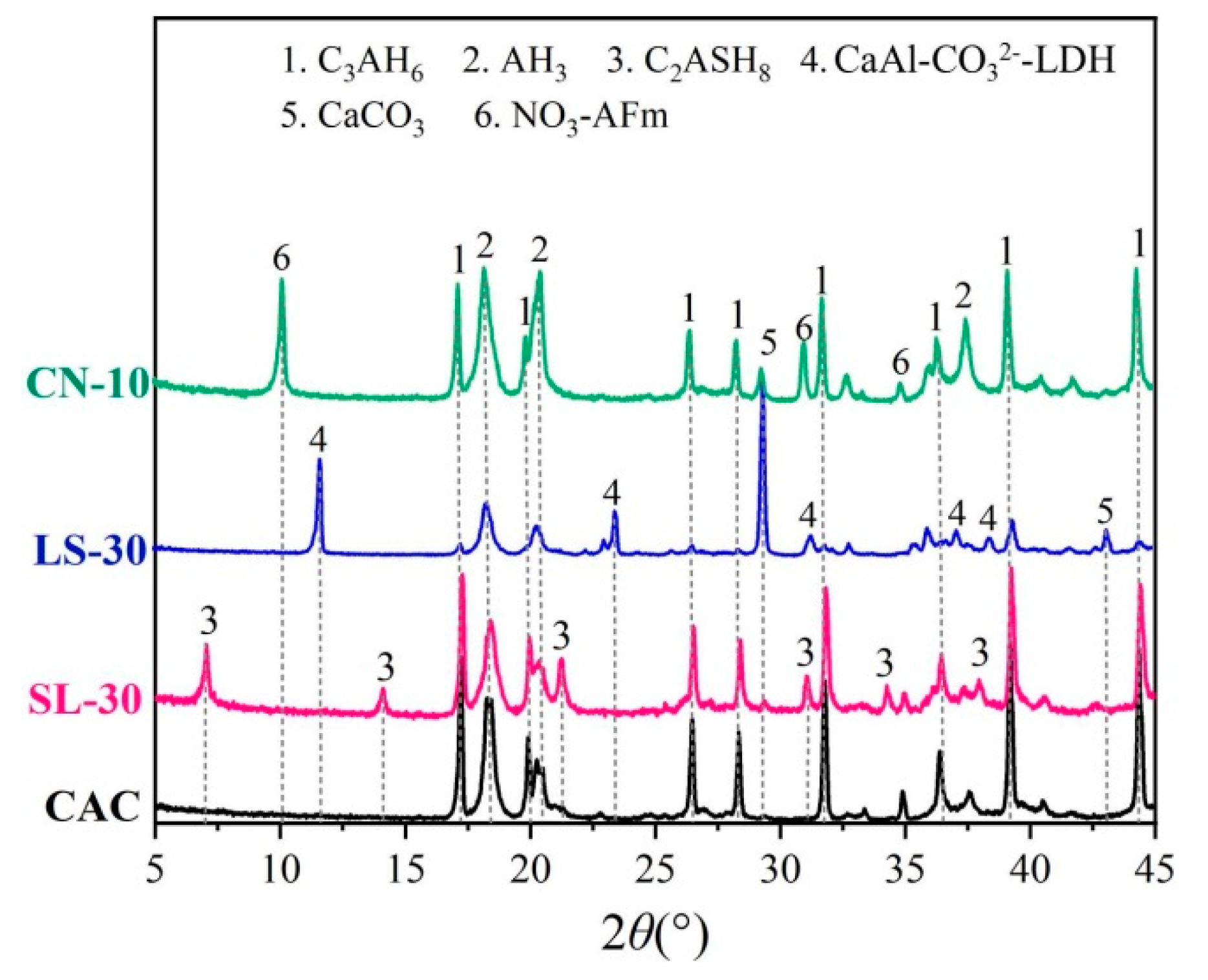
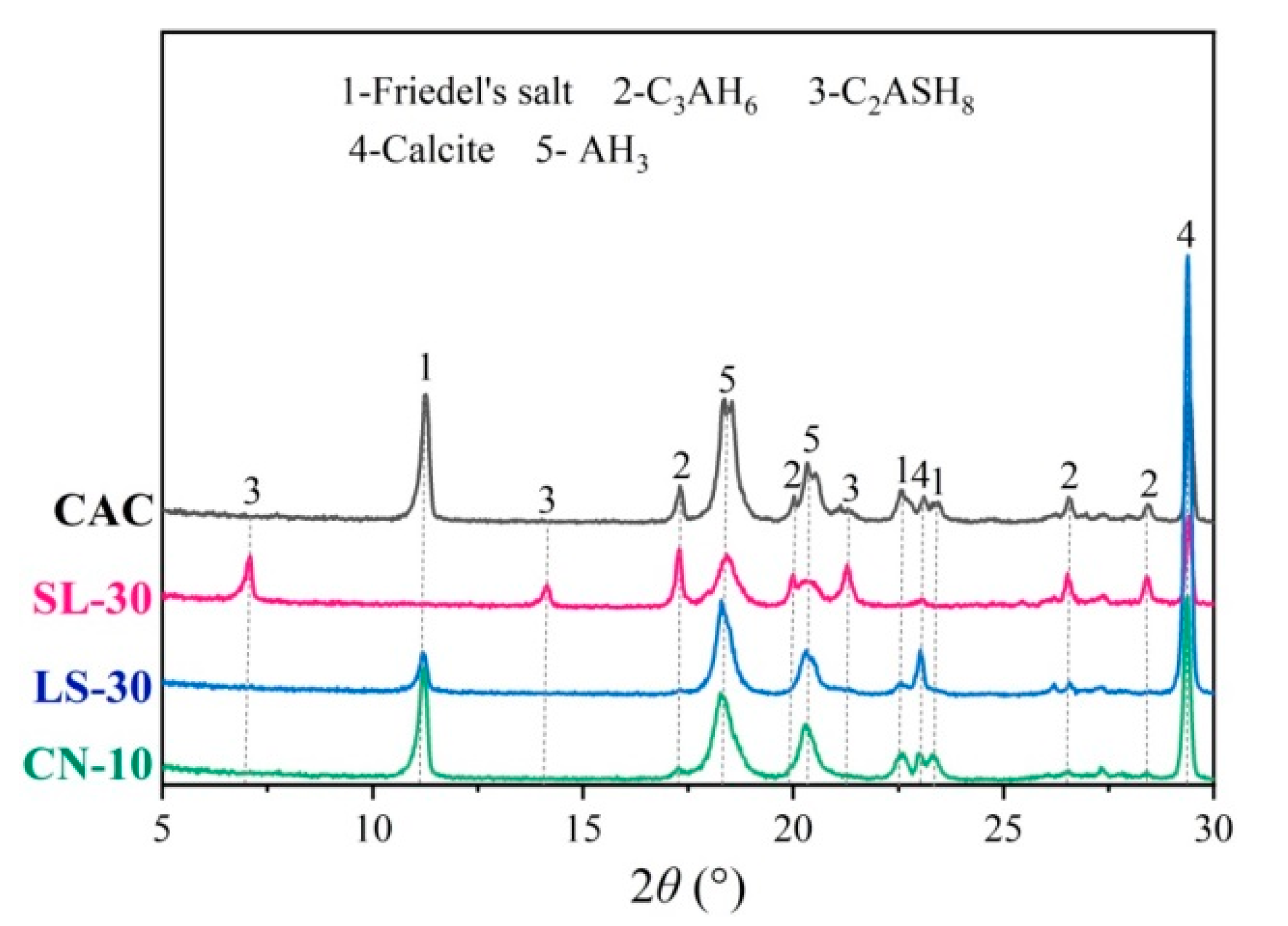
3.4. Phase Composition
4. Conclusions
- The electrochemical parameters of calcium nitrate-modified CAC specimens show a slow downward trend within 90 days, earlier than the unmodified CAC specimen. This indicates that calcium nitrate can improve the corrosion resistance of steel reinforcements in CAC mortar. However, slag and limestone powder show adverse effects on the corrosion resistance of steel bars in CAC mortar. The relevant electrochemical parameters drop suddenly at 14 days, leading to early corrosion.
- The NO3-AFm in the calcium nitrate-modified CAC specimen was found to have great chloride-binding ability by generating substantial amount of Friedel’s salt, which improves the chloride resistance of the CAC paste. The CO3-AFm in the limestone powder-modified CAC specimen also can combine with chloride to form Friedel’s salt. However, the chloride-binding ability of C2ASH8 in the slag-modified CAC specimen is poor, and thus not conducive to corrosion resistance of the CAC paste.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Polder, R. Corrosion of steel in concrete: Prevention, diagnosis, repair. Wiley-VCH Verlag GmbH Co. KGaA. 2013, 49, 4113–4133. [Google Scholar]
- Bagheri, A.; Rastegar, M.M. Investigation of passive layer formation on steel rebars in foamed concrete. Mater. Corros. 2019, 70, 1252–1261. [Google Scholar] [CrossRef]
- Tang, F.J.; Chen, G.D.; Brow, R.K. Chloride-induced corrosion mechanism and rate of enamel- and epoxy-coated deformed steel bars embedded in mortar. Cem. Concr. Res. 2016, 82, 58–73. [Google Scholar] [CrossRef] [Green Version]
- Morris, W.; Vazquez, M. Corrosion of reinforced concrete exposed to marine environment. Corros. Rev. 2002, 20, 469–508. [Google Scholar] [CrossRef]
- Yu, H.; Chiang, K.T.K.; Yang, L.T. Threshold chloride level and characteristics of reinforcement corrosion initiation in simulated concrete pore solutions. Constr. Build. Mater. 2012, 26, 723–729. [Google Scholar] [CrossRef]
- Ann, K.Y.; Song, H.W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.Z.; Dong, C.F.; Li, X. Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl. Surf. Sci. 2017, 400, 38–48. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Ltd.: London, UK, 1997; pp. 295–312. [Google Scholar]
- Arya, C.; Buenfeld, N.R.; Newman, J.B. Assessment of simple methods of determining the free chloride ion content of cement paste. Cem. Concr. Res. 1987, 17, 907–918. [Google Scholar] [CrossRef]
- Ann, K.Y.; Cho, C.G. Corrosion resistance of calcium aluminate cement concrete exposed to a chloride environment. Materials 2014, 7, 887–898. [Google Scholar] [CrossRef] [Green Version]
- Ukrainczyk, N.; Matusinovic, T. Thermal properties of hydrating calcium aluminate cement pastes. Cem. Concr. Res. 2010, 40, 128–136. [Google Scholar] [CrossRef]
- Bensted, J.; Barnes, P. Structure and Performance of Cements, 2nd ed.; Spon Press: London, UK, 2008; pp. 116–130. [Google Scholar]
- KıRCA, Ö.; ÖZGüR YAMAN, İ.; TOKYAY, M. Compressive strength development of calcium aluminate cement–GGBFS blends. Cement Concrete Comp. 2013, 35, 163–170. [Google Scholar] [CrossRef]
- Zao-Yuan, L.I.; Peng, W.U.; Cheng, X.W.; Wang, Y.; Guo, X.Y. Influence study on the performance of aluminate cement with slag. Bull. Chin. Ceram. Soc. 2014, 12, 3338–3342. [Google Scholar]
- Xing, H. Influence of Limestone Powder on the Performance of High Alumina Cement. Ph.D. Thesis, Central South University, Changsha, China, 2010. [Google Scholar]
- Piasta, J.; Sawica, Z.; Piasta, W.G. Durability of high alumina cement pastes with mineral additions in water sulfate environment. Cem. Concr. Res. 1989, 19, 103–113. [Google Scholar] [CrossRef]
- Yang, H.; Sun, J.; Zhang, R. High alumina cement modified by limestone powders and its heat-resistant property. J. Chin. Ceram. Soc. 2006, 34, 452–457. [Google Scholar]
- Xiao, J.; Gou, C.F.; Xing, H.; Xu, C.Y.; Jin, Y.G. Effect of ground limestone on performance of high alumina cement. J. Build. Mater. 2011, 14, 366–370. [Google Scholar]
- Yao, Y.; Wang, H.; Diao, G.; Liu, G. Effect of calcium nitrate on strength and hydration of calcium aluminate cement. J. Chin. Ceram. Soc. 2019, 47, 207–213. [Google Scholar]
- Bernard, S.; Cédric, P.; Laurent, S.; Fabrice, D.; Martin, C. Study on corrosion durability with electrochemical tests of GGBS/Portland blends activated by chlorides. In Proceedings of the XIV DBMC—14th International Conference on Durability of Building Materials and Components, Ghent University, Ghent, Belgium, 30 May 2017. [Google Scholar]
- Monticelli, C.; Natali, M.E.; Balbo, A.; Chiavari, C.; Zanotto, F.; Manzi, S.; Bignozzi, M.C. A study on the corrosion of reinforcing bars in alkali-activated fly ash mortars under wet and dry exposures to chloride solutions. Cem. Concr. Res. 2016, 87, 53–63. [Google Scholar] [CrossRef]
- Hoshi, Y.; Hasegawa, C.; Okamoto, T.; Soukura, M.; Tokieda, H.; Shitanda, I.; Itagaki, M.; Kato, Y. Electrochemical impedance analysis of corrosion of reinforcing bars in concrete. Electrochemistry 2019, 87, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.Q.; Wu, Y.S.; Teng, X.J.; Zhuang, Z.T.; Gu, Z.T.; Zhang, J.C.; Xing, F.; Hong, S.X. Investigation of the Cl− migration behavior of cement materials blended with fly ash or/and slag via the electrochemical impedance spectroscopy method. Constr. Build. Mater. 2019, 211, 261–270. [Google Scholar] [CrossRef]
- Nguyen, W.; Duncan, J.F.; Devine, T.M.; Ostertag, C.P. Electrochemical polarization and impedance of reinforced concrete and hybrid fiber-reinforced concrete under cracked matrix conditions. Electrochim. Acta 2018, 271, 319–336. [Google Scholar] [CrossRef] [Green Version]
- Diaz, B.; Guitian, B.; Novoa, X.R.; Perez, M.C. The effect of long-term atmospheric aging and temperature on the electrochemical behaviour of steel rebars in mortar. Corros. Sci. 2018, 140, 143–150. [Google Scholar]
- Ann, K.Y.; Kim, T.S.; Kim, J.H.; Kim, S.H. The resistance of high alumina cement against corrosion of steel in concrete. Constr. Build. Mater. 2010, 24, 1502–1510. [Google Scholar] [CrossRef]
- Macias, A.; Kindness, A.; Glasser, F.P. Corrosion behaviour of steel in high alumina cement mortar cured at 5,25 and 55 °C: Chemical and physical factors. J. Mater. Sci. 1996, 31, 2279–2289. [Google Scholar] [CrossRef]
- Wei, J.; Bian, X.; Huang, W. Passivation behavior of steel bar in sea sand ultra-high performance cement evaluated using electrochemical method. J. Chin. Ceram. Soc. 2020, 48, 1223–1232. [Google Scholar]
- Szabados, M.; Mészáros, R.; Erdei, S.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Ultrasonically-enhanced mechanochemical synthesis of CaAl-layered double hydroxides intercalated by a variety of inorganic anions. Ultrason. Sonochem. 2016, 31, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Szabados, M.; Varga, G.; Kónya, Z.; Kukovecz, Á.; Carlson, S.; Sipos, P.; Pálinkó, I. Ultrasonically-enhanced preparation, characterization of CaFe-layered double hydroxides with various interlayer halide, azide and oxo anions (CO32, NO3, ClO4). Ultrason. Sonochem. 2018, 40, 853. [Google Scholar] [CrossRef]



| MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl | K2O | CaO | TiO2 | MnO | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.46 | 48.45 | 7.33 | 0.15 | 0.46 | 0.01 | 0.45 | 37.87 | 2.52 | 0.04 | 1.90 |
| CA | CA2 | C2AS | CT |
|---|---|---|---|
| 51.12 | 4.31 | 35.15 | 4.86 |
| C | Si | Mn | P | S | Ni | Cr | Cu | Fe |
| 0.140 | 0.180 | 0.330 | 0.0170 | 0.004 | 0.010 | 0.010 | 0.010 | 99.0 |
| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | K2O | SO3 | Na2O |
|---|---|---|---|---|---|---|---|
| 32.22 | 39.03 | 17.01 | 0.43 | 7.67 | 0.27 | 2.08 | 0.42 |
| CAC | Slag | Limestone | Calcium Nitrate | |
|---|---|---|---|---|
| CAC | 100 | 0 | 0 | 0 |
| SL-30 | 70 | 30 | 0 | 0 |
| LS-30 | 70 | 0 | 30 | 0 |
| CN-10 | 90 | 0 | 0 | 10 |
| Samples | Pore Volume Fraction/% | Porosity/% | Average Pore Diameter/nm | ||
|---|---|---|---|---|---|
| <100 nm | 100~1000 nm | >1000 nm | |||
| CAC | 7.40 | 23.86 | 68.74 | 11.82 | 400.20 |
| SL-30 | 16.65 | 26.32 | 57.03 | 13.11 | 79.00 |
| LS-30 | 28.32 | 28.97 | 42.71 | 16.63 | 40.90 |
| CN-10 | 41.99 | 45.78 | 12.23 | 15.02 | 30.70 |
| Samples | Fitting Terms | Immersion Time | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 d | 7 d | 14 d | 28 d | 42 d | 56 d | 90 d | ||
| CAC | Rp (KΩ·cm2) | 7.81 | 6.55 | 4.93 | 2.43 | 1.87 | 1.76 | 1.86 |
| R2 | 0.84 | 0.83 | 0.86 | 0.89 | 0.93 | 0.94 | 0.94 | |
| SL-30 | Rp (KΩ·cm2) | 4.15 | 1.98 | 1.49 | 0.93 | 0.80 | 0.59 | 0.52 |
| R2 | 0.79 | 0.81 | 0.85 | 0.93 | 0.93 | 0.94 | 0.95 | |
| LS-30 | Rp (KΩ·cm2) | 10.81 | 4.29 | 2.32 | 2.24 | 1.72 | 1.60 | 1.46 |
| R2 | 0.84 | 0.90 | 0.92 | 0.92 | 0.92 | 0.92 | 0.93 | |
| CN-10 | Rp (KΩ·cm2) | 7.05 | 6.15 | 5.50 | 4.88 | 3.68 | 2.87 | 2.50 |
| R2 | 0.90 | 0.92 | 0.89 | 0.89 | 0.90 | 0.93 | 0.91 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, Y.; Zhu, Z.; Peng, X.; Wu, K.; Xu, L. Steel Corrosion Behavior of Reinforced Calcium Aluminate Cement-Mineral Additions Modified Mortar. Materials 2021, 14, 4053. https://doi.org/10.3390/ma14144053
Wang Z, Chen Y, Zhu Z, Peng X, Wu K, Xu L. Steel Corrosion Behavior of Reinforced Calcium Aluminate Cement-Mineral Additions Modified Mortar. Materials. 2021; 14(14):4053. https://doi.org/10.3390/ma14144053
Chicago/Turabian StyleWang, Zhongping, Yuting Chen, Zheyu Zhu, Xiang Peng, Kai Wu, and Linglin Xu. 2021. "Steel Corrosion Behavior of Reinforced Calcium Aluminate Cement-Mineral Additions Modified Mortar" Materials 14, no. 14: 4053. https://doi.org/10.3390/ma14144053






