Wear and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy in Electrolytes with Aluminate and Phosphate
Abstract
:1. Introduction
2. Experimental Details
2.1. PEO Process
2.2. Surface Characterization of the Coatings
3. Results and Discussions
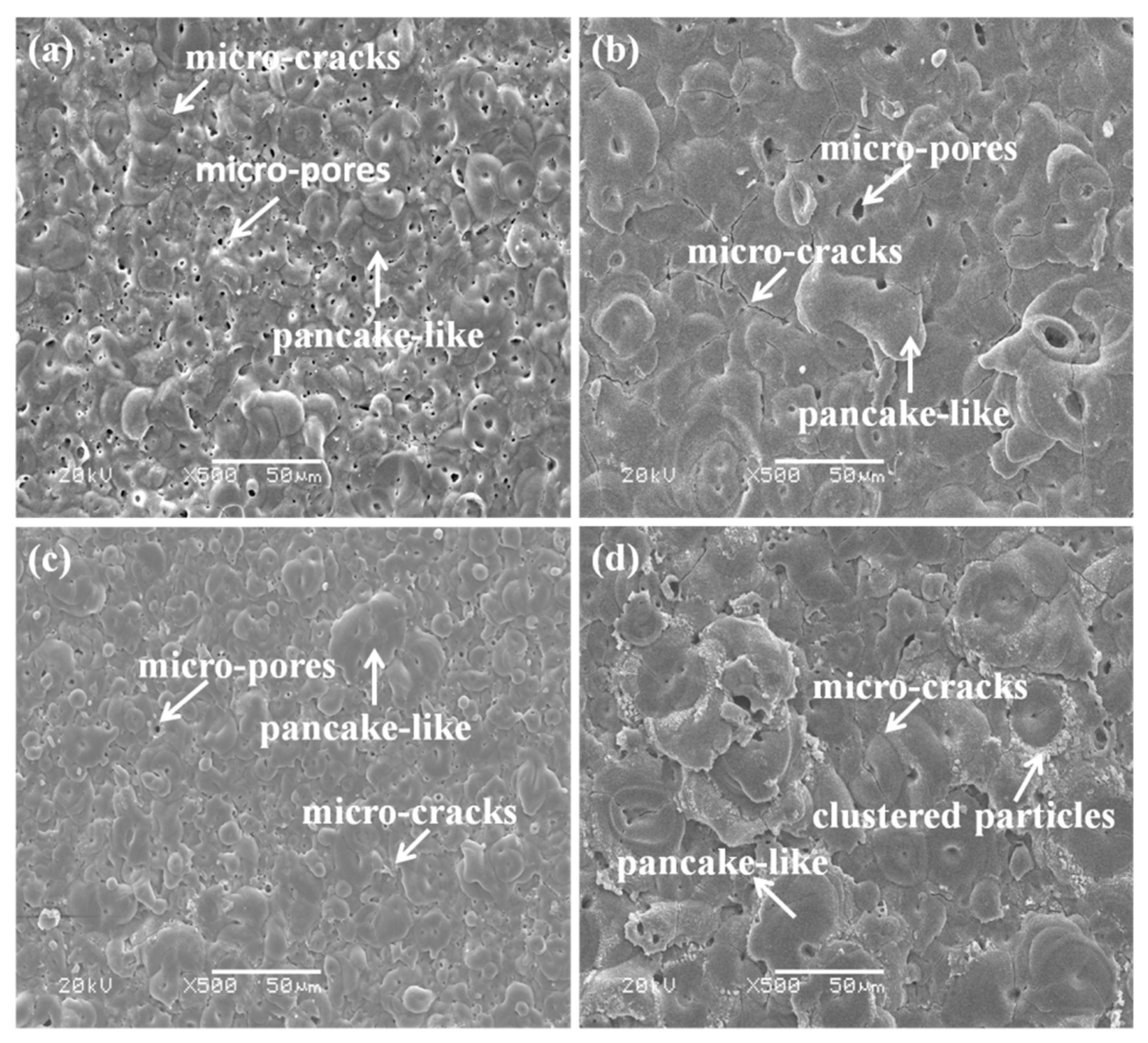
3.1. Morphologies of the Coatings
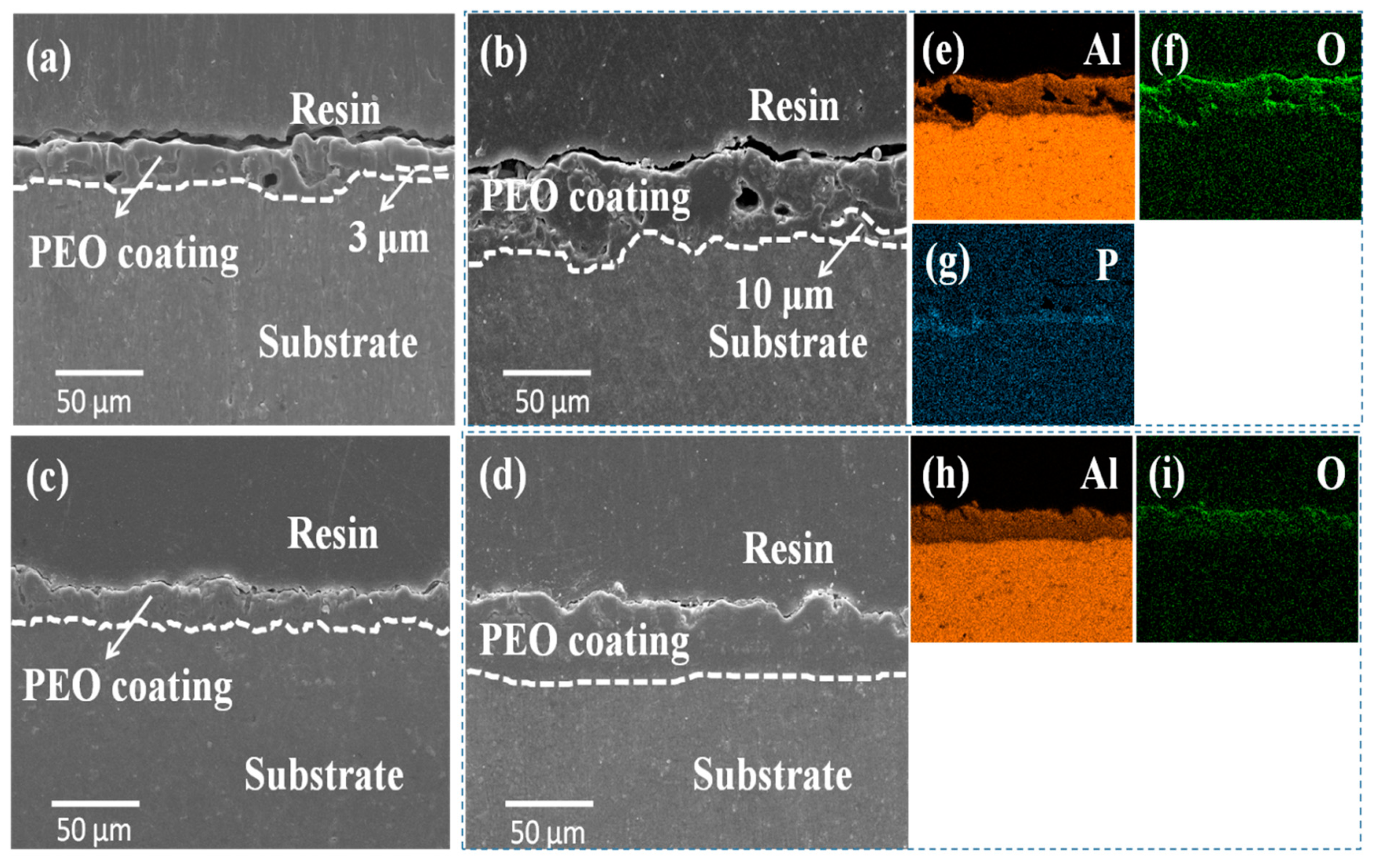
3.2. Cross-Sectional Morphologies and Elemental Distributions of the Coatings
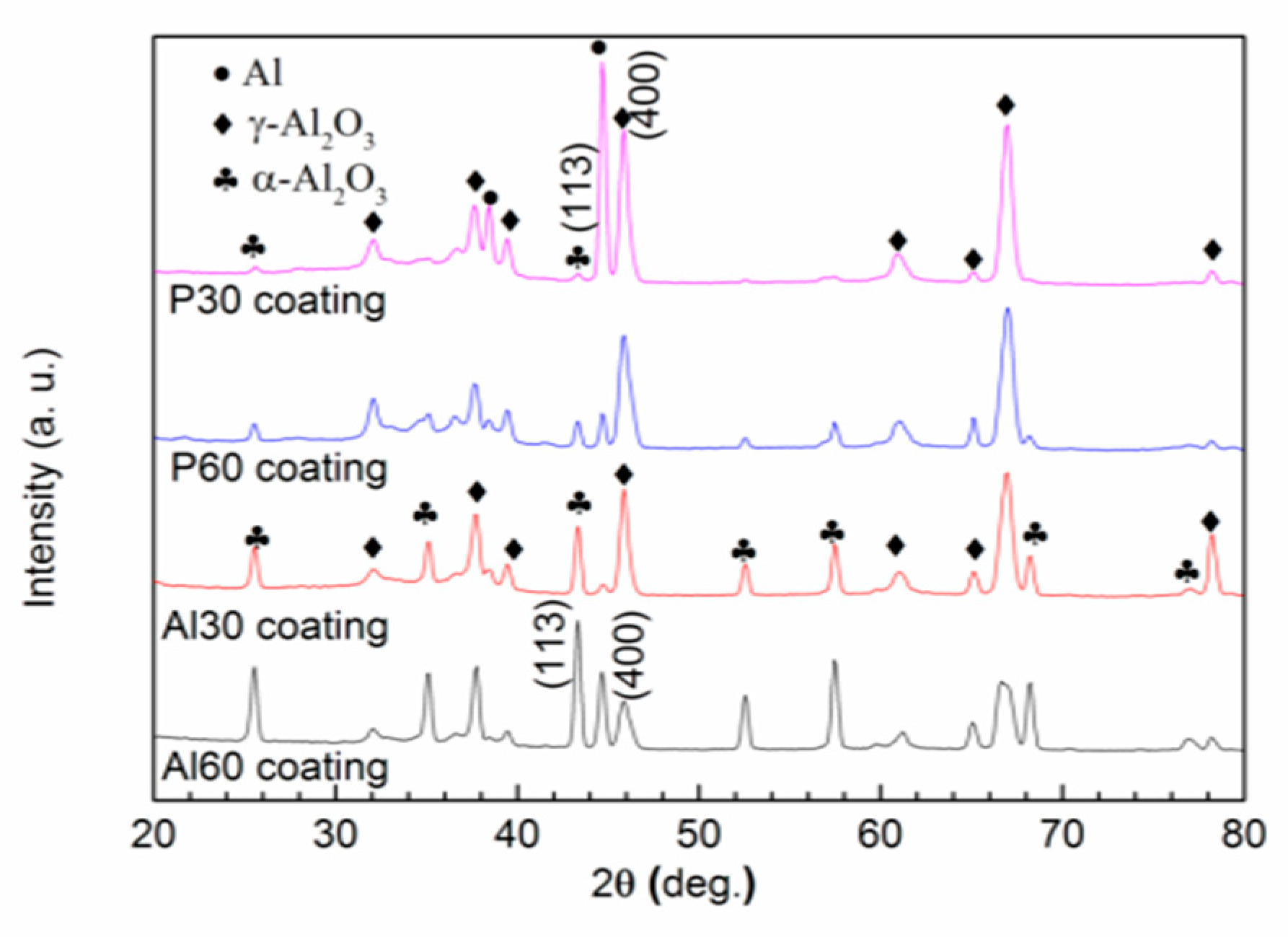
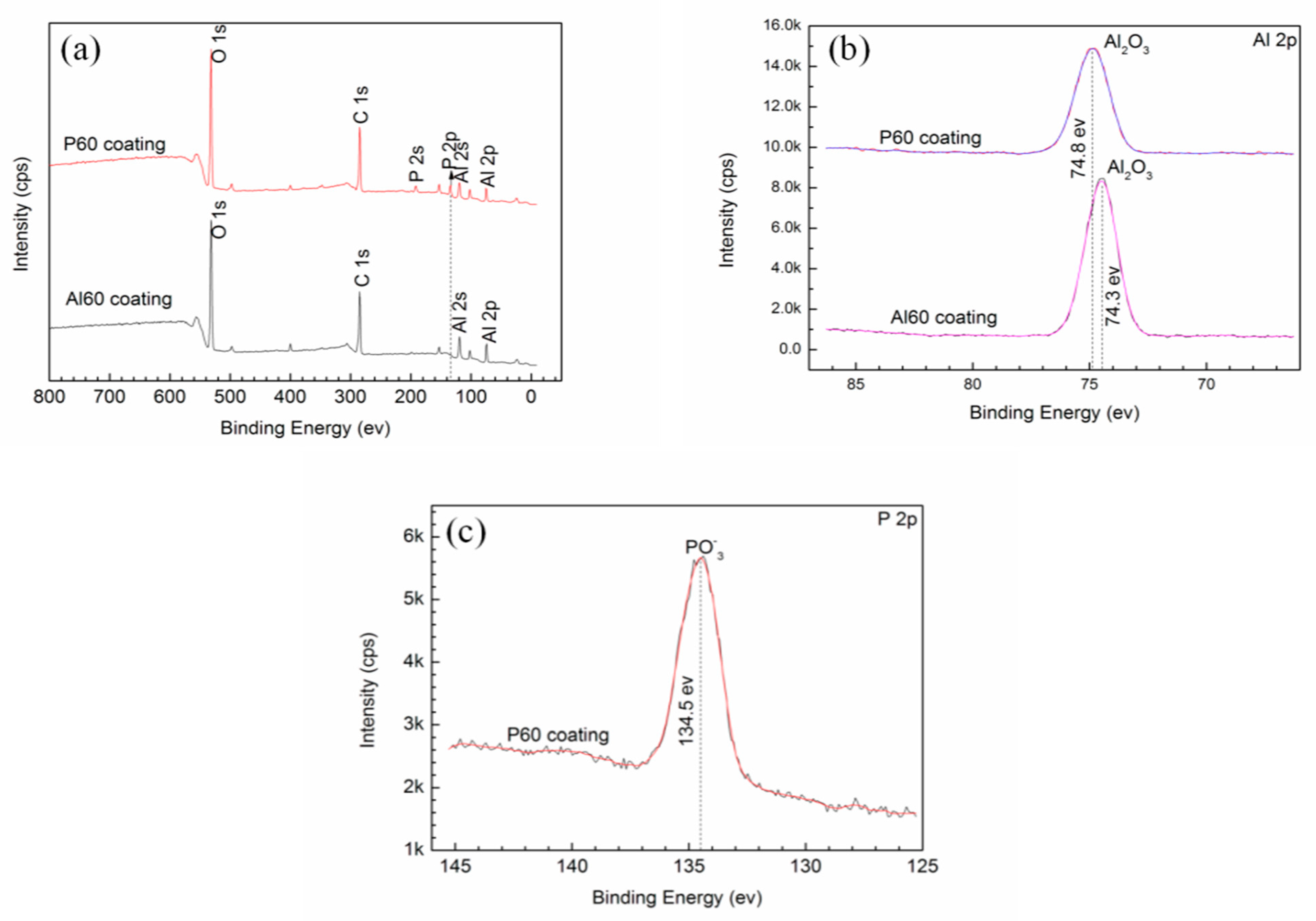
3.3. Phase and Chemical Composition of the Coatings
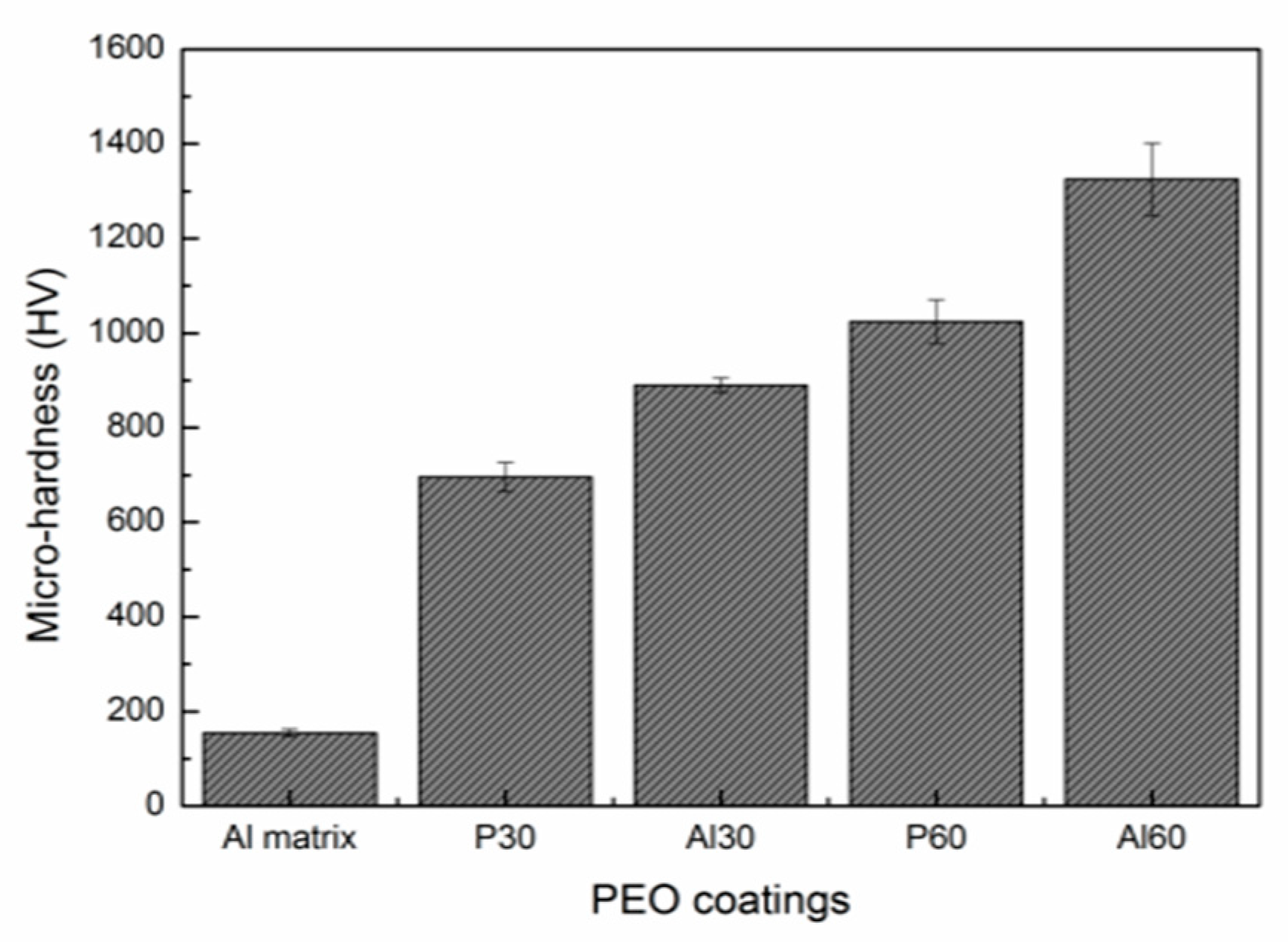
3.4. Micro-Hardness of the Coatings
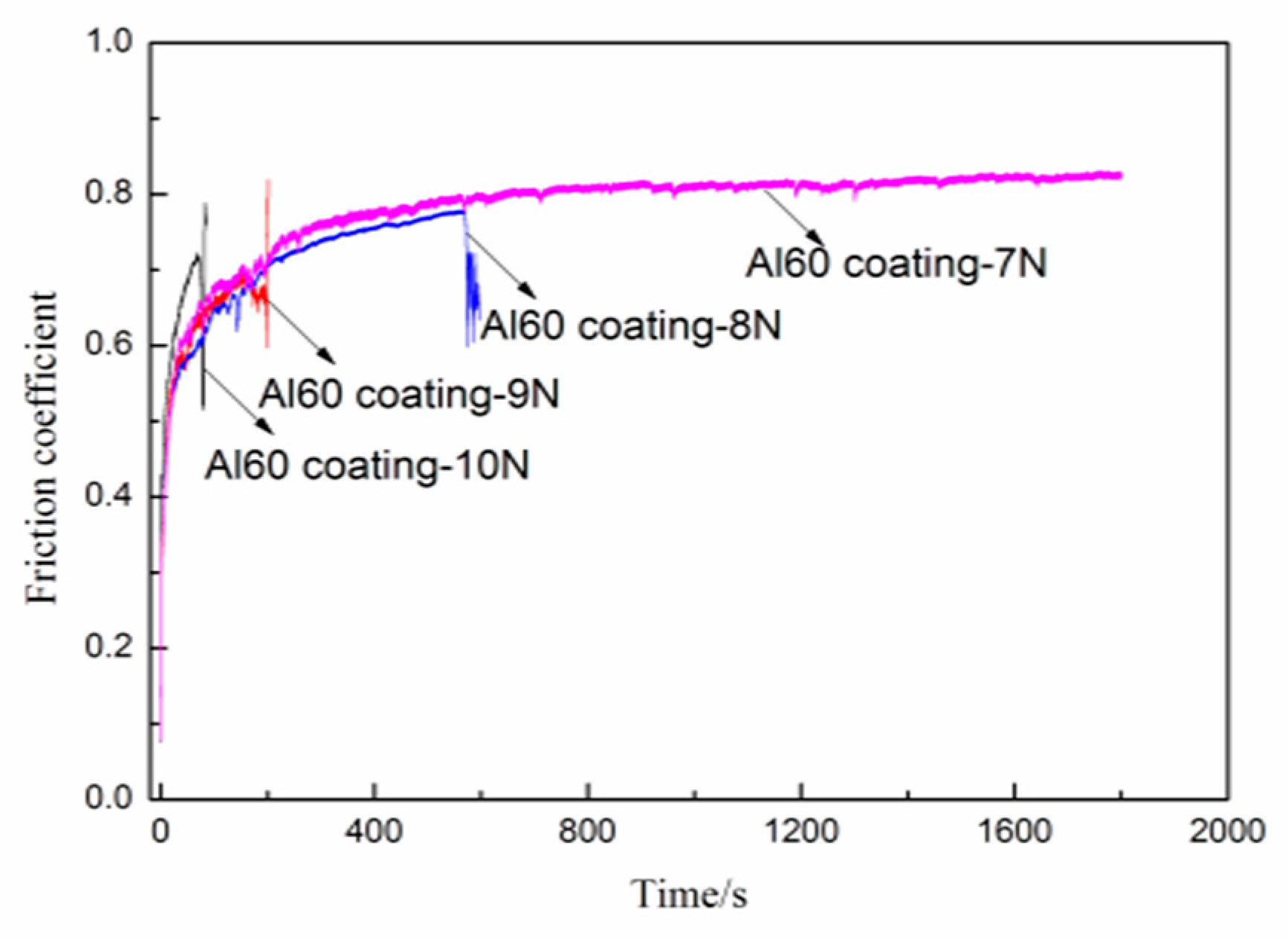
3.5. Friction and Wear Properties of the Coatings
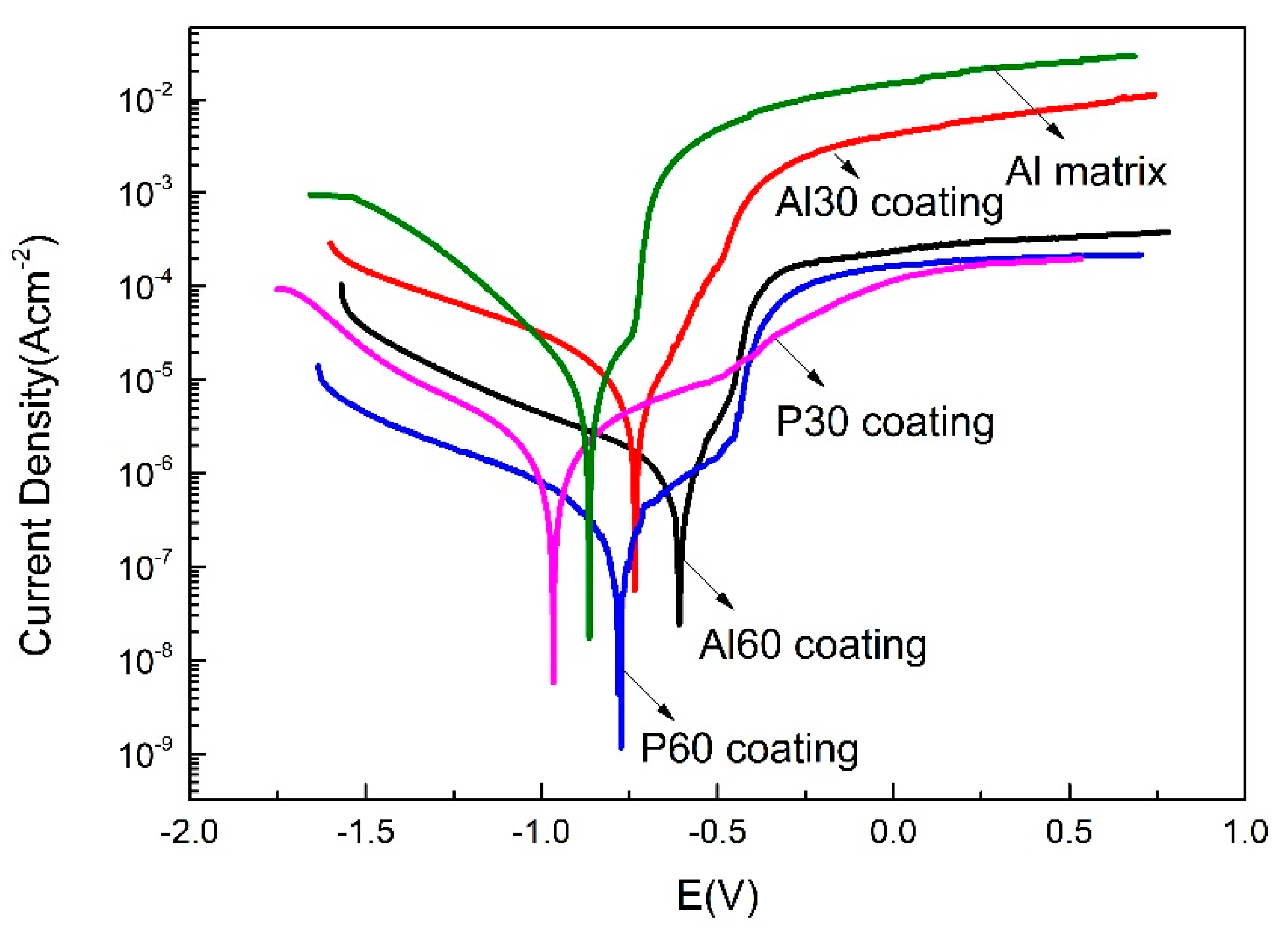
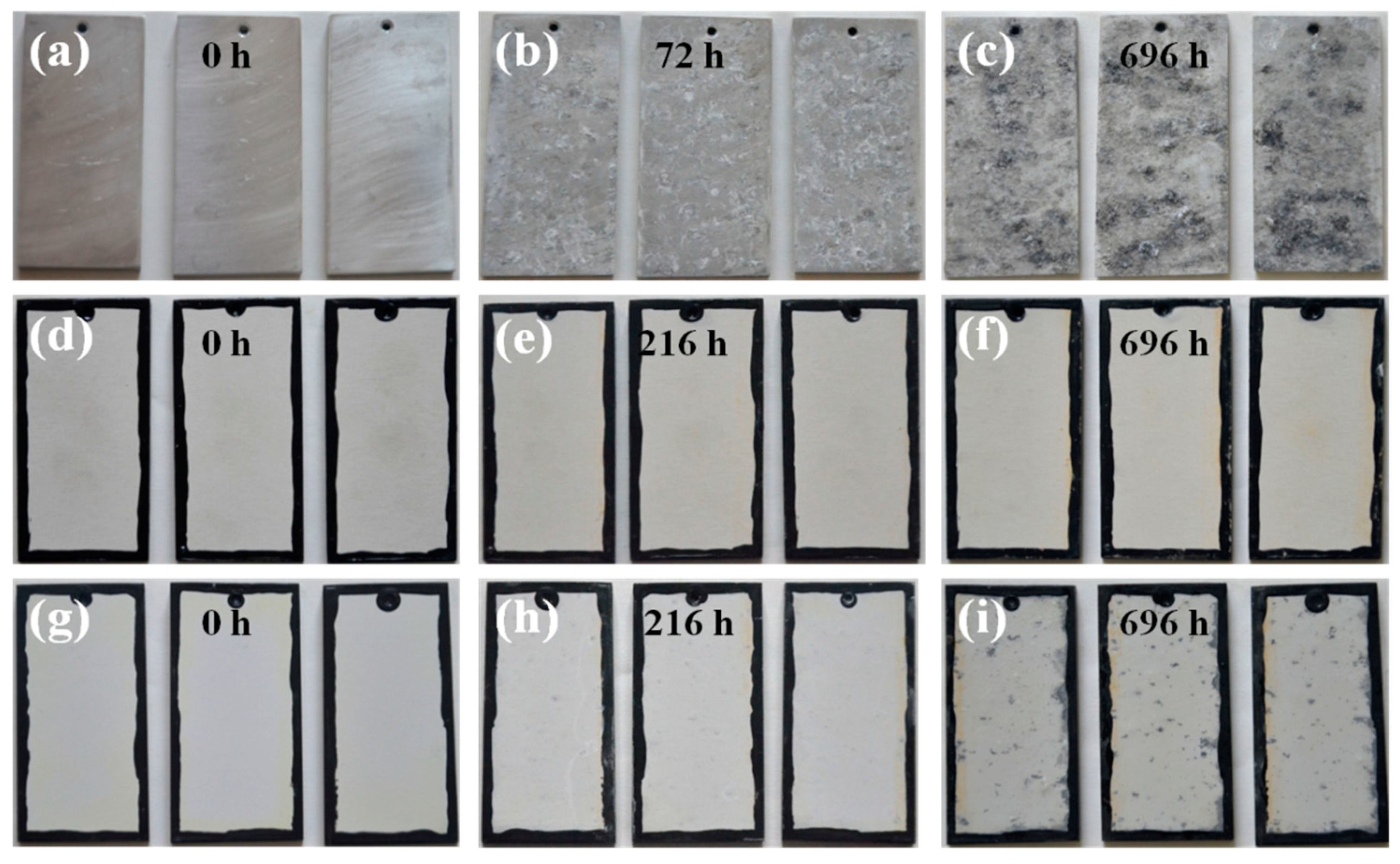
3.6. Corrosion Behavior of the PEO Coatings
4. Conclusions
- γ-Al2O3 and α-Al2O3 are the main crystal phases of the PEO coatings, accompanied by a small amount amorphous of phosphorus-rich oxide formed in phosphate electrolyte. With the increase of PEO treating time, the ratios of Iα/Iγ increased and the surface hardness increased.
- The single-layered coatings formed in the aluminate electrolyte exhibit more desirable wear resistance than that in the phosphate electrolyte, which is relatively high α-Al2O3 content.
- The bi-layered coatings formed in the phosphate electrolyte have better excellent acid corrosion resistance, which is attributed to the dense phosphorus-rich oxide of the inner layer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Qu, R.T.; Scudino, S.; Sun, B.A.; Prashanth, K.G.; Louzguine-Luzgin, D.V.; Chen, M.W.; Zhang, Z.F.; Eckert, J. Hybrid nanostructured aluminum alloy with super-high strength. NPG Asia Mater. 2015, 7, 229. [Google Scholar] [CrossRef]
- Park, G.H.; Kim, J.T.; Park, H.J.; Kim, Y.S.; Jeong, H.J.; Lee, N.; Seo, Y.; Suh, J.Y.; Son, H.T.; Wang, W.M.; et al. Development of lightweight Mg-Li-Al alloys with high specifific strength. J. Alloys Compd. 2016, 680, 116–120. [Google Scholar] [CrossRef]
- Shin, J.; Kim, T.; Kim, D.E.; Kim, D.; Kim, K. Castability and mechanical properties of new 7xxx aluminum alloys for automotive chassis/body applications. J. Alloys Compd. 2017, 698, 577–590. [Google Scholar] [CrossRef]
- Torre, A.D.L.; Afeltra, U.; Gomez-Esparza, C.D.; Camarillo-Cisneros, J.; Martinez-Sanchez, J. Grain refiner effect on the microstructure and mechanical properties of the A356 automotive wheels. J. Mater. Eng. Perform. 2014, 23, 581–587. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Snizhko, L.O.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Spatial characteristics of discharge phenomena in plasma electrolytic oxidation of aluminium alloy. Surf. Coat. Technol. 2004, 177, 779–783. [Google Scholar] [CrossRef]
- Wang, J.H.; Du, M.H.; Han, F.Z.; Yang, J. Effects of the ratio of anodic and cathodic currents on the characteristics of micro-arc oxidation ceramic coatings on Al-alloys. Appl. Surf. Sci. 2014, 292, 658–664. [Google Scholar] [CrossRef]
- Abreu, C.M.; Cristobal, M.J.; Figueroa, R.; Pena, G. Wear and corrosion performance of two different tempers (T6 and T73) of AA7075 aluminium alloy after nitrogen implantation. Appl. Surf. Sci. 2015, 327, 51–61. [Google Scholar] [CrossRef]
- Yin, B.; Peng, Z.J.; Liang, J.; Jin, K.J.; Zhu, S.Y.; Yang, J.; Qiao, Z.H. Tribological behavior and mechanism of self-lubricating wear-resistant composite coatings fabricated by one-step plasma electrolytic oxidation. Tribol. Int. 2016, 97, 97–107. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Ogino, T. Control of the spatial distribution of porous alumina micro-domes formed during anodic oxidation. Electrochim. Acta 2009, 54, 4712–4717. [Google Scholar] [CrossRef]
- Chen, R.; Iwabuchi, A.; Shimizu, T. The effect of a T6 heat treatment on the fretting wear of a SiC particle-reinforced A356 aluminum alloy matrix composite. Wear 2000, 238, 110–119. [Google Scholar] [CrossRef]
- Bolelli, G.; Bonferroni, B.; Coletta, G.; Lusvarghi, L.; Pitacco, F. Wear and corrosion behaviour of HVOF WCe-CoCr/CVD DLC hybrid coating systems deposited onto aluminium substrate. Surf. Coat. Technol. 2011, 205, 4211–4220. [Google Scholar] [CrossRef]
- Wang, S.X.; Liu, X.H.; Yin, X.L.; Du, N. Influence of electrolyte components on the microstructure and growth mechanism of plasma electrolytic oxidation coatings on 1060 aluminum alloy. Surf. Coat. Technol. 2020, 381, 125–214. [Google Scholar] [CrossRef]
- Li, Q.B.; Liang, J.; Liu, B.X.; Peng, Z.J.; Wang, Q. Effects of cathodic voltages on structure and wear resistance of plasma electrolytic oxidation coatings formed on aluminium alloy. Appl. Surf. Sci. 2014, 297, 176–181. [Google Scholar] [CrossRef]
- Fadaee, H.; Javidi, M. Investigation on the corrosion behaviour and microstructure of 2024-T3 Al alloy treated via plasma electrolytic oxidation. J. Alloys Compd. 2014, 604, 36–42. [Google Scholar] [CrossRef]
- Monfort, F.; Berkani, A.; Matykina, E.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K. Development of anodic coatings on aluminium under sparking conditions in silicate electrolyte. Corros. Sci. 2007, 49, 672–693. [Google Scholar] [CrossRef]
- Wang, D.D.; Liu, X.T.; Wu, Y.K.; Han, H.P.; Shen, D.J. Evolution process of the plasma electrolytic oxidation (PEO) coating formed on aluminum in an alkaline sodium hexametaphosphate ((NaPO3)6) electrolyte. J. Alloys Compd. 2019, 798, 129–143. [Google Scholar] [CrossRef]
- Xie, H.J.; Cheng, Y.L.; Li, S.X.; Cao, J.H.; Cao, L. Wear and corrosion resistant coatings on surface of cast A356 aluminum alloy by plasma electrolytic oxidation in moderately concentrated aluminate electrolytes. Trans. Nonferrous Met. Soc. China 2017, 27, 336–351. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Xue, Z.G.; Wang, Q.; Wu, X.Q.; Matykina, E.; Skeldon, P.; Thompson, G.E. New findings on properties of plasma electrolytic oxidation coatings from study of an Al-Cu-Li alloy. Electrochimi. Acta 2013, 107, 358–378. [Google Scholar] [CrossRef]
- Lugovskoy, A.; Zinigrad, M.; Kossenko, A.; Kazanski, B. Production of ceramic layers on aluminum alloys by plasma electrolytic oxidation in alkaline silicate electrolytes. Appl. Surf. Sci. 2013, 264, 743–747. [Google Scholar] [CrossRef]
- Aliramezani, R.; Raeissi, K.; Santamaria, M.; Hakimizad, A. Characterization and properties of PEO coatings on 7075 Al alloy grown in alkaline silicate electrolyte containing KMnO4 additive. Surf. Coat. Technol. 2017, 329, 250–261. [Google Scholar] [CrossRef]
- Wu, Y.-K.; Yang, Z.; Wang, R.Q.; Wu, G.R.; Chen, D.; Wang, D.D.; Liu, X.T.; Li, D.L.; Guo, C.H.; Yu, S.X.; et al. An investigation of microstructure evolution for plasma electrolytic oxidation (PEO) coated Al in an alkaline silicate electrolyte. Surf. Coat. Technol. 2018, 351, 136–152. [Google Scholar] [CrossRef]
- Rahimi, S.; Khiabani, A.B.; Yarmand, B.; Kolahi, A. Comparison of corrosion and antibacterial properties of Al alloy treated by plasma electrolytic oxidation and anodizing methods. Mater. Today Proc. 2018, 5, 15667–15676. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.K.; Zhang, X.Z.; Wang, D.D.; Liu, X.T.; Wu, G.R.; Li, D.L.; Yu, S.X.; Shen, D.J. An interesting anodic oxidation behavior of plasma electrolytic oxidation coatings fabricated on aluminum in alkaline phosphate electrolyte. Surf. Interfaces 2019, 16, 199–205. [Google Scholar] [CrossRef]
- Nakajima, M.; Miura, Y.; Fushimi, K.; Habazaki, H. Spark anodizing behaviour of titanium and its alloys in alkaline aluminate electrolyte. Corros. Sci. 2019, 51, 1534–1539. [Google Scholar] [CrossRef]
- Koo, B.H.; Lee, C.G.; Kim, U.J.; Lee, S.H.; Byon, E. Effects of electrolytes variation on formation of oxide layers of 6061 Al alloys by plasma electrolytic oxidation. Trans. Nonferrous Met. Soc. China 2009, 19, 866–870. [Google Scholar]
- Sang, S.B.; Kai, W.; Jung, Y.G.; Koo, B.H. Characteristic of AlON-Al2O3 coatings on Al6061 alloy by electrolytic plasma processing in aluminate and nitride electrolytes. Surf. Coat. Technol. 2010, 204, 3196–3199. [Google Scholar]
- Yerokhin, A.L.; Snizhko, L.O.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Discharge characterization in plasma electrolytic oxidation of aluminium. J. Phys. D Appl. Phys. 2003, 36, 2110–2120. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, M.; Wen, S.; Mao, X.; Huo, W.G.; Guo, Y.Y.; Wang, Y.X. Microstructure and wear resistance of micro-arc oxidation ceramic coatings prepared on 2A50 aluminum alloys. Surf. Coat. Technol. 2020, 394, 125853. [Google Scholar] [CrossRef]
- Xue, W.B.; Deng, Z.W.; Lai, Y.C.; Chen, R.Y. Analysis of Phase Distribution for Ceramic Coatings Formed by Microarc Oxidation on Aluminum Alloy. J. Am. Ceram. Soc. 1998, 81, 1365–1368. [Google Scholar] [CrossRef]
- Dehnavi, V.; Liu, X.Y.; Luan, B.L.; Shoesmith, D.W.; Rohani, S. Phase transformation in plasma electrolytic oxidation coatings on 6061 aluminum alloy. Surf. Coat. Technol. 2014, 251, 106–114. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Cao, J.H.; Mao, M.-K.; Peng, Z.M.; Skeldon, P.; Thompson, G.E. High growth rate, wear resistant coatings on an Al-Cu-Li alloy by plasma electrolytic oxidation in concentrated aluminate electrolytes. Surf. Coat. Technol. 2015, 269, 74–82. [Google Scholar] [CrossRef]
- Hughes, A.E.; Hedges, M.M.; Sexton, B.A. Reactions at the Al/SiO2/SiC layered interface. J. Mater. Sci. 1990, 25, 4856–4865. [Google Scholar] [CrossRef]
- Wagner, C.D. X-ray photoelectron spectroscopy with X-ray photons of higher energy. J. Vac. Sci. Technol. 1978, 15, 518–523. [Google Scholar] [CrossRef]
- Wang, Y.M.; Jiang, B.L.; Lei, T.Q.; Guo, L.X. Dependence of growth features of microarc oxidation coatings. Mater. Lett. 2004, 58, 1907–1911. [Google Scholar] [CrossRef]
- Muhaffel, F.; Cimenoglu, H. Development of corrosion and wear resistant micro-arc oxidation coating on a magnesium alloy. Surf. Coat. Technol. 2019, 357, 822–832. [Google Scholar] [CrossRef]


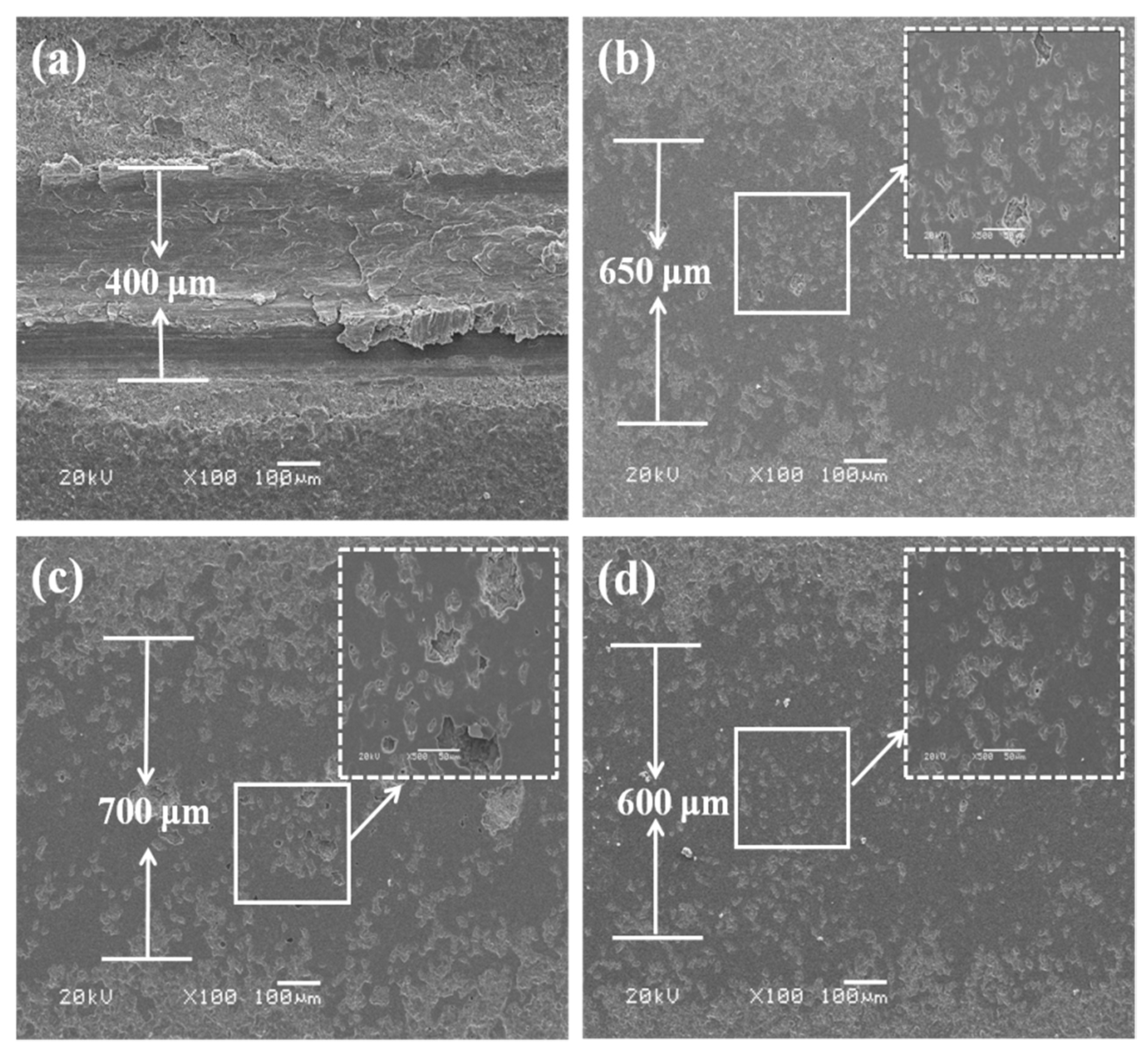
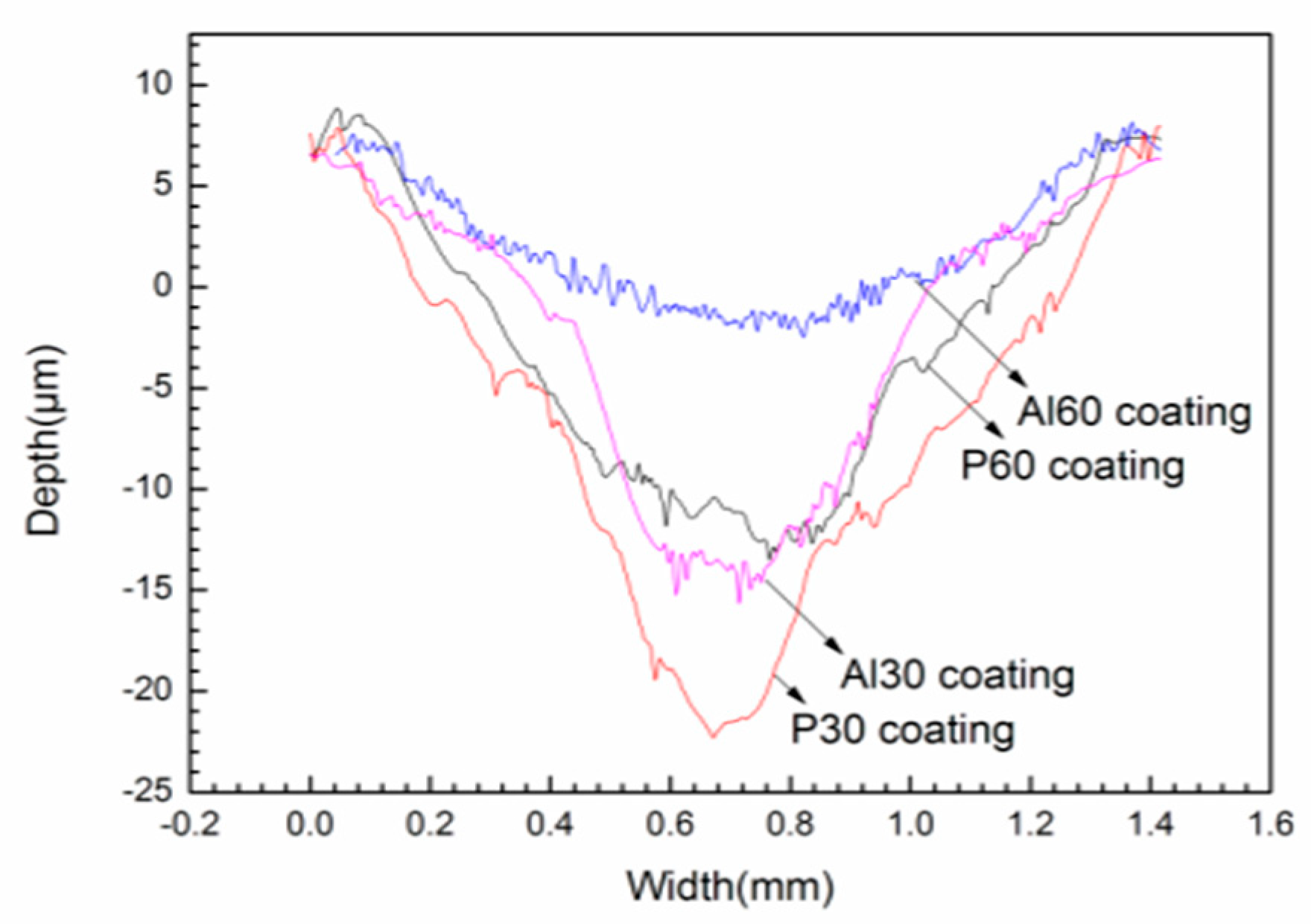

| Coatings | Coefficient of Friction | Width of Wear Track (μm) | Depth of Wear Track (μm) | Wear Rate (mm3/N·m) |
|---|---|---|---|---|
| P30 | 0.78 ± 0.01 | 400 | 29 | 6.46 × 10−4 |
| P60 | 0.81 ± 0.01 | 650 | 18 | 1.66 × 10−4 |
| Al30 | 0.83 ± 0.02 | 700 | 20 | 1.37 × 10−4 |
| Al60 | 0.82 ± 0.01 | 600 | 8 | 8.24 × 10−5 |
| Sample | Ecorr (V vs. AgCl) | icorr (A·cm−2) |
|---|---|---|
| Al alloy | −0.874 | 9.28 × 10−6 |
| P30 coating | −0.964 | 1.22 × 10−6 |
| Al30 coating | −0.739 | 4.77 × 10−6 |
| P60 coating | −0.784 | 1.09 × 10−7 |
| Al60 coating | −0.610 | 5.61 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Xu, H.; Liu, S.; Qi, Y.; Liang, J. Wear and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy in Electrolytes with Aluminate and Phosphate. Materials 2021, 14, 4037. https://doi.org/10.3390/ma14144037
Peng Z, Xu H, Liu S, Qi Y, Liang J. Wear and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy in Electrolytes with Aluminate and Phosphate. Materials. 2021; 14(14):4037. https://doi.org/10.3390/ma14144037
Chicago/Turabian StylePeng, Zhenjun, Hui Xu, Siqin Liu, Yuming Qi, and Jun Liang. 2021. "Wear and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy in Electrolytes with Aluminate and Phosphate" Materials 14, no. 14: 4037. https://doi.org/10.3390/ma14144037






