Microstructure and Optical Properties of Nanostructural Thin Films Fabricated through Oxidation of Au–Sn Intermetallic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Li, l.; Ao, S.; Wang, J.; Li, G. Interfacial Doping of Heteroatom in Porous SnO2 for Highly Sensitive Surface Properties. ACS Omega 2018, 3, 6988–6997. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, J.S.; Zheng, Y.H.; Xu, X.L.; Liu, M.W.; Fang, X.X. Tuning Al2O3 Surface with SnO2 to Prepare Improved Supports for Pd for CO Oxidation. ChemCatChem 2014, 6, 1604–1611. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.P.; Wang, B.B.; Zhao, M.G.; Liu, F.; Zhang, X.B. Nickel-doped tin oxide hollow nanofibers prepared by electrospinning for acetone sensing. Sens. Actuators B 2014, 190, 78–85. [Google Scholar] [CrossRef]
- Ding, L.X.; Wang, A.L.; Ou, Y.N.; Li, Q.; Guo, R.; Zhao, W.X.; Tong, y.X.; Li, G.R. Hierarchical Pd-Sn alloy nanosheet dendrites: An economical and highly active catalyst for ethanol electrooxidation. Sci. Rep. 2013, 3, 1181. [Google Scholar] [CrossRef]
- Liu, W.-L.; Lin, F.C.; Yang, Y.-C.; Huang, C.-H.; Gwo, S.; Huang, M.H.; Huang, J.-S. The influence of shell thickness of Au@TiO2 core–shell nanoparticles on the plasmonic enhancement effect in dye-sensitized solar cells. Nanoscale 2013, 5, 7953–7962. [Google Scholar] [CrossRef]
- Hosseini, Z.S.; Zad, A.I.; Mortezaali, A. Room temperature H2S gas sensor based on rather aligned ZnO nanorods with flower-like structures. Sens. Actuators B 2015, 207, 865–871. [Google Scholar] [CrossRef]
- Song, W.; Wu, H.; Wang, J.; Lin, Y.; Song, J.; Xie, Y.; Li, L.; Shi, K. A CH3NH3PbI3 film for a room-temperature NO2 gas sensor with quick response and high selectivity. Aust. J. Chem. 2015, 68, 1569. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, G.; Zhu, Y.; Zhang, H.; Xu, Z.; Dai, Z.; Cai, W. Room temperature H2S gas sensing properties of In2O3 micro/nanostructured porous thin film and hydrolyzation-induced enhanced sensing mechanism. Sens. Actuators B 2016, 228, 74–84. [Google Scholar] [CrossRef]
- Bhati, V.S.; Ranwa, S.; Rajamani, S.; Kumari, K.; Raliya, R.; Biswas, P.; Kumar, M. Improved sensitivity with low limit of detection of a hydrogen gas sensor based on rGO-loaded Ni-doped ZnO nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 11116–11124. [Google Scholar] [CrossRef]
- Kou, X.; Xie, N.; Chen, F.; Wang, T.; Guo, L.; Wang, C.; Wang, Q.; Ma, J.; Sun, Y.; Zhang, H.; et al. Superior acetone gas sensor based on electrospun SnO2 nanofibers by Rh doping. Sens. Actuators B 2018, 256, 861–869. [Google Scholar] [CrossRef]
- Kim, H.; Pak, Y.; Jeong, Y.; Kim, W.; Kim, J.; Jung, G.Y. Amorphous Pd–assisted H2 detection of ZnO nanorod gas sensor with enhanced sensitivity and stability. Sens. Actuators B 2018, 262, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Sankar Ganesh, R.; Navaneethan, M.; Patil, V.L.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.S.; Hayakawa, Y. Sensitivity enhancement of ammonia gas sensor based on Ag/ZnO flower and nanoellipsoids at low temperature. Sens. Actuators B 2018, 255, 672–683. [Google Scholar]
- Park, S.; Kim, S.; Kheel, H.; Hyun, S.K.; Jin, C.; Lee, C. Enhanced H2S gas sensing performance of networked CuO–ZnO composite nanoparticle sensor. Mater. Res. Bull. 2016, 82, 130–135. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, L.; Wang, L.; Sun, P.; Lu, H.; Liu, F.; Chuai, X.; Lu, G. Ultrasensitive and low detection limit of toluene gas sensor based on SnO2-decorated NiO nanostructure. Sens. Actuators B 2018, 255, 3505–3515. [Google Scholar] [CrossRef]
- Chen, H.; Hu, J.; Li, G.-D.; Gao, Q.; Wei, C.; Zou, X. Porous Ga–In Bimetallic Oxide Nanofibers with Controllable Structures for Ultrasensitive and Selective Detection of Formaldehyde. ACS Appl. Mater. Interfaces 2017, 9, 4692–4700. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wu, Y.; Wu, Z.; Yin, L.; Weng, Z.; Zhong, Y.; Xu, W.; Sun, X.; Wang, H. Unlocking Bifunctional Electrocatalytic Activity for CO2 Reduction Reaction by Win-Win Metal–Oxide Cooperation. ACS Energy Lett. 2018, 3, 2816–2822. [Google Scholar] [CrossRef]
- Fang, M.; Dong, G.; Wei, R.; Ho, J.C. Hierarchical Nanostructures: Design for Sustainable Water Splitting. Adv. Energy Mater. 2017, 7, 1700559. [Google Scholar] [CrossRef] [Green Version]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.T.; Lee, Y.-L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.M.; Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Liu, C.; Liu, Y.; Qiao, Z.-A.; Huo, Q. A controllable asymmetrical/symmetrical coating strategy for architectural mesoporous organosilica nanostructures. Nanoscale 2016, 8, 13581–13588. [Google Scholar] [CrossRef]
- Jo, Y.-M.; Kim, T.-H.; Lee, C.-S.; Lim, K.; Na, C.; Abdel-Hady, F.; Wazzan, A.A.; Lee, J.-H. Metal–Organic Framework–Derived Hollow Hierarchical Co3O4 Nanocages with Tunable Size and Morphology: Ultrasensitive and Highly Selective Detection of Methylbenzenes. ACS Appl. Mater. Interfaces 2018, 10, 8860–8868. [Google Scholar] [CrossRef]
- Choi, J.; Kim, W.-S.; Hong, S.-H. Highly stable SnO2-Fe+2O3-C hollow spheres for reversible lithium storage with extremely long cycle life. Nanoscale 2018, 10, 4370–4376. [Google Scholar] [CrossRef]
- Rai, P.; Yoon, J.-W.; Jeong, H.-M.; Hwang, S.-J.; Kwak, C.-H.; Lee, J.-H. Design of highly sensitive and selective Au@NiO yolk-shell nanoreactors for gas sensor applications. Nanoscale 2014, 6, 8292–8299. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Y.; Xu, S.; Li, G. A wrinkle to sub–100 nm yolk/shell Fe3O4@SiO2 nanoparticles. Nano Res. 2016, 9, 3632–3643. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Dong, J.; Ding, F.; Li, X.; Zhang, B.; Yang, S.; Zhang, K. Facile general strategy toward hierarchical mesoporous transition metal Oxides Arrays on three-dimensional macroporous foam with superior lithium storage Properties. Nano Energy 2015, 13, 77–91. [Google Scholar] [CrossRef]
- Zheng, N.; Stucky, G.D. A General Synthetic Strategy for Oxide–Supported Metal Nanoparticle Catalysts. J. Am. Chem. Soc. 2006, 128, 14278–14280. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yao, M.-S.; Lin, Y.-H.; Nan, C.-W. A comprehensive review on synthesis methods for transition-metal oxide nanostructures. CrystEngComm 2015, 17, 3551–3585. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition–Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Carretero-Genevriera, A.; Mestres, N. Growth of 1-D Oxide Nanostructures. In Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2015; pp. 1–17. [Google Scholar]
- Liang, Y.; Chen, Z.; Yao, W.; Wang, P.; Yu, S.; Wang, X. Decorating of Ag and CuO on Cu Nanoparticles for Enhanced High Catalytic Activity to the Degradation of Organic Pollutants. Langmuir 2017, 33, 7606–7614. [Google Scholar] [CrossRef]
- Bertuna, A.; Faglia, G.; Ferroni, M.; Kaur, N.; Arachchige, H.M.M.M.; Sberveglieri, G.; Comini, E. Metal Oxide Nanowire Preparation and Their Integration into Chemical Sensing Devices at the SENSOR Lab in Brescia. Sensors 2017, 17, 1000. [Google Scholar] [CrossRef]
- Yu, K.; Wu, Z.; Zhao, Q.; Li, B.; Xie, Y. High-Temperature-Stable Au@SnO2 Core/Shell Supported Catalyst for CO Oxidatio. J. Phys. Chem. C 2008, 112, 2244–2247. [Google Scholar] [CrossRef]
- Louis, C. Chemical Preparation of Supported Bimetallic Catalysts. Gold-Based Bimetallic, a Case Study. Catalysts 2016, 6, 110. [Google Scholar] [CrossRef]
- Pan, X.; Zheng, J.; Zhang, L.; Yi, Z. Core-Shell Au@SnO2 Nanostructures Supported on Na2Ti4O9 Nanobelts as a Highly Active and Deactivation-Resistant Catalyst toward Selective Nitroaromatics Reduction. Inorg. Chem. 2019, 58, 11164–11171. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Samu, G.F.; Balog, Á.; Csapoó, E.; Janaáky, C. Composition-Dependent Electrocatalytic Behavior of Au-Sn Bimetallic Nanoparticles in Carbon Dioxide Reduction. ACS Energy Lett. 2019, 4, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Corma, A.; Serna, P. Chemoselective Hydrogenation of Nitro Compounds with Supported Gold Catalysts. Science 2006, 313, 332–334. [Google Scholar] [CrossRef]
- Zhong, Z.; Lin, J.; Teh, S.-P.; Teo, J.; Dautzenberg, F.M. A Rapid and Efficient Method to Deposit Gold Particles on Catalyst Supports and Its Application for CO Oxidation at Low Temperatures. Adv. Funct. Mater. 2007, 17, 1402–1408. [Google Scholar] [CrossRef]
- Zhong, C.J.; Maye, M.M. Core–Shell Assembled Nanoparticles as Catalysts. Adv. Mater. 2001, 13, 1507–1511. [Google Scholar] [CrossRef]
- Cui, Z.M.; Li, L.J.; Manthiram, A.; Goodenough, J.B. A Surfactant-Free Strategy for Synthesizing and Processing Intermetallic Platinum-Based Nanoparticle Catalysts. J. Am. Chem. Soc. 2015, 137, 7278–7281. [Google Scholar] [CrossRef]
- Cui, Z.M.; Chen, H.; Zhao, M.T.; Marshall, D.; Yu, Y.C.; Abruna, H.; DiSalvo, F.J. Synthesis of structurally ordered Pt3Ti and Pt3V nanoparticles as methanol oxidation catalysts. J. Am. Chem. Soc. 2014, 136, 10206–10209. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Pyo, J.B.; Ye, X.C.; Gordon, T.R.; Murray, C.B. Synthesis, Shape Control, and Methanol Electro-oxidation Properties of Pt–Zn Alloy and Pt3Zn Intermetallic Nanocrystals. Acs Nano 2012, 6, 5642–5647. [Google Scholar] [CrossRef] [PubMed]
- Rerek, T.; Skowronski, L.; Szczesny, R.; Naparty, M.K.; Derkowska-Zielinska, B. The effect of the deposition rate on morphology, opto-electronic properties and formation intermetallic compounds of Au–Sn alloys. J. Alloys Compd. 2020, 849, 156041. [Google Scholar] [CrossRef]
- Rerek, T.; Skowronski, L.; Kobierski, M.; Naparty, M.K.; Derkowska-Zielinska, B. Microstructure and opto-electronic properties of Sn-rich Au-Sn diffusive solders. Appl. Surf. Sci. 2018, 451, 32–39. [Google Scholar] [CrossRef]
- Wronkowska, A.A.; Wronkowski, A.; Bukaluk, A.; Trzcinski, M.; Okulewicz, K.; Skowronski, L. Structural analysis of In/Ag, In/Cu and In/Pd thin films on tungsten by ellipsometric, XRD and AES methods. Appl. Surf. Sci. 2008, 254, 4401–4407. [Google Scholar] [CrossRef]
- Wronkowska, A.A.; Wronkowski, A.; Skowronski, L. Non-destructive characterization of In/Ag and In/Cu diffusive layers. J. Alloys Compd. 2009, 479, 583–588. [Google Scholar] [CrossRef]
- Wronkowska, A.A.; Wronkowski, A.; Kukliński, K.; Senski, M.; Skowronski, L. Spectroscopic ellipsometry study of the dielectric response of Au-In and Ag-Sn thin-film couples. Appl. Surf. Sci. 2010, 256, 4839–4844. [Google Scholar] [CrossRef]
- Wronkowska, A.A.; Czerniak, G.; Wronkowski, A.; Skowronski, L. Optical and microstructural characterisation of Au–Sn and Cu–Sn diffusive layers. Appl. Surf. Sci. 2013, 281, 30–37. [Google Scholar] [CrossRef]
- Kasprzak, W.; Amirkhiz, B.S.; Niewczas, M. Structure and properties of cast Al–Si based alloy with Zr–V–Ti additions and its evaluation of high temperature performance. J. Alloys Compd. 2014, 595, 67–79. [Google Scholar] [CrossRef]
- Chookajorn, T.; Murdoch, H.A.; Schuh, C.A. Design of Stable Nanocrystalline Alloys. Science 2012, 337, 951–954. [Google Scholar] [CrossRef] [Green Version]
- Mosby, J.M.; Prieto, A.L. Direct electrodeposition of Cu2Sb for lithium-ion battery anodes. J. Am. Chem. Soc. 2008, 130, 10656–10661. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, M. Intermetallic compound catalysts: Synthetic scheme, structure characterization and catalytic application. J. Mater. Chem. A 2020, 8, 2207–2221. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Li, Q.; Fan, J.; Zheng, J.; Li, G. Smart Solution Chemistry to Sn-Containing Intermetallic Compounds through a Self-Disproportionation Process. Chem. A Eur. J. 2016, 22, 14196–14204. [Google Scholar] [CrossRef]
- Guizard, C.; Princivalle, A. Preparation and characterization of catalyst thin films. Catal. Today 2009, 146, 367–377. [Google Scholar] [CrossRef]
- Mehla, S.; Das, J.; Jampaiah, D.; Periasamy, S.; Nafady, A.; Bhargava, S.K. Recent advances in preparation methods for catalytic thin films and coatings. Catal. Sci. Technol. 2019, 9, 3582–3602. [Google Scholar] [CrossRef]
- Sutter, P.; Tenney, S.A.; Ivars-Barcelo, F.; Wu, L.; Zhu, Y.; Sutter, E. Alloy oxidation as a route to chemically active nanocomposites of gold atoms in a reducible oxide matrix. Nanoscale Horiz. 2016, 1, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-G.; Tian, M.-L.; Mallouk, T.E.; Chan, M.H.W. Microstructure and interdiffusion of template-synthesized Au/Sn/Au junction nanowires. Nano Lett. 2004, 4, 1113–1118. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Miura, K.; Kajihara, M.; Kurokawa, N.; Sakamoto, K. Kinetics of reactive diffusion between Au and Sn during annealing at solid-state temperatures. Mater. Sci. Eng. A 2005, 390, 118–126. [Google Scholar] [CrossRef]
- Woollam, J.A. Guide to Using WVASE32®; Wextech Systems Inc.: New York, NY, USA, 2010. [Google Scholar]
- Fujiwara, H. Spectroscopic Ellipsometry. Principles and Applications; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Rerek, T.; Skowronski, L.; Szczesny, R.; Naparty, M.K.; Derkowska-Zielinska, B. The effect of the deposition rate on microstructural and opto-electronic properties of β-Sn layers. Thin Solid Films 2019, 670, 86–92. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Barman, S.; Roy, T.; Singh, V.K.; Sarkar, S.; Chakrabarti, A.; Barman, S.R. Electronic structure of Au-Sn compounds grown on Au(111). Phys. Rev. B 2019, 100, 235404. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; He, A.; Liu, Q.; Ivey, D.G. Room temperature interfacial reactions in electrodeposited Au/Sn couples. Acta Mater. 2008, 56, 5818–5827. [Google Scholar] [CrossRef]
- Taylor, J.A.; Merchant, S.M.; Perry, D.L. Study of the oxidation of gold-tin performs using x-ray photoelectron spectroscopy. J. Appl. Phys. 1995, 78, 5356–5361. [Google Scholar] [CrossRef]
- Ciulik, J.; Notis, M.R. The Au-Sn phase diagram. J. Alloys Compd. 1993, 191, 71–78. [Google Scholar] [CrossRef]
- Bolzan, A.A.; Fong, C.; Kennedy, B.J.; Howard, C.J. Structural studies of rutile-type metal dioxides. Acta Crystallogr. Sect. B Struct. Sci. 1997, 53, 373–380. [Google Scholar] [CrossRef]
- Suh, I.-K.; Ohta, H.; Waseda, Y. High-temperature thermal expansion of six metallic elements measured by dilatation method and X-ray diffraction. J. Mater. Sci. 1988, 23, 757–760. [Google Scholar] [CrossRef]
- Hanfland, M.; Schwarz, U.; Syassen, K.; Takemura, K. Crystal structure of the high-pressure phase silicon VI. Phys. Rev. Lett. 1999, 82, 1197–1200. [Google Scholar] [CrossRef]
- Izumi, F. Pattern-fitting structure refinement of tin(II) oxide. J. Solid State Chem. 1981, 38, 381–385. [Google Scholar] [CrossRef]
- Bhatta, U.M.; Dash, J.K.; Rath, A.; Satyam, P.V. Structural phase transitions in Au thin films on Si (110): An in situ temperature dependent transmission electron microscopy study. Appl. Surf. Sci. 2009, 256, 567–571. [Google Scholar] [CrossRef]
- Li, Y.; Shi, W.; Gupta, A.; Chopra, N. Morphological evolution of gold nanoparticles on silicon nanowires and their plasmonics. RSC Adv. 2015, 5, 49708–49718. [Google Scholar] [CrossRef]
- Bhatta, U.M.; Dash, J.K.; Rath, A.; Satyam, P.V. Formation of aligned nanosilicide structures in a MBE-grown Au/Si(110) system: A real-time temperature-dependent TEM study. J. Phys. Condens. Matter 2009, 21, 205403. [Google Scholar] [CrossRef] [PubMed]
- Rath, A.; Juluri, R.R.; Satyam, P.V. Real time nanoscale structural evaluation of gold structures on Si (100) surface using in-situ transmission electron microscopy. J. Appl. Phys. 2014, 115, 184303. [Google Scholar] [CrossRef] [Green Version]
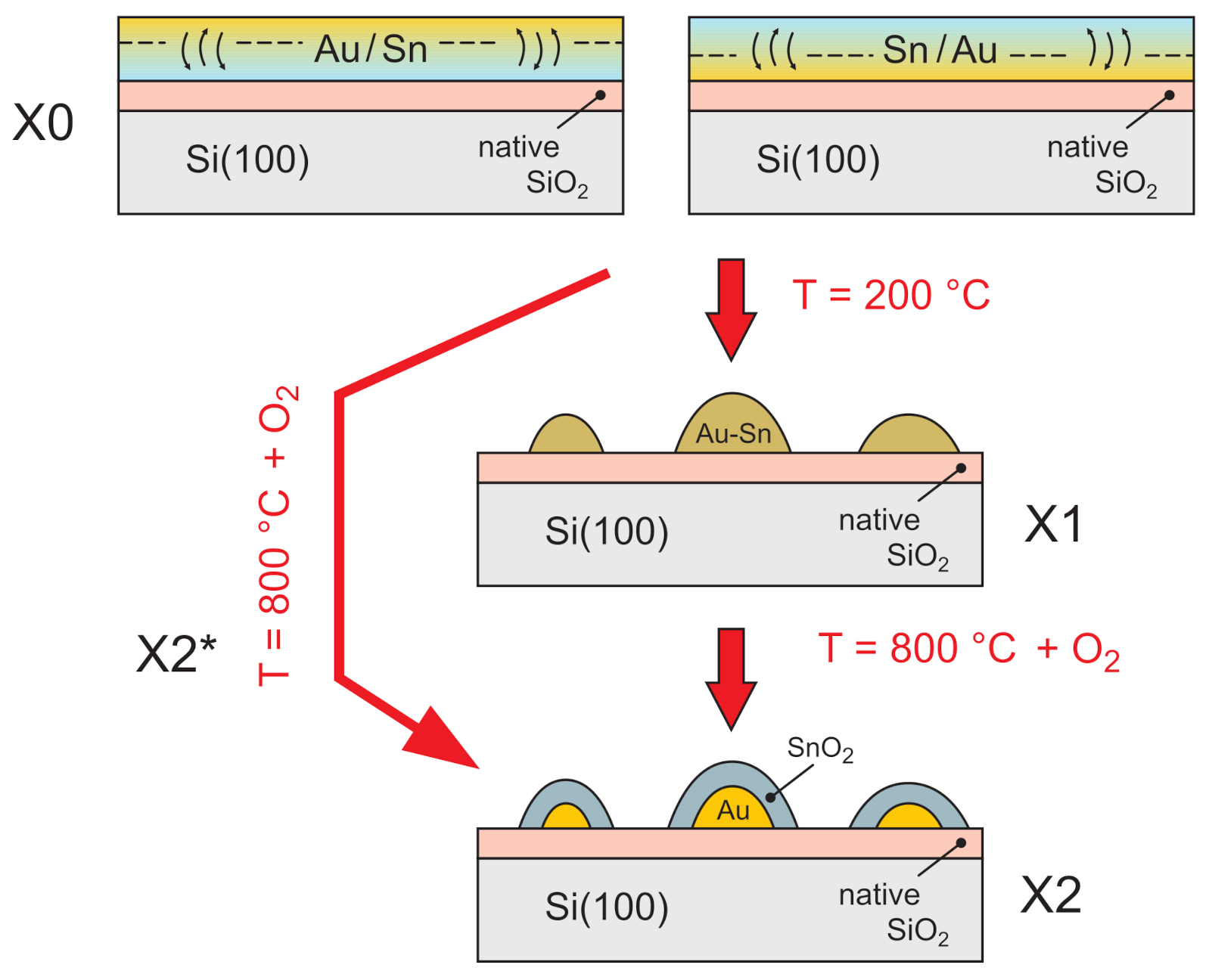
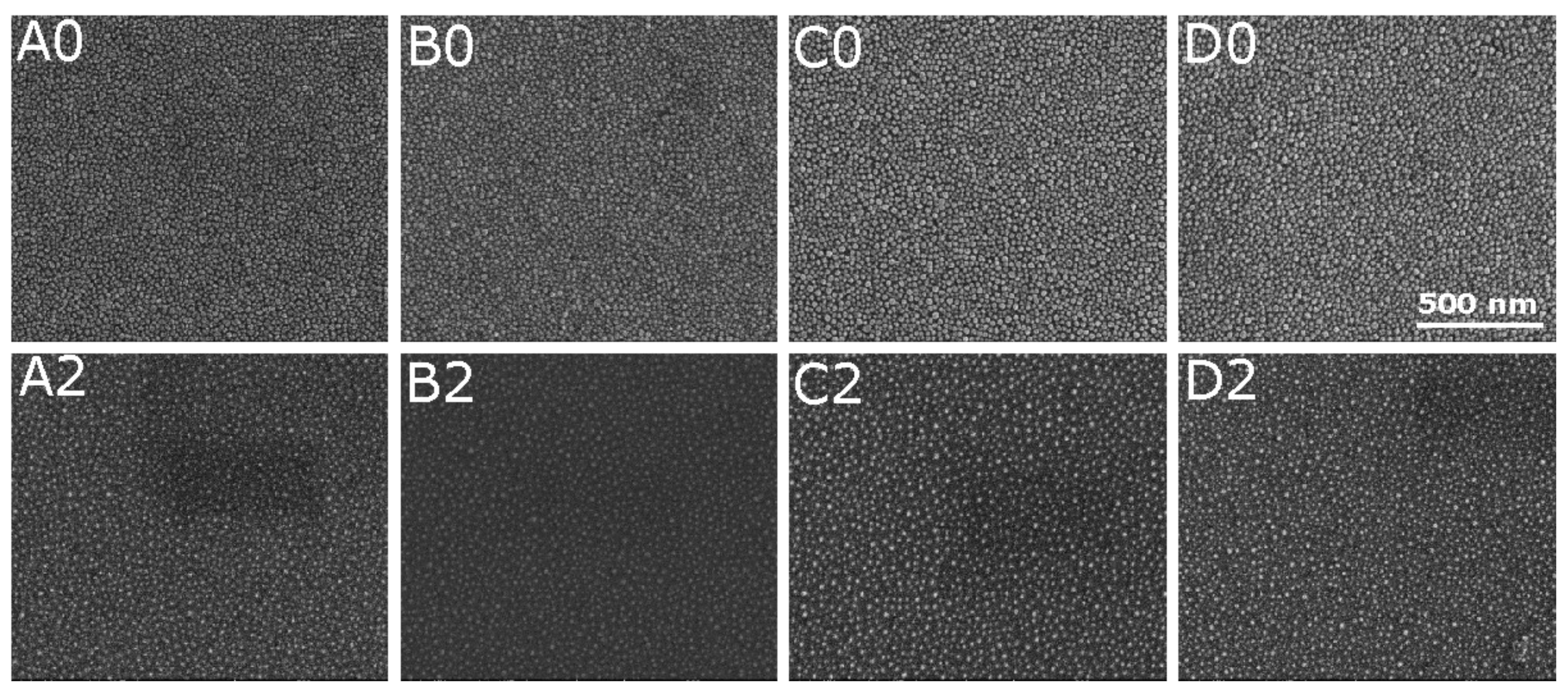
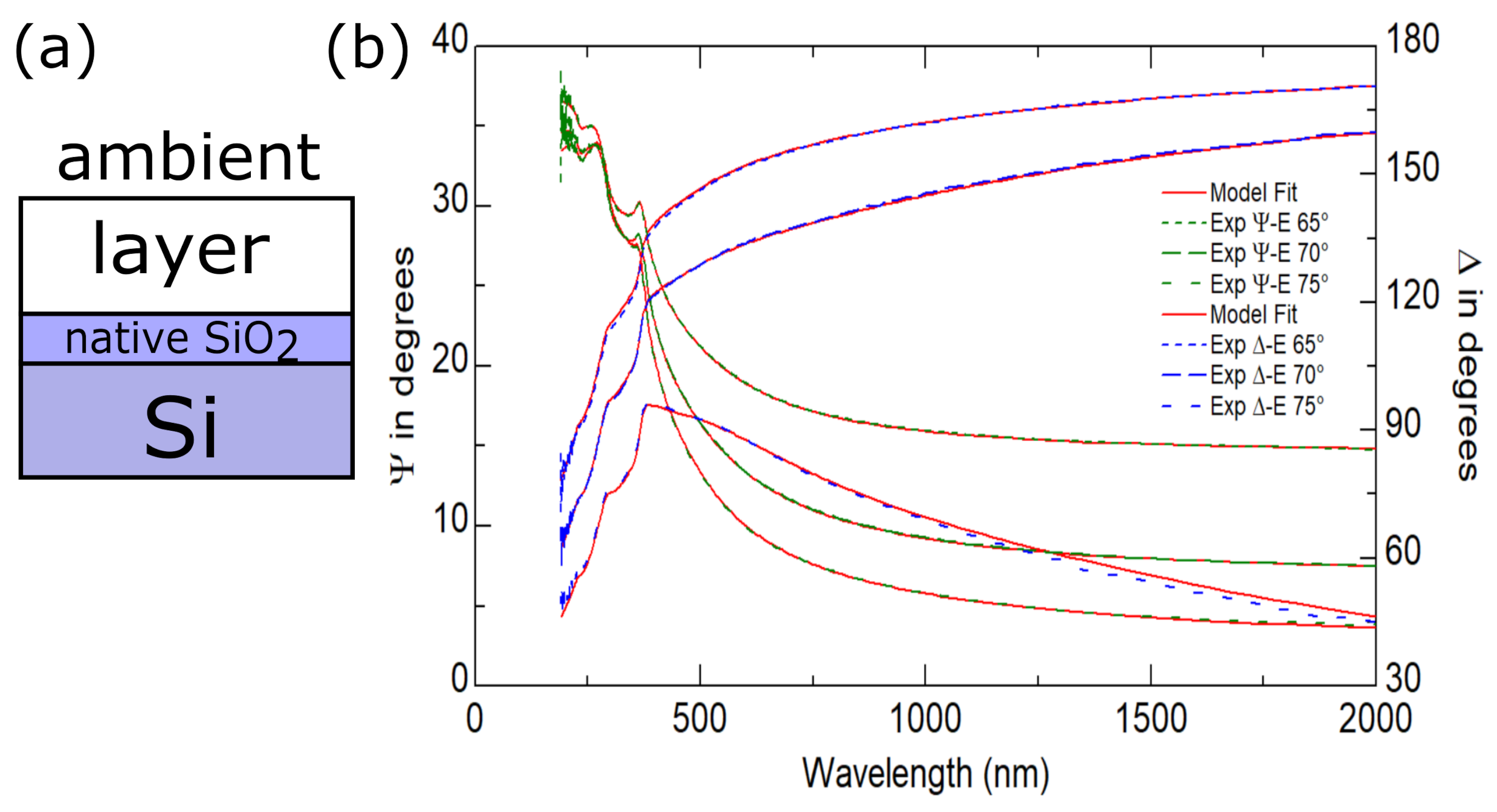
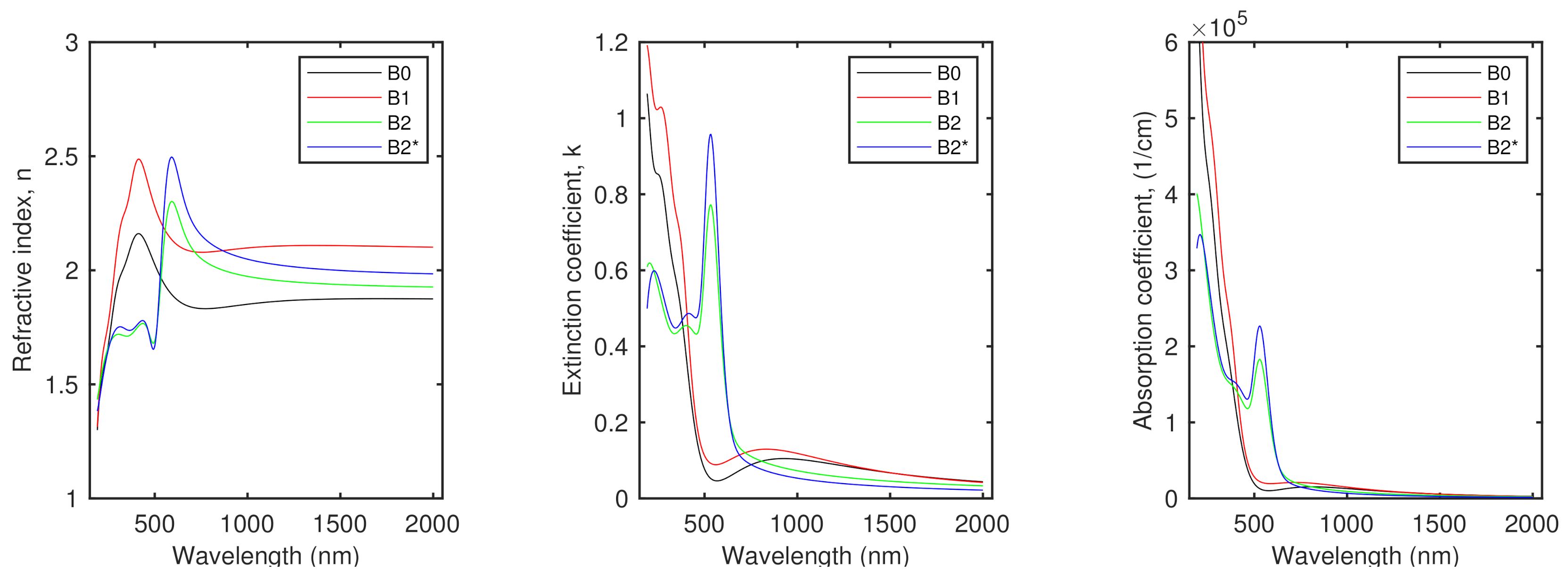

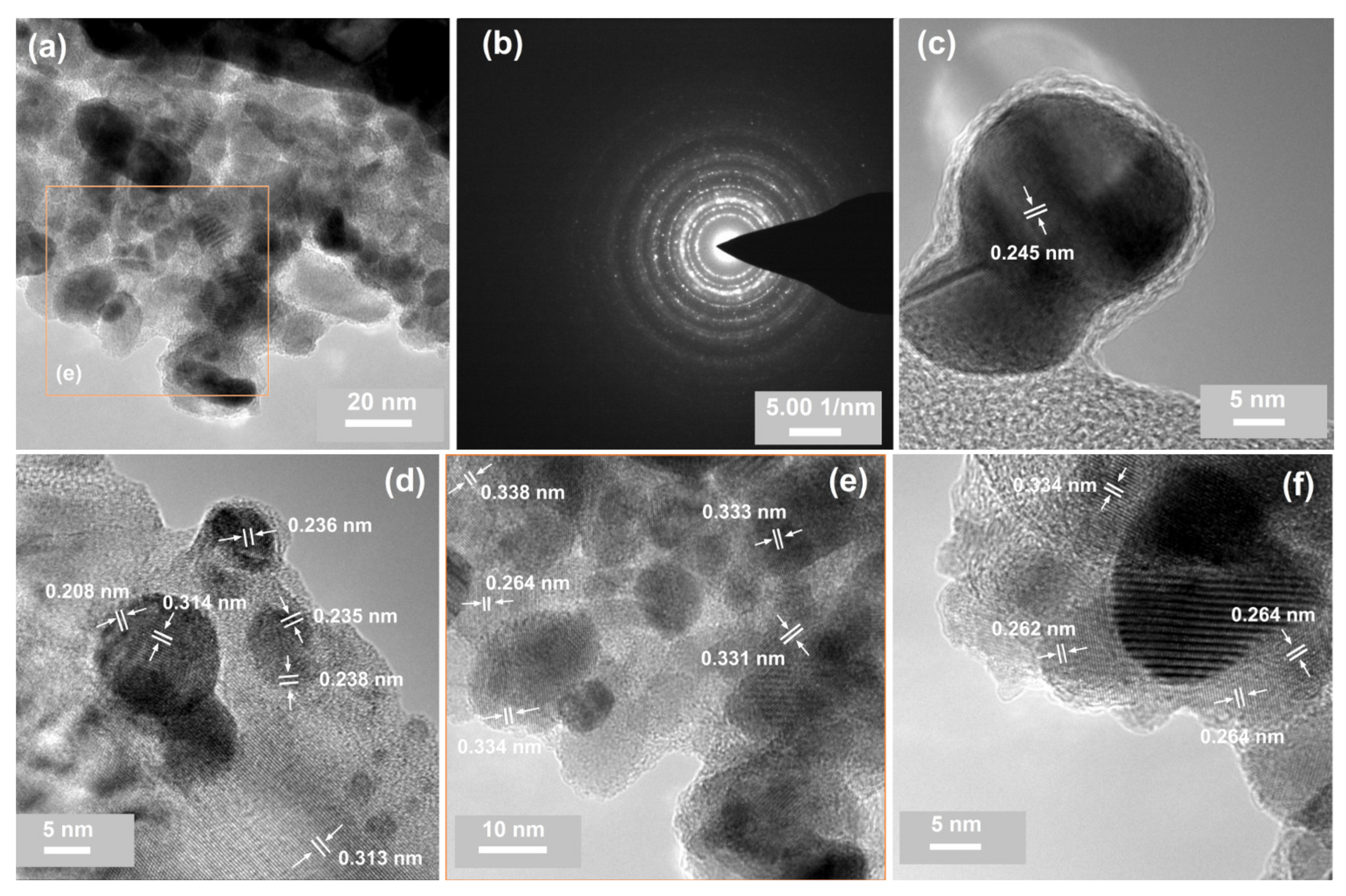
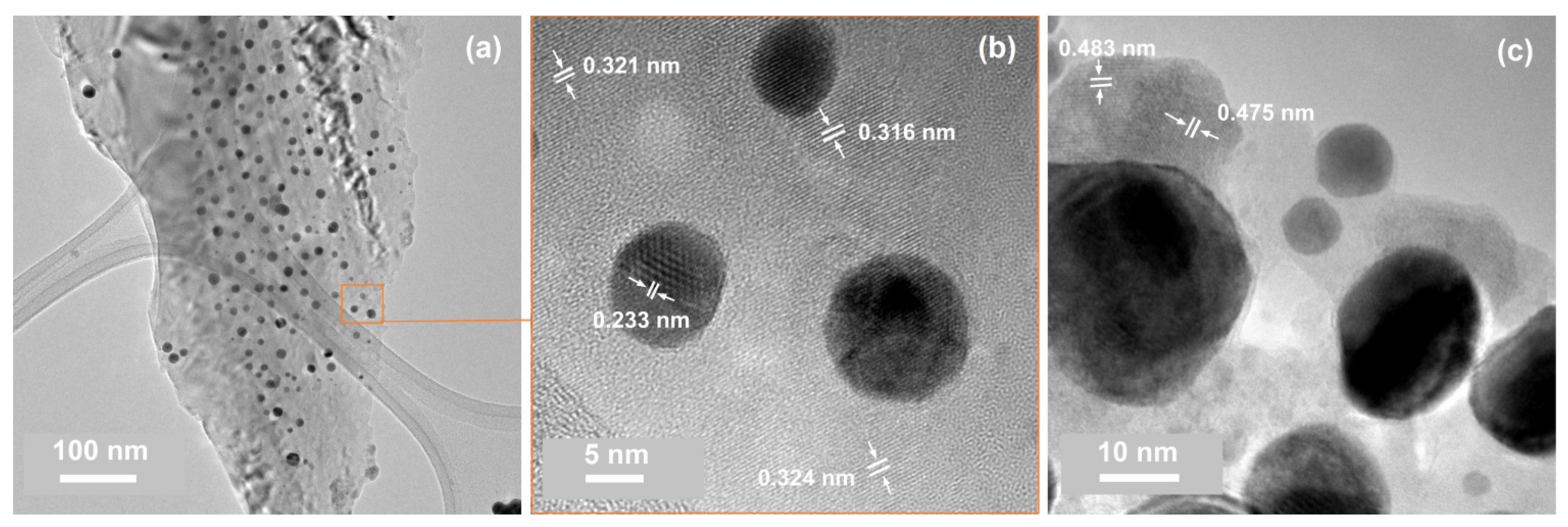
| No. | Id. | Sequence of Deposition | Au:Sn | (°C) | (°C) |
|---|---|---|---|---|---|
| 1. | A0 | Si\Au(19)\Sn(31) | 1:1 | - | - |
| 2. | A1 | Si\Au(19)\Sn(31) | 1:1 | 200 | - |
| 3. | A2 | Si\Au(19)\Sn(31) | 1:1 | 200 | 800 |
| 4. | A2* | Si\Au(19)\Sn(31) | 1:1 | - | 800 |
| 5. | B0 | Si\Sn(31)\Au(19) | 1:1 | - | - |
| 6. | B1 | Si\Sn(31)\Au(19) | 1:1 | 200 | - |
| 7. | B2 | Si\Sn(31)\Au(19) | 1:1 | 200 | 800 |
| 8. | B2* | Si\Sn(31)\Au(19) | 1:1 | - | 800 |
| 9. | C0 | Si\Au(12)\Sn(38) | 1:2 | - | - |
| 10. | C1 | Si\Au(12)\Sn(38) | 1:2 | 200 | - |
| 11. | C2 | Si\Au(12)\Sn(38) | 1:2 | 200 | 800 |
| 12. | C2* | Si\Au(12)\Sn(38) | 1:2 | - | 800 |
| 13. | D0 | Si\Sn(38)\Au(12) | 1:2 | - | |
| 14. | D1 | Si\Sn(38)\Au(12) | 1:2 | 200 | - |
| 15. | D2 | Si\Sn(38)\Au(12) | 1:2 | 200 | 800 |
| 16. | D2* | Si\Sn(38)\Au(12) | 1:2 | - | 800 |
| No. | Id. | (nm) | (nm) | (eV) |
|---|---|---|---|---|
| 1. | A0 | 18.1 ± 0.9 | - | - |
| 2. | A1 | 17.4 ± 1.6 | - | - |
| 3. | A2 | 8.3 ± 0.1 | 530 ± 4 | 2.34 ± 0.02 |
| 4. | A2* | 18.5 ± 0.1 | 530 ± 5 | 2.34 ± 0.02 |
| 5. | B0 | 14.3 ± 0.8 | - | - |
| 6. | B1 | 14.2 ± 0.9 | - | - |
| 7. | B2 | 10.2 ± 0.2 | 530 ± 5 | 2.34 ± 0.02 |
| 8. | B2* | 14.4 ± 0.1 | 530 ± 5 | 2.34 ± 0.02 |
| 9. | C0 | 15.5 ± 0.6 | - | - |
| 10. | C1 | 16.5 ± 0.8 | - | - |
| 11. | C2 | 4.8 ± 0.1 | 521 ± 4 | 2.38 ± 0.02 |
| 12. | C2* | 4.0 ± 0.1 | 521 ± 4 | 2.38 ± 0.02 |
| 13. | D0 | 13.5 ± 0.5 | - | - |
| 14. | D1 | 14.1 ± 0.6 | - | - |
| 15. | D2 | 4.5 ± 0.1 | 521 ± 4 | 2.38 ± 0.02 |
| 16. | D2* | 5.3 ± 0.1 | 521 ± 4 | 2.38 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowronski, L.; Trzcinski, M.; Olszewska, A.; Szczesny, R. Microstructure and Optical Properties of Nanostructural Thin Films Fabricated through Oxidation of Au–Sn Intermetallic Compounds. Materials 2021, 14, 4034. https://doi.org/10.3390/ma14144034
Skowronski L, Trzcinski M, Olszewska A, Szczesny R. Microstructure and Optical Properties of Nanostructural Thin Films Fabricated through Oxidation of Au–Sn Intermetallic Compounds. Materials. 2021; 14(14):4034. https://doi.org/10.3390/ma14144034
Chicago/Turabian StyleSkowronski, Lukasz, Marek Trzcinski, Aleksandra Olszewska, and Robert Szczesny. 2021. "Microstructure and Optical Properties of Nanostructural Thin Films Fabricated through Oxidation of Au–Sn Intermetallic Compounds" Materials 14, no. 14: 4034. https://doi.org/10.3390/ma14144034






