Phase Transition and Coefficients of Thermal Expansion in Al2−xInxW3O12 (0.2 ≤ x ≤ 1)
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Al2−xInxW3O12 Powders through Modified Reverse-Strike Co-Precipitation
2.2. Characterization of Al2−xInxW3O12 Powders
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Sun, W.; Zhang, Z.; Lovings, L.; Lind, C. Thermal Expansion Behavior in the A2M3O12 Family of Materials. Solids 2021, 2, 87–107. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Ari, M.; de Avillez, R.; Rizzo, F.; Ferreira, F.F.; Miller, K.J.; Johnson, M.B.; White, M.A. Correlation between AO6 Polyhedral Distortion and Negative Thermal Expansion in Orthorhombic Y2Mo3O12 and Related Materials. Chem. Mater. 2009, 21, 2886–2894. [Google Scholar] [CrossRef]
- Romao, C.P.; Perras, F.A.; Werner-Zwanziger, U.; Lussier, J.A.; Miller, K.J.; Calahoo, C.M.; Zwanziger, J.W.; Bieringer, M.; Marinkovic, B.A.; Bryce, D.L.; et al. Zero Thermal Expansion in ZrMgMo3O12: NMR Crystallography Reveals Origins of Thermoelastic Properties. Chem. Mater. 2015, 27, 2633–2646. [Google Scholar] [CrossRef]
- Sleight, A.; Brixner, L. A new ferroelastic transition in some A2(MO4)3 molybdates and tungstates. J. Solid State Chem. 1973, 7, 172–174. [Google Scholar] [CrossRef]
- Woodcock, D.A.; Lightfoot, P.; Ritterb, C. Negative Thermal Expansion in Y2(WO4)3. J. Solid State Chem. 2000, 149, 92–98. [Google Scholar] [CrossRef]
- Imanaka, N.; Hiraiwa, M.; Adachi, G.; Dabkowska, H.; Dabkowski, A. Thermal contraction behavior in Al2(WO4)3 single crystal. J. Cryst. Growth 2000, 220, 176–179. [Google Scholar] [CrossRef]
- Sivasubramanian, V.; Ravindran, T.R.; Nithya, R.; Arora, A.K. Structural phase transition in indium tungstate. J. Appl. Phys. 2004, 96, 387–392. [Google Scholar] [CrossRef]
- Hashimoto, T.; Sugimoto, T.; Omoto, K.; Kineri, N.; Ogata, Y.; Tsuda, K. Analysis of phase transition and expansion behaviour of Al2(WO4)3 by temperature-regulated X-ray diffraction. Phys. Status Solidi B 2008, 245, 2504–2508. [Google Scholar] [CrossRef]
- Baiz, T.I.; Heinrich, C.P.; Banek, N.A.; Vivekens, B.L.; Lind, C. In-situ non-ambient X-ray diffraction studies of indium tungstate. J. Solid State Chem. 2012, 187, 195–199. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Ma, J.; Jun, Z.; Zeng, X. Effect of isovalent substitution on phase transition and negative thermal expansion of In2−xScxW3O12 ceramics. Ceram. Int. 2015, 41, 9873–9877. [Google Scholar] [CrossRef]
- Evans, J.; Mary, T.A. Sleight, Negative thermal expansion in a large molybdate and tungstate family. J. Solid State Chem. 1997, 275, 61–65. [Google Scholar] [CrossRef]
- Mary, T.A.; Sleight, A.W. Bulk thermal expansion for tungstate and molybdates of the type A2M3O12. J. Mater. Res. 1999, 14, 912–915. [Google Scholar] [CrossRef]
- Tzvetkov, P.; Ivanova, D.; Kovacheva, D.; Nikolov, V. Synthesis and powder XRD characterization of Al2−xInx(WO4)3 solid solutions. J. Alloy. Compd. 2009, 470, 492–496. [Google Scholar] [CrossRef]
- Zhecheva, E.; Stoyanova, R.; Ivanova, S.; Nikolov, V. On the preparation of nanosized Al2(WO4)3 by a precipitation method. Solid State Sci. 2010, 12, 2010–2014. [Google Scholar] [CrossRef]
- Yordanova, A.S.; Nikolov, V.S.; Koseva, I.I. Fabrication of high density ceramic from Al2−XInX(WO4)3 solid solutions. J. Chem. Technol. Metall. 2015, 50, 537–542. [Google Scholar]
- Pontón, P.I.; Prisco, L.P.; Dosen, A.; Faro, G.S.; de Abreu, M.A.; Marinkovic, B.A. Co-precipitation synthesis of Y2W3O12 submicronic powder. Ceram. Int. 2017, 43, 4222–4228. [Google Scholar] [CrossRef]
- Miller, K.J.; Johnson, M.B.; White, M.A.; Marinkovic, B.A. Low-temperature investigations of the open-framework material HfMgMo3O12. Solid State Commun. 2012, 152, 1748–1752. [Google Scholar] [CrossRef]
- Costa, I.L.M.; Blair, V.L.; Paraguassu, W.; Marinkovic, B.A. Evaluating Al2−xGaxW3O12 system for thermal shock resistance. J. Solid State Chem. 2019, 277, 149–158. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Aoki, T.; Niwa, Y.; Hashimoto, E.; Morito, T. Thermal Expansion and Phase Transition Behavior of Al2−xMx(WO4)3 (M = Y, Ga and Sc) Ceramics. J. Ceram. Soc. Jap. 2007, 115, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Jacob, K.T.; Raj, S.; Rannesh, L. Vegard’s law: A fundamental relation or an approximation? Int. J. Mater. Res. 2007, 98, 776–779. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. J. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Evans, J.; Mary, T. Structural phase transitions and negative thermal expansion in Sc2(MoO4)3. Int. J. Inorg. Mater. 2000, 2, 143–151. [Google Scholar] [CrossRef]
- Marinkovic, B.A.; Ari, M.; Jardim, P.; de Avillez, R.R.; Rizzo, F.; Ferreira, F.F. In2Mo3O12: A low negative thermal expansion compound. Thermochim. Acta 2010, 499, 48–53. [Google Scholar] [CrossRef]
- Romao, C.P.; Donegan, S.P.; Zwanziger, J.W.; White, M.A. Relationships between elastic anisotropy and thermal expansion in A2Mo3O12 materials. Phys. Chem. Chem. Phys. 2016, 18, 30652–30661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prisco, L.P.; Pontón, P.; Paraguassu, W.; Romao, C.; White, M.A.; Marinkovic, B.A. Near-zero thermal expansion and phase transition in In0.5(ZrMg)0.75Mo3O12. J. Mater. Res. 2016, 31, 3240–3248. [Google Scholar] [CrossRef]
- Varga, T.; Moats, J.L.; Ushakov, S.; Navrotsky, A. Thermochemistry of A2M3O12 negative thermal expansion materials. J. Mater. Res. 2007, 22, 2512–2521. [Google Scholar] [CrossRef]
- Truitt, R.; Hermes, I.; Main, A.; Sendecki, A.; Lind, C. Low Temperature Synthesis and Characterization of AlScMo3O12. Materials 2015, 8, 700–716. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.Y.; Song, W.B.; Liang, E.J. Structures, Phase Transition, and Crystal Water of Fe2–xYxMo3O12. J. Phys. Chem. C 2011, 115, 17806–17811. [Google Scholar] [CrossRef]
- Ari, M.; Jardim, P.; Marinkovic, B.; Rizzo, F.; Ferreira, F.F. Thermal expansion of Cr2xFe2−2xMo3O12, Al2xFe2−2xMo3O12 and Al2xCr2−2xMo3O12 solid solutions. J. Solid State Chem. 2008, 181, 1472–1479. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Cheng, X. Synthesis and tunable thermal expansion property of Al2−δScδW3O12. Solid State Sci. 2012, 14, 187–190. [Google Scholar] [CrossRef]
- Dasgupta, N.; Sorge, E.; Butler, B.; Wen, T.-C.; Shetty, D.K.; Cambrea, L.R.; Harris, D.C. Synthesis and characterization of Al2−xScx(WO4)3 ceramics for low-expansion infrared-transmitting windows. J. Mater. Sci. 2012, 47, 6286–6296. [Google Scholar] [CrossRef]
- Liu, J.; Maynard-Casely, H.E.; Brand, H.E.A.; Sharma, N. Sc1.5Al0.5W3O12 Exhibits Zero Thermal Expansion between 4 and 1400 K. Chem. Mater. 2021, 33, 3823–3831. [Google Scholar] [CrossRef]

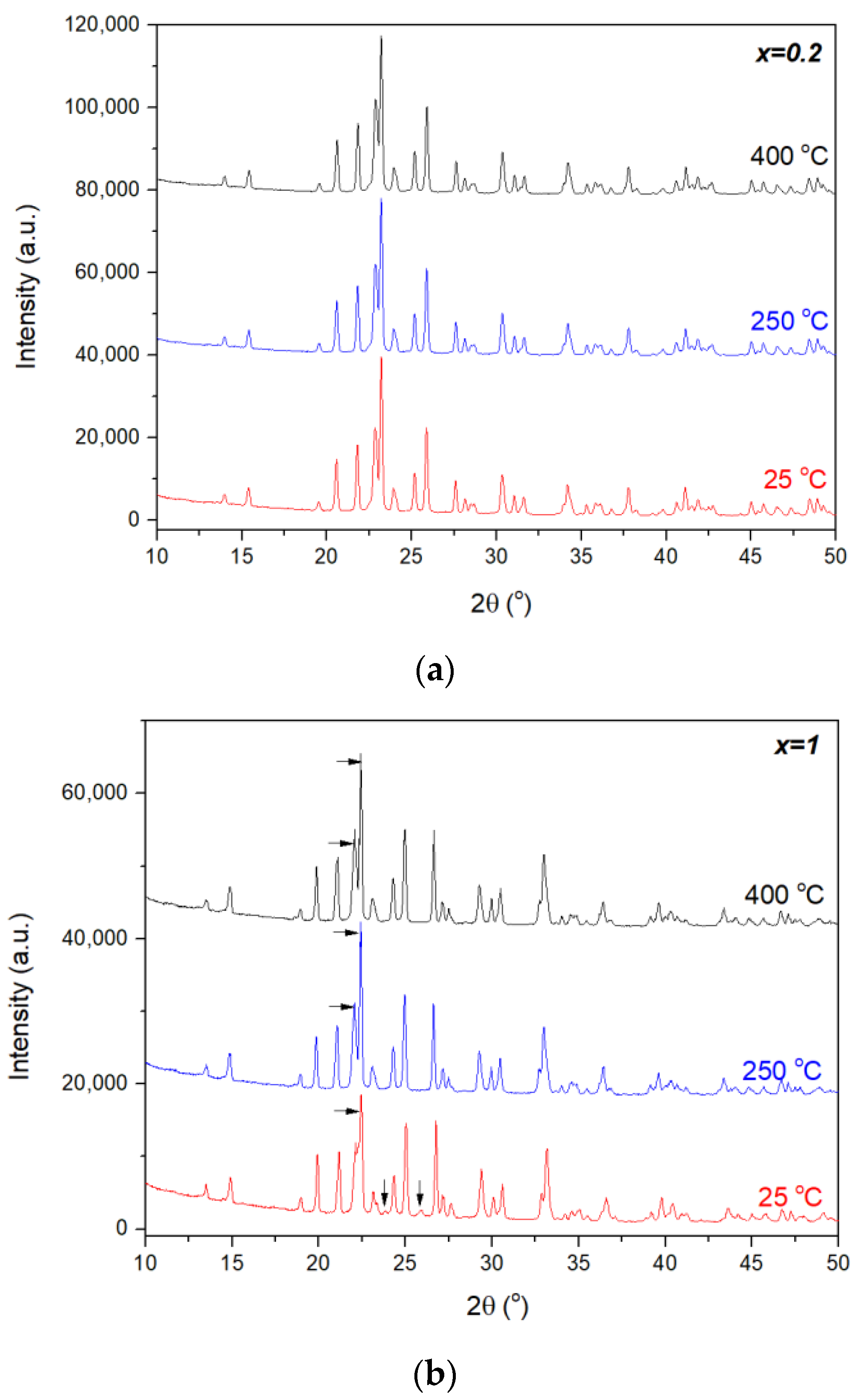
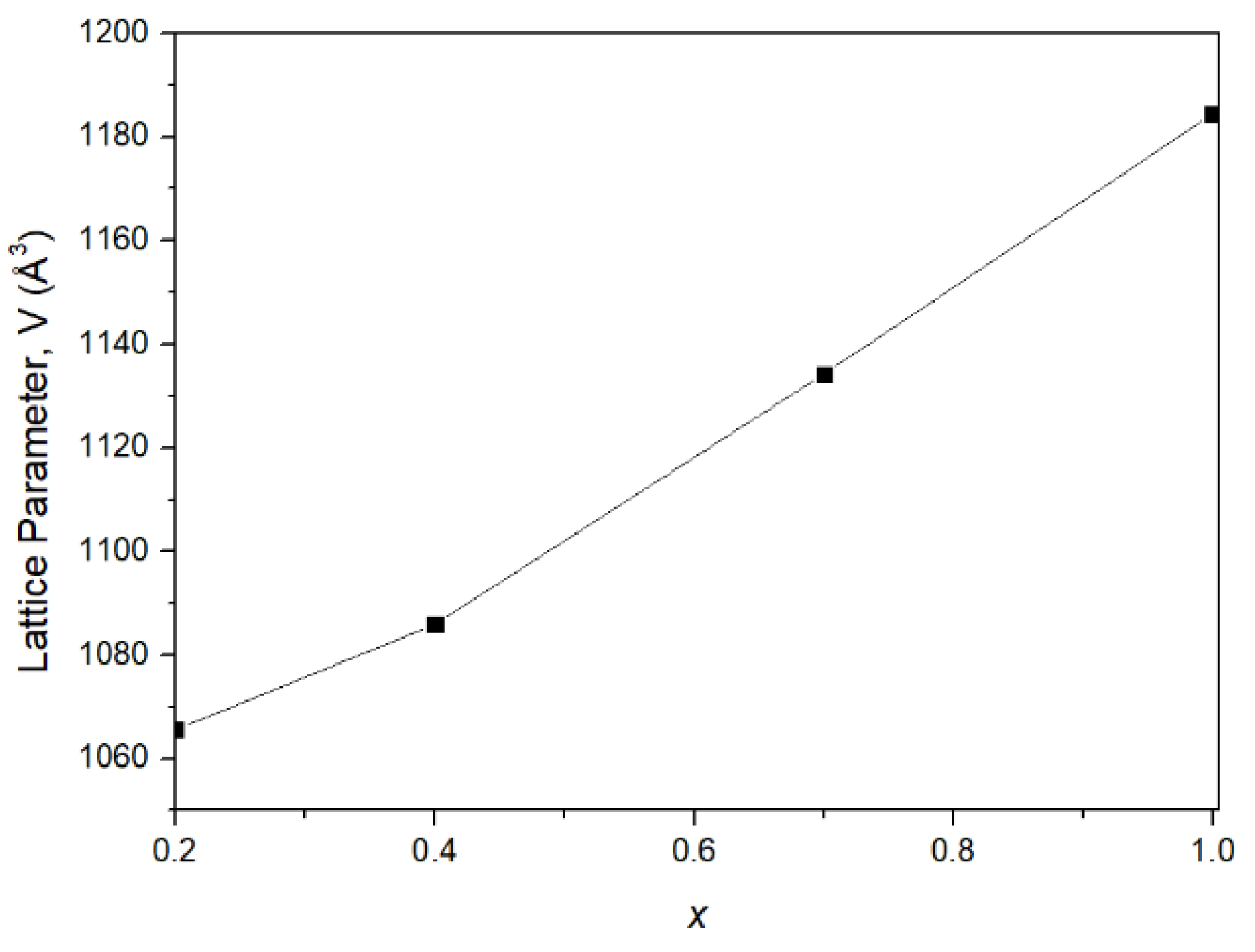

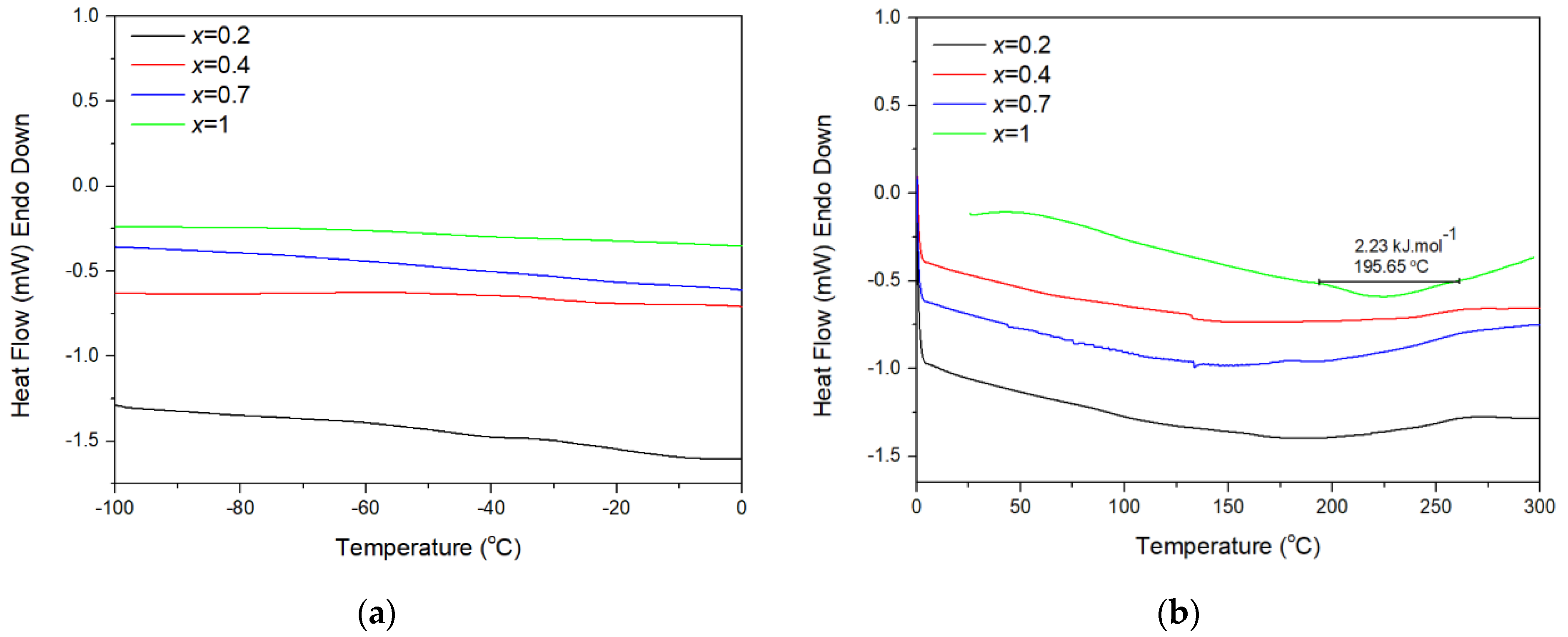
| x (In3+ Content in Chemical Formula) | αa [K−1] | αb [K−1] | αc [K−1] | αl [K−1] | Temperature Range [°C] | Ref. |
|---|---|---|---|---|---|---|
| 0 | 5.94 × 10−6 | −0.994 × 10−6 | −1.31 × 10−6 | 1.51 × 10−6 | RT–800 (XRPD) | [5] |
| 0.2 | 4.98 × 10−6 ±1.3 × 10−7 | 9.47 × 10−7 ±1.3 × 10−7 | −2.93 × 10−7 ±1.5 × 10−7 | 1.88 × 10−6 ±1.4 × 10−7 4.96 × 10−6 ±1.8 × 10−7 | RT–400 (XRPD) RT–500 (dilatometry) | - |
| 0.4 | 3.80 × 10−6 ±2.2 × 10−7 | 2.09 × 10−6 ±2.4 × 10−7 | 8.77 × 10−7 ±2.7 × 10−7 | 2.25 × 10−6 ±2.4 × 10−7 6.29 × 10−6 ±7.0 × 10−7 | RT–400 (XRPD) RT–500 (dilatometry) | - |
| 0.7 | 5.52 × 10−6 ±1.2 × 10−7 | 3.92 × 10−6 ±3.2 × 10−7 | 2.22 × 10−6 ±2.3 × 10−7 | 3.88 × 10−6 ±2.2 × 10−7 6.00 × 10−6 ±7.0 × 10−7 | RT–400 (XRPD) RT–500 (dilatometry) | - |
| 1 (P21/a)) | 1.83 × 10−5 ±7.5 × 10−6 | 1.47 × 10−5 ±4.5 × 10−6 | 2.63 × 10−5 ±8.1 × 10−6 | 1.88 × 10−5 ±7.7 × 10−6 | RT–100 (XRPD) | - |
| 1 (Pbcn) | 8.47 × 10−7 ±2.9 × 10−7 | −9.32 × 10−7 ±4.0 × 10−7 | −2.27 × 10−6 ±4.7 × 10−7 | −7.87 × 10−7 ±3.9 × 10−7 3.18 × 10−6 ±1.6 × 10−7 | 250–400 (XRPD) 250–500 (dilatometry) | - |
| 2 (Pbcn) | −3.1 × 10−6 | 11 × 10−6 | 1.6 × 10−6 | 3.1 × 10−6 | 250–600 (XRPD) | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerón Cortés, A.E.; Dosen, A.; Blair, V.L.; Johnson, M.B.; White, M.A.; Marinkovic, B.A. Phase Transition and Coefficients of Thermal Expansion in Al2−xInxW3O12 (0.2 ≤ x ≤ 1). Materials 2021, 14, 4021. https://doi.org/10.3390/ma14144021
Cerón Cortés AE, Dosen A, Blair VL, Johnson MB, White MA, Marinkovic BA. Phase Transition and Coefficients of Thermal Expansion in Al2−xInxW3O12 (0.2 ≤ x ≤ 1). Materials. 2021; 14(14):4021. https://doi.org/10.3390/ma14144021
Chicago/Turabian StyleCerón Cortés, Andrés Esteban, Anja Dosen, Victoria L. Blair, Michel B. Johnson, Mary Anne White, and Bojan A. Marinkovic. 2021. "Phase Transition and Coefficients of Thermal Expansion in Al2−xInxW3O12 (0.2 ≤ x ≤ 1)" Materials 14, no. 14: 4021. https://doi.org/10.3390/ma14144021
APA StyleCerón Cortés, A. E., Dosen, A., Blair, V. L., Johnson, M. B., White, M. A., & Marinkovic, B. A. (2021). Phase Transition and Coefficients of Thermal Expansion in Al2−xInxW3O12 (0.2 ≤ x ≤ 1). Materials, 14(14), 4021. https://doi.org/10.3390/ma14144021






