Phase Stability of Iron Nitride Fe4N at High Pressure—Pressure-Dependent Evolution of Phase Equilibria in the Fe–N System
Abstract
:1. Introduction
2. Materials and Methods
2.1. DFT Calculations
2.2. Starting Materials
2.3. Multi-Anvil Experiments
2.4. Sample Analysis
3. Results
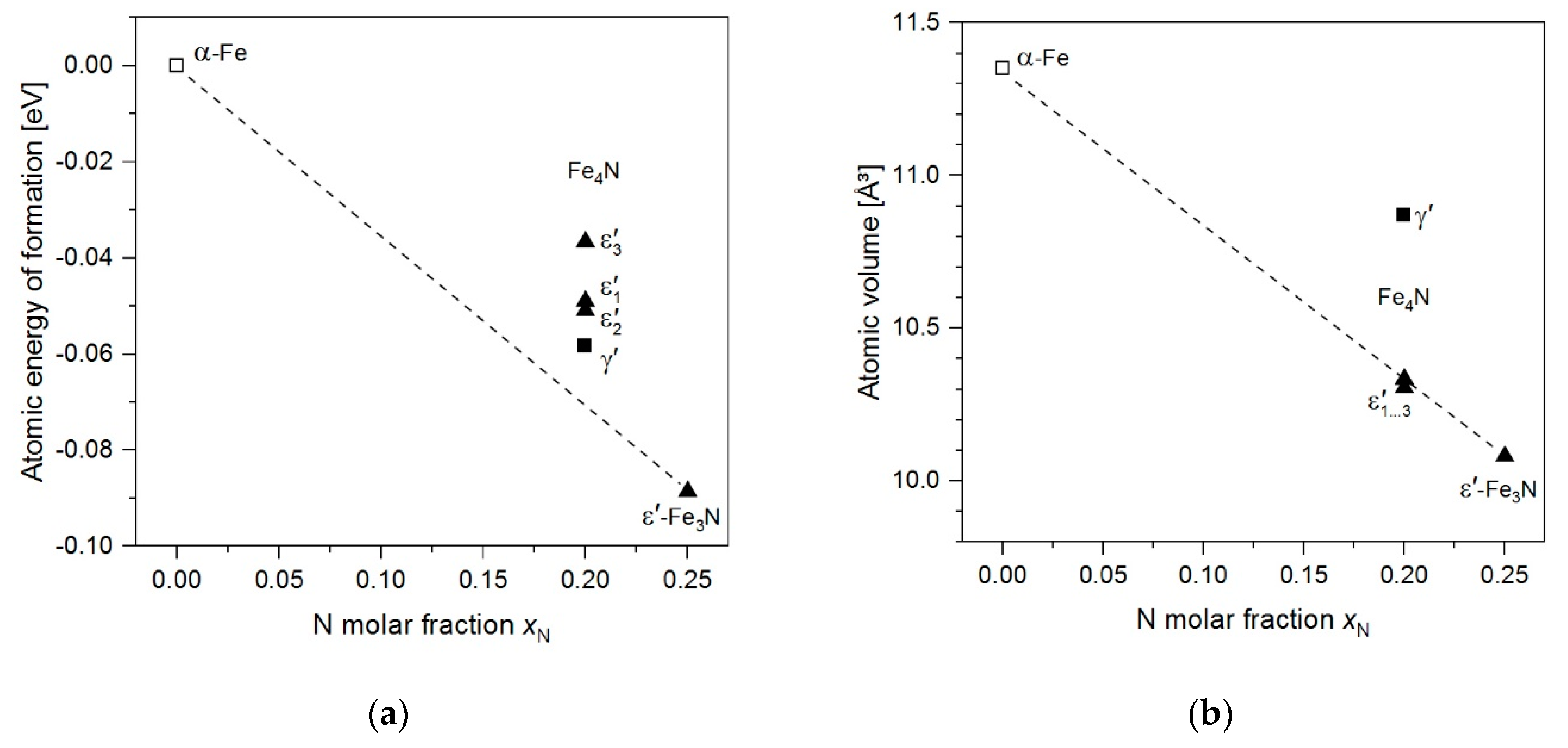
3.1. Analysis of DFT Calculations
3.2. Analysis of Starting Materials
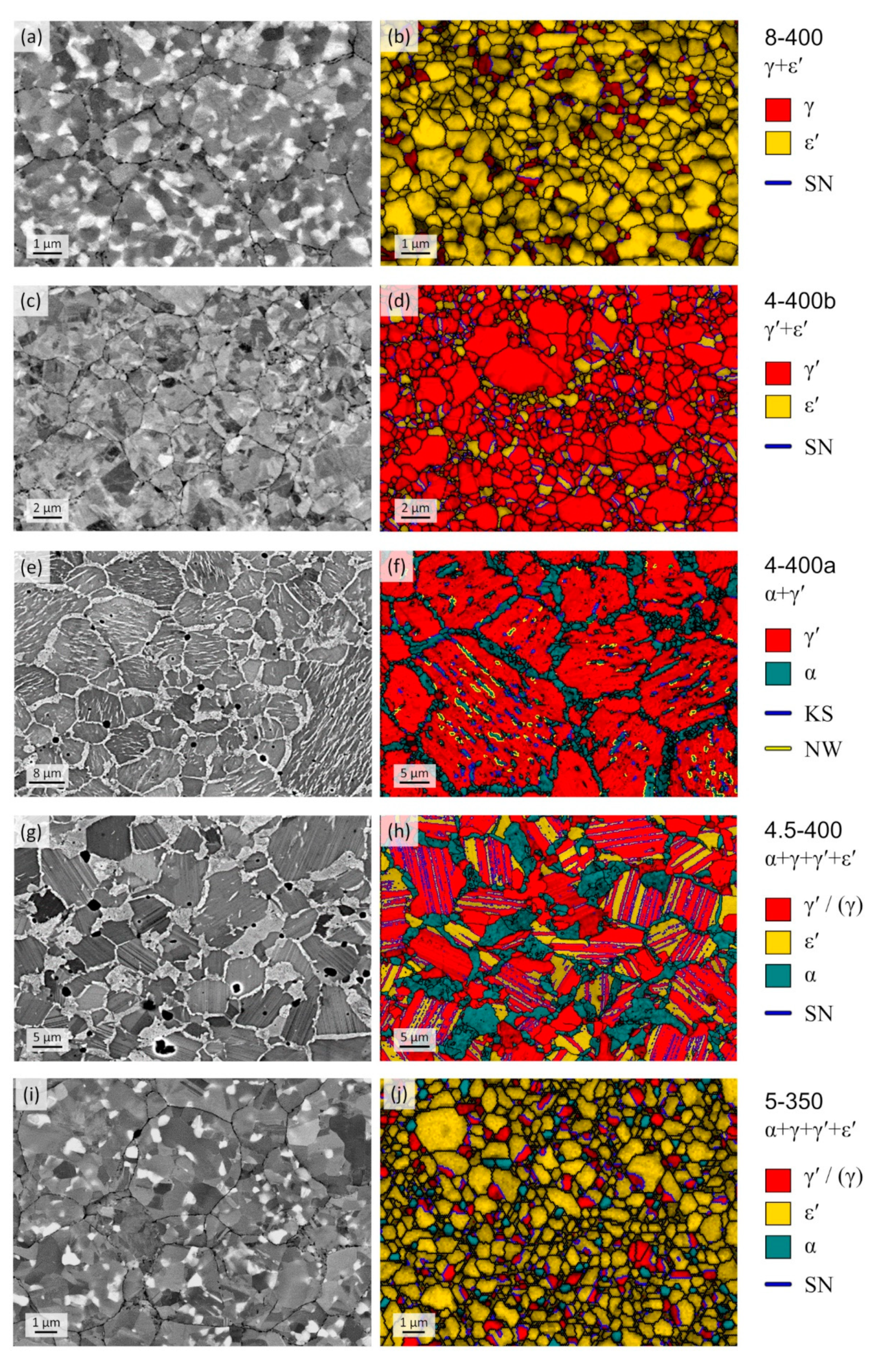
3.3. Analysis of Phase Constitution and Microstructures of Quenched Samples
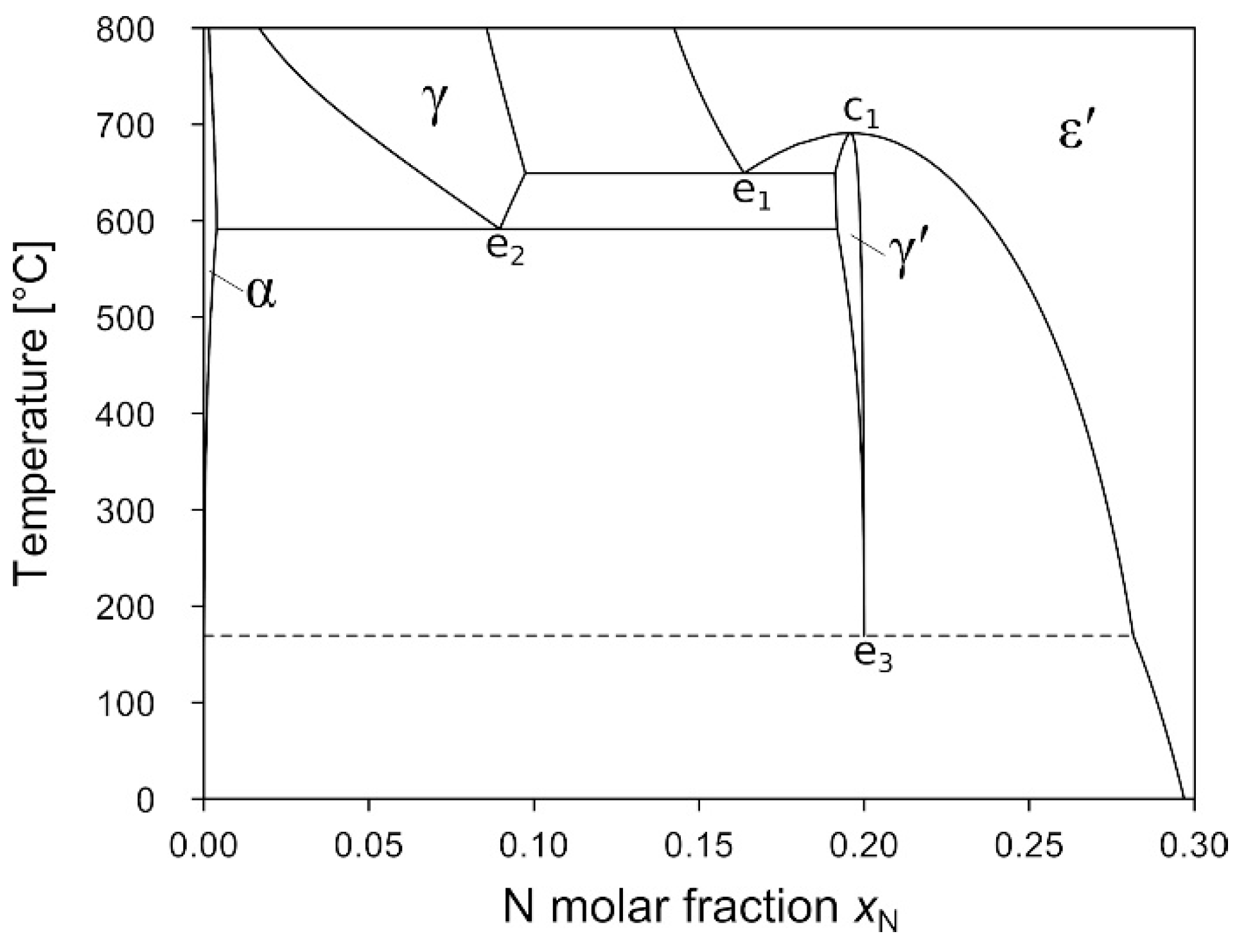
3.4. Phase Equilibria in the Fe-Rich Part of Fe–N System at High Pressure
3.4.1. Isobaric Sections at 2 GPa and 4 GPa
3.4.2. Isothermal Section at 400 °C
3.4.3. Low-Temperature Eutectoid Decomposition of γ′-Fe4N
4. Discussion
4.1. Evolution of the Phase Equilibria in the Fe–N System Subjected to High Pressure
4.2. Discussion of the P–T-Dependent Phase Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittemeijer, E.J. Fundamentals of Nitriding and Nitrocarburizing. In Steel Heat Treating Fundamentals and Processes; Dossett, J.L., Totten, G.E., Eds.; ASM International: Materials Park, OH, USA, 2013; Volume 4A, pp. 619–646. [Google Scholar]
- Jack, K.H. Binary and ternary interstitial alloys I. The iron-nitrogen system: The structures of Fe4N and Fe2N. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1948, 195, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Jack, K.H. The iron–nitrogen system: The crystal structures of ε-phase iron nitrides. Acta Crystallogr. 1952, 5, 404–411. [Google Scholar] [CrossRef]
- Leineweber, A.; Jacobs, H.; Hüning, F.; Lueken, H.; Schilder, H.; Kockelmann, W. ε-Fe3N: Magnetic structure, magnetization and temperature dependent disorder of nitrogen. J. Alloys Compd. 1999, 288, 79–87. [Google Scholar] [CrossRef]
- Leineweber, A.; Jacobs, H.; Hüning, F.; Lueken, H.; Kockelmann, W. Nitrogen ordering and ferromagnetic properties of ε-Fe3N1+x (0.10 ≤ x ≤ 0.39) and ε-Fe3(N0.80C0.20)1.38. J. Alloys Compd. 2001, 316, 21–38. [Google Scholar] [CrossRef]
- Wriedt, H.A.; Gokcen, N.A.; Nafziger, R.H. The Fe-N (Iron-Nitrogen) System. Bull. Alloy Phase Diagr. 1987, 8, 355–377. [Google Scholar] [CrossRef]
- Göhring, H.; Fabrichnaya, O.; Leineweber, A.; Mittemeijer, E.J. Thermodynamics of the Fe-N and Fe-N-C Systems: The Fe-N and Fe-N-C Phase Diagrams Revisited. Metall. Mater. Trans. A 2016, 47, 6173–6186. [Google Scholar] [CrossRef] [Green Version]
- You, Z.; Paek, M.K.; Jung, I.H. Critical Evaluation and Optimization of the Fe-N, Mn-N and Fe-Mn-N Systems. J. Phase Equilibria Diffus. 2018, 39, 650–677. [Google Scholar] [CrossRef] [Green Version]
- Malinov, S.; Böttger, A.J.; Mittemeijer, E.J.; Pekelharing, M.I.; Somers, M.A.J. Phase transformations and phase equilibria in the Fe-N system at temperatures below 573 K. Metall. Mater. Trans. A 2001, 32, 59–73. [Google Scholar] [CrossRef]
- Du Marchie van Voorthuysen, E.H.; Boerma, D.O.; Chechenin, N.C. Low-temperature extension of the Lehrer diagram and the iron-nitrogen phase diagram. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2002, 33, 2593–2598. [Google Scholar] [CrossRef]
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Dewaele, A.; Denoual, C.; Anzellini, S.; Occelli, F.; Mezouar, M.; Cordier, P.; Merkel, S.; Véron, M.; Rausch, E. Mechanism of the α-ϵ phase transformation in iron. Phys. Rev. B 2015, 91, 174105. [Google Scholar] [CrossRef]
- Dewaele, A.; Svitlyk, V.; Bottin, F.; Bouchet, J.; Jacobs, J. Iron under conditions close to the α-γ-ϵ triple point. Appl. Phys. Lett. 2018, 112. [Google Scholar] [CrossRef]
- Ackermann, S.; Martin, S.; Schwarz, M.; Schimpf, C.; Kulawinski, D.; Lathe, C.; Henkel, S.; Rafaja, D.; Biemann, H.; Weidner, A. Investigation of phase transformations in high-alloy austenitic TRIP steel under high pressure (up to 18 GPa) by in situ synchrotron X-ray diffraction and scanning electron microscopy. Metall. Mater. Trans. A 2016, 47, 95–111. [Google Scholar] [CrossRef]
- Dupé, B.; Amadon, B.; Pellegrini, Y.P.; Denoual, C. Mechanism for the α → ϵ phase transition in iron. Phys. Rev. B Condens. Matter Mater. Phys. 2013, 87, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Luu, H.-T.; Veiga, R.G.A.; Gunkelmann, N. Atomistic Study of the Role of Defects on α → ϵ Phase Transformations in Iron under Hydrostatic Compression. Metals 2019, 9, 1040. [Google Scholar] [CrossRef] [Green Version]
- Mankovsky, S.; Polesya, S.; Ebert, H.; Bensch, W.; Mathon, O.; Pascarelli, S.; Minár, J. Pressure-induced bcc to hcp transition in Fe: Magnetism-driven structure transformation. Phys. Rev. B Condens. Matter Mater. Phys. 2013, 88, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lord, J.S.; Armitage, J.G.M.; Riedi, P.C.; Matar, S.F.; Demazeau, G. The volume dependence of the magnetization and NMR of Fe4N and Mn4N. J. Phys. Condens. Matter 1994, 6, 1779–1790. [Google Scholar] [CrossRef]
- Yang, C.L.; Abd-Elmeguid, M.M.; Micklitz, H.; Michels, G.; Otto, J.W.; Kong, Y.; Xue, D.S.; Li, F.S. Pressure effects on the electronic properties and the magnetic ground state of γ′-Fe4N. J. Magn. Magn. Mater. 1995, 8853, 2–6. [Google Scholar] [CrossRef]
- Ishimatsu, N.; Maruyama, H.; Kawamura, N.; Suzuki, M.; Ohishi, Y.; Ito, M.; Nasu, S.; Kawakami, T.; Shimomura, O. Pressure-Induced Magnetic Transition in Fe4N Probed by Fe K-edge XMCD Measurement. J. Phys. Soc. Jpn. 2003, 72, 2372–2376. [Google Scholar] [CrossRef]
- Lv, M.; Liu, J.; Zhu, F.; Li, J.; Zhang, D.; Xiao, Y.; Dorfman, S.M. Spin Transitions and Compressibility of ε-Fe7N3 and γ′-Fe4N: Implications for Iron Alloys in Terrestrial Planet Cores. J. Geophys. Res. Solid Earth 2020, 125, 1–15. [Google Scholar] [CrossRef]
- Adler, J.F.; Williams, Q. A high-pressure X-ray diffraction study of iron nitrides: Implications for Earth’s core. J. Geophys. Res. 2005, 110, B01203. [Google Scholar] [CrossRef]
- Litasov, K.D.; Shatskiy, A.; Ponomarev, D.S.; Gavryushkin, P.N. Equations of state of iron nitrides ε-Fe3Nx and γ-Fe4Ny to 30 GPa and 1200 K and implication for nitrogen in the Earth’s core. J. Geophys. Res. Solid Earth 2017, 122, 3574–3584. [Google Scholar] [CrossRef] [Green Version]
- Breton, H.; Komabayashi, T.; Thompson, S.; Potts, N.; McGuire, C.; Suehiro, S.; Anzellini, S.; Ohishi, Y. Static compression of Fe4N to 77 GPa and its implications for nitrogen storage in the deep Earth. Am. Mineral. 2019, 104, 1781–1787. [Google Scholar] [CrossRef]
- Minobe, S.; Nakajima, Y.; Hirose, K.; Ohishi, Y. Stability and compressibility of a new iron-nitride β-Fe7N3 to core pressures. Geophys. Res. Lett. 2015, 42, 5206–5211. [Google Scholar] [CrossRef]
- Zhuang, Y.; Su, X.; Salke, N.P.; Cui, Z.; Hu, Q.; Zhang, D.; Liu, J. The effect of nitrogen on the compressibility and conductivity of iron at high pressure. Geosci. Front. 2020. [Google Scholar] [CrossRef]
- Niewa, R.; Rau, D.; Wosylus, A.; Meier, K.; Wessel, M.; Hanfland, M.; Dronskowski, R.; Schwarz, U. High-pressure high-temperature phase transition of γ′-Fe4N. J. Alloys Compd. 2009, 480, 76–80. [Google Scholar] [CrossRef]
- Schwarz, U.; Wosylus, A.; Wessel, M.; Nskowski, R.D.; Hanfland, M.; Rau, D.; Niewa, R. High-pressure-high-temperature behavior of ζ-Fe2N and phase transition to ε-Fe3N1.5. Eur. J. Inorg. Chem. 2009, 1634–1639. [Google Scholar] [CrossRef]
- Clark, W.P.; Steinberg, S.; Dronskowski, R.; McCammon, C.; Kupenko, I.; Bykov, M.; Dubrovinsky, L.; Akselrud, L.G.; Schwarz, U.; Niewa, R. High-Pressure NiAs-Type Modification of FeN. Angew. Chem. Int. Ed. 2017, 56, 7302–7306. [Google Scholar] [CrossRef] [Green Version]
- Laniel, D.; Dewaele, A.; Garbarino, G. High Pressure and High Temperature Synthesis of the Iron Pernitride FeN2. Inorg. Chem. 2018, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Bykov, M.; Khandarkhaeva, S.; Fedotenko, T.; Sedmak, P.; Dubrovinskaia, N.; Dubrovinsky, L. Synthesis of FeN4 at 180 GPa and its crystal structure from a submicron-sized grain. Acta Crystallogr. Sect. E Crystallogr. Commun. 2018, 74, 1392–1395. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, M.H.; Schwarz, M.R.; Leineweber, A. High-pressure high-temperature study of the pressure induced decomposition of the iron nitride γ′-Fe4N. J. Alloys Compd. 2019, 801, 438–448. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Zhou, Y.; Huo, C.; Guo, W.; Yang, Y.; Li, Y.; Wen, X. Crystal Structure Prediction Approach to Explore the Iron Carbide Phases: Novel Crystal Structures and Unexpected Magnetic Properties. J. Phys. Chem. C 2020, 124, 17244–17254. [Google Scholar] [CrossRef]
- Shatskiy, A.; Katsura, T.; Litasov, K.D.; Shcherbakova, A.V.; Borzdov, Y.M.; Yamazaki, D.; Yoneda, A.; Ohtani, E.; Ito, E. High pressure generation using scaled-up Kawai-cell. Phys. Earth Planet. Int. 2011, 189, 92–108. [Google Scholar] [CrossRef]
- Walker, D.; Carpenter, M.A.; Hitch, C.M. Some simplifications to multianvil devices for high pressure experiments. Am. Mineral. 1990, 75, 1020–1028. [Google Scholar]
- Rubie, D.C. Characterising the sample environment in multianvil high-pressure experiments. Phase Transit. 1999, 68, 431–451. [Google Scholar] [CrossRef]
- Bundy, F.P. Phase Diagram of Bismuth to 130,000 kg/cm2, 500 °C. Phys. Rev. 1958, 110, 314–318. [Google Scholar] [CrossRef]
- Ohtaka, O.; Fukui, H.; Kunisada, T.; Fujisawa, T.; Funakoshi, K.; Utsumi, W.; Irifune, T.; Kuroda, K.; Kikegawa, T. Phase relations and equations of state of ZrO2 under high temperature and high pressure. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 63, 1741081–1741088. [Google Scholar] [CrossRef]
- Schwarz, M.R. Multianvil calibration and education: A four probe method to measure the entire force-versus-pressure curve in a single run—Performed as an interdisciplinary lab-course for students. J. Phys. Conf. Ser. 2010, 215. [Google Scholar] [CrossRef]
- Nishihara, Y.; Doi, S.; Kakizawa, S.; Higo, Y.; Tange, Y. Effect of pressure on temperature measurements using WRe thermocouple and its geophysical impact. Phys. Earth Planet. Int. 2020, 298, 106348. [Google Scholar] [CrossRef]
- Nishihara, Y.; Matsukage, K.N.; Karato, S.I. Effects of metal protection coils on thermocouple EMF in multi-anvil high-pressure experiments. Am. Mineral. 2006, 91, 111–114. [Google Scholar] [CrossRef]
- Stokes, H.T.; Hatch, D.M. FINDSYM: Program for identifying the space-group symmetry of a crystal. J. Appl. Crystallogr. 2005, 38, 237–238. [Google Scholar] [CrossRef] [Green Version]
- Somers, M.A.J.; Pers, N.M.; Schalkoord, D.; Mittemeijer, E.J. Dependence of the lattice parameter of γ′ iron nitride, Fe4N1−x, on nitrogen content; Accuracy of the nitrogen absorption data. Metall. Trans. A 1989, 20, 1533–1539. [Google Scholar] [CrossRef]
- Imai, Y.; Izumlyama, M.; Tsuchiya, M. Thermodynamic Study on the Transformation of Austenite into Martensite in Iron-High Nitrogen and Iron-Carbon Binary System. Sci. Rep. Res. Inst. Tohoku Univ. Ser. A Phys. Chem. Metall. 1965, 17, 173–192. [Google Scholar]
- Gerardin, D.; Morniroli, J.P.; Michel, H.; Gantois, M. Microstructural study of ε-iron-carbonitrides formed by a glow discharge technique. J. Mater. Sci. 1981, 16, 159–169. [Google Scholar] [CrossRef]
- Göhring, H.; Kante, S.; Leineweber, A.; Mittemeijer, E.J. Microstructural development and crystallographic properties of decomposing Fe-N-C compound layers. Int. J. Mater. Res. 2016, 107, 203–216. [Google Scholar] [CrossRef]
- Nishiyama, Z. Martensitic Transformation, 1st ed.; Academic Press: London, UK, 1978; ISBN 9780323148818. [Google Scholar]
- Kral, M.V. Proeutectoid ferrite and cementite transformations in steels. In Phase Transformations in Steels; Pereloma, E., Edmonds, D.V., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2012; Volume 1, pp. 225–275. ISBN 9781845699703. [Google Scholar]
- Yang, J.R.; Chang, L.C. The effect of stress on the Widmanstätten ferrite transformation. Mater. Sci. Eng. A 1997, 223, 158–167. [Google Scholar] [CrossRef]
- Cheng, L.; Böttger, A.; de Keijser, T.H.; Mittemeijer, E.J. Lattice parameters of iron-carbon and iron-nitrogen martensites and austenites. Scr. Metall. Mater. 1990, 24, 509–514. [Google Scholar] [CrossRef]
- Liapina, T.; Leineweber, A.; Mittemeijer, E.J.; Kockelmann, W. The lattice parameters of ε-iron nitrides: Lattice strains due to a varying degree of nitrogen ordering. Acta Mater. 2004, 52, 173–180. [Google Scholar] [CrossRef]
- Burdese, A. Structural Relations and Equilibria between the ε and ζ Phases of the System Iron-Nitrogen. Metall. Ital. 1957, 49, 195–199. [Google Scholar]
- Guo, K.; Rau, D.; von Appen, J.; Prots, Y.; Schnelle, W.; Dronskowski, R.; Niewa, R.; Schwarz, U. High pressure high-temperature behavior and magnetic properties of Fe4N: Experiment and theory. High Press. Res. 2013, 33, 684–696. [Google Scholar] [CrossRef]
- Paranjpe, V.G.; Cohen, M.; Bever, M.B.; Floe, C.F. The iron-nitrogen system. J. Met. 1950, 2, 261–267. [Google Scholar] [CrossRef]
- Bouchard, R.J.; Frederick, C.G.; Johnson, V. Preparation and properties of submicron hexagonal FexN, 2 < x < 3. J. Appl. Phys. 1974, 45, 4067–4070. [Google Scholar] [CrossRef]
- Rauschenbach, B.; Kolitsch, A.; Hohmuth, K. Iron nitride phases formed by nitrogen ion implantation and thermal treatment. Phys. Status Solidi 1983, 80, 471–482. [Google Scholar] [CrossRef]
- Somers, M.A.J.; Kooi, B.J.; Maldzinski, L.; Mittemeijer, E.J.; Van Der Horst, A.A.; Van Der Kraan, A.M.; Van Der Pers, N.M. Thermodynamics and long-range order of interstitials in an h.c.p. Lattice: Nitrogen in ε-Fe2N1−z. Acta Mater. 1997, 45, 2013–2025. [Google Scholar] [CrossRef] [Green Version]
- Dubrovinsky, L.S.; Saxena, S.K.; Tutti, F.; Rekhi, S.; LeBehan, T. In situ X-ray study of thermal expansion and phase transition of iron at multimegabar pressure. Phys. Rev. Lett. 2000, 84, 1720–1723. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P.; Occelli, F.; Mezouar, M.; Dorogokupets, P.I.; Torrent, M. Quasihydrostatic equation of state of Iron above 2 Mbar. Phys. Rev. Lett. 2006, 97, 29–32. [Google Scholar] [CrossRef]
- Boehler, R.; Santamaría-Pérez, D.; Errandonea, D.; Mezouar, M. Melting, density, and anisotropy of iron at core conditions: New X-ray measurements to 150 GPa. J. Phys. Conf. Ser. 2008, 121, 022018. [Google Scholar] [CrossRef]
- Lu, X.G.; Selleby, M.; Sundman, B. Implementation of a new model for pressure dependence of condensed phases in Thermo-Calc. Calphad Comput. Coupling Phase Diagr. Thermochem. 2005, 29, 49–55. [Google Scholar] [CrossRef]
- Hillert, M. Phase Equilibria, Phase Diagrams and Phase Transformations, 2nd ed.; Cambridge University Press: Cambridge, UK, 2007; ISBN 9780511812781. [Google Scholar]
- Acet, M.; Gehrmann, B.; Wassermann, E.F.; Bach, H.; Pepperhoff, W. Relevance of magnetic instabilities to the properties of interstitial solid solutions and compounds of Fe. J. Magn. Magn. Mater. 2001, 232, 221–230. [Google Scholar] [CrossRef]
- Hernlund, J.; Leinenweber, K.; Locke, D.; Tyburczy, J.A. A numerical model for steady-state temperature distributions in solid-medium high-pressure cell assemblies. Am. Mineral. 2006, 91, 295–305. [Google Scholar] [CrossRef]
- Wang, K.; Reeber, R.R. Thermal Expansion of Caesium Halides with the CsCl Structure. J. Appl. Crystallogr. 1995, 28, 306–313. [Google Scholar] [CrossRef]
- Köhler, U.; Johannsen, P.G.; Holzapfel, W.B. Equation-of-state data for CsCl-type alkali halides. J. Phys. Condens. Matter 1997, 9, 5581–5592. [Google Scholar] [CrossRef]
- Leineweber, A.; Hickel, T.; Azimi-Manavi, B.; Maisel, S.B. Crystal structures of Fe4C vs. Fe4N analysed by DFT calculations: Fcc-based interstitial superstructures explored. Acta Mater. 2017, 140, 433–442. [Google Scholar] [CrossRef]
- De Waele, S.; Lejaeghere, K.; Leunis, E.; Duprez, L.; Cottenier, S. A first-principles reassessment of the Fe-N phase diagram in the low-nitrogen limit. J. Alloys Compd. 2019, 775, 758–768. [Google Scholar] [CrossRef]
- Imai, Y.; Sohma, M.; Suemasu, T. Energetic stability and magnetic moment of tri-, tetra-, and octa-ferromagnetic element nitrides predicted by first-principle calculations. J. Alloys Compd. 2014, 611, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Billingham, J.; Lewis, M.H. Dislocation mechanisms for the nucleation of transformations in vanadium carbide. Philos. Mag. J. Theor. Exp. Appl. Phys. 1971, 24, 231–240. [Google Scholar] [CrossRef]
- Leineweber, A. Mobility of nitrogen in ε-Fe3N below 150 °C: The activation energy for reordering. Acta Mater. 2007, 55, 6651–6658. [Google Scholar] [CrossRef]
- Liapina, T.; Leineweber, A.; Mittemeijer, E.J.; Knapp, M.; Baehtz, C.; Liu, Z.Q.; Mitsuishi, K.; Furuya, K. γ′-Fe4N formation in decomposing ε-Fe3N: A powder diffraction study using synchrotron radiation. Z. Krist. Suppl. 2006, 2006, 449–454. [Google Scholar] [CrossRef]
- Foct, J.; de Figueiredo, R.S. Mössbauer study of phase transformation induced by mechanosynthesis in Fe4N nitrides. Nanostruct. Mater. 1994, 4, 685–697. [Google Scholar] [CrossRef]
- Foct, J.; de Figueiredo, R.S.; Richard, O.; Morniroli, J.P. Mechanical Alloying of Interstitial Solid Solutions and Compounds. Mater. Sci. Forum 1996, 225–227, 409–416. [Google Scholar] [CrossRef]
- De Wit, L.; Weber, T.; Custer, J.S.; Saris, F.W. Thermodynamic stability of iron nitrides at temperatures below 350 °C. Phys. Rev. Lett. 1994, 72, 3835–3838. [Google Scholar] [CrossRef]
- Pochet, P.; Bellon, P.; Chaffron, L.; Martin, G. Phase Transformations under Ball Milling: Theory versus Experiment. Mater. Sci. Forum 1996, 225–227, 207–216. [Google Scholar] [CrossRef]
- Zarkevich, N.A.; Johnson, D.D. Coexistence pressure for a martensitic transformation from theory and experiment: Revisiting the bcc-hcp transition of iron under pressure. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 91, 1–9. [Google Scholar] [CrossRef] [Green Version]
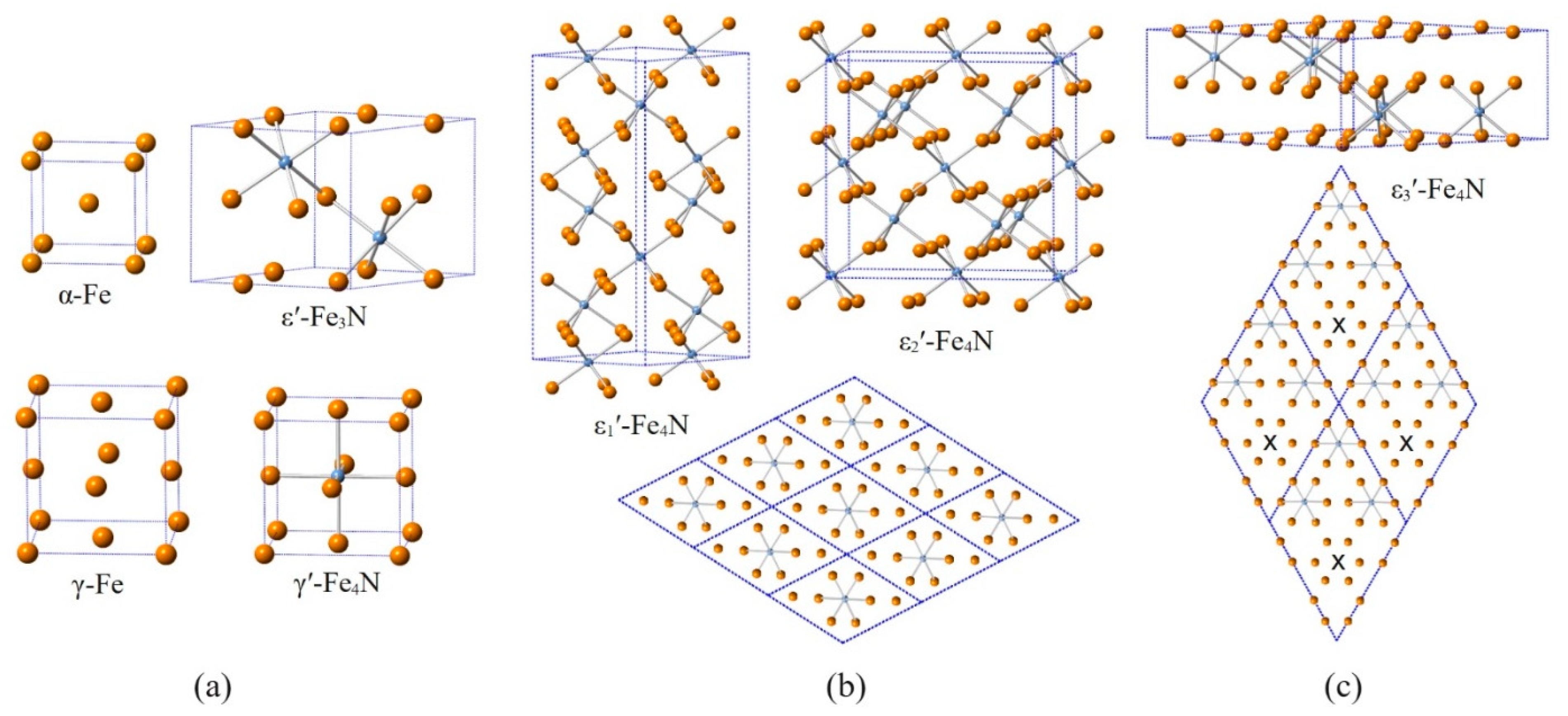
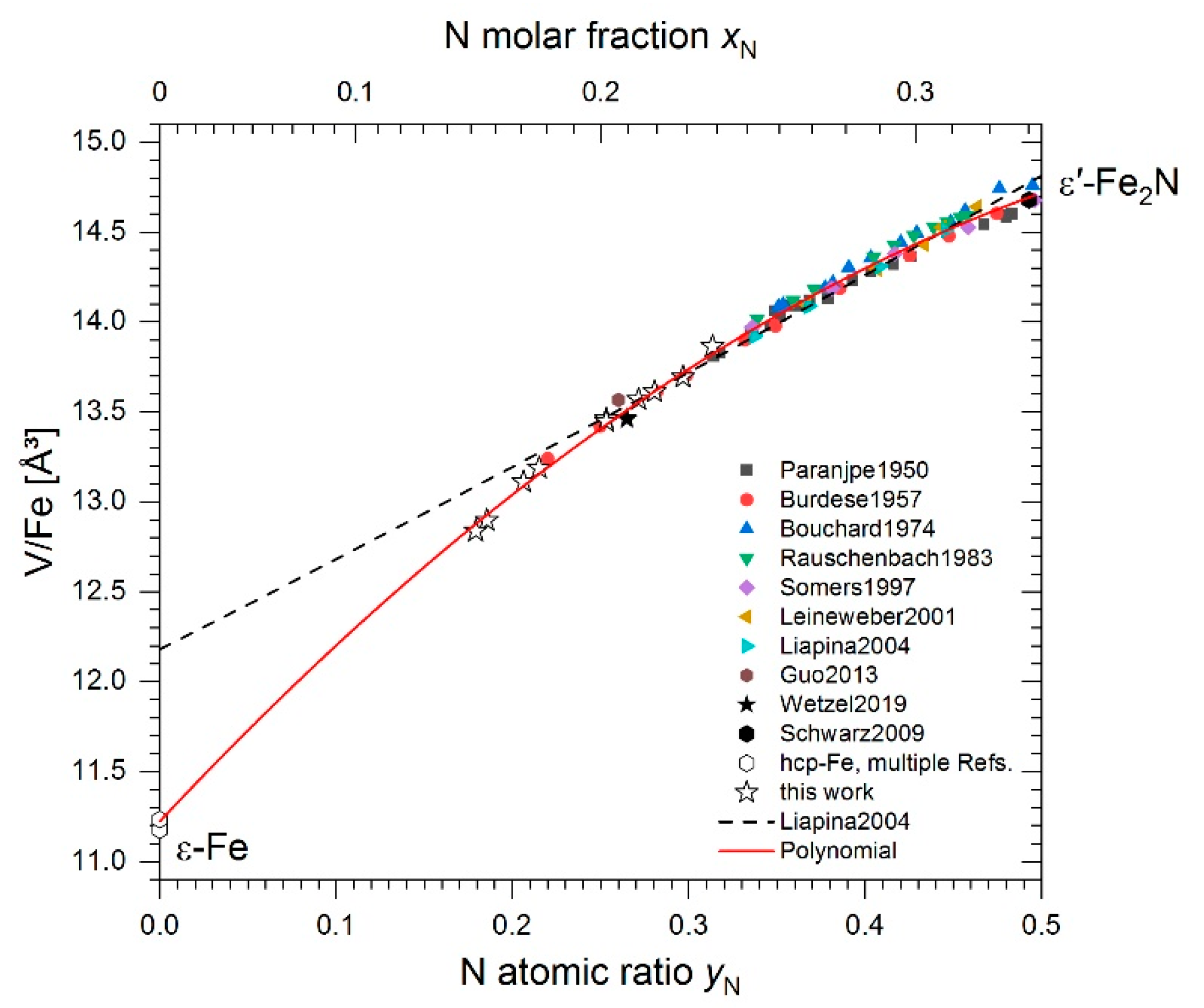
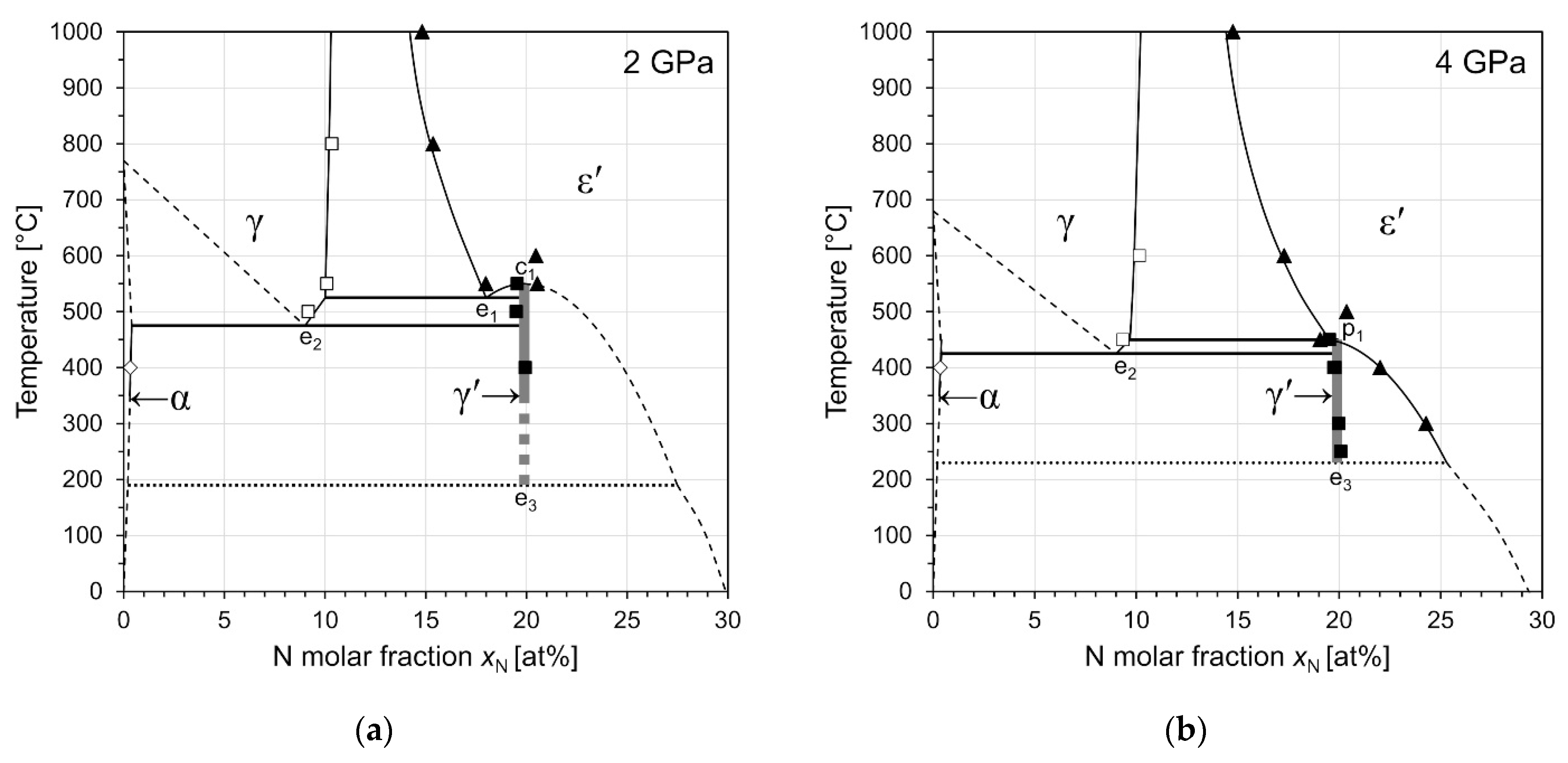
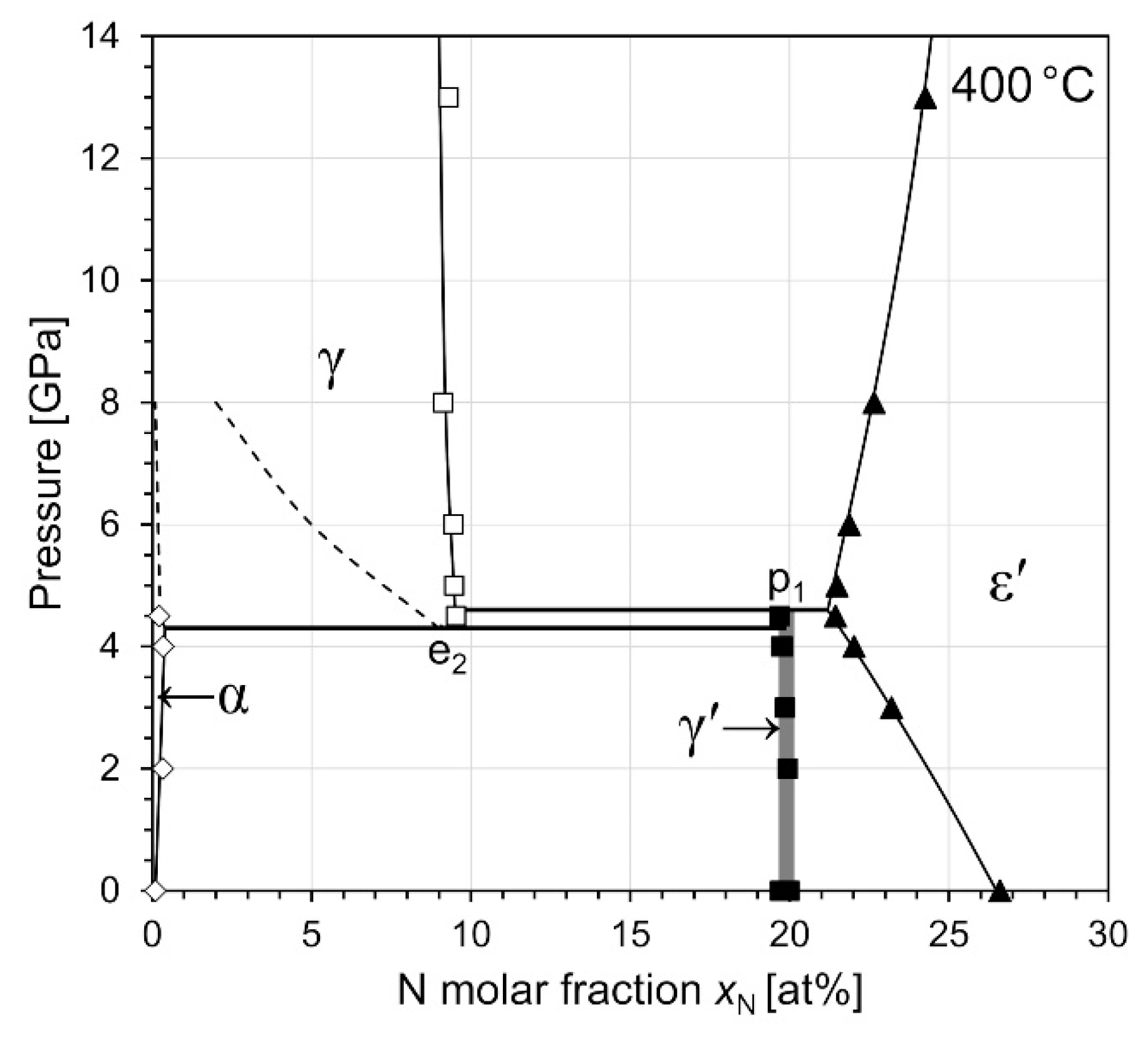
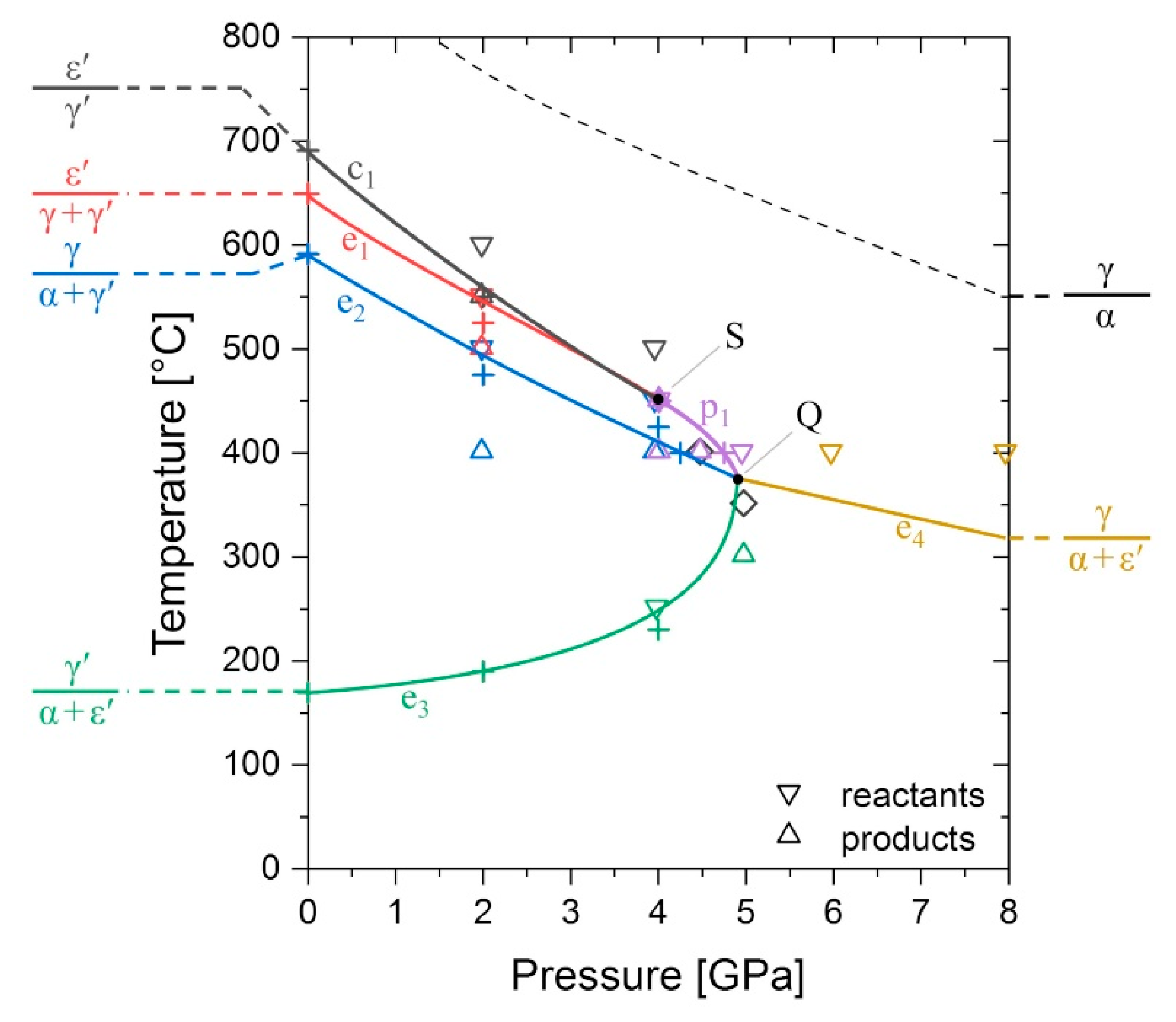
| Phase Identifier | Fe Basis Structure | Space Group | Fe Atoms per Employed Unit Cell | Lattice Parameters of the Employed Unit Cell | Reference for Structure Model |
|---|---|---|---|---|---|
| α/α-Fe(N) | bcc | Imm | 2 | aα = abcc | [6] |
| γ/γ-Fe(N) | fcc | Fmm | 4 | aγ = afcc | [6] |
| γ′/γ′-Fe4N | fcc | Pmm | 4 | aγ′ = afcc | [2] |
| ε′/ε′-Fe3N1+x a | hcp | P6322 | 6 | aε′ = 31/2 ahcp, cε′ = chcp | [4,5] |
| Model Structure for DFT | Space Group | Fe/N Atoms per Unit Cell | Lattice Parameters | Δu a [meV] | ΔV a [Å3] | µb [μB] |
|---|---|---|---|---|---|---|
| α-Fe | Imm | 2/0 | aα = 2.8315 Å = abcc | 0 | 0 | 2.20 |
| γ′-Fe4N | Pmm | 4/1 | aγ = 3.7877 Å = afcc | +12.5 | +0.53 | 2.48 |
| -Fe4N | P6122 | 24/6 | = 5.2541 Å = 2 ahcp, | +21.9 | −0.03 | 1.97 |
| 12.8796 Å = 3chcp | ||||||
| -Fe4N | F2dd | 32/8 | = 5.2705 Å ≈ 2ahcp, | +20.0 | −0.00 | 1.99 |
| = 9.1055 ≈ 2 × 31/2ahcp, | ||||||
| = 8.61456 = 2chcp | ||||||
| -Fe4N | P6322 | 24/6 | = 2 × 31/2ahcp = 9.1409 Å, | +34.3 | −0.00 | 2.02 |
| = 4.2839 Å = chcp | ||||||
| ε′-Fe3N | P6322 | 6/2 | aε′ = 4.6475 Å = 31/2ahcp, | 0 | 0 | 2.04 |
| cε′ = 4.3119 Å = chcp |
| Sample ID | P [GPa] | T1 [°C] | t1 [h] | T2 [°C] | t2 [h] | Initial Phases | Product Phases |
|---|---|---|---|---|---|---|---|
| 2-400 | 2 | 900 | 1 | 400 | 4 | α + γ′ | α + γ′ |
| 2-500 | 2 | – | – | 500 | 4 | α + γ′ | γ + γ′ |
| 2-550a | 2 | – | – | 550 | 4 | α + γ′ | γ + ε′ |
| 2-550b | 2 | – | – | 550 | 4 | γ′ | γ′ + ε′ |
| 2-600 | 2 | – | – | 600 | 4 | γ′ | ε′ |
| 2-800 | 2 | – | – | 800 | 1 | α + γ′ | γ + ε′ |
| 2-1000 | 2 | – | – | 1000 | 0.25 | α + γ′ | ε′ |
| 3-400 | 3 | – | – | 400 | 1 | γ′ | γ′ + ε′ |
| 4-250 | 4 | – | – | 250 | 4 | γ′ | γ′ + (ε′) |
| 4-300 | 4 | – | – | 300 | 4 | γ′ | γ′ + ε′ |
| 4-400a | 4 | 1000 | 1 | 400 | 4 | α + γ′ | α + γ′ |
| 4-400b | 4 | – | – | 400 | 4 | γ′ | γ′ + ε′ |
| 4-450 | 4 | 1000 | 0.5 | 450 | 4 | α + γ′ | γ + γ′ + ε′ |
| 4-500 | 4 | – | – | 500 | 1 | γ′ | ε′ |
| 4-600 | 4 | – | – | 600 | 1 | α + γ′ | γ + ε′ |
| 4-1000 | 4 | – | – | 1000 | 1 | α + γ′ | ε′ |
| 4.5-400 | 4.5 | 1000 | 0.5 | 400 | 4 | α + γ′ | α + γ + γ′ + ε′ |
| 5-300 | 5 | – | – | 300 | 4 | γ′ | α + γ′ + ε′ |
| 5-350 | 5 | – | – | 350 | 4 | γ′ | α + γ + γ′ + ε′ |
| 5-400 | 5 | – | – | 400 | 4 | γ′ | γ + ε′ |
| 6-400 | 6 | – | – | 400 | 4 | γ′ | γ + ε′ |
| 8-400 | 8 | – | – | 400 | 4 | γ′ | γ + ε′ |
| ID | α | γ | γ′ | ε′ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w | a | VFe | w | a | VFe | w | a | VFe | w | a | c | VFe | |
| [wt%] | [Å] | [ų] | [wt%] | [Å] | [ų] | [wt%] | [Å] | [ų] | [wt%] | [Å] | [Å] | [ų] | |
| 2-400 | 30.1 | 2.8687 | 11.80 | 69.9 | 3.7968 | 13.68 | |||||||
| 2-500 | 40.4 | 3.6443 | 12.10 | 59.6 | 3.7912 | 13.62 | |||||||
| 2-550a | 34.4 | 3.6521 | 12.18 | 65.6 | 4.5912 | 4.3342 | 13.19 | ||||||
| 2-550b | 18.8 | 3.7920 | 13.63 | 81.2 | 4.6283 | 4.3555 | 13.47 | ||||||
| 2-600 | 100 | 4.6271 | 4.3554 | 13.46 | |||||||||
| 2-800 | 8.9 | 3.6542 | 12.20 | 91.1 | 4.5573 | 4.3019 | 12.90 | ||||||
| 2-1000 | 100 | 4.5500 | 4.2959 | 12.84 | |||||||||
| 3-400 | 84.0 | 3.7967 | 13.68 | 16.0 | 4.6685 | 4.3715 | 13.75 | ||||||
| 4-250 a | 100 | 3.7999 | 13.72 | ||||||||||
| 4-300 | 93.6 | 3.7984 | 13.70 | 6.4 | 4.6832 | 4.3802 | 13.87 | ||||||
| 4-400a | 26.3 | 2.8688 | 11.81 | 73.7 | 3.7948 | 13.66 | |||||||
| 4-400b | 79.3 | 3.7957 | 13.67 | 20.7 | 4.6504 | 4.3654 | 13.63 | ||||||
| 4-450 | 37.8 | 3.6459 | 12.12 | 30.0 | 3.7916 | 13.63 | 32.2 | 4.6056 | 4.3456 | 13.30 | |||
| 4-500 | 100.0 | 4.6254 | 4.3546 | 13.45 | |||||||||
| 4-600 | 28.6 | 3.6527 | 12.18 | 71.4 | 4.5814 | 4.3274 | 13.11 | ||||||
| 4-1000 | 100.0 | 4.5492 | 4.2949 | 12.83 | |||||||||
| 4.5-400 | 23.2 | 2.8679 | 11.79 | 8.0 | 3.6474 | 12.13 | 44.7 | 3.7941 | 13.65 | 24.1 | 4.6430 | 4.3595 | 13.56 |
| 5-300 | 7.1 | 2.8692 | 11.81 | 55.1 | 3.7967 | 13.68 | 37.8 | 4.6724 | 4.3763 | 13.79 | |||
| 5-350 | 5.9 | 2.8686 | 11.80 | 3.0 | 3.6456 | 12.11 | 22.8 | 3.7946 | 13.66 | 68.3 | 4.6503 | 4.3661 | 13.63 |
| 5-400 | 10.6 | 3.6470 | 12.13 | 89.4 | 4.6420 | 4.3627 | 13.57 | ||||||
| 6-400 | 15.0 | 3.6468 | 12.13 | 85.0 | 4.6475 | 4.3658 | 13.61 | ||||||
| 8-400 | 21.4 | 3.6440 | 12.10 | 78.6 | 4.6591 | 4.3706 | 13.69 | ||||||
| Sample ID | α | γ | γ′ | ε′ | Average | |
|---|---|---|---|---|---|---|
| xN [at%] | xN [at%] | xN [at%] | xN [at%] | xN,LR [at%] | xN [at%] | |
| 2-400 | 0.3 | 19.9 | 14.7 | |||
| 2-500 | 9.2 | 19.5 | 15.5 | |||
| 2-550a | 10.1 | 18.0 | 17.7 | 15.4 | ||
| 2-550b | 19.5 | 20.5 | 20.4 | |||
| 2-600 | 20.5 | 20.2 | 20.5 | |||
| 2-800 | 10.3 | 15.4 | 15.6 | 14.9 | ||
| 2-1000 | 14.8 | 15.2 | 14.8 | |||
| 3-400 | 19.9 | 23.2 | 20.4 | |||
| 4-250 | 20.1 | 20.1 | ||||
| 4-300 | 20.0 | 24.3 | 20.3 | |||
| 4-400a | 0.3 | 19.7 | 15.2 | |||
| 4-400b | 19.8 | 22.0 | 20.3 | |||
| 4-450 | 9.4 | 19.5 | 19.1 | 15.7 | ||
| 4-500 | 20.4 | 20.2 | 20.4 | |||
| 4-600 | 10.2 | 17.3 | 17.1 | 15.3 | ||
| 4-1000 | 14.8 | 15.2 | 14.8 | |||
| 4.5-400 | 0.2 | 9.5 | 19.7 | 21.4 | 15.4 | |
| 5-300 | 0.4 | 19.9 | 23.6 | 20.2 | ||
| 5-350 | 0.3 | 9.3 | 19.7 | 22.0 | 20.1 | |
| 5-400 | 9.5 | 21.5 | 21.3 | 20.3 | ||
| 6-400 | 9.5 | 21.9 | 21.9 | 20.2 | ||
| 8-400 | 9.1 | 22.6 | 22.9 | 20.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wetzel, M.H.; Rabending, T.T.; Friák, M.; Všianská, M.; Šob, M.; Leineweber, A. Phase Stability of Iron Nitride Fe4N at High Pressure—Pressure-Dependent Evolution of Phase Equilibria in the Fe–N System. Materials 2021, 14, 3963. https://doi.org/10.3390/ma14143963
Wetzel MH, Rabending TT, Friák M, Všianská M, Šob M, Leineweber A. Phase Stability of Iron Nitride Fe4N at High Pressure—Pressure-Dependent Evolution of Phase Equilibria in the Fe–N System. Materials. 2021; 14(14):3963. https://doi.org/10.3390/ma14143963
Chicago/Turabian StyleWetzel, Marius Holger, Tina Trixy Rabending, Martin Friák, Monika Všianská, Mojmír Šob, and Andreas Leineweber. 2021. "Phase Stability of Iron Nitride Fe4N at High Pressure—Pressure-Dependent Evolution of Phase Equilibria in the Fe–N System" Materials 14, no. 14: 3963. https://doi.org/10.3390/ma14143963
APA StyleWetzel, M. H., Rabending, T. T., Friák, M., Všianská, M., Šob, M., & Leineweber, A. (2021). Phase Stability of Iron Nitride Fe4N at High Pressure—Pressure-Dependent Evolution of Phase Equilibria in the Fe–N System. Materials, 14(14), 3963. https://doi.org/10.3390/ma14143963







