Autologous Platelet Gel (APG): A Preliminary Evaluation of the Mechanical Properties after Activation with Autologous Thrombin and Calcium Chloride
Abstract
1. Introduction
2. Materials and Methods
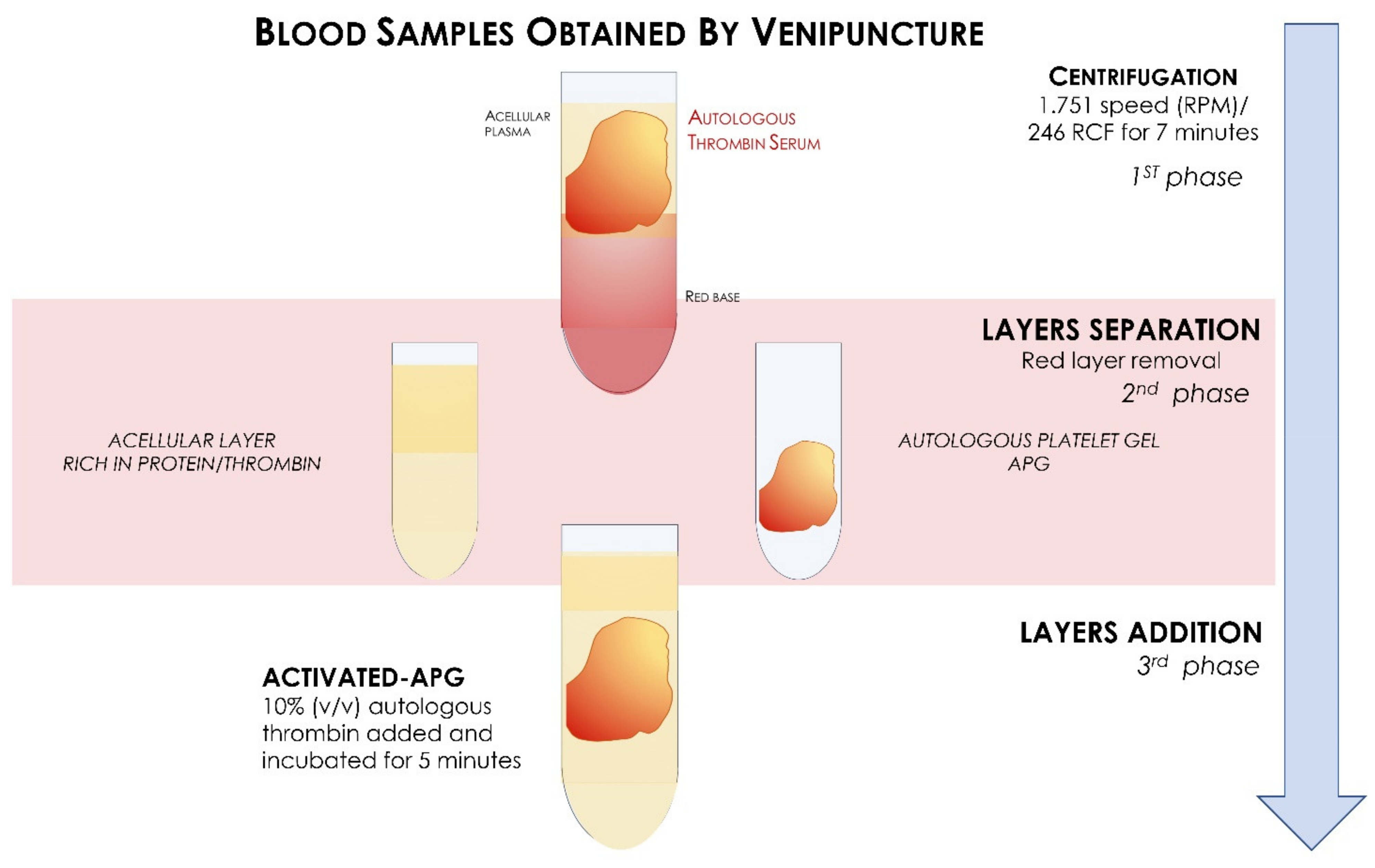
2.1. Preparation APG
2.2. Mechanical Investigations
2.3. Statistical Evaluation
3. Results
Mechanical Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scarano, A.; Carinci, F.; Assenza, B.; Piattelli, M.; Murmura, G.; Piattelli, A. Vertical Ridge Augmentation of Atrophic Posterior Mandible Using an Inlay Technique with a Xenograft without Miniscrews and Miniplates: Case Series. Clin. Oral Implant. Res. 2011, 22, 1125–1130. [Google Scholar] [CrossRef]
- Mavriqi, L.; Mortellaro, C.; Scarano, A. Inferior Alveolar Nerve Mobilization Using Ultrasonic Surgery with Crestal Approach Technique, Followed by Immediate Implant Insertion: Evaluation of Neurosensory Disturbance. J. Craniofac. Surg. 2016, 27, 1209–1211. [Google Scholar] [CrossRef]
- Fugazzotto, P.A. Success and Failure Rates of 1,344 6-to 9-Mm-Length Rough-Surface Implants Placed at the Time of Transalveolar Sinus Elevations, Restored with Single Crowns, and Followed for 60 to 229 Months in Function. Int. J. Oral Maxillofac. Implant. 2017, 32, 1359–1363. [Google Scholar] [CrossRef][Green Version]
- Prasadh, S.; Wong, R.C.W. Unraveling the Mechanical Strength of Biomaterials Used as a Bone Scaffold in Oral and Maxillofacial Defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. JFB 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Hannink, G.; Arts, J.J.C. Bioresorbability, Porosity and Mechanical Strength of Bone Substitutes: What Is Optimal for Bone Regeneration? Injury 2011, 42 (Suppl. S2), S22–S25. [Google Scholar] [CrossRef]
- Draenert, K.; Draenert, M.; Erler, M.; Draenert, A.; Draenert, Y. How Bone Forms in Large Cancellous Defects: Critical Analysis Based on Experimental Work and Literature. Injury 2011, 42 (Suppl. S2), S47–S55. [Google Scholar] [CrossRef]
- Patel, P.P.; Buckley, C.; Taylor, B.L.; Sahyoun, C.C.; Patel, S.D.; Mont, A.J.; Mai, L.; Patel, S.; Freeman, J.W. Mechanical and Biological Evaluation of a Hydroxyapatite-Reinforced Scaffold for Bone Regeneration. J. Biomed. Mater. Res. A 2019, 107, 732–741. [Google Scholar] [CrossRef]
- Wang, H.-L.; Boyapati, L. “PASS” Principles for Predictable Bone Regeneration. Implant. Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef]
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone Grafting Materials in Dentoalveolar Reconstruction: A Comprehensive Review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef]
- Buck, D.W.; Dumanian, G.A. Bone Biology and Physiology: Part, I. The Fundamentals. Plast. Reconstr. Surg. 2012, 129, 1314–1320. [Google Scholar] [CrossRef]
- Frost, H.M. Bone’s Mechanostat: A 2003 Update. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 275, 1081–1101. [Google Scholar] [CrossRef]
- Malchiodi, L.; Scarano, A.; Quaranta, M.; Piattelli, A. Rigid Fixation by Means of Titanium Mesh in Edentulous Ridge Expansion for Horizontal Ridge Augmentation in the Maxilla. Int. J. Oral Maxillofac. Implant. 1998, 13, 701–705. [Google Scholar]
- Degidi, M.; Scarano, A.; Piattelli, A. Regeneration of the Alveolar Crest Using Titanium Micromesh with Autologous Bone and a Resorbable Membrane. J. Oral Implant. 2003, 29, 86–90. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, A.; Murmura, G.; Iezzi, G.; Assenza, B.; Mancino, C. Delayed Expansion of the Atrophic Mandible by Ultrasonic Surgery: A Clinical and Histologic Case Series. Int. J. Oral Maxillofac. Implant. 2015, 30, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.J.; Zhou, J.N.; Guo, L.H. Impact of Different Regenerative Techniques and Materials on the Healing Outcome of Endodontic Surgery: A Systematic Review and Meta-Analysis. Int. Endod. J. 2020, 52, 536–555. [Google Scholar] [CrossRef]
- Stumbras, A.; Galindo-Moreno, P.; Januzis, G.; Juodzbalys, G. Three-Dimensional Analysis of Dimensional Changes after Alveolar Ridge Preservation with Bone Substitutes or Plasma Rich in Growth Factors: Randomized and Controlled Clinical Trial. Clin. Implant. Dent. Relat. Res. 2020, 23, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Guida, A.; Cecoro, G.; Rullo, R.; Laino, L.; Del Fabbro, M.; Annunziata, M. A Systematic Critical Appraisal of the Methodological Quality of Systematic Reviews on the Effect of Autologous Platelet Concentrates in the Treatment of Periodontal Intraosseous Defects. Materials 2020, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-Rich Plasma: Growth Factor Enhancement for Bone Grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Anitua, E. Plasma Rich in Growth Factors: Preliminary Results of Use in the Preparation of Future Sites for Implants. Int. J. Oral Maxillofac. Implant. 1999, 14, 529–535. [Google Scholar]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part V: Histologic Evaluations of PRF Effects on Bone Allograft Maturation in Sinus Lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Scarano, A.; Valbonetti, L.; Marchetti, M.; Lorusso, F.; Ceccarelli, M. Soft Tissue Augmentation of the Face with Autologous Platelet-Derived Growth Factors and Tricalcium Phosphate. Microtomography Evaluation of Mice. J. Craniofacial Surg. 2016, 27, 1212–1214. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Didembourg, M.; Wood, A.; Devi, A.; Dinsdale, R.; Hazeldine, J.; Alsousou, J.; Keene, D.J.; Hulley, P.; Wagland, S.; et al. Characteristics of L-PRP Preparations for Treating Achilles Tendon Rupture within the PATH-2 Study. Platelets 2020, 32, 273–279. [Google Scholar] [CrossRef]
- Xu, P.-C.; Xuan, M.; Cheng, B. Effects and Mechanism of Platelet-Rich Plasma on Military Drill Injury: A Review. Mil. Med. Res. 2020, 7, 56. [Google Scholar] [CrossRef]
- Nadal, J.; Figueroa, M.S.; Carreras, E.; Pujol, P.; Canut, M.I.; Barraquer, R.I. Autologous Platelet Concentrate in Surgery for Macular Detachment Associated with Congenital Optic Disc Pit. Clin. Ophthalmol. 2015, 9, 1965–1971. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scarano, A.; Inchingolo, F.; Rapone, B.; Lucchina, A.G.; Qorri, E.; Lorusso, F. Role of Autologous Platelet Gel (APG) in Bone Healing: A Rabbit Study. Appl. Sci. 2021, 11, 395. [Google Scholar] [CrossRef]
- Alston, S.M.; Solen, K.A.; Broderick, A.H.; Sukavaneshvar, S.; Mohammad, S.F. New Method to Prepare Autologous Fibrin Glue on Demand. Transl. Res. 2007, 149, 187–195. [Google Scholar] [CrossRef]
- Silva, R.F.; Carmona, J.U.; Rezende, C.M.F. Comparison of the Effect of Calcium Gluconate and Batroxobin on the Release of Transforming Growth Factor Beta 1 in Canine Platelet Concentrates. BMC Vet. Res. 2012, 8, 121. [Google Scholar] [CrossRef]
- Cavallo, C.; Roffi, A.; Grigolo, B.; Mariani, E.; Pratelli, L.; Merli, G.; Kon, E.; Marcacci, M.; Filardo, G. Platelet-Rich Plasma: The Choice of Activation Method Affects the Release of Bioactive Molecules. Biomed. Res. Int. 2016, 2016, 6591717. [Google Scholar] [CrossRef]
- Inchingolo, F.; Ballini, A.; Cagiano, R.; Inchingolo, A.D.; Serafini, M.; De Benedittis, M.; Cortelazzi, R.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; et al. Immediately Loaded Dental Implants Bioactivated with Platelet-Rich Plasma (PRP) Placed in Maxillary and Mandibular Region. Clin. Ter. 2015, 166, e146–e152. [Google Scholar] [CrossRef]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 1: Periodontal and Dentoalveolar Surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef]
- Scarano, A.; Ceccarelli, M.; Marchetti, M.; Piattelli, A.; Mortellaro, C. Soft Tissue Augmentation with Autologous Platelet Gel and β-TCP: A Histologic and Histometric Study in Mice. Biomed. Res. Int. 2016, 2016, 2078104. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, K.; Bhattacharyya, M. Overview of Platelet Physiology: Its Hemostatic and Nonhemostatic Role in Disease Pathogenesis. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Inchingolo, F.; Murmura, G.; Traini, T.; Piattelli, A.; Lorusso, F. Three-Dimensional Architecture and Mechanical Properties of Bovine Bone Mixed with Autologous Platelet Liquid, Blood, or Physiological Water: An In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1230. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Franco, T.; Schettino, A.M.; Filho, J.M.T.; Vendramin, F.S. Protocol for Obtaining Platelet-Rich Plasma (PRP), Platelet-Poor Plasma (PPP), and Thrombin for Autologous Use. Aesthetic Plast. Surg. 2012, 36, 1254–1259. [Google Scholar] [CrossRef]
- Brugnami, F.; Corsi, A.; Riminucci, M.; Caiazzo, A. A Case Report of Bilateral Mandibular Vertical Guided Bone Regeneration with and without Bovine Thrombin/Calcium Chloride Activated Platelet-Rich Plasma. J. Oral Implantol. 2011, 37, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Velier, M.; Magalon, J.; Daumas, A.; Cassar, M.; Francois, P.; Ghazouane, A.; Philandrianos, C.; Bertrand, B.; Frere, C.; Bernot, D.; et al. Production of Platelet-Rich Plasma Gel from Elderly Patients under Antithrombotic Drugs: Perspectives in Chronic Wounds Care. Platelets 2018, 29, 496–503. [Google Scholar] [CrossRef]
- Hartwig, J.H. Mechanisms of Actin Rearrangements Mediating Platelet Activation. J. Cell Biol. 1992, 118, 1421–1442. [Google Scholar] [CrossRef]
- Simion, M.; Jovanovic, S.A.; Trisi, P.; Scarano, A.; Piattelli, A. Vertical Ridge Augmentation around Dental Implants Using a Membrane Technique and Autogenous Bone or Allografts in Humans. Int. J. Periodontics Restor. Dent. 1998, 18, 8–23. [Google Scholar]
- Betoni-Junior, W.; Dechichi, P.; Esteves, J.C.; Zanetta-Barbosa, D.; Magalhães, A.E.O. Evaluation of the Bone Healing Process Utilizing Platelet-Rich Plasma Activated by Thrombin and Calcium Chloride: A Histologic Study in Rabbit Calvaria. J. Oral Implantol. 2013, 39, 14–21. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sánchez, M.; Nurden, A.T.; Nurden, P.; Orive, G.; Andía, I. New Insights into and Novel Applications for Platelet-Rich Fibrin Therapies. Trends Biotechnol. 2006, 24, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous Platelets as a Source of Proteins for Healing and Tissue Regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J. Platelets in Wound Healing and Regenerative Medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; D’Ascenzo, S.; Macchiarelli, G.; Dolo, V. In Vitro Evidence Supporting Applications of Platelet Derivatives in Regenerative Medicine. Blood Transfus. 2020, 18, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Santhanakrishnan, M. Mechanical, Chemical, Structural Analysis and Comparative Release of PDGF-AA from L-PRF, A-PRF and T-PRF—An In Vitro Study. Biomater. Res. 2020, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Brass, E.P.; Forman, W.B.; Edwards, R.V.; Lindan, O. Fibrin Formation: Effect of Calcium Ions. Blood 1978, 52, 654–658. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Mozzati, M.; Martinasso, G.; Pol, R.; Polastri, C.; Cristiano, A.; Muzio, G.; Canuto, R. The Impact of Plasma Rich in Growth Factors on Clinical and Biological Factors Involved in Healing Processes after Third Molar Extraction. J. Biomed. Mater. Res. A 2010, 95, 741–746. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Gallesio, G.; Mozzati, M. Autologous Platelet Concentrates for Bisphosphonate-Related Osteonecrosis of the Jaw Treatment and Prevention. A Systematic Review of the Literature. Eur. J. Cancer 2015, 51, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Woodell-May, J.; Ponticiello, M.; Yang, Z.; Nimni, M. The Effect of Thrombin Activation of Platelet-Rich Plasma on Demineralized Bone Matrix Osteoinductivity. J. Bone Joint Surg. Am. 2009, 91, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Vang, S.; Brady, C.; Isler, J.; Allen, K.; Anderson, J.; Holt, D. Autologous Platelet Gel: An In Vitro Analysis of Platelet-Rich Plasma Using Multiple Cycles. J. Extra Corpor. Technol. 2006, 38, 249–253. [Google Scholar]
- Marchetti, E.; Mancini, L.; Bernardi, S.; Bianchi, S.; Cristiano, L.; Torge, D.; Marzo, G.; Macchiarelli, G. Evaluation of Different Autologous Platelet Concentrate Biomaterials: Morphological and Biological Comparisons and Considerations. Materials 2020, 13, 2282. [Google Scholar] [CrossRef] [PubMed]
- Nelb, G.W.; Gerth, C.; Ferry, J.D. Rheology of Fibrin Clots. III. Shear Creep and Creep Recovery of Fine Ligated and Coarse Unligated Closts. Biophys. Chem. 1976, 5, 377–387. [Google Scholar] [CrossRef]
- Storm, C.; Pastore, J.J.; MacKintosh, F.C.; Lubensky, T.C.; Janmey, P.A. Nonlinear Elasticity in Biological Gels. Nature 2005, 435, 191–194. [Google Scholar] [CrossRef]
- Taylor, B.L.; Limaye, A.; Yarborough, J.; Freeman, J.W. Investigating Processing Techniques for Bovine Gelatin Electrospun Scaffolds for Bone Tissue Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1131–1140. [Google Scholar] [CrossRef]
- Mockros, L.F.; Roberts, W.W.; Lorand, L. Viscoelastic Properties of Ligation-Inhibited Fibrin Clots. Biophys. Chem. 1974, 2, 164–169. [Google Scholar] [CrossRef]
- Gersh, K.C.; Nagaswami, C.; Weisel, J.W. Fibrin Network Structure and Clot Mechanical Properties Are Altered by Incorporation of Erythrocytes. Thromb. Haemost. 2009, 102, 1169. [Google Scholar] [CrossRef] [PubMed]




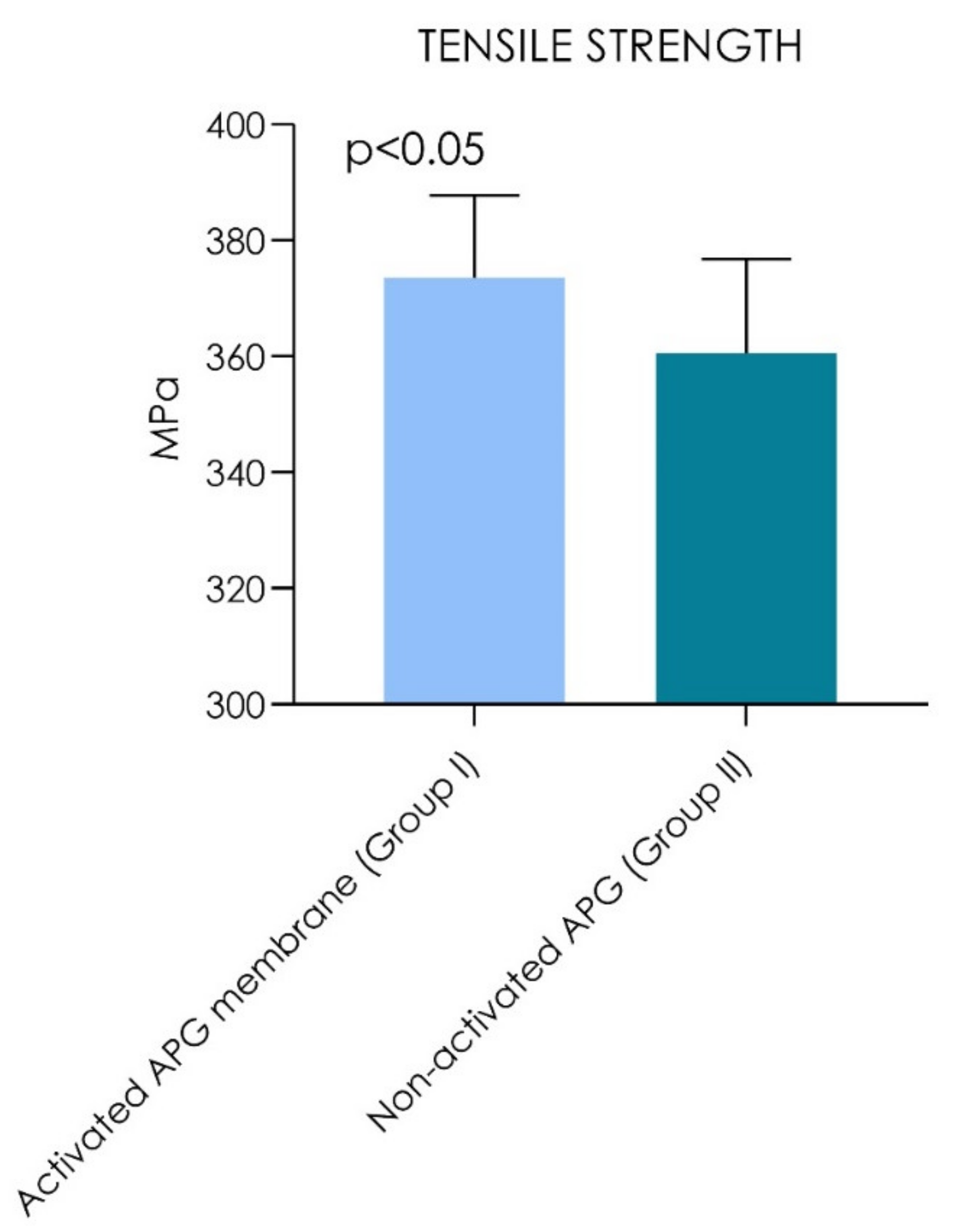
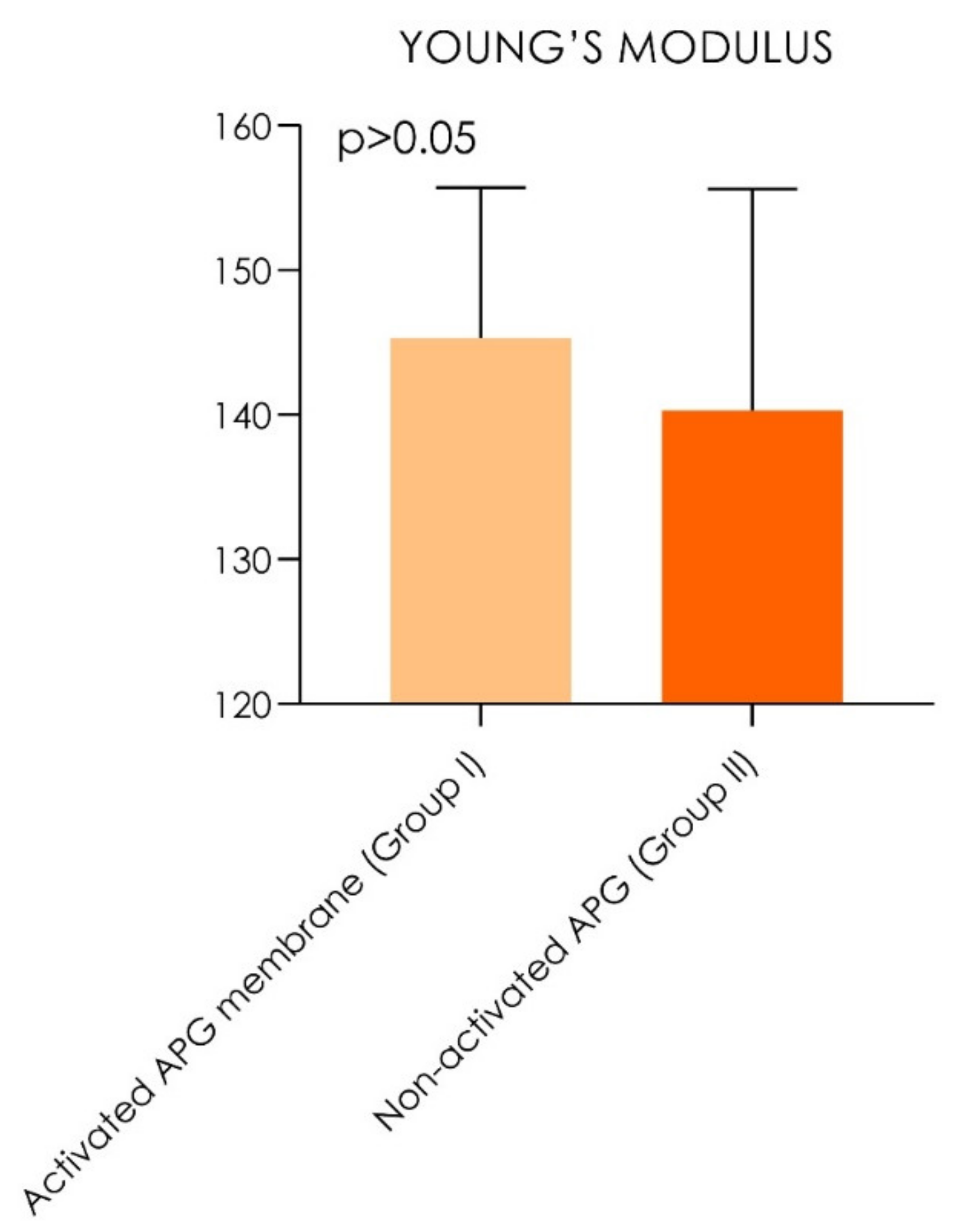
| Tensile Strength | Young’s Modulus | |||||
|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | |
| Activated APG membrane (Group I) | 373.5 MPa | (±14.3) | (348.2–372.8) | 145.3 MPa | (±10.4) | 128.8–151.8 |
| Non-activated APG (Group II) | 360.5 MPa | (±16.3) | (362.7–384.3) | 140.3 MPa | (±15.3) | 132.5–148.1 |
| p values | p = 0.0020 | p = 0.40 | ||||
| Multiple Linear Regression for Tensile Strength | |||||
|---|---|---|---|---|---|
| Activated APG membrane (Group I) | |||||
| Variable | |t| | p value | Estimate | Standard error | 95% confidence interval |
| Intercept | 15.56 | <0.0001 | 15.56 | 22.94 | 302.7 to 411.1 |
| age | 0.95 | 0.443 | 0.8926 | 0.8019 | −1.180 to 2.612 |
| Gender (m/f) | 0.28 | 0.804 | 0.2417 | 10.64 | −27.73 to 22.58 |
| Non-activated APG (Group II) | |||||
| Variable | |t| | p value | Estimate | Standard error | 95% confidence interval |
| Intercept | 13.07 | <0.0001 | 341.6 | 26.14 | 279.7 to 403.4 |
| Age | 0.8926 | 0.452 | 0.8158 | 0.9140 | −1.345 to 2.977 |
| Gender (m/f) | 0.2417 | 0.871 | -2.930 | 12.13 | −31.60 to 25.74 |
| Multiple Linear Regression for Young’s Modulus | |||||
|---|---|---|---|---|---|
| Activated APG membrane (Group I) | |||||
| Variable | |t| | p value | Estimate | Standard error | 95% confidence interval |
| Intercept | 7.687 | <0.0001 | 128.2 | 16.68 | 88.78 to 167.7 |
| Age | 0.8926 | 0.579 | 0.5205 | 0.5832 | −0.8584 to 1.899 |
| Gender (m/f) | 0.2417 | 0.874 | −1.870 | 7.737 | −20.16 to 16.42 |
| Non-activated APG (Group II) | |||||
| Variable | |t| | p value | Estimate | Standard error | 95% confidence interval |
| Intercept | 4.993 | <0.0001 | 122.5 | 24.54 | 64.50 to 180.6 |
| age | 0.8926 | 0.401 | 0.7658 | 0.8579 | −1.263 to 2.794 |
| Gender (m/f) | 0.2417 | 0.733 | −2.751 | 11.38 | −29.66 to 24.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarano, A.; Bugea, C.; Leo, L.; Santos de Oliveira, P.; Lorusso, F. Autologous Platelet Gel (APG): A Preliminary Evaluation of the Mechanical Properties after Activation with Autologous Thrombin and Calcium Chloride. Materials 2021, 14, 3941. https://doi.org/10.3390/ma14143941
Scarano A, Bugea C, Leo L, Santos de Oliveira P, Lorusso F. Autologous Platelet Gel (APG): A Preliminary Evaluation of the Mechanical Properties after Activation with Autologous Thrombin and Calcium Chloride. Materials. 2021; 14(14):3941. https://doi.org/10.3390/ma14143941
Chicago/Turabian StyleScarano, Antonio, Calogero Bugea, Lucia Leo, Pablo Santos de Oliveira, and Felice Lorusso. 2021. "Autologous Platelet Gel (APG): A Preliminary Evaluation of the Mechanical Properties after Activation with Autologous Thrombin and Calcium Chloride" Materials 14, no. 14: 3941. https://doi.org/10.3390/ma14143941
APA StyleScarano, A., Bugea, C., Leo, L., Santos de Oliveira, P., & Lorusso, F. (2021). Autologous Platelet Gel (APG): A Preliminary Evaluation of the Mechanical Properties after Activation with Autologous Thrombin and Calcium Chloride. Materials, 14(14), 3941. https://doi.org/10.3390/ma14143941








