Sol-Gel Synthesis of the Double Perovskite Sr2FeMoO6 by Microwave Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sol-Gel Method
2.3. Sol-Gel Method assisted by Microwave Radiation
2.4. X-ray Diffraction
2.5. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
2.6. Thermogravimetric Analysis and Differential Scanning Calorimetry
3. Results
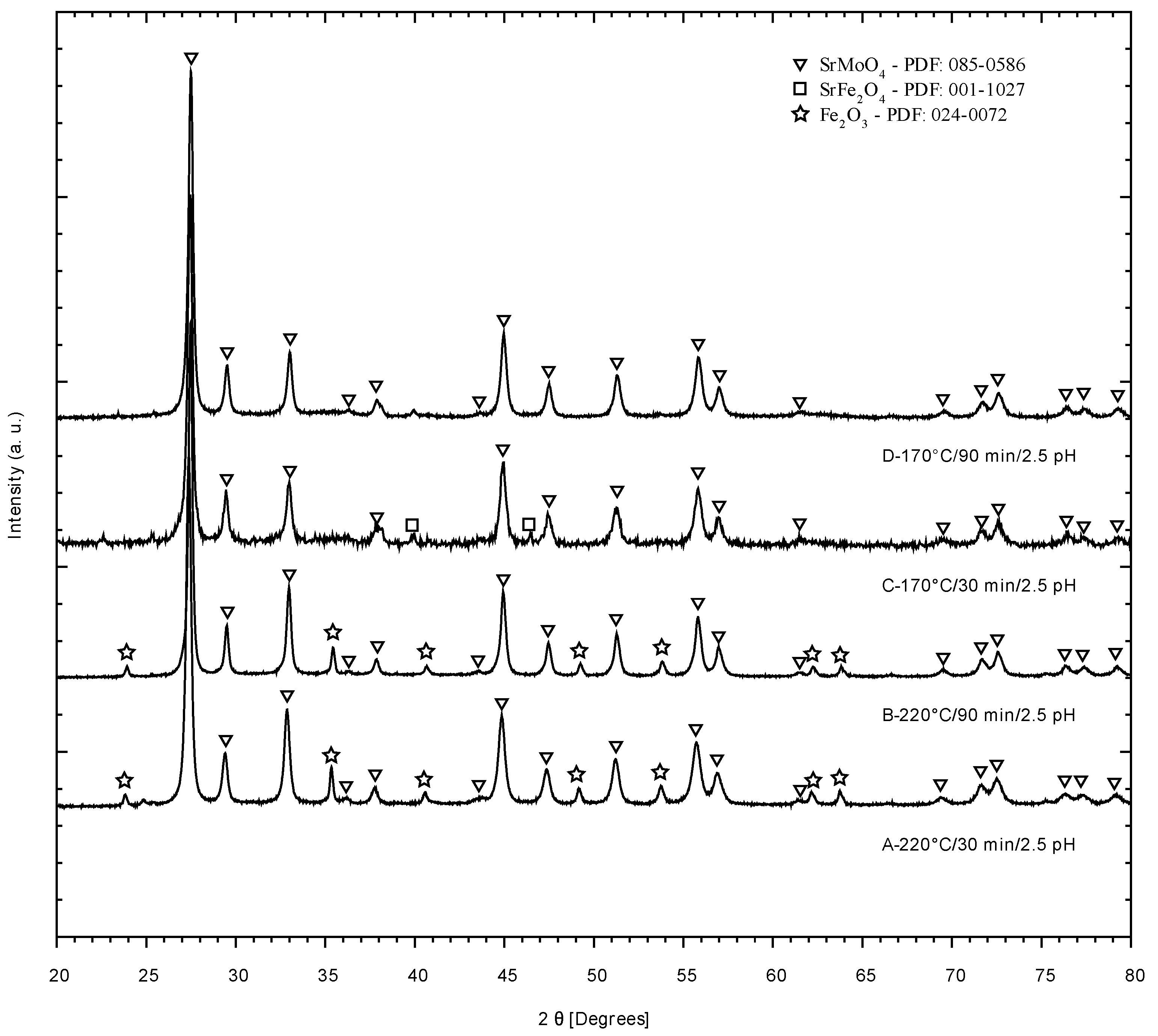
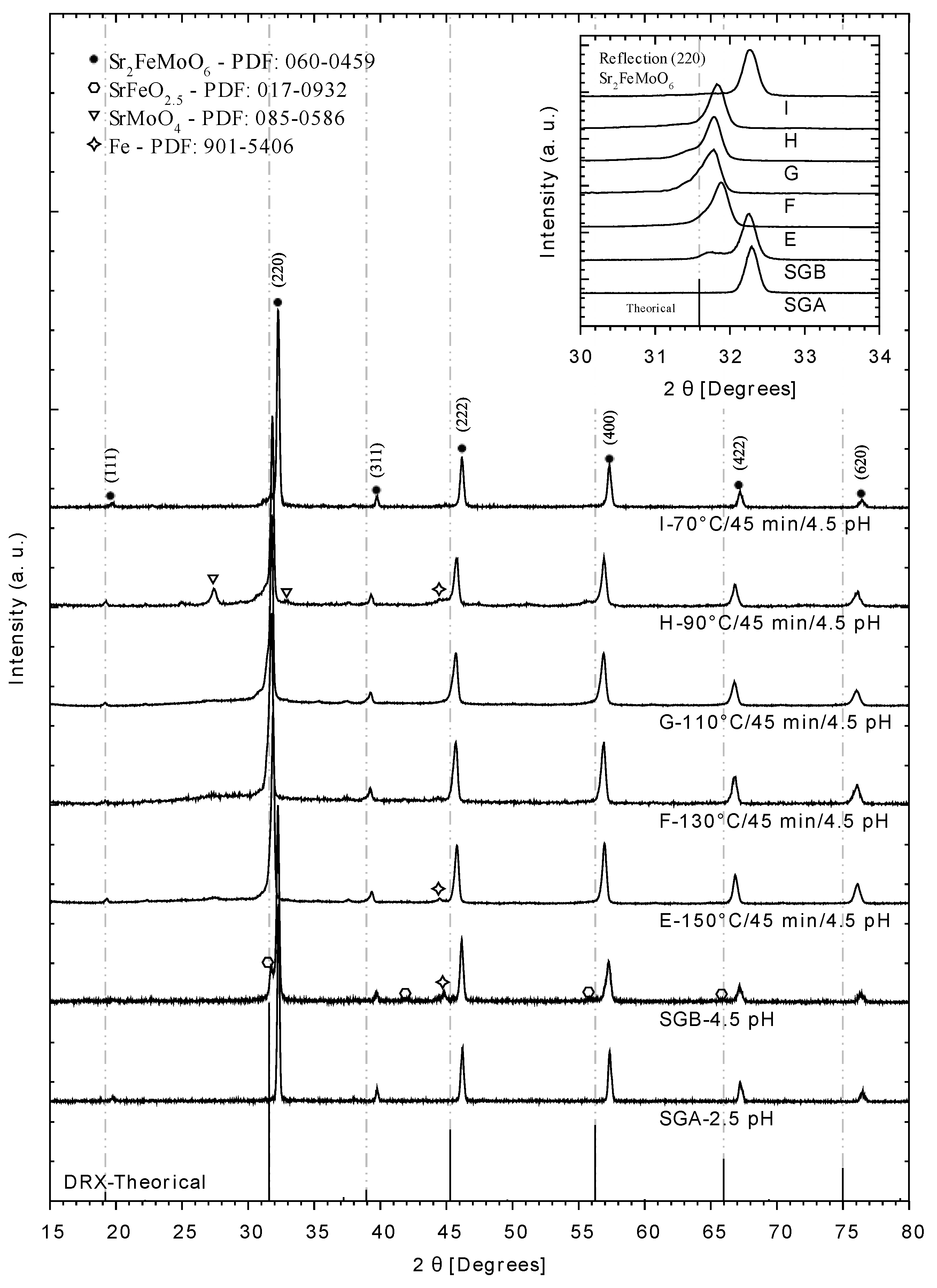
3.1. X-ray Diffraction
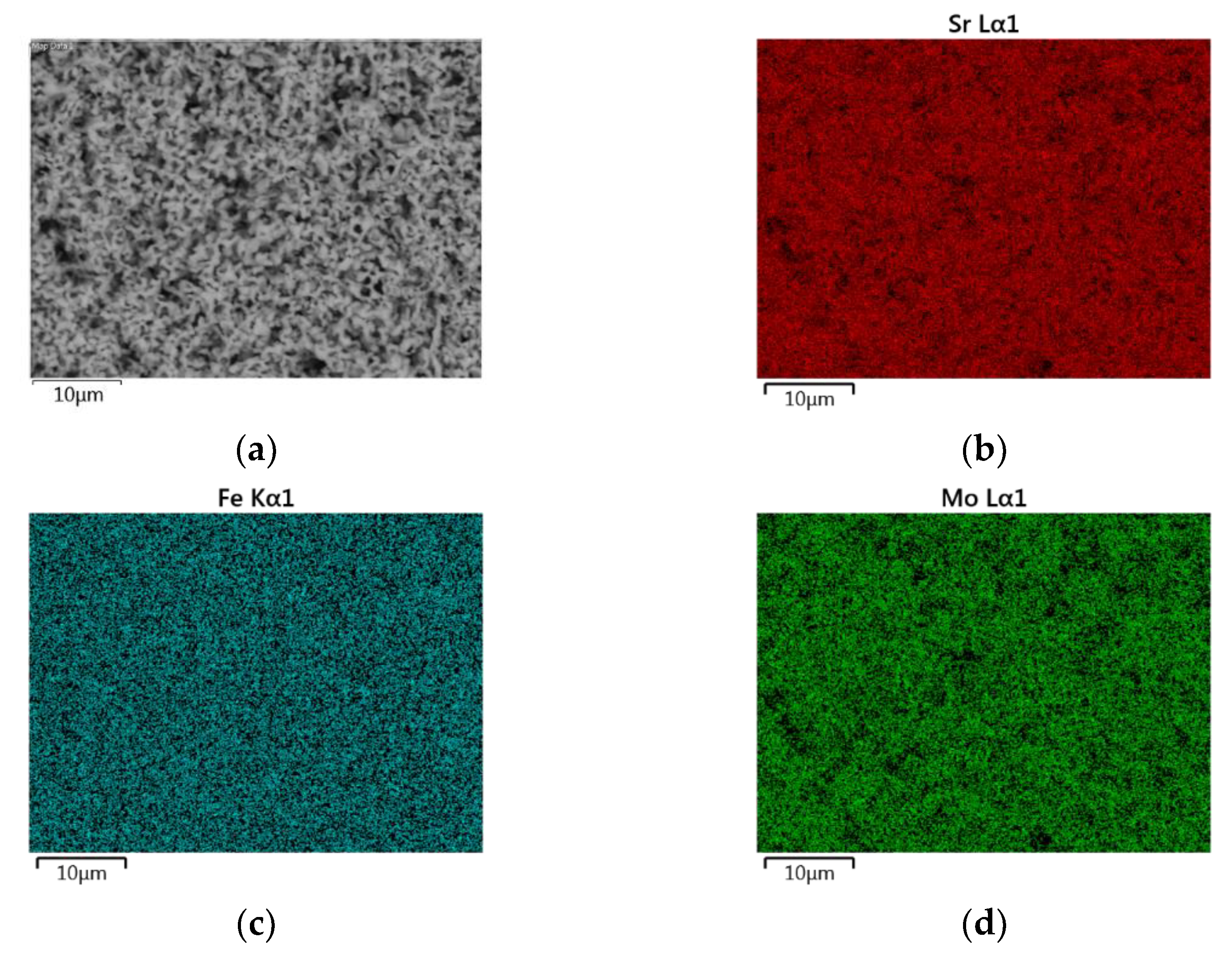
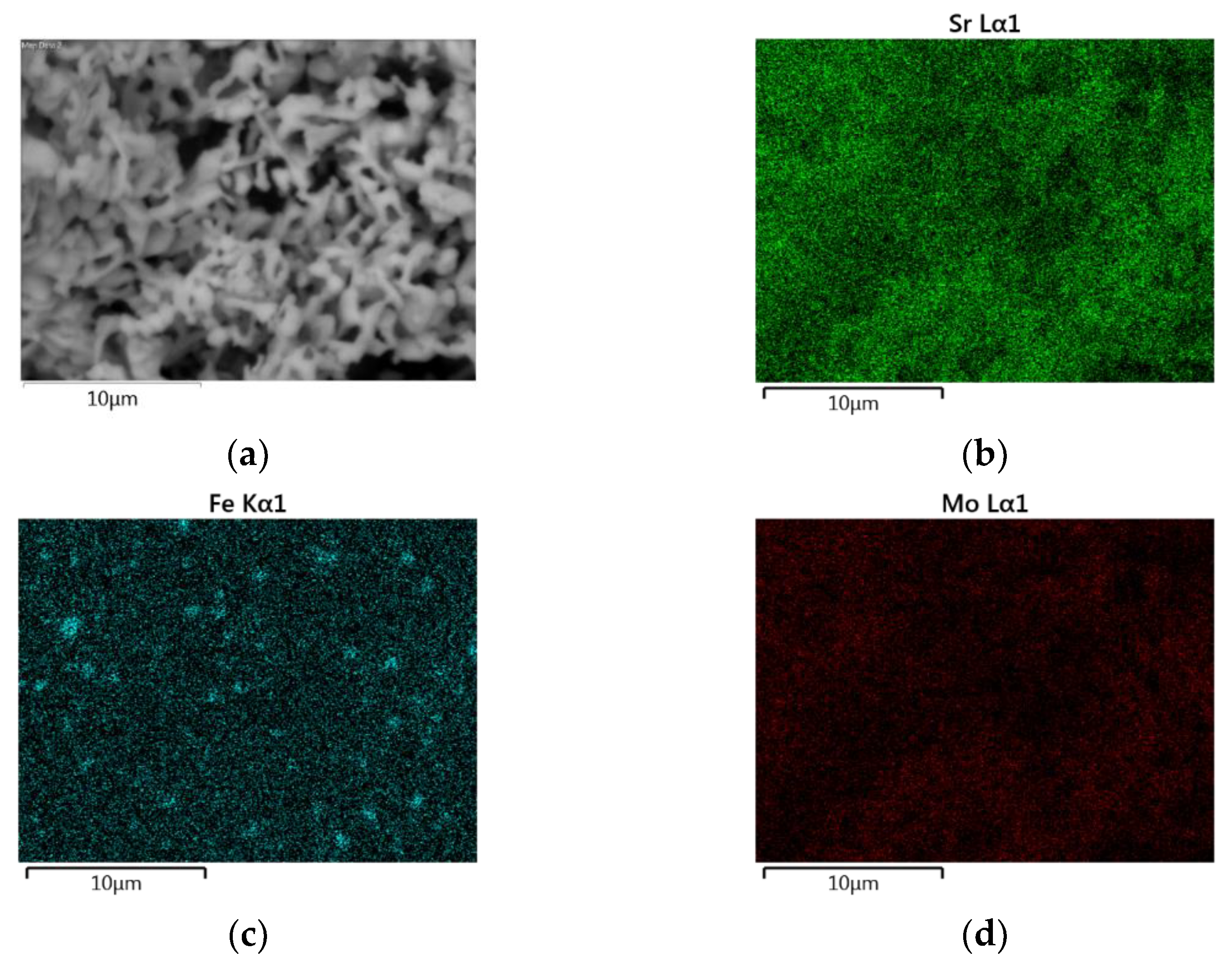
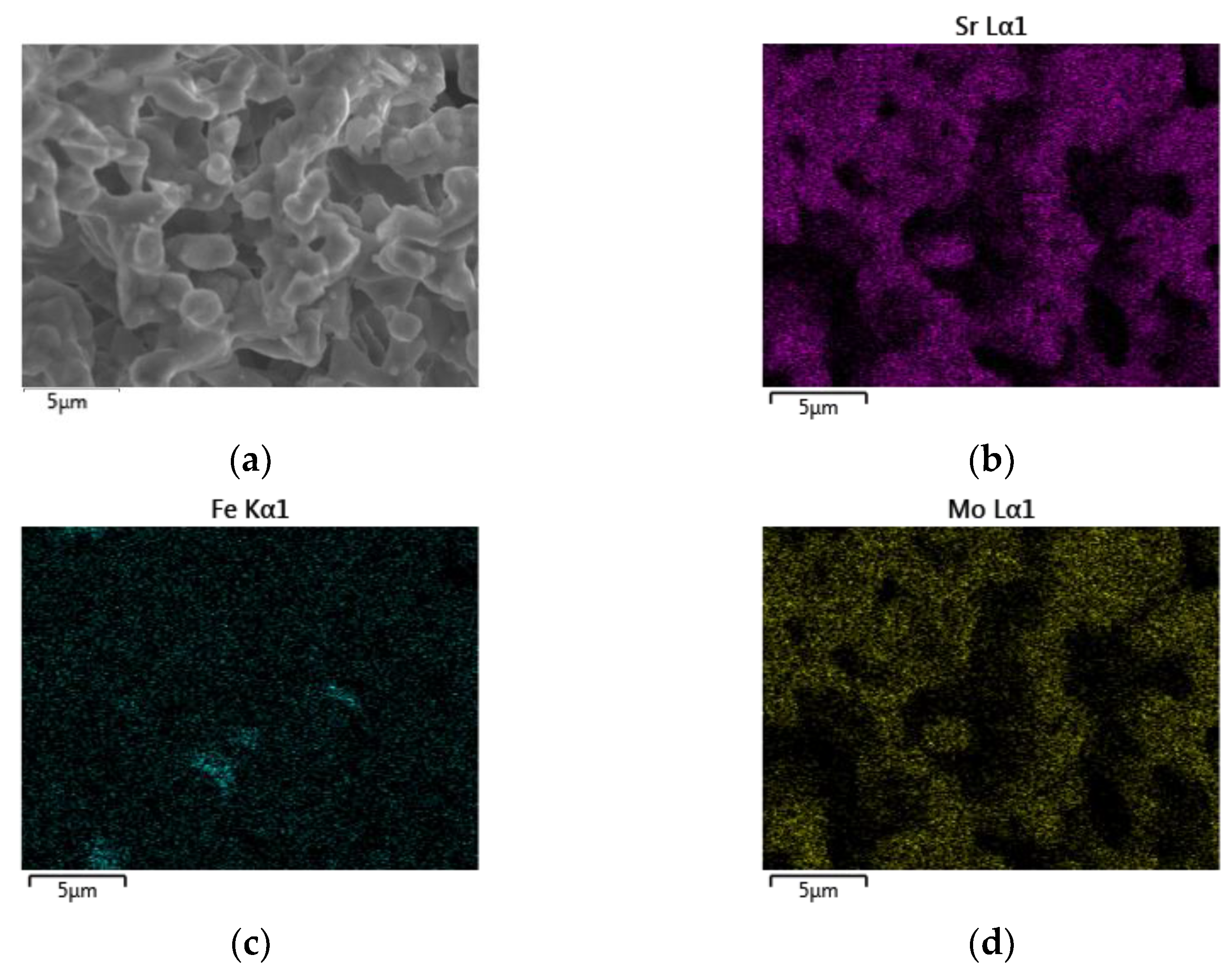
3.2. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
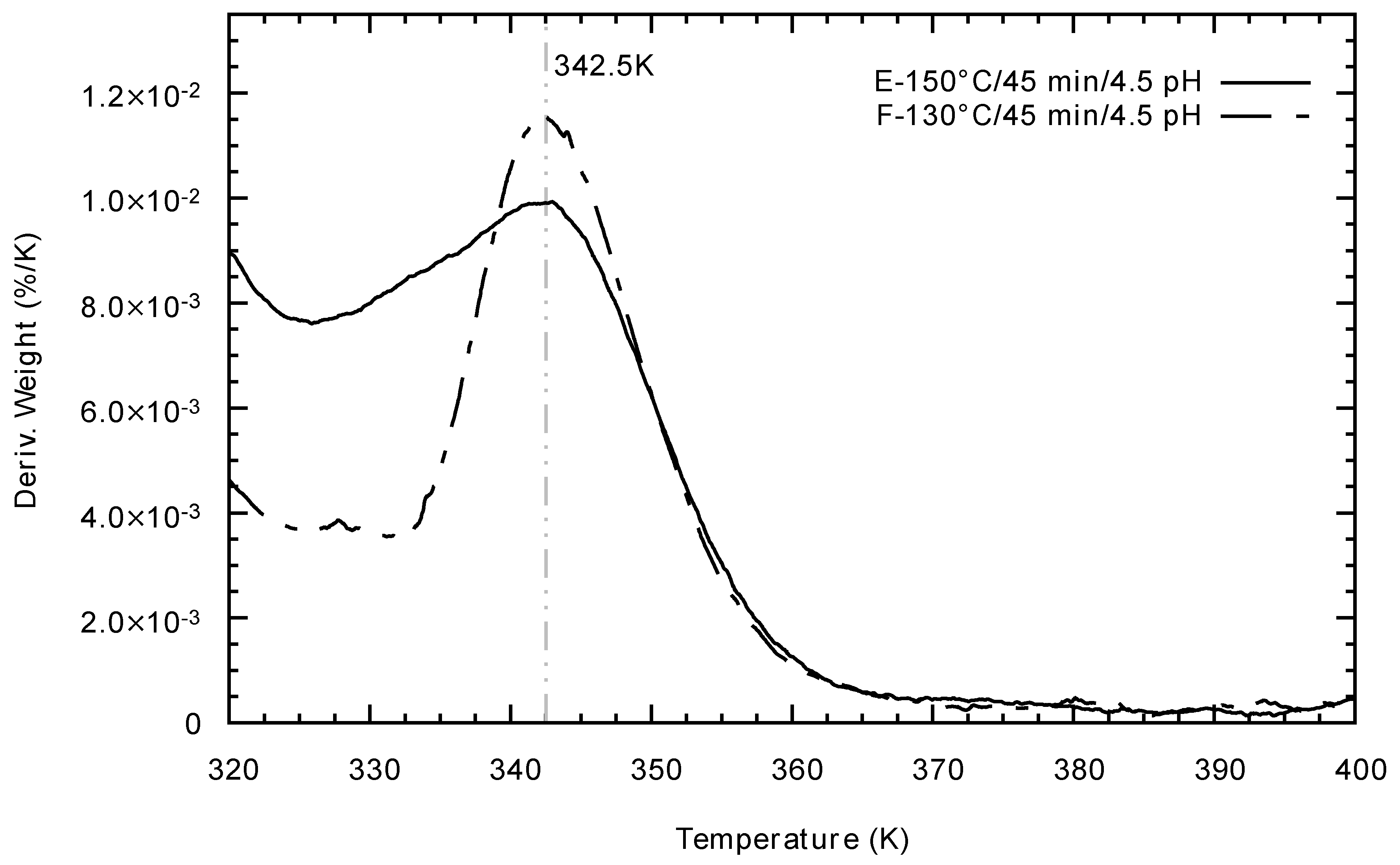
3.3. Thermogravimetric Analysis and Differential Scanning Calorimetry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valenzuela, J.L.; Soto, T.E.; Lemus, J.; Navarro, O.; Morales, R. Reaction kinetics of the double perovskite Sr2FeMoO6 by gas-solid reactions. Phys. B Condens. Matter 2014, 455, 10–13. [Google Scholar] [CrossRef]
- Kobayashi, K.I.; Kimura, T.; Sawada, H.; Terakura, K.; Tokura, Y. Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure. Nature 1998, 395, 677–680. [Google Scholar] [CrossRef]
- Kumar, N.; Khurana, G.; Katiyar, R.S.; Gaur, A.; Kotnala, R.K. Double perovskite Sr2FeMoO6: A potential candidate for room temperature magnetoresistance device applications. Magn. Sens. Dev. Trends Appl. 2017, 89. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhong, Y.; Shao, Z. Double perovskites in catalysis, electrocatalysis, and photo (electro) catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Soto, T.E.; Valenzuela, J.L.; Mondragón, R.; Morales, R.; Lemus-Ruz, J.; Navarro, O. Synthesis of the off-stoichiometric oxide system Sr2Fe1+xMo1-xO6 with -1 <= x <= 0.25. Phys. B Condens. Matter 2014, 455, 6–9. [Google Scholar] [CrossRef]
- Zhai, Y.; Qiao, J.; Huo, G.; Han, S. Synthesis, magnetic and electrical transport properties of magnetoresistance material Sr2FeMoO6 by microwave sintering. J. Magn. Magn. Mater. 2012, 324, 2006–2010. [Google Scholar] [CrossRef]
- Das, R.; Chaudhuri, U.; Mahendiran, R. Ac magnetoresistance and microwave absorption in Sr2FeMoO6. AIP Adv. 2021, 11, 015026. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol-gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Ghadiri, M.; Kang, A.K.; Gorji, N.E. XRD characterization of graphene-contacted perovskite solar cells: Moisture degradation and dark-resting recovery. Superlattices Microstruct. 2020, 146, 106677. [Google Scholar] [CrossRef]
- Li, X.Y.; Yao, Z.F.; Zhang, L.Y.; Zheng, G.H.; Dai, Z.X.; Chen, K.Y. Generation of oxygen vacancies on Sr2FeMoO6 to improve its photocatalytic performance through a novel preparation method involving pH adjustment and use of surfactant. Appl. Surf. Sci. 2019, 480, 262–275. [Google Scholar] [CrossRef]
- Alvarado-Flores, J.J.; Mondragón-Sánchez, R.; Ávalos-Rodrguez, M.L.; Alcaraz-Vera, J.V.; Rutiaga-Quiñones, J.G.; Guevara-Martnez, S.J. Synthesis, characterization and kinetic study of the Sr2FeMoO6-δ double perovskite: New findings on the calcination of one of its precursors. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Liu, S.; Qian, X.; Xiao, J. Synthesis and characterization of La0.8Sr0.2Co0.5Fe0.5O3+δ nanopowders by microwave assisted sol–gel route. J. Sol-Gel Sci. Technol. 2007, 44, 187–193. [Google Scholar] [CrossRef]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Puga, F.; Navó, J.A.; Jaramillo-Páez, C.; Sánchez-Cid, P.; Hidalgo, M.C. Microwave-assisted sol-gel synthesis of TiO2 in the presence of halogenhydric acids. Characterization and photocatalytic activity. J. Photochem. Photobiol. A Chem. 2020, 394, 112457. [Google Scholar] [CrossRef]
- Tran, T.H.; Bach, T.C.; Pham, N.H.; Nguyen, Q.H.; Sai, C.D.; Nguyen, H.N.; Nguyen, V.T.; Nguyen, T.T.; Ho, K.H.; Doan, Q.K. Phase transition of LaMnO3 nanoparticles prepared by microwave assisted combustion method. Mater. Sci. Semicond. Process. 2019, 89, 121–125. [Google Scholar] [CrossRef]
- Majid, F.; Riaz, S.; Naseem, S. Microwave-assisted sol–gel synthesis of BiFeO3 nanoparticles. J. Sol-Gel Sci. Technol. 2015, 74, 310–319. [Google Scholar] [CrossRef]
- Junliang, L.; Yanwei, Z.; Cuijing, G.; Wei, Z.; Xiaowei, Y. One-step synthesis of barium hexaferrite nano-powders via microwave-assisted sol–gel auto-combustion. J. Eur. Ceram. Soc. 2010, 30, 993–997. [Google Scholar] [CrossRef]
- Komarneni, S.; Roy, R.; Li, Q.H. Microwave-hydrothermal synthesis of ceramic powders. Mater. Res. Bull. 1992, 27, 1393–1405. [Google Scholar] [CrossRef]
- Li, X.H.; Sun, Y.P.; Lu, W.J.; Ang, R.; Zhang, S.B.; Zhu, X.B.; Song, W.H.; Dai, J.M. Size dependence of electronic and magnetic properties of double-perovskite Sr2FeMoO6. Solid State Commun. 2008, 145, 98–102. [Google Scholar] [CrossRef]
- Cernea, M.; Vasiliu, F.; Plapcianu, C.; Bartha, C.; Mercioniu, I.; Pasuk, I.; Lowndes, R.; Trusca, R.; Aldica, G.V.; Pintilie, L. Preparation by sol–gel and solid state reaction methods and properties investigation of double perovskite Sr2FeMoO6. J. Eur. Ceram. Soc. 2013, 33, 2483–2490. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, W.; Au, C.T.; Du, Y.W. Tunnelling magnetoresistance of double perovskite Sr2FeMoO6 enhanced by grain boundary adjustment. Nanotechnology 2005, 17, 250. [Google Scholar] [CrossRef]
- Reyes, A.M.; Arredondo, Y.; Navarro, O. First principles study of the effects of disorder in the Sr2FeMoO6 perovskite. Rev. Mex. Física 2016, 62, 160–163. [Google Scholar]
- Varaprasad, S.; Thyagarajan, K.; Markandeya, Y.; Suresh, K.; Bhikshamaiah, G. Characterization and magnetic studies of Sr2FexMo2-xO6 (0.8 < x < 1.5) double perovskites prepared by sonochemical method. J. Mater. Sci. Mater. Electron. 2018, 29, 13606–13613. [Google Scholar] [CrossRef]
- Cernea, M.; Vasiliu, F.; Bartha, C.; Plapcianu, C.; Mercioniu, I. Characterization of ferromagnetic double perovskite Sr2FeMoO6 prepared by various methods. Ceram. Int. 2014, 40, 11601–11609. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.H.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, B.; Soto, T.E.; de la Torre Medina, J.; Navarro, O. Curie temperature enhancement in the double perovskite Sr2-xLaxFeMoO6 system: An experimental study. Phys. B Condens. Matter 2019, 556, 108–113. [Google Scholar] [CrossRef]
- Kittel, C.; Galt, J.K. Ferromagnetic domain theory. Solid State Phys. 1956, 3, 437–564. [Google Scholar] [CrossRef]




| Experiment | Temperature (°C) | Pressure (Bar) | Reaction Time (min) | pH |
|---|---|---|---|---|
| A | 220 | 80 | 30 | 2.5 |
| B | 220 | 80 | 90 | 2.5 |
| C | 170 | 46 | 30 | 2.5 |
| D | 170 | 45 | 90 | 2.5 |
| E | 150 | 42 | 45 | 4.5 |
| F | 130 | 30 | 45 | 4.5 |
| G | 110 | 35 | 45 | 4.5 |
| H | 90 | 33 | 45 | 4.5 |
| I | 70 | 20 | 45 | 4.5 |
| Experiment | Crystallite Size (nm) | Temperature (°C) |
|---|---|---|
| SGA | 420 | 80 |
| SGB | 345 | 80 |
| E | 378 | 150 1 |
| F | 310 | 130 1 |
| G | 401 | 110 1 |
| H | 352 | 90 1 |
| I | 318 | 70 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, J.; Reséndiz, D.; Cuán, Á.; Nava, R.; Aguilar, B.; Cortés-Romero, C.M.; Navarro, O. Sol-Gel Synthesis of the Double Perovskite Sr2FeMoO6 by Microwave Technique. Materials 2021, 14, 3876. https://doi.org/10.3390/ma14143876
Valdés J, Reséndiz D, Cuán Á, Nava R, Aguilar B, Cortés-Romero CM, Navarro O. Sol-Gel Synthesis of the Double Perovskite Sr2FeMoO6 by Microwave Technique. Materials. 2021; 14(14):3876. https://doi.org/10.3390/ma14143876
Chicago/Turabian StyleValdés, Jesús, Daniel Reséndiz, Ángeles Cuán, Rufino Nava, Bertha Aguilar, Carlos M. Cortés-Romero, and Oracio Navarro. 2021. "Sol-Gel Synthesis of the Double Perovskite Sr2FeMoO6 by Microwave Technique" Materials 14, no. 14: 3876. https://doi.org/10.3390/ma14143876
APA StyleValdés, J., Reséndiz, D., Cuán, Á., Nava, R., Aguilar, B., Cortés-Romero, C. M., & Navarro, O. (2021). Sol-Gel Synthesis of the Double Perovskite Sr2FeMoO6 by Microwave Technique. Materials, 14(14), 3876. https://doi.org/10.3390/ma14143876







