Optical Transitions and Excited State Absorption Cross Sections of SrLaGaO4 Doped with Ho3+ Ions
Abstract
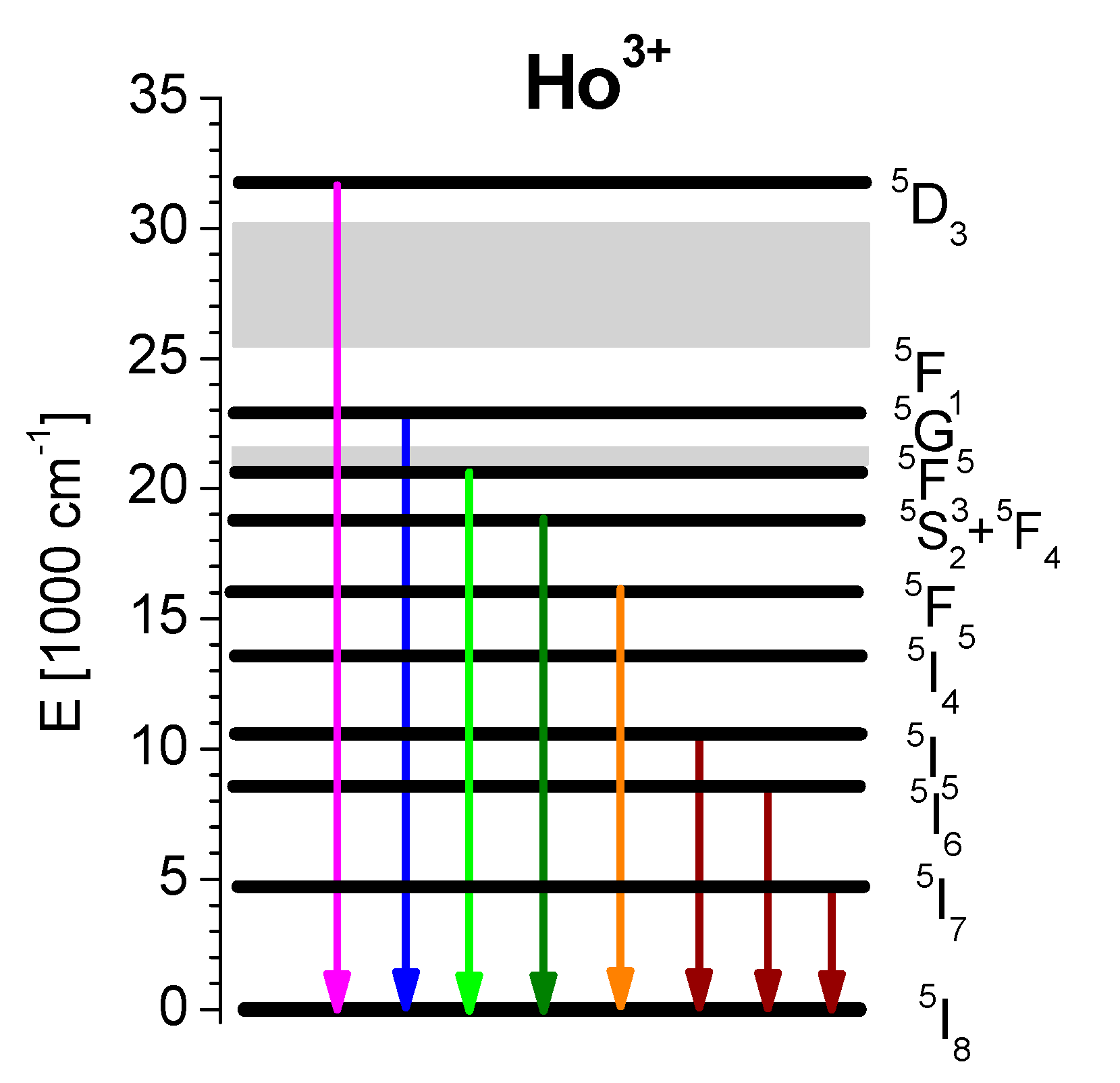
:1. Introduction
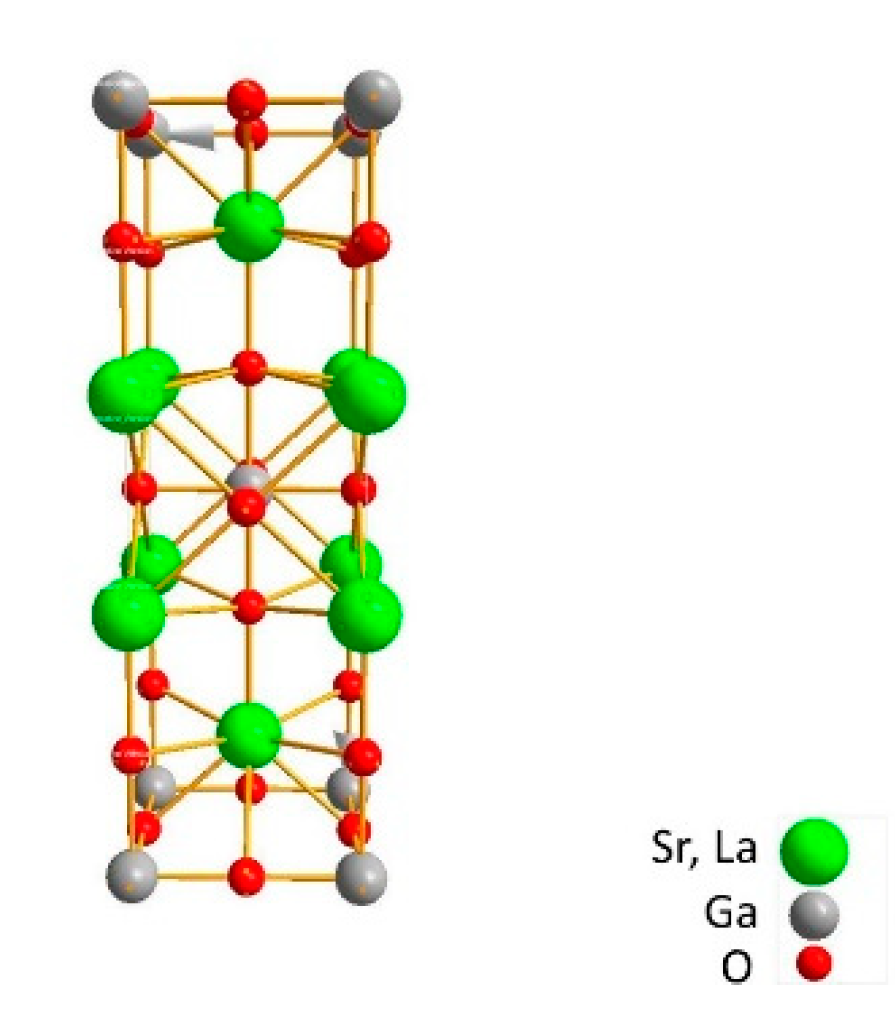
2. Materials and Experiment
3. Results and Discussion
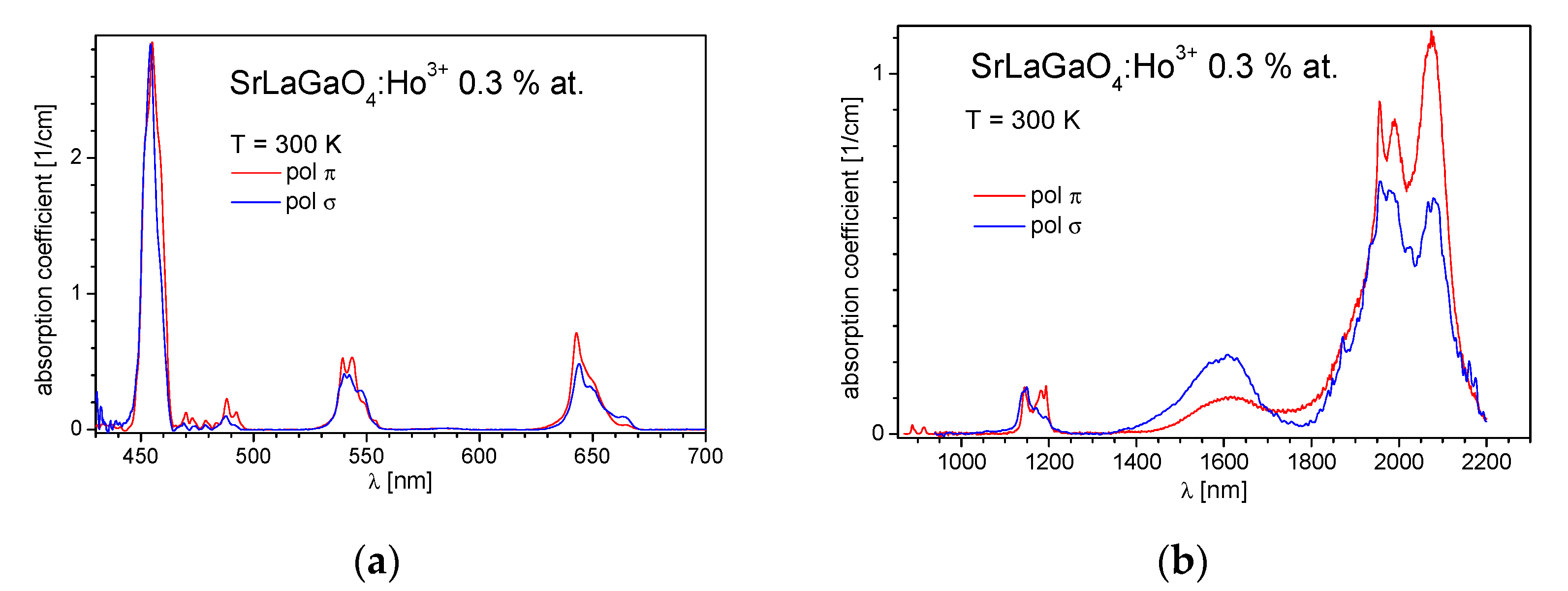
3.1. Absorption Spectra and Optical Transition Intensity Analysis
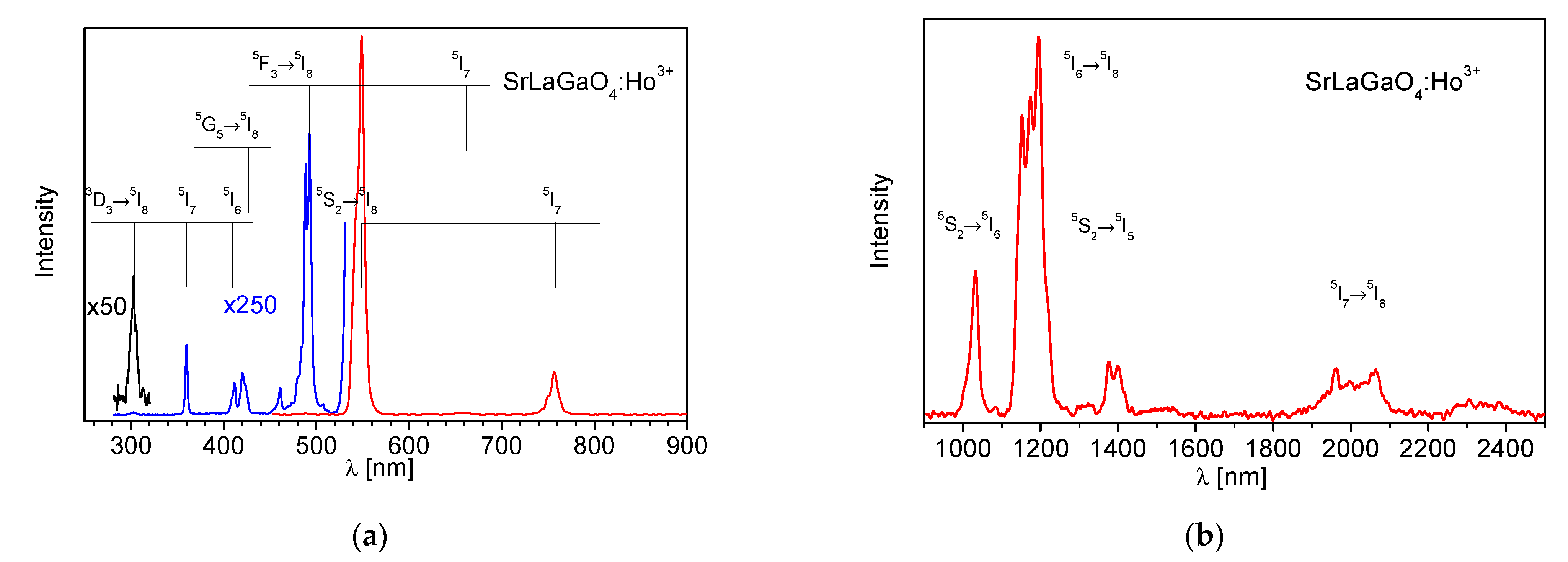
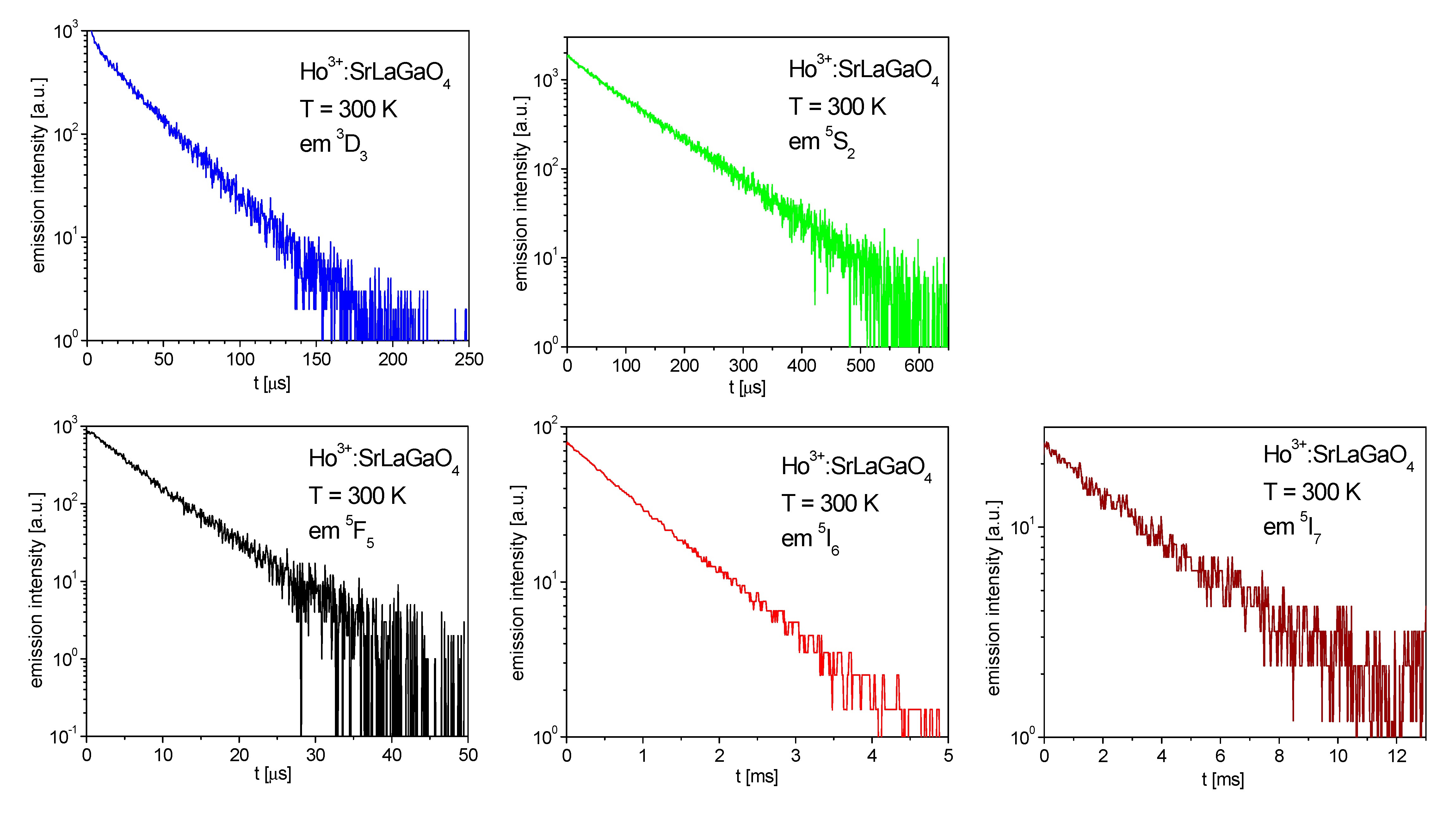
3.2. Luminescence Dynamics
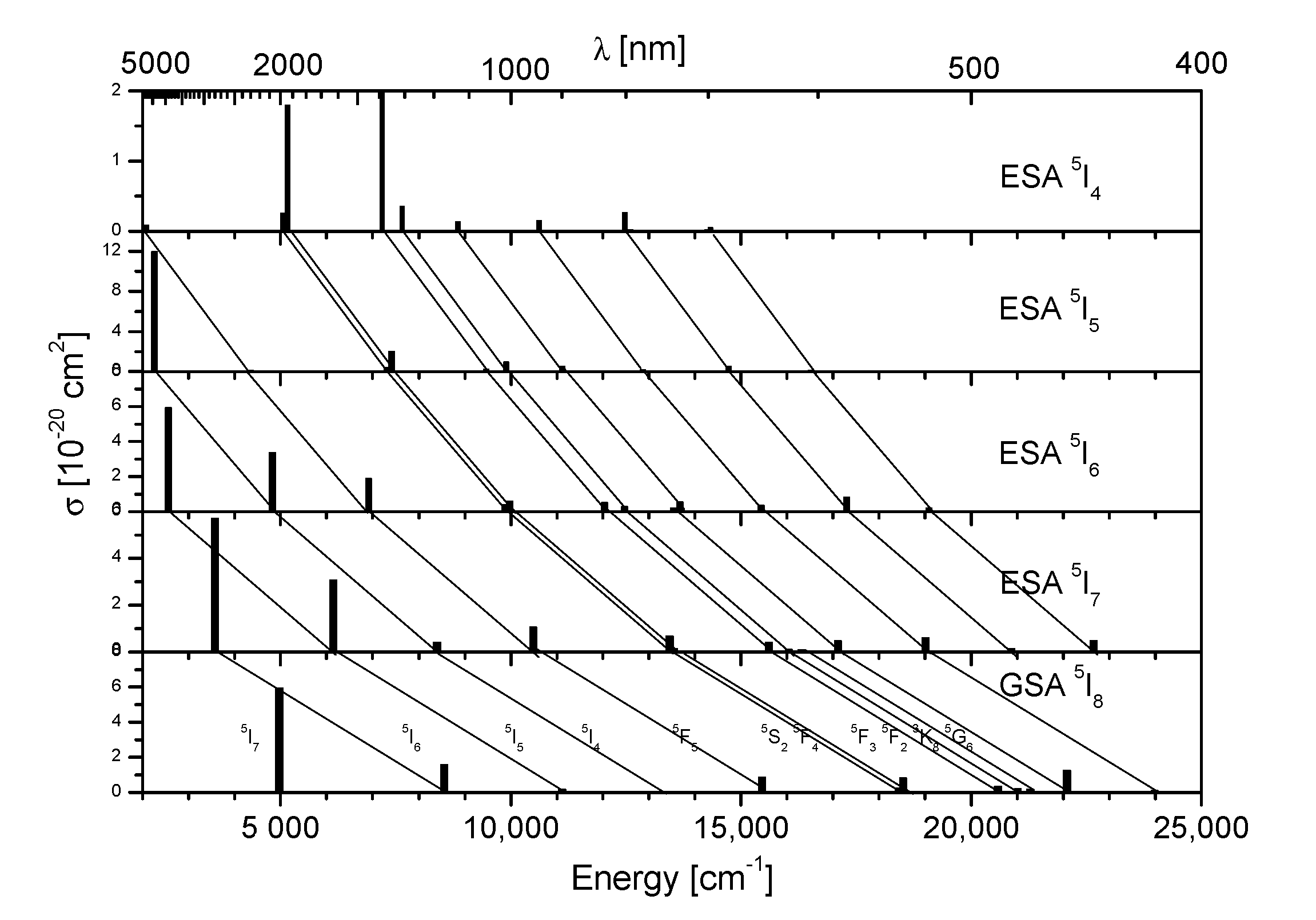
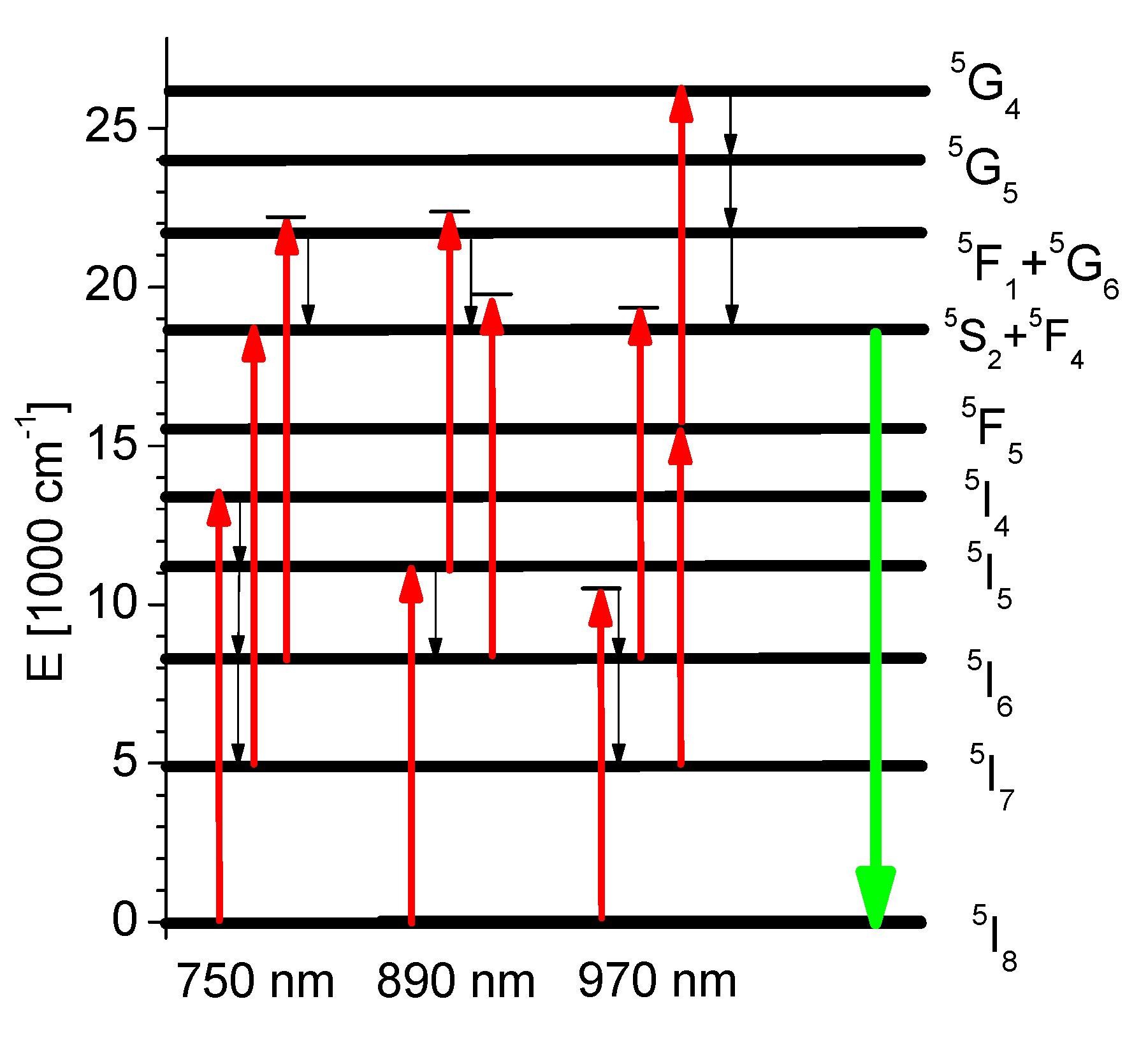
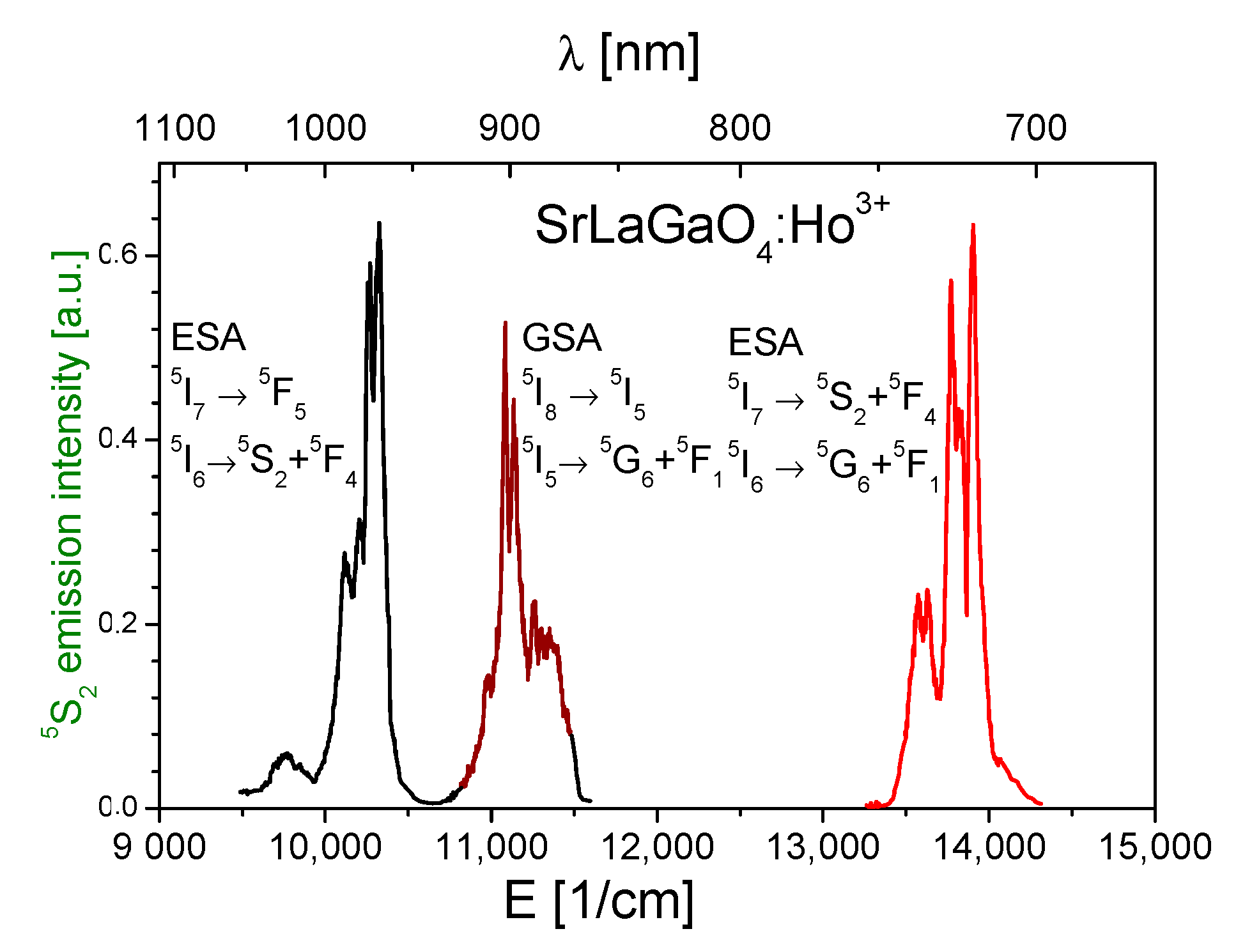
3.3. Excited State Absorption Transitions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uecker, R.; Reiche, P.; Ganschow, S.; Uecker, D.-C.; Schultze, D. Investigation of crystal growth of SrΡrGaO4 and SrLaGaO4. Acta Phys. Pol. A 1997, 92, 23–34. [Google Scholar] [CrossRef]
- Drozdowski, M.; Kasprowicz, D.; Paja̧czkowska, A. Characterization of the elastic and elasto-optic properties of SrLaAlO4 and SrLaGaO4 crystals using the Brillouin scattering method. J. Mol. Struct. 2000, 555, 119–129. [Google Scholar] [CrossRef]
- Fu, X.; Zheng, S.; Shi, J.; Li, Y.; Zhang, H. Long persistent luminescence property of a novel green emitting SrLaGaO4: Tb3+ phosphor. J. Lumin. 2017, 184, 199–204. [Google Scholar] [CrossRef]
- Grinberg, M.; Barzowska, J.; Shen, Y.; Pajączkowska, A. Spectral properties of Cr3+ in SrLaGaO4 under high hydrostatic pressure. Photonics Lett. Pol. 2011, 3, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Zhang, X.; Wang, J.; Zhao, Q.; Wong, K.-L.; Peng, M. Synthesis and photoluminescence properties of a novel red phosphor SrLaGaO4:Mn4+. J. Am. Ceram. Soc. 2018, 102, 1269–1276. [Google Scholar] [CrossRef]
- Karbowiak, M.; Rudowicz, C. Crystal-field analysis for RE3+ ions in laser materials: I. Absorption spectra and energy levels calculations for Nd3+ and Pr3+ ions in ABCO4 crystals. Chem. Phys. 2011, 383, 68–82. [Google Scholar] [CrossRef]
- Pan, Z.; Dai, X.; Lei, Y.; Cai, H.; Serres, J.M.; Aguiló, M.; Díaz, F.; Ma, J.; Tang, D.; Vilejshikova, E.; et al. Crystal growth and properties of the disordered crystal Yb:SrLaAlO4: A promising candidate for high-power ultrashort pulse lasers. CrystEngComm 2018, 20, 3388–3395. [Google Scholar] [CrossRef]
- Weber, M.J. Handbook of Optical Materials; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2003. [Google Scholar]
- Singh, V.; Rai, V.K.; Voss, B.; Haase, M.; Chakradhar, R.P.S.; Naidu, D.S.; Kim, S.H. Photoluminescence study of nanocrystalline Y2O3:Ho3+ phosphor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 109, 206–212. [Google Scholar] [CrossRef]
- Mariselvam, K.; Liu, J. Green emission and laser properties of Ho3+ doped titano lead borate (TLB) glasses for colour display applications. J. Solid State Chem. 2021, 293, 121793. [Google Scholar] [CrossRef]
- An, N.; Zhou, H.; Zhu, K.; Ye, L.; Qiu, J.; Wang, L.-G. Improved temperature sensing performance of YAG: Ho3+/Yb3+ by doping Ce3+ ions based on up-conversion luminescence. J. Alloys Compd. 2020, 843, 156057. [Google Scholar] [CrossRef]
- Piatkowski, D.; Wisniewski, K.; Rozanski, M.; Koepke, C.; Kaczkan, M.; Klimczak, M.; Piramidowicz, R.; Malinowski, M. Excited state absorption spectroscopy of ZBLAN:Ho3+ glass—Experiment and simulation. J. Phys. Condens. Matter 2008, 20, 155201. [Google Scholar] [CrossRef]
- Kshetria, Y.K.; Joshib, B.; Leeb, S.W. Intense visible upconversion emission in transparent (Ho3+, Er3+)-α-Sialon ceramics under 980 nm laser excitation. J. Eur. Ceram. Soc. 2016, 36, 4215–4224. [Google Scholar] [CrossRef]
- Wnuk, A.; Kaczkan, M.; Frukacz, Z.; Pracka, I.; Chadeyron, G.; Joubert, M.-F.; Malinowski, M. Upconversion processes in holmium activated laser crystals. J. Alloys Compd. 2002, 341, 353–357. [Google Scholar] [CrossRef]
- Wang, J.; Bae, N.; Lee, S.B.; Lee, K. Effects of ion clustering and excited state absorption on the performance of Ho-doped fiber lasers. Opt. Express 2019, 27, 14283–14297. [Google Scholar] [CrossRef]
- Ryba-Romanowski, W.; Gołąb, S.; Pisarski, W.A.; Dominiak-Dzik, G.; Berkowski, M.; Pajączkowska, A. Investigation of Eu3+ sites in SrLaGa3O7, SrLaGaO4 and SrLaAlO4 crystals. J. Phys. Chem. Solids 1997, 58, 639–645. [Google Scholar] [CrossRef]
- Kaczkan, M.; Malinowski, M. Inhomogeneity of Ho3+ activated SrLaGa3O7 and SrLaGaO4 crystals studied by fluorescence line narrowing technique. Opt. Mater. 2006, 28, 119–122. [Google Scholar] [CrossRef]
- Kaczkan, M.; Malinowski, M. Upconversion-induced ultraviolet emission in Ho3+ doped SrLaGa3O7 and SrLaGaO4 crystals. J. Alloys Compd. 2004, 380, 201–204. [Google Scholar] [CrossRef]
- Pajaczkowska, A.; Novosselov, A.V.; Zimina, G.V. On the dissociation and growth of SrLaGaO4 and SrLaAlO4 single crystals. J. Cryst. Growth 2001, 223, 169–174. [Google Scholar] [CrossRef]
- Shannon, R.; Oswald, R.; Parise, J.; Chai, B.; Byszewski, P.; Pajaczkowska, A.; Sobolewski, R. Dielectric constants and crystal structures of CaYAlO4, CaNdAlO4, and SrLaAlO4, and deviations from the oxide additivity rule. J. Solid State Chem. 1992, 98, 90–98. [Google Scholar] [CrossRef]
- Lomheim, T.S.; DeShazer, L.G. Optical-absorption intensities of trivalent neodymium in the uniaxial crystal yttrium orthovanadate. J. Appl. Phys. 1978, 49, 5517–5522. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Walsh, B. Judd-Ofelt Theory: Principles and Practices; Di Bartolo, B., Forte, O., Eds.; Advances in Spectroscopy for Lasers and Sensing; Springer: Dordrecht, The Netherlands, 2006; pp. 403–433. [Google Scholar]
- Carnall, W.T.; Crosswhite, H.; Crosswhite, H.M. Energy Level Structure and Transition Probabilities of the Trivalent Lanthanides in LaF3; Argonne National Laboratory: Argonne, IL, USA, 1975. [Google Scholar]
- Kaminskii, A.A. Crystalline Lasers; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Kaczkan, M.; Pracka, I.; Malinowski, M. Optical transitions of Ho3+ in SrLaGa3O7. Opt. Mater. 2004, 25, 345–352. [Google Scholar] [CrossRef]
- Walsh, B.M.; Grew, G.W.; Barnes, N.P. Energy levels and intensity parameters of Ho3+ ions in Y3Al5O12 and Lu3Al5O12. J. Phys. Chem. Solids 2006, 67, 1567–1582. [Google Scholar] [CrossRef]
- Walsh, B.M.; Grew, G.W.; Barnes, N.P. Energy levels and intensity parameters of Ho3+ ions in GdLiF4, YLiF4 and LuLiF4. J. Phys. Condens. Matter 2005, 17, 7643–7665. [Google Scholar] [CrossRef]
- Riseberg, A.; Moos, H.W. Multiphonon Orbit-Lattice Relaxation of Excited States of Rare-Earth Ions in Crystals. Phys. Rev. 1968, 157, 429–438. [Google Scholar] [CrossRef]
- Mobini, E.; Peysokhan, M.; Abaie, B.; Hehlen, M.P.; Mafi, A. Spectroscopic Investigation of Yb -Doped Silica Glass for Solid-State Optical Refrigeration. Phys. Rev. Appl. 2019, 11, 014066. [Google Scholar] [CrossRef] [Green Version]
- Kamba, S.; Buixaderas, E.; Pajaczkowska, A. Polarized IR Spectra of SrLaAlO4 and SrLaGaO4 Single Crystals. Phys. Stat. Sol. 1998, 168, 317–324. [Google Scholar] [CrossRef]
- Liu, C.; Heo, J. Up-conversion and photon avalanche in oxy-fluoride nano-structured glasses doped with Ho3+. J. Non-Cryst. Solids 2006, 352, 5325–5330. [Google Scholar] [CrossRef]
- Auzel, F. Multiphonon-assisted anti-Stokes and Stokes fluorescence of triply ionized rare-earth ions. Phys. Rev. B 1976, 13, 2809–2817. [Google Scholar] [CrossRef]
- Kück, S.; Osiac, E.; Sokólska, I. Determination of excited state absorption cross sections for spectrally overlapping transitions via phase sensitive detection. In Proceedings of the CLEO/Europe. 2005 Conference on Lasers and Electro-Optics Europe, Munich, Germany, 12–17 June 2005; p. 47. [Google Scholar]
- Goldner, P.; Pelle, F. Photon avalanche fluorescence and lasers. Opt. Mater. 1996, 5, 239–249. [Google Scholar] [CrossRef]


| Transition 5I8 → | Wavelength λ [nm] | Oscillator Strengths | |
|---|---|---|---|
| [10−6] | [10−6] | ||
| 5I7 | 2011 | 2.19 | 1.73 |
| 5I6 | 1169 | 1.13 | 1.34 |
| 5I5 | 902 | 0.33 | 0.24 |
| 5I4 | 748 | 0.03 | 0.02 |
| 5F5 | 647 | 3.37 | 2.29 |
| 5S2 + 5F4 | 541 | 3.86 | 4.04 |
| 5F3 | 488 | 0.92 | 1.58 |
| 5F2 | 476 | 0.10 | 0.92 |
| 3K8 | 470 | 0.29 | 0.84 |
| 5G6 + 5F1 | 454 | 20.7 | 6.68 |
| RMS = 5.3 × 10−6 | |||
| Material | Ω2 [10−20 cm2] | Ω4 [10−20 cm2] | Ω6 [10−20 cm2] | Ref. |
|---|---|---|---|---|
| SrLaGaO4 (SLO) | 1.25 | 0.42 | 1.80 | This work |
| SrLaGa3O7 (SLG) | 2.23 | 0.85 | 1.81 | [27] |
| ZBLAN glass | 2.46 | 2.02 | 1.71 | [12] |
| Y3Al5O12 (YAG) | 0.10 | 2.09 | 1.72 | [28] |
| LiYF4 (YLF) | 1.16 | 2.24 | 2.09 | [29] |
| Transition | SrLaGaO4:Ho3+ | |||
|---|---|---|---|---|
| A [1/s] | β | τR | ||
| 3D3 → 3G5 | 22 | 0.011 | ||
| 5G5 | 70 | 0.034 | ||
| 3K7 | 32 | 0.015 | ||
| 5G6 | 20 | 0.009 | ||
| 3K8 | 821 | 0.374 | ||
| 5F3 | 25 | 0.011 | ||
| 5F4 | 35 | 0.016 | ||
| 5F5 | 11 | 0.005 | ||
| 5I4 | 50 | 0.023 | ||
| 5I6 | 106 | 0.141 | ||
| 5I7 | 794 | 0.361 | ||
| 5I8 | 87 | 0.039 | ||
| ∑A = 2097 | 1 | 477 μs | ||
| 5F3 → 5F4 | 0 | 0.000 | ||
| 5S2 | 0 | 0.000 | ||
| 5F5 | 1 | 0.000 | ||
| 5I4 | 112 | 0.024 | ||
| 5I5 | 50 | 0.011 | ||
| 5I6 | 265 | 0.057 | ||
| 5I7 | 761 | 0.165 | ||
| 5I8 | 3427 | 0.742 | ||
| ∑A = 4615 | 1 | 217 μs | ||
| 5S2 → 5F5 | 0 | 0.000 | ||
| 5I4 | 64 | 0.013 | ||
| 5I5 | 64 | 0.014 | ||
| 5I6 | 256 | 0.054 | ||
| 5I7 | 1835 | 0.388 | ||
| 5I8 | 2511 | 0.531 | ||
| ∑A = 4731 | 1 | 211 μs | ||
| 5F5 → 5I4 | 0 | 0.000 | ||
| 5I5 | 11 | 0.004 | ||
| 5I6 | 140 | 0.052 | ||
| 5I7 | 471 | 0.175 | ||
| 5I8 | 2068 | 0.769 | ||
| ∑A = 2690 | 1 | 372 μs | ||
| 5I4 → 5I5 | 9 | 0.050 | ||
| 5I6 | 70 | 0.371 | ||
| 5I7 | 90 | 0.478 | ||
| 5I8 | 19 | 0.100 | ||
| ∑A = 187 | 1 | 5.34 ms | ||
| 5I5 → 5I6 | 9 | 0.031 | ||
| 5I7 | 173 | 0.583 | ||
| 5I8 | 115 | 0.386 | ||
| ∑A = 297 | 1 | 3.37 ms | ||
| 5I6 → 5I7 | 30 | 0.084 | ||
| 5I8 | 324 | 0.916 | ||
| ∑A = 353 | 1 | 2.83 ms | ||
| 5I7 → 5I8 | 125 | 1 | 8.02 ms | |
| Manifold | SrLaGaO4:Ho3+ | |||
|---|---|---|---|---|
| τR [µs] | τobs [µs] | ΔEi(i−1) [cm−1] | Wnr [s−1] | |
| 3D3 | 477 | 35 | 2100 | 26,500 |
| 5S2 | 211 | 151 | 2794 | 1883 |
| 5F5 | 372 | 7 | ~1950 | 140,169 |
| 5I6 | 2833 | 1200 | 3426 | 480 |
| 5I7 | 8015 | 4000 | 4225 | 125 |
| Material | 5I8 → 5I4 | 5I7 → 5S2 + 5F4 | 5I6 → 5G6 + 5F1 | Ref. |
|---|---|---|---|---|
| σ (GSA) [10−20 cm2] | σ (ESA) [10−20 cm2] | σ (ESA) [10−20 cm2] | ||
| SLO | 0.0053 | 1.242 | 0.903 | This work |
| SLG | 0.0037 | 1.318 | 0.116 | [27] |
| ZBLAN | 0.0025 | 1.220 | 0.942 | [12] |
| YAG | 0.0036 | 1.309 | 1.202 | [28] |
| YLF | 0.0037 | 1.405 | 1.080 | [29] |
| |Ut|2 | 5I8 → 5I4 | 5I7 → 5F4 | 5I7 → 5S2 | 5I7 → 5S2 + 5F4 | 5I6 → 5G6 | 5I6 → 5F1 | 5I6 → 5G6 + 5F1 |
|---|---|---|---|---|---|---|---|
| |U2|2 | 0 | 0 | 0 | 0 | 0.0093 | 0 | 0.0093 |
| |U4|2 | 0 | 0.1965 | 0 | 0.1965 | 0.0837 | 0 | 0.0837 |
| |U6|2 | 0.0082 | 0.0349 | 0.4195 | 0.4544 | 0.1094 | 0.2383 | 0.3477 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczkan, M.; Malinowski, M. Optical Transitions and Excited State Absorption Cross Sections of SrLaGaO4 Doped with Ho3+ Ions. Materials 2021, 14, 3831. https://doi.org/10.3390/ma14143831
Kaczkan M, Malinowski M. Optical Transitions and Excited State Absorption Cross Sections of SrLaGaO4 Doped with Ho3+ Ions. Materials. 2021; 14(14):3831. https://doi.org/10.3390/ma14143831
Chicago/Turabian StyleKaczkan, Marcin, and Michał Malinowski. 2021. "Optical Transitions and Excited State Absorption Cross Sections of SrLaGaO4 Doped with Ho3+ Ions" Materials 14, no. 14: 3831. https://doi.org/10.3390/ma14143831
APA StyleKaczkan, M., & Malinowski, M. (2021). Optical Transitions and Excited State Absorption Cross Sections of SrLaGaO4 Doped with Ho3+ Ions. Materials, 14(14), 3831. https://doi.org/10.3390/ma14143831






