Electric Field Assisted Femtosecond Laser Preparation of Au@TiO2 Composites with Controlled Morphology and Crystallinity for Photocatalytic Degradation
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Au3+ and TiO2 Hydrate Suspensions
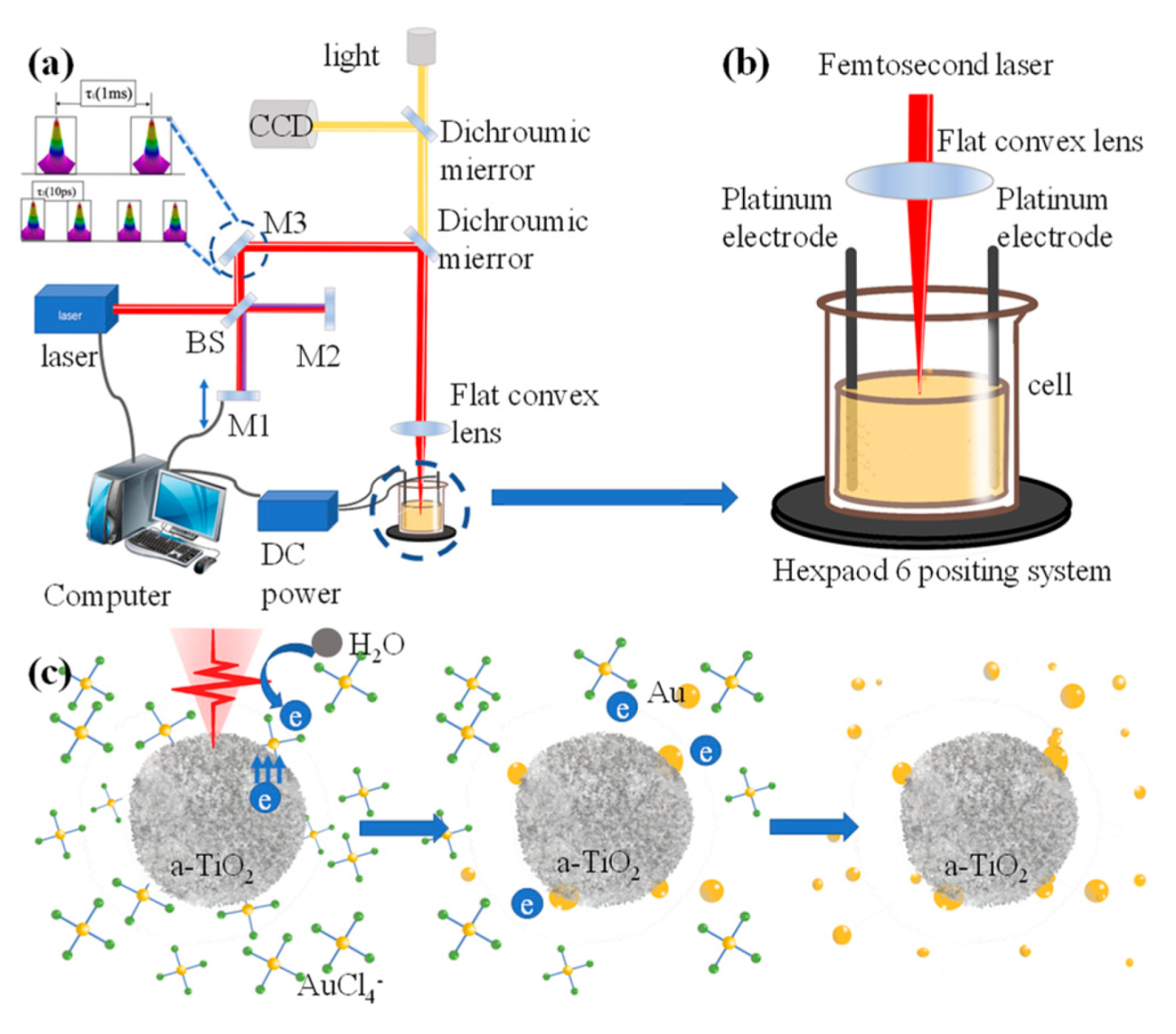
2.2. Electric Field Assisted Laser Processing System
2.3. Characterization of Au@TiO2
2.4. Crystallinity for Photocatalytic Degradation
3. Results and Discussion
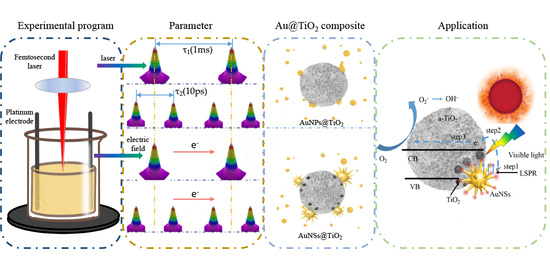
3.1. Electric-Field-Assisted Femtosecond Laser Preparation of Au@TiO2 Composites
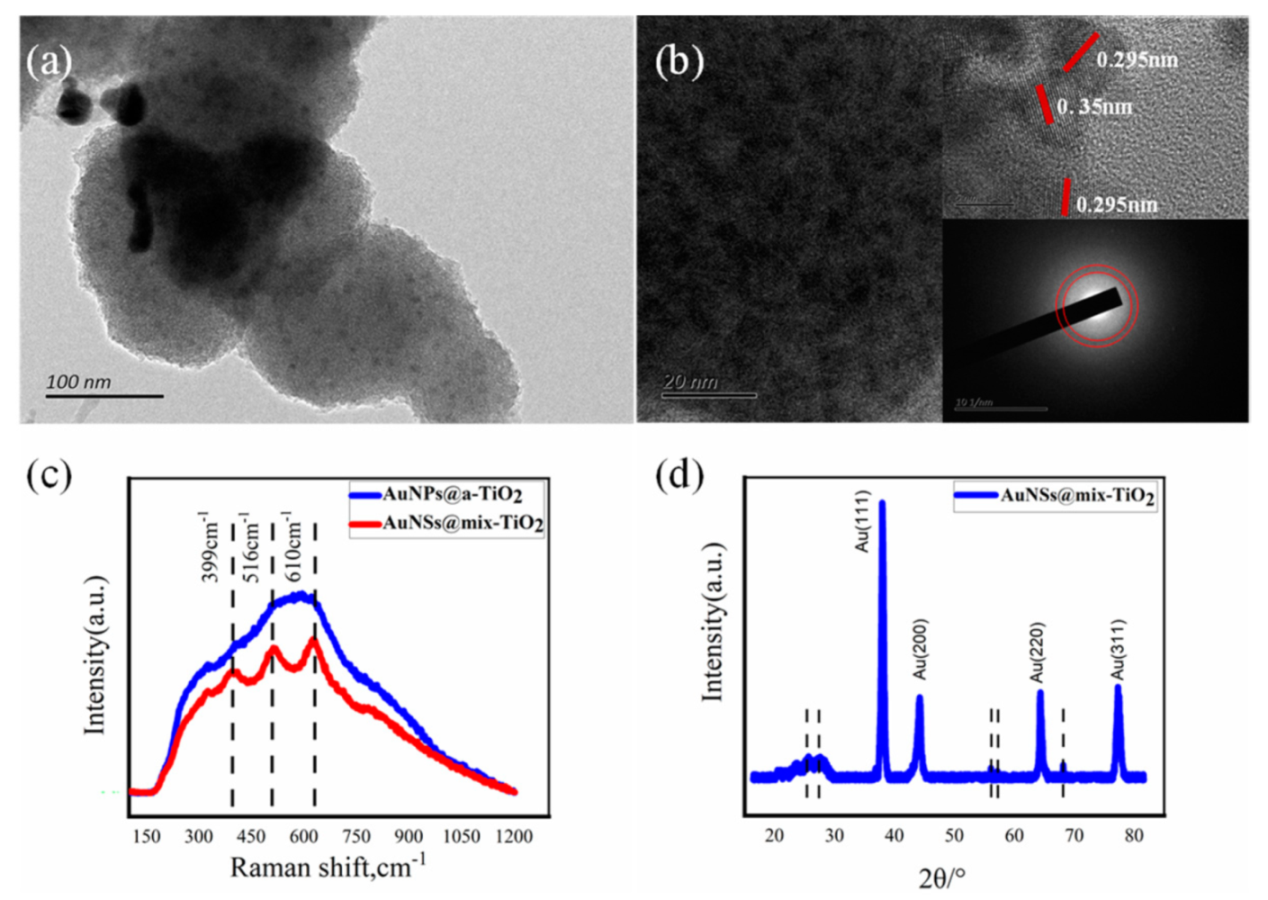
3.2. Morphology Characterization of Au @TiO2 Composite
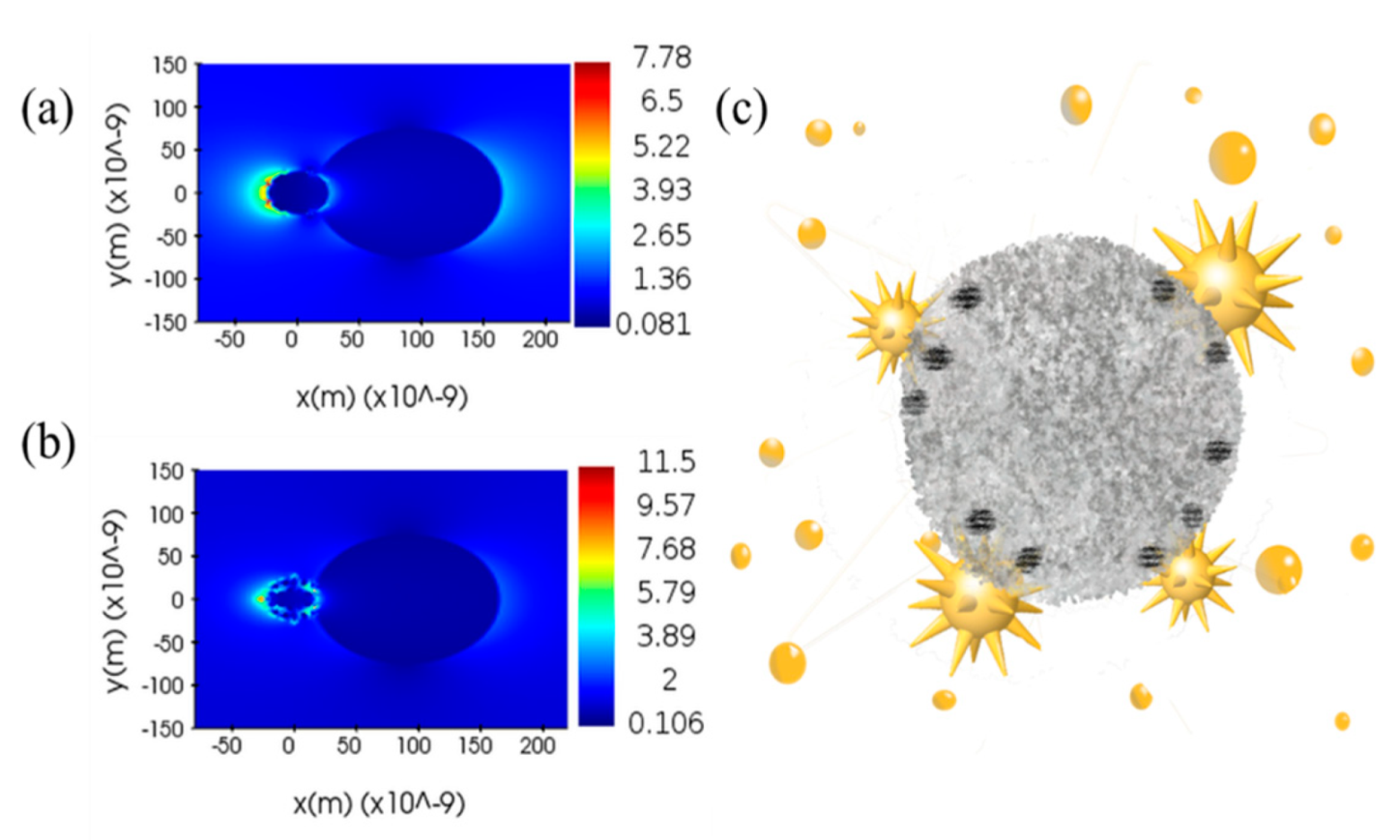
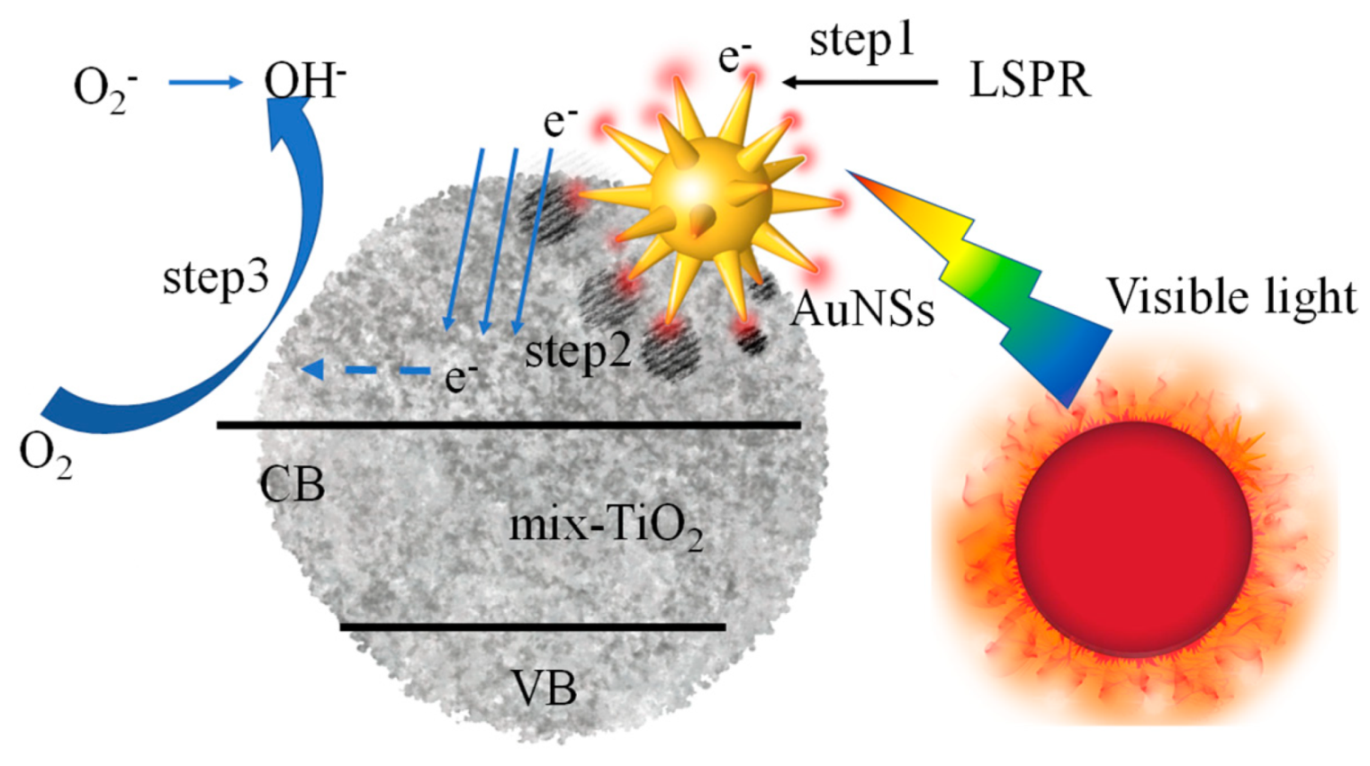
3.3. Au Nanostar Induces LSPR to Crystallize a-TiO2
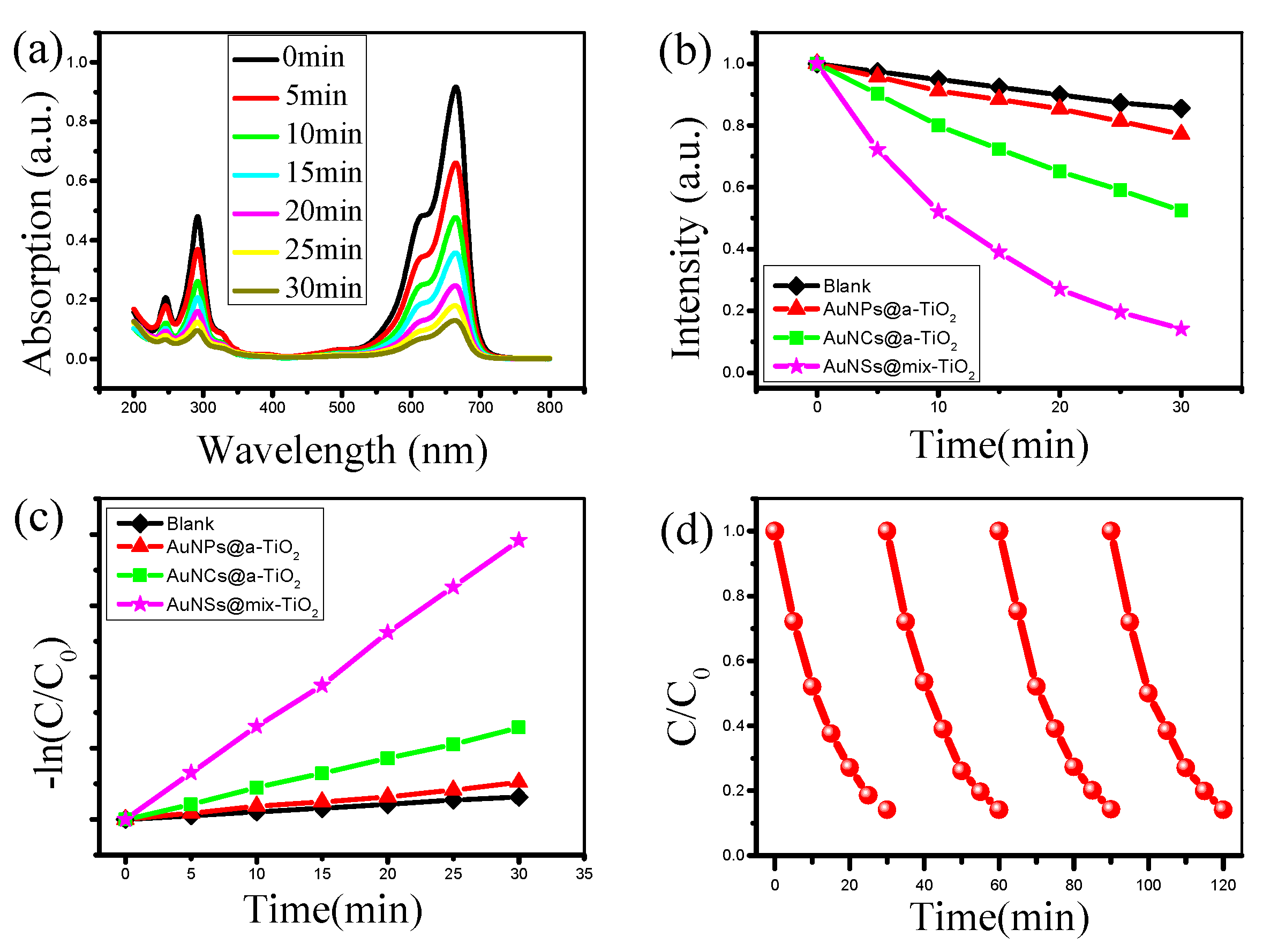
3.4. Au@TiO2 Composites for Photocatalytic Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.-L.; Tsai, H.-R.; Hung, Y.-T.; Lao, K.-U.; Liao, C.-W.; Chung, P.-J.; Huang, J.-S.; Chen, I.-C.; Huang, M.H. A Comparative Study of Gold Nanocubes, Octahedra, and Rhombic Dodecahedra as Highly Sensitive SERS Substrates. Inorg. Chem. 2011, 50, 8106–8111. [Google Scholar] [CrossRef]
- Wu, L.; Fu, C.; Huang, W. Surface chemistry of TiO2 connecting thermal catalysis and photocatalysis. Phys. Chem. Chem. Phys. 2020, 22, 9875–9909. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Gao, X.; Kang, S.; Xiong, R.; Chen, M. Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals. Sustainability 2020, 12, 7615. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-Light Photocatalysts and Their Perspectives for Building Photocatalytic Mem-brane Reactors for Various Liquid Phase Chemical Conversions. Catalysts 2020, 10, 1334. [Google Scholar] [CrossRef]
- Zhou, H.; Qu, Y.; Zeid, T.; Duan, X. Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy Environ. Sci. 2012, 5, 6732–6743. [Google Scholar] [CrossRef]
- Arcanjo, G.S.; Mounteer, A.H.; Bellato, C.R.; da Silva, L.M.M.; Dias, S.H.B.; da Silva, P.R. Heterogeneous photocataly-sis using TiO2 modified with hydrotalcite and iron oxide under UV-visible irradiation for color and toxicity reduction in sec-ondary textile mill effluent. J. Environ. Manag. 2018, 211, 154–163. [Google Scholar] [CrossRef]
- Sun, S.; Song, P.; Cui, J.; Liang, S. Amorphous TiO2 nanostructures: Synthesis, fundamental properties and photocatalytic ap-plications. Catal. Sci. Technol. 2019, 9, 4198–4215. [Google Scholar] [CrossRef]
- Lopez, E.R.; Morales-Luna, M.; Gonzalez, M.V.; Aruna-Devi, R.; de Moure-Flores, F.; Hernandez, S.A.M.; Cruz, J.S. Bandgap modi-fication of titanium dioxide doped with rare earth ions for luminescent processes. J. Appl. Phys. 2020, 128, 175106. [Google Scholar] [CrossRef]
- Li, C.; Rao, Y.; Zhang, B.; Huang, K.; Cao, X.; Peng, D.; Wu, J.; Xiao, L.; Huang, Y. Extraordinary catalysis induced by titanium foil cathode plasma for degradation of water pollutant. Chemosphere 2019, 214, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef]
- Dodekatos, G.; Schufcnemann, S.; Tüysüz, H. Surface Plasmon-Assisted Solar Energy Conversion. Solar Energy for Fuels 2015, 371, 215–252. [Google Scholar]
- Andreeva, Y.; Sharma, N.; Rudenko, A.; Mikhailova, J.; Sergeev, M.; Veiko, V.P.; Vocanson, F.; Lefkir, Y.; Destouches, N.; Itina, T.E. Insights into Ultrashort Laser-Driven Au:TiO2 Nanocomposite Formation. J. Phys. Chem. C 2020, 124, 10209–10219. [Google Scholar] [CrossRef]
- Olvera-Rodríguez, I.; Hernández, R.; Medel, A.; Guzmán, C.; Escobar-Alarcón, L.; Brillas, E.; Sirés, I.; Esquivel, K. TiO2/Au/TiO2 multilayer thin-film photoanodes synthesized by pulsed laser deposition for photoelectrochemical degradation of organic pollutants. Sep. Purif. Technol. 2019, 224, 189–198. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Development and characterization of the kefiran-whey protein iso-late-TiO2 nanocomposite films. Int. J. Biol. Macromol. 2014, 65, 340–345. [Google Scholar] [CrossRef]
- Long, R.; Mao, K.; Gong, M.; Zhou, S.; Hu, J.; Zhi, M.; You, Y.; Bai, S.; Jiang, J.; Zhang, Q.; et al. Tunable Oxygen Activation for Catalytic Organic Oxidation: Schottky Junction versus Plasmonic Effects. Angew. Chem. Int. Ed. 2014, 53, 3205–3209. [Google Scholar] [CrossRef]
- Raffi, M.; Batool, Z.; Ahmad, M.; Zakria, M.; Shakoor, R.I.; Mirza, M.A.; Mahmood, A. Synthesis of Ag-Loaded TiO2 Electrospun Nano-fibers for Photocatalytic Decolorization of Methylene Blue. Fibers Polym. 2018, 19, 1930–1939. [Google Scholar] [CrossRef]
- He, Z.; Xue, W.; Cui, W.; Li, C.; Li, Z.; Pu, L.; Feng, J.; Xiao, X.; Wang, X.; Li, A.G. Tunable Fano Resonance and Enhanced Sensing in a Simple Au/TiO2 Hybrid Metasurface. Nanomaterials 2020, 10, 687. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Huang, J.; Jing, X.; Sun, D.; Li, Q. Alternative method for preparation of Au/TiO2 with precise Au0/Auδ+. J. Chem. Technol. Biotechnol. 2016, 91, 2125–2130. [Google Scholar] [CrossRef]
- Asapu, R.; Claes, N.; Ciocarlan, R.-G.; Minjauw, M.; Detavernier, C.; Cool, P.; Bals, S.; Verbruggen, S.W. Electron Transfer and Near-Field Mecha-nisms in Plasmonic Gold-Nanoparticle-Modified TiO2 Photocatalytic Systems. ACS Appl. Nano Mater. 2019, 2, 4067–4074. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocata-lytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Wu, K.; Rodríguez-Córdoba, W.E.; Yang, Y.; Lian, T. Plasmon-Induced Hot Electron Transfer from the Au Tip to CdS Rod in CdS-Au Nanoheterostructures. Nano Lett. 2013, 13, 5255–5263. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Chen, H.; Jiang, R.; Sun, L.-D.; Li, Q.; Wang, J.; Yu, J.C.; Yan, C.-H. Plasmonic Harvesting of Light Energy for Suzuki Coupling Reactions. J. Am. Chem. Soc. 2013, 135, 5588–5601. [Google Scholar] [CrossRef] [PubMed]
- Petridis, C.; Savva, K.; Kymakis, E.; Stratakis, E. Laser generated nanoparticles based photovoltaics. J. Colloid Interface Sci. 2017, 489, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J.; Tian, Z.; Ye, Y.; Cai, Y.; Liang, C.; Terabe, K. A general strategy toward transition metal carbide/carbon core/shell nanospheres and their application for supercapacitor electrode. Carbon 2016, 100, 590–599. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, P.; Wang, C.; Yang, G. External field-assisted laser ablation in liquid: An efficient strategy for nanocrystal synthesis and nanostructure assembly. Prog. Mater. Sci. 2017, 87, 140–220. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.-W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via Laser Ablation/Irradiation in Liquid: A Review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Fan, K.; Peng, T.; Chen, J.; Dai, K. Effects of tetrabutoxytitanium on photoelectrochemical properties of plastic-based TiO2 film electrodes for flexible dye-sensitized solar cells. J. Power Sources 2011, 196, 2939–2944. [Google Scholar] [CrossRef]
- Luna, M.; Mosquera, M.J.; Vidal, H.; Gatica, J.M. Au-TiO2/SiO2 photocatalysts for building materials: Self-cleaning and de-polluting performance. Build. Environ. 2019, 164, 106347. [Google Scholar] [CrossRef]
- Duran-Alvarez, J.C.; Santiago, A.L.; Ramirez-Ortega, D.; Acevedo-Pena, P.; Castillon, F.; Ramirez-Zamora, R.M.; Zanella, R. Surface modification of B-TiO2 by deposition of Au nanoparticles to increase its photocatalytic activity under simulated sunlight irra-diation. J. Sol-Gel Sci. Technol. 2018, 88, 474–487. [Google Scholar] [CrossRef]
- Yang, G. Laser ablation in liquids: Applications in the synthesis of nanocrystals. Prog. Mater. Sci. 2007, 52, 648–698. [Google Scholar] [CrossRef]
- Banfield, J.F.; Welch, S.A.; Zhang, H.Z.; Ebert, T.T.; Penn, R.L. Aggregation-based crystal growth and microstructure develop-ment in natural iron oxyhydroxide biomineralization products. Science 2000, 289, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Wackerow, S.; Abdolvand, A. Generation of silver nanoparticles with controlled size and spatial distribution by pulsed laser irradiation of silver ion-doped glass. Opt. Express 2014, 22, 5076–5085. [Google Scholar] [CrossRef] [PubMed]
- Kalyva, M.; Kumar, S.; Brescia, R.; Petroni, S.; la Tegola, C.; Bertoni, G.; de Vittorio, M.; Cingolani, R.; Athanassiou, A. Electrical response from nanocomposite PDMS-Ag NPs generated by in situ laser ablation in solution. Nanotechnology 2013, 24, 035707. [Google Scholar] [CrossRef]
- Veziroglu, S.; Obermann, A.-L.; Ullrich, M.; Hussain, M.; Kamp, M.; Kienle, L.; Leißner, T.; Rubahn, H.-G.; Polonskyi, O.; Strunskus, T.; et al. Photodeposition of Au Nanoclusters for Enhanced Photocatalytic Dye Degradation over TiO2 Thin Film. ACS Appl. Mater. Interfaces 2020, 12, 14983–14992. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Ohhashi, S.; Kameoka, S.; Tsai, A.P. Highly dispersive Au nanoparticles on TiO2 nanofibers as a supported catalyst synthesized from Al-Ti-Au intermetallic compound. Chem. Eng. Sci. 2020, 211, 115249. [Google Scholar] [CrossRef]
- Chansa, J.L.; Wewers, F.; Mthethwa, T. Plasmonic photocatalysts consisting of a mixture of Au nanospheres and Au nanorods on TiO2 support for improved visible light induced photocatalytic degradation properties. J. Photochem. Photobiol. A Chem. 2020, 401, 112740. [Google Scholar] [CrossRef]
- Rudenko, A.; Colombier, J.-P.; Itina, T.E. Femtosecond laser irradiation of dielectric materials containing randomly-arranged nanoparticles. Synth. Photonics Nanoscale Mater. 2016, 9737. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, W.; Yu, C.; Zhang, H. Influence of TiO2 morphology on adsorption-photocatalytic efficiency of TiO2-graphene composites for methylene blue degradation. J. Environ. Chem. Eng. 2018, 6, 4899–4907. [Google Scholar] [CrossRef]
- Zarkov, S.V.; Avetisyan, Y.A.; Akchurin, G.G.; Akchurin, G.G.; Bibikova, O.A.; Tuchin, V.V.; Yakunin, A.N. Numerical modeling of plas-monic properties of gold nanostars to prove the threshold nature of their modification under laser pulse. Opt. Eng. 2020, 59, 061628. [Google Scholar] [CrossRef]
- Pylaev, T.; Vanzha, E.; Avdeeva, E.; Khlebtsov, B.; Khlebtsov, N. A novel cell transfection platform based on laser optoporation mediated by Au nanostar layers. J. Biophotonics 2019, 12, e201800166. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, X.; Zuo, P.; Chen, X.; Liang, M.; Ma, L. Electric Field Assisted Femtosecond Laser Preparation of Au@TiO2 Composites with Controlled Morphology and Crystallinity for Photocatalytic Degradation. Materials 2021, 14, 3816. https://doi.org/10.3390/ma14143816
Li X, Li X, Zuo P, Chen X, Liang M, Ma L. Electric Field Assisted Femtosecond Laser Preparation of Au@TiO2 Composites with Controlled Morphology and Crystallinity for Photocatalytic Degradation. Materials. 2021; 14(14):3816. https://doi.org/10.3390/ma14143816
Chicago/Turabian StyleLi, Xiaojie, Xin Li, Pei Zuo, Xiaozhe Chen, Misheng Liang, and Le Ma. 2021. "Electric Field Assisted Femtosecond Laser Preparation of Au@TiO2 Composites with Controlled Morphology and Crystallinity for Photocatalytic Degradation" Materials 14, no. 14: 3816. https://doi.org/10.3390/ma14143816
APA StyleLi, X., Li, X., Zuo, P., Chen, X., Liang, M., & Ma, L. (2021). Electric Field Assisted Femtosecond Laser Preparation of Au@TiO2 Composites with Controlled Morphology and Crystallinity for Photocatalytic Degradation. Materials, 14(14), 3816. https://doi.org/10.3390/ma14143816