Experimental Study on Activated Carbon-MIL-101(Cr) Composites for Ethanol Vapor Adsorption
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Synthesis of MIL-101(Cr)
2.3. Synthesis of AC-MIL-101(Cr)
2.4. Characterization
3. Results and Discussion
3.1. Sample Yield
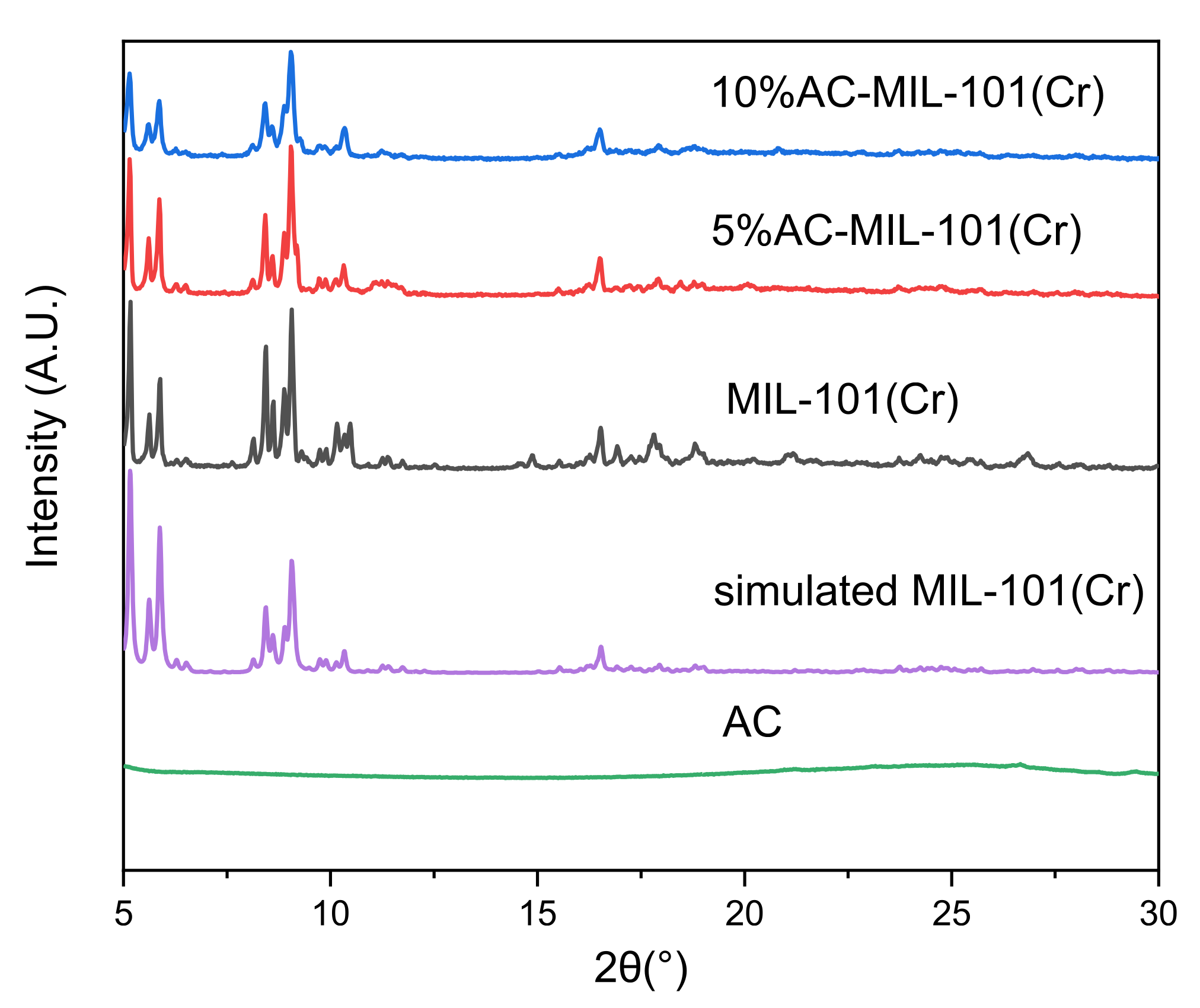
3.2. XRD Analysis
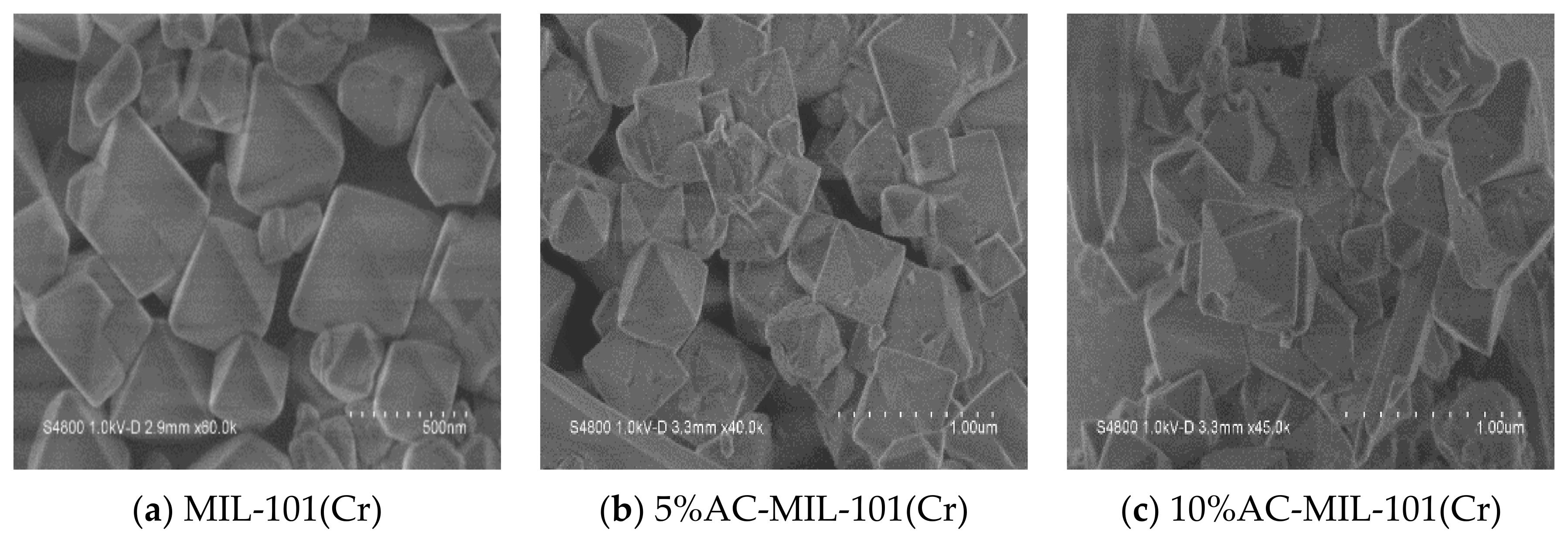
3.3. SEM Analysis
3.4. Thermogravimetric Analysis
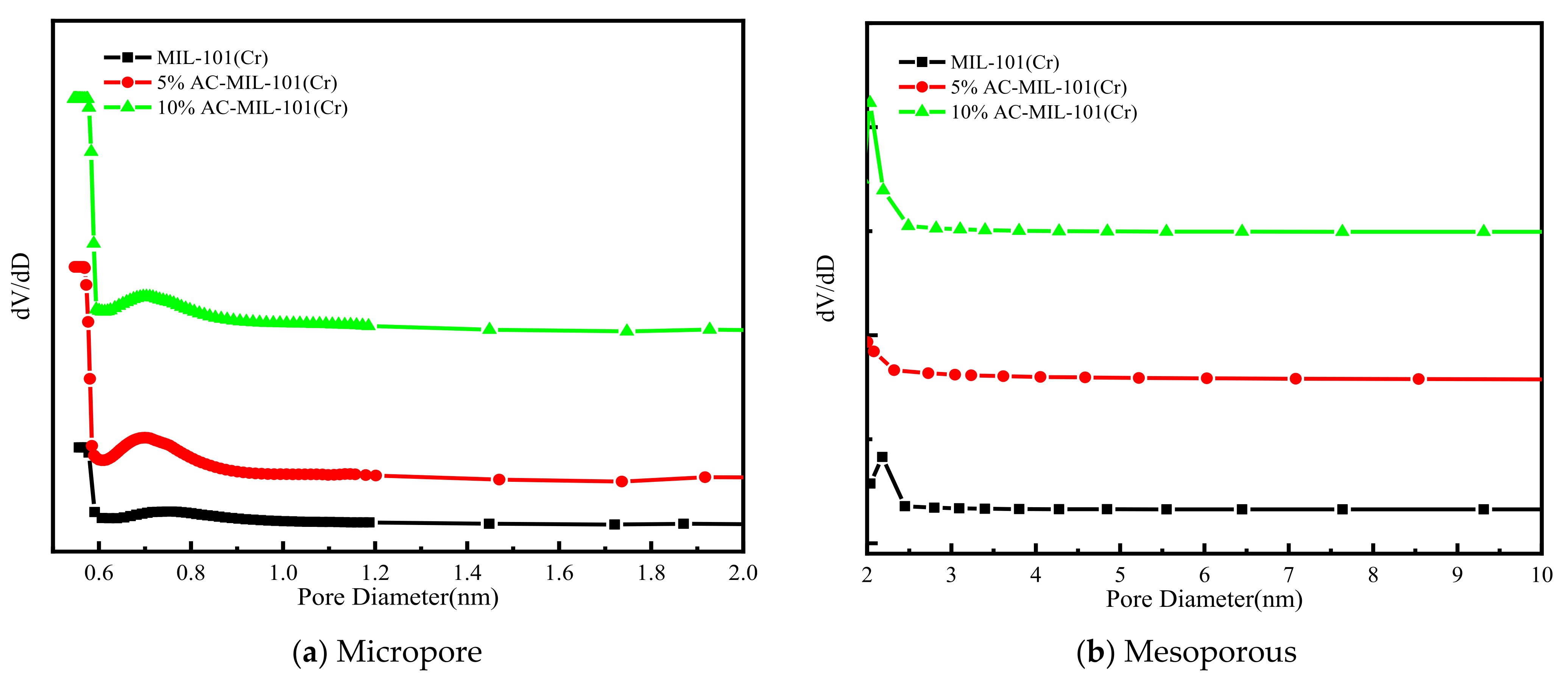
3.5. Surface and Pore Analysis
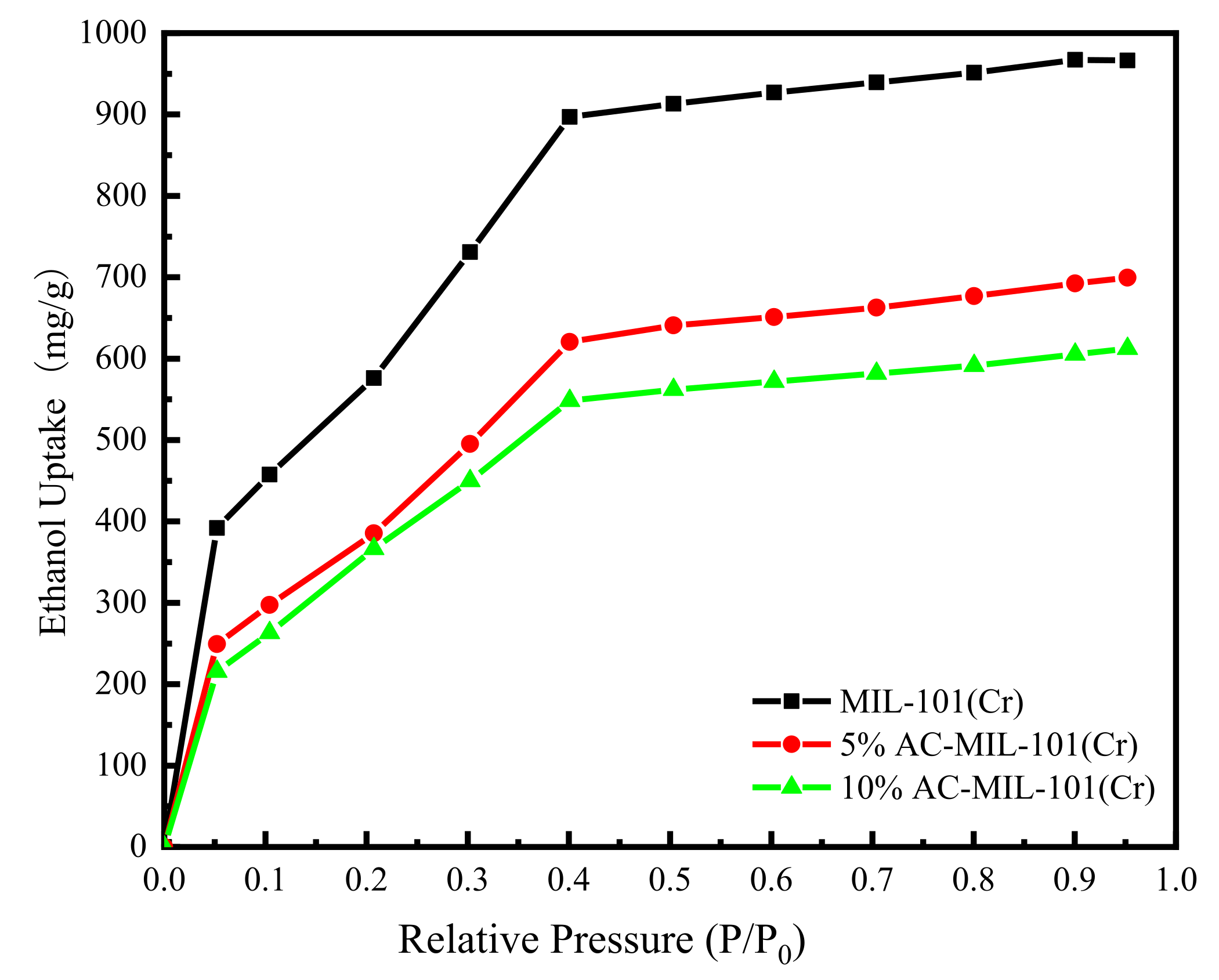
3.6. Ethanol Vapor Adsorption Isotherm
3.7. Adsorption Kinetics of Ethanol Vapor
4. Conclusions
- (1)
- The XRD results showed that the three samples were successfully prepared, and the addition of AC did not affect the skeletal crystal structure of MIL-101(Cr).
- (2)
- SEM results showed that the three samples had similar regular octahedral structures, but the addition of AC would cause certain defects on the crystal surface of AC-MIL-101(Cr).
- (3)
- Compared with MIL-101(Cr), the thermal stability of AC-MIL-101(Cr) improved.
- (4)
- At 77K, N2 adsorption and desorption isotherm results showed that the specific surface area and pore volume of AC-MIL-101(Cr) significantly increased, and the three samples had two different cage structures. The addition of AC is conducive to the opening of the mesopores.
- (5)
- When the relative pressure P/P0 ≤ 0.4, the three samples showed rapid adsorption of ethanol vapor. Although the ethanol vapor adsorption capacity of AC-MIL-101(Cr) decreased, AC-MIL-101(Cr) exhibited a more uniform adsorption rate. This illustrates the application prospect of AC-MIL-101(Cr) in adsorption refrigeration systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, L.; Eom, J.; Marten, E.H.; Horowitz, R.; Kyle, P.; Link, R.; Mignone, B.K.; Mundra, A.; Zhou, Y. Effects of long-term climate change on global building energy expenditures. Energy Econ. 2018, 72, 667–677. [Google Scholar] [CrossRef]
- Yan, J.; Yu, Y.; Xiao, J.; Li, Y.; Li, Z. Improved Ethanol Adsorption Capacity and Coefficient of Performance for Adsorption Chillers of Cu-BTC@GO Composite Prepared by Rapid Room Temperature Synthesis. Ind. Eng. Chem. Res. 2016, 55, 11767–11774. [Google Scholar] [CrossRef]
- De Lange, M.F.; Verouden, K.J.; Vlugt, T.J.; Gascon, J.; Kapteijn, F. Adsorption-driven heat pumps: The potential of metal–organic frameworks. Chem. Rev. 2015, 115, 12205–12250. [Google Scholar] [CrossRef] [PubMed]
- Frazzicaa, A.; Palombaa, V.; Dawoud, B. Design, realization and testing of an adsorption refrigerator based on activated carbon/ethanol working pair. Appl. Energy 2016, 174, 15–24. [Google Scholar] [CrossRef]
- El-Sharkawy, I.I.; Saha, B.B.; Koyama, S.; He, J.; Ng, K.C.; Yap, C. Experimental investigation on activated carbon–ethanol pair for solar powered adsorption cooling applications. Int. J. Refrig. 2008, 31, 1407–1413. [Google Scholar] [CrossRef]
- Farha, O.K.; Yazaydin, A.O.; Eryazici, I.; Malliakas, C.D.; Hauser, B.G.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010, 2, 944–948. [Google Scholar] [CrossRef]
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral metal-organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 2012, 112, 1196–1231. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Betard, A.; Fischer, R.A. Metal-organic framework thin films: From fundamentals to applications. Chem. Rev. 2012, 112, 1055–1083. [Google Scholar] [CrossRef]
- Rezk, A.; Al-Dadah, R.; Mahmoud, S.; Elsayed, A. Investigation of Ethanol/metal organic frameworks for low temperature adsorption cooling applications. Appl. Energy 2013, 112, 1025–1031. [Google Scholar] [CrossRef]
- Saha, B.B.; El-Sharkawy, I.I.; Miyazaki, T.; Koyama, S.; Henninger, S.K.; Herbst, A.; Janiak, C. Ethanol adsorption onto metal organic framework: Theory and experiments. Energy 2015, 79, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Rui, Z.; Wu, Q.; Yang, H.; Yin, Y.; Liu, Z.; Cui, Q.; Wang, H. Performance evaluation of shaped MIL-101–ethanol working pair for adsorption refrigeration. Appl. Therm. Eng. 2016, 95, 223–228. [Google Scholar] [CrossRef]
- de Lange, M.F.; van Velzen, B.L.; Ottevanger, C.P.; Verouden, K.J.F.M.; Lin, L.C.; Vlugt, T.J.H.; Gascon, J.; Kapteijn, F. Metal-Organic Frameworks in Adsorption-Driven Heat Pumps: The Potential of Alcohols as Working Fluids. Langmuir 2015, 31, 12783–12796. [Google Scholar] [CrossRef] [PubMed]
- Teo, H.W.B.; Chakraborty, A.; Kayal, S. Evaluation of CH4 and CO2 adsorption on HKUST-1 and MIL-101(Cr) MOFs employing Monte Carlo simulation and comparison with experimental date. Appl. Therm. Eng. 2017, 110, 891–900. [Google Scholar] [CrossRef]
- Yu, Z.; Deschamps, J.; Hamon, L.; Prabhakaran, P.K.; Pré, P. Modeling hydrogen diffusion in hybrid activated carbon-MIL-101(Cr) considering temperature variations and surface loading changes. Microporous Mesoporous Mat. 2017, 248, 72–83. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Q.; Li, J.; Dong, J. Synthesis of metal–organic framework MIL-101 in TMAOH-Cr(NO3)3-H2BDC-H2O and its hydrogen-storage behavior. Microporous Mesoporous Mat. 2010, 130, 174–179. [Google Scholar] [CrossRef]
- Gao, S.; Feng, T.; Feng, C.; Shang, N.; Wang, C. Novel visible-light responsive Ag/Ag Cl@MIL-101 hybrid materials with synergistic photocatalytic activity. J. Colloid Interface Sci. 2016, 466, 284–290. [Google Scholar] [CrossRef]
- Wu, H.; Chua, Y.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and highly tunable missing-linker defects in zirconium metaleorganic framework UiO-66 and their important effects on gas adsorption. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef]
- Rallapalli, P.B.S.; Raj, M.C.; Patil, D.V.; Prasanth, K.P.; Somani, R.S.; Bajaj, H.C. Activated carbon @ MIL-101(Cr): A potential metal-organic framework composite material for hydrogen storage. Int. J. Energy Res. 2013, 37, 746–753. [Google Scholar] [CrossRef]
- Yu, Z.; Deschamps, J.; Hamon, L.; Prabhakaran, P.K.; Pré, P. Hydrogen adsorption and kinetics in MIL-101(Cr) and hybrid activated carbon-MIL-101(Cr) materials. Int. J. Hydrog. Energy 2017, 42, 8021–8031. [Google Scholar] [CrossRef]
- Sun, X.; Xia, Q.; Zhao, Z.; Li, Y.; Li, Z. Synthesis and adsorption performance of MIL-101(Cr)/graphite oxide composites with high capacities of n-hexane. Chem. Eng. J. 2014, 239, 226–232. [Google Scholar] [CrossRef]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Janiak, C. MOFs for Use in Adsorption Heat Pump Processes. Eur. J. Inorg. Chem. 2012, 16, 2625–2634. [Google Scholar] [CrossRef]
- Hong, D.; Hwang, Y.; Serre, C.; Férey, G.; Chang, J. Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]



| Sample | SBET m2/g | Smi m2/g | Sme m2/g | Vtotal cm3/g | Vmi cm3/g | Vme cm3/g |
|---|---|---|---|---|---|---|
| MIL-101(Cr) | 814 | 145 | 319 | 0.475 | 0.101 | 0.202 |
| 5%AC-MIL-101(Cr) | 1863 | 508 | 686 | 1.340 | 0.245 | 0.776 |
| 10%AC-MIL-101(Cr) | 2018 | 643 | 734 | 1.466 | 0.330 | 0.745 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Gao, J.; Qi, X.; Zhao, Z.; Sun, H. Experimental Study on Activated Carbon-MIL-101(Cr) Composites for Ethanol Vapor Adsorption. Materials 2021, 14, 3811. https://doi.org/10.3390/ma14143811
Liu Z, Gao J, Qi X, Zhao Z, Sun H. Experimental Study on Activated Carbon-MIL-101(Cr) Composites for Ethanol Vapor Adsorption. Materials. 2021; 14(14):3811. https://doi.org/10.3390/ma14143811
Chicago/Turabian StyleLiu, Zhongbao, Jiayang Gao, Xin Qi, Zhi Zhao, and Han Sun. 2021. "Experimental Study on Activated Carbon-MIL-101(Cr) Composites for Ethanol Vapor Adsorption" Materials 14, no. 14: 3811. https://doi.org/10.3390/ma14143811
APA StyleLiu, Z., Gao, J., Qi, X., Zhao, Z., & Sun, H. (2021). Experimental Study on Activated Carbon-MIL-101(Cr) Composites for Ethanol Vapor Adsorption. Materials, 14(14), 3811. https://doi.org/10.3390/ma14143811






