Applications of PERALS-Alpha Spectrometry for the Investigation of Radionuclides in Water Samples
Abstract
:1. Introduction
1.1. The Legal Basis in Switzerland
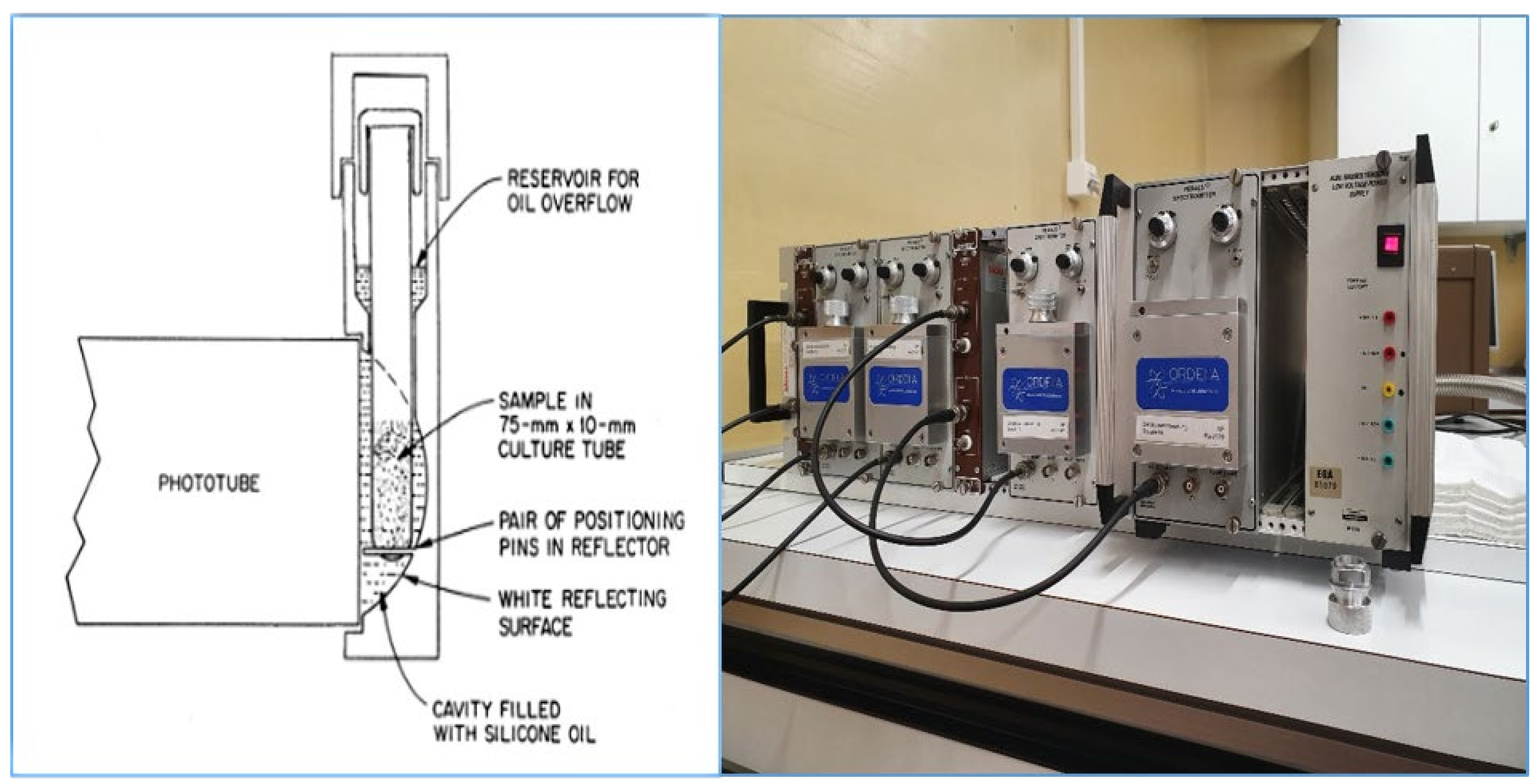
1.2. The PERALS Method
1.3. Selective Extraction
1.4. General Procedure for the Extraction of Radionuclides from Water Samples
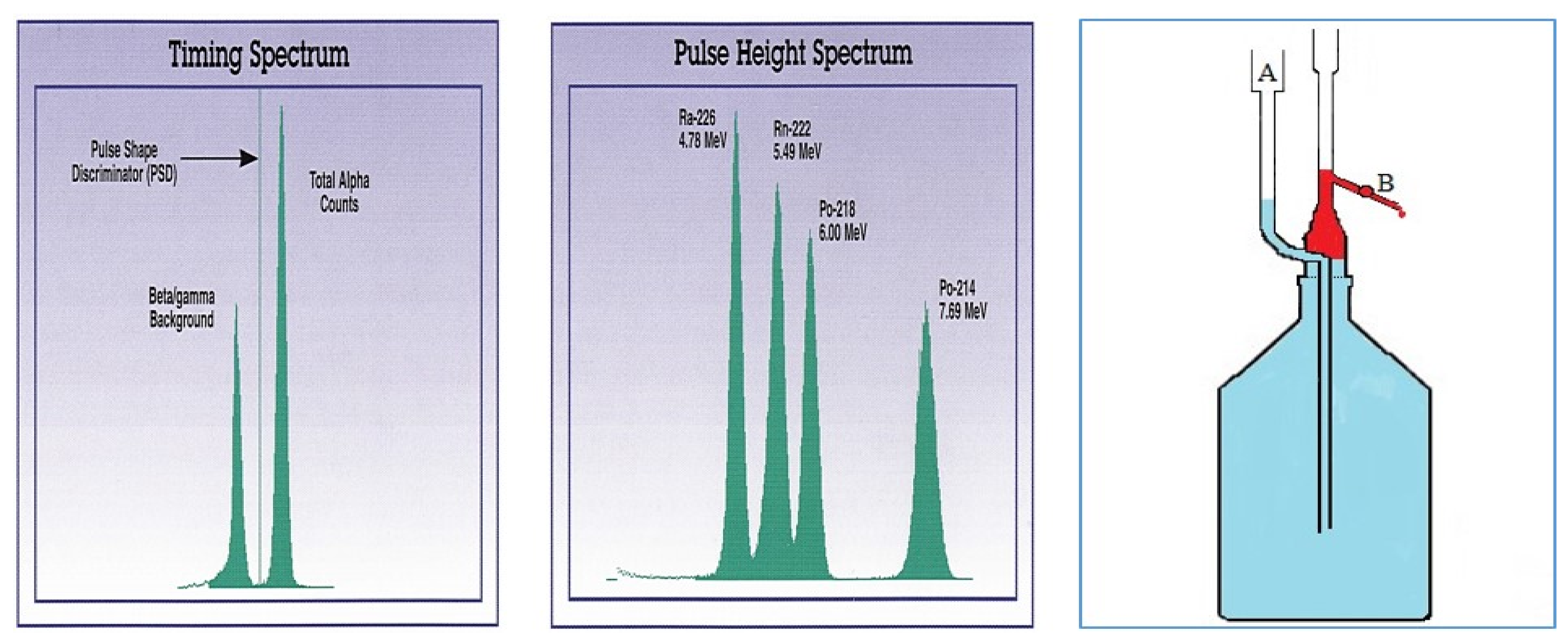
1.5. Alpha Spectrometry, PSD
2. Polonium
2.1. General Methods
2.2. Polonium Trace-Analyses
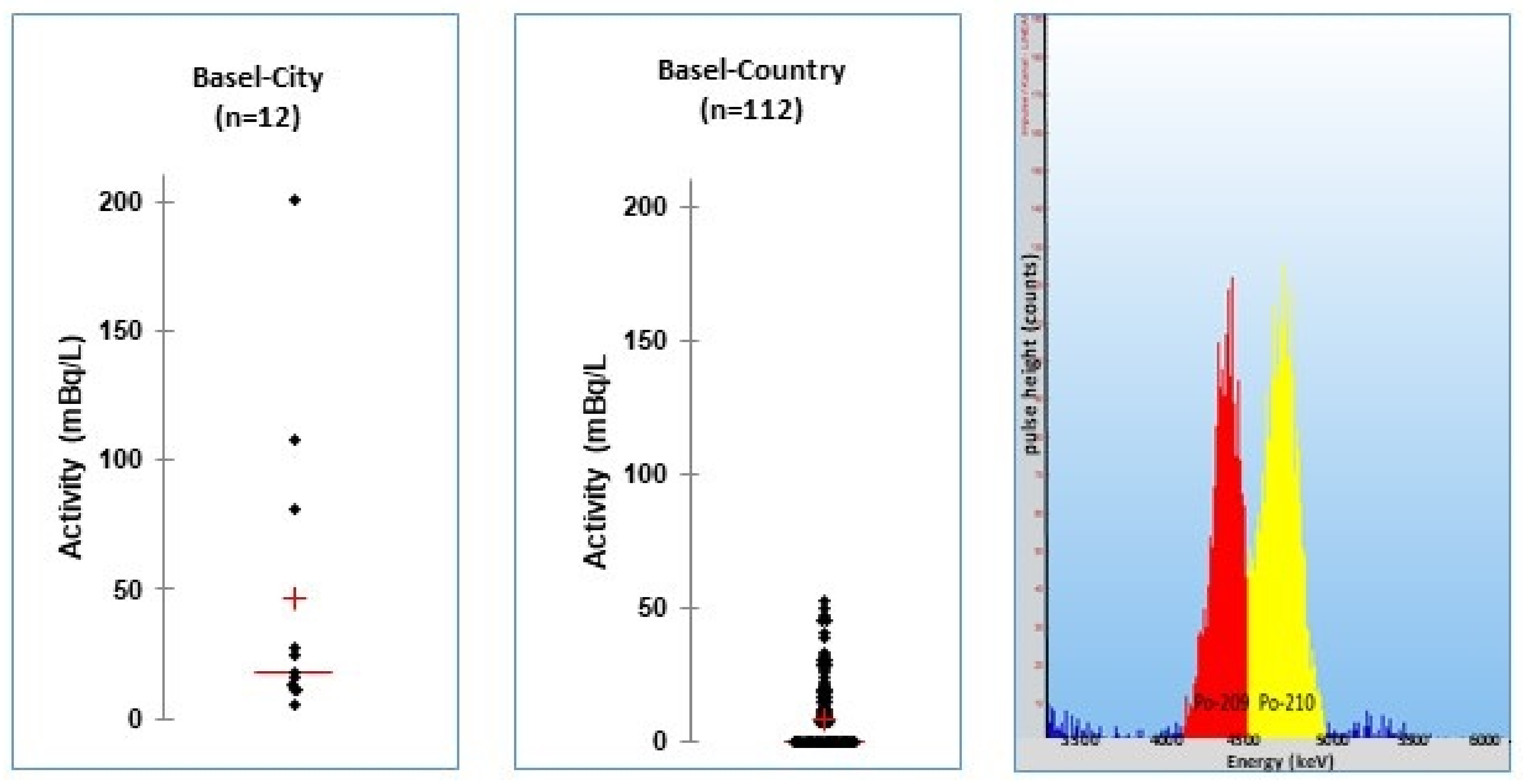
2.3. Drinking Water Monitoring
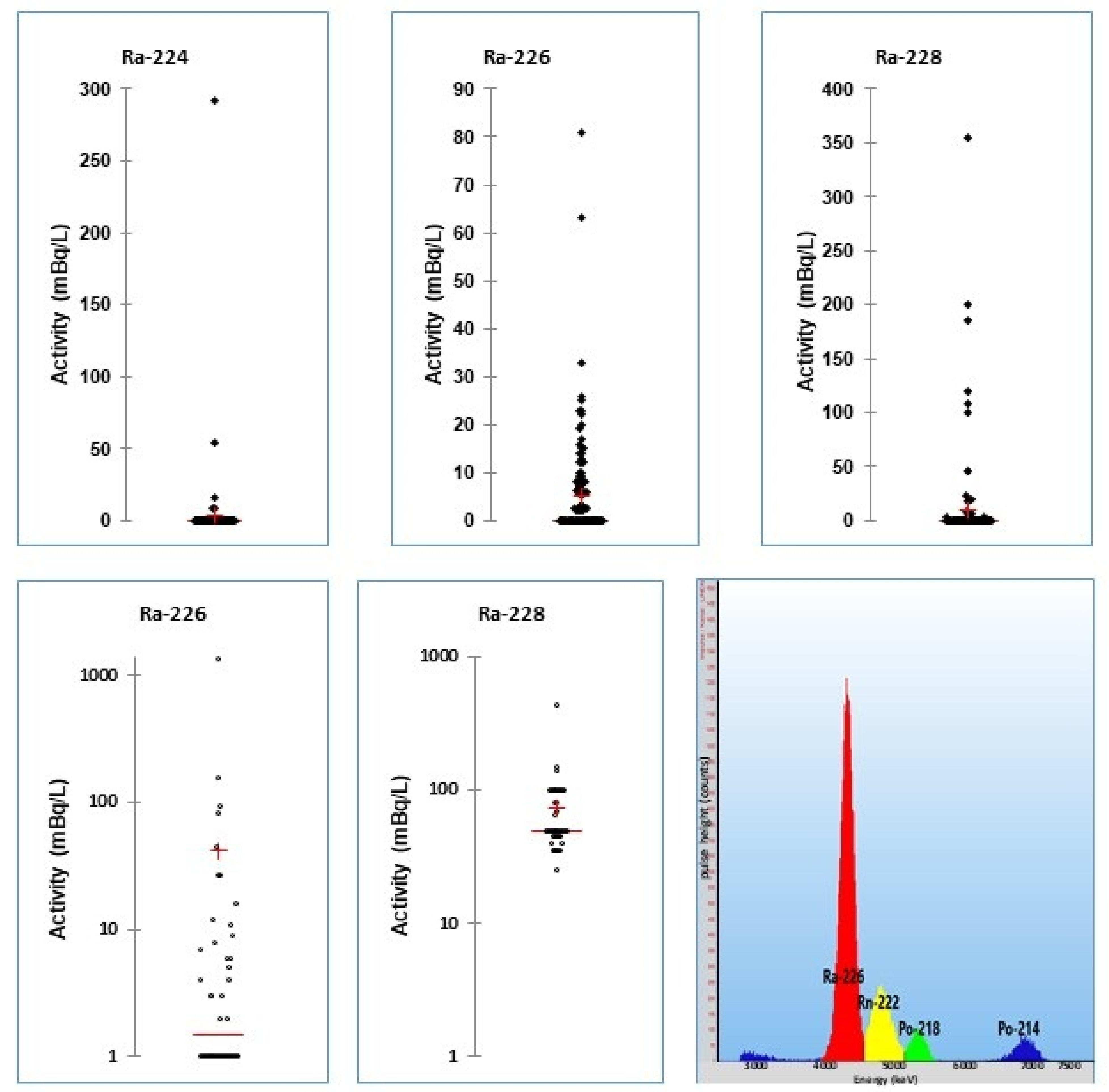
3. Radium
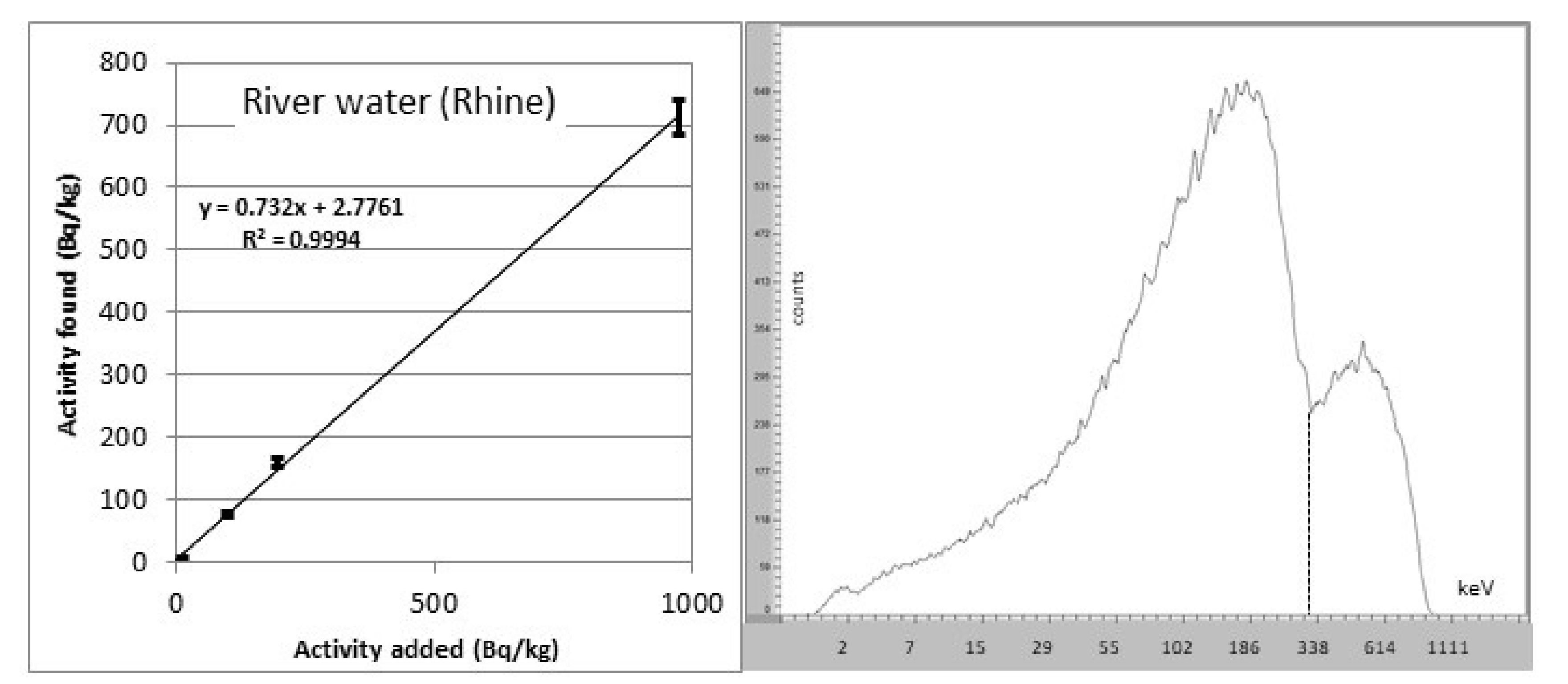
3.1. General Methods
3.2. Radium Trace Analyses
3.3. Drinking Water Monitoring
3.4. Radium Monitoring Campaigns
4. Radon
4.1. General Methods
4.2. Radon Trace Analyses
4.3. Drinking Water Monitoring
5. Strontium
5.1. General Methods
5.2. Strontium Trace Analyses
6. Thorium
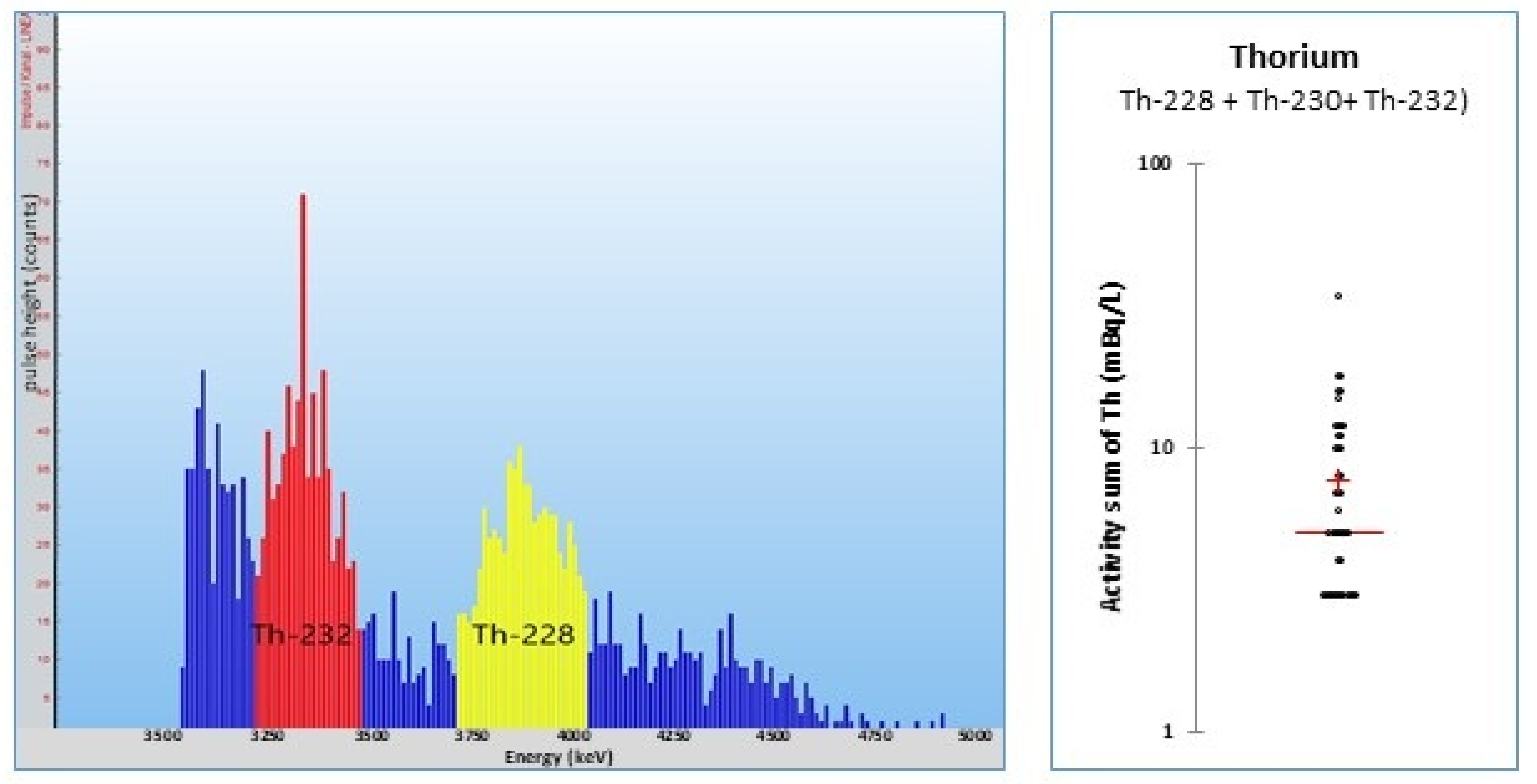
6.1. General Methods
6.2. Thorium Trace Analyses
6.3. Thorium in Mineral Waters
7. Uranium
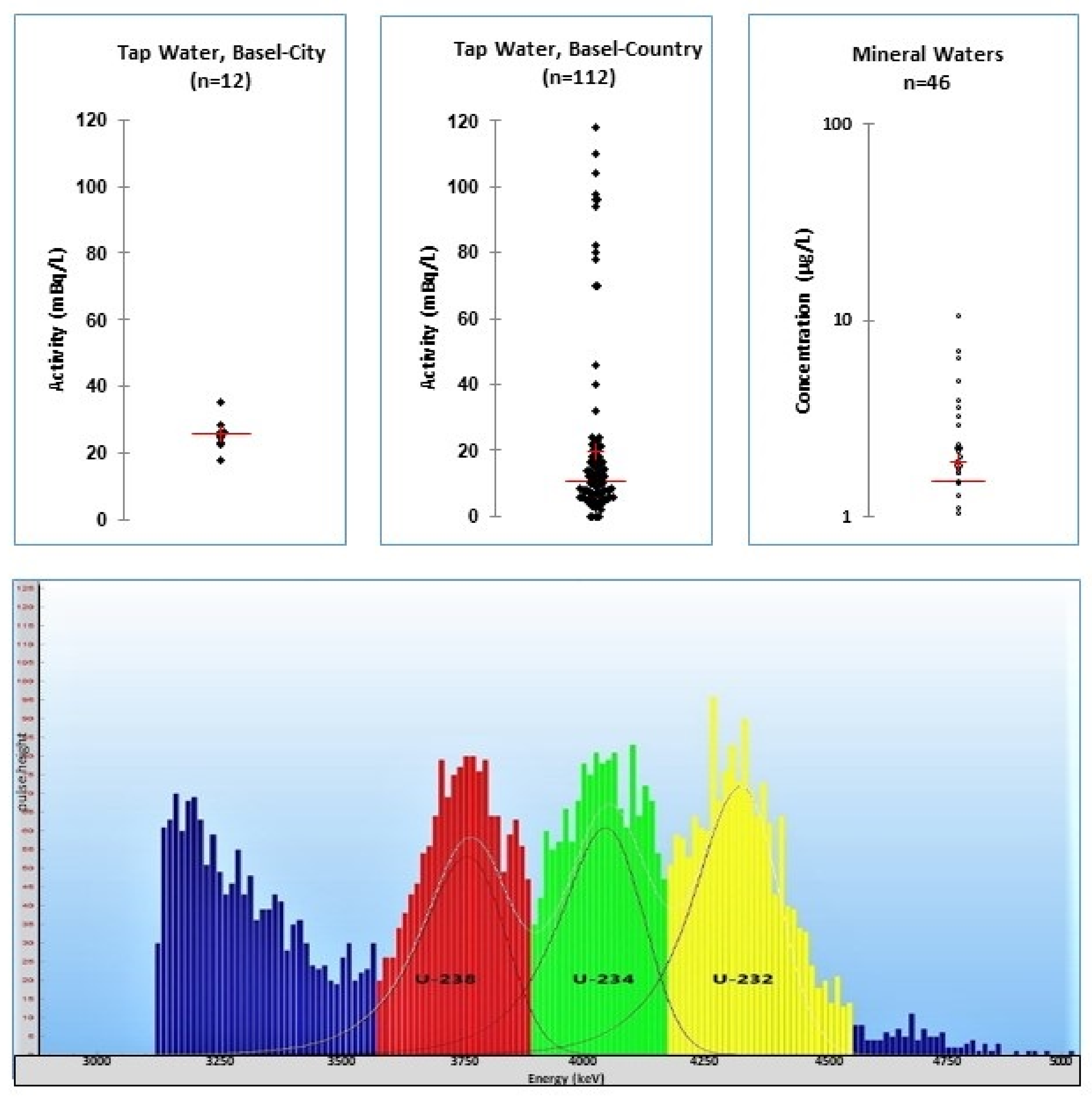
7.1. General Methods
7.2. Uranium Trace Analyses
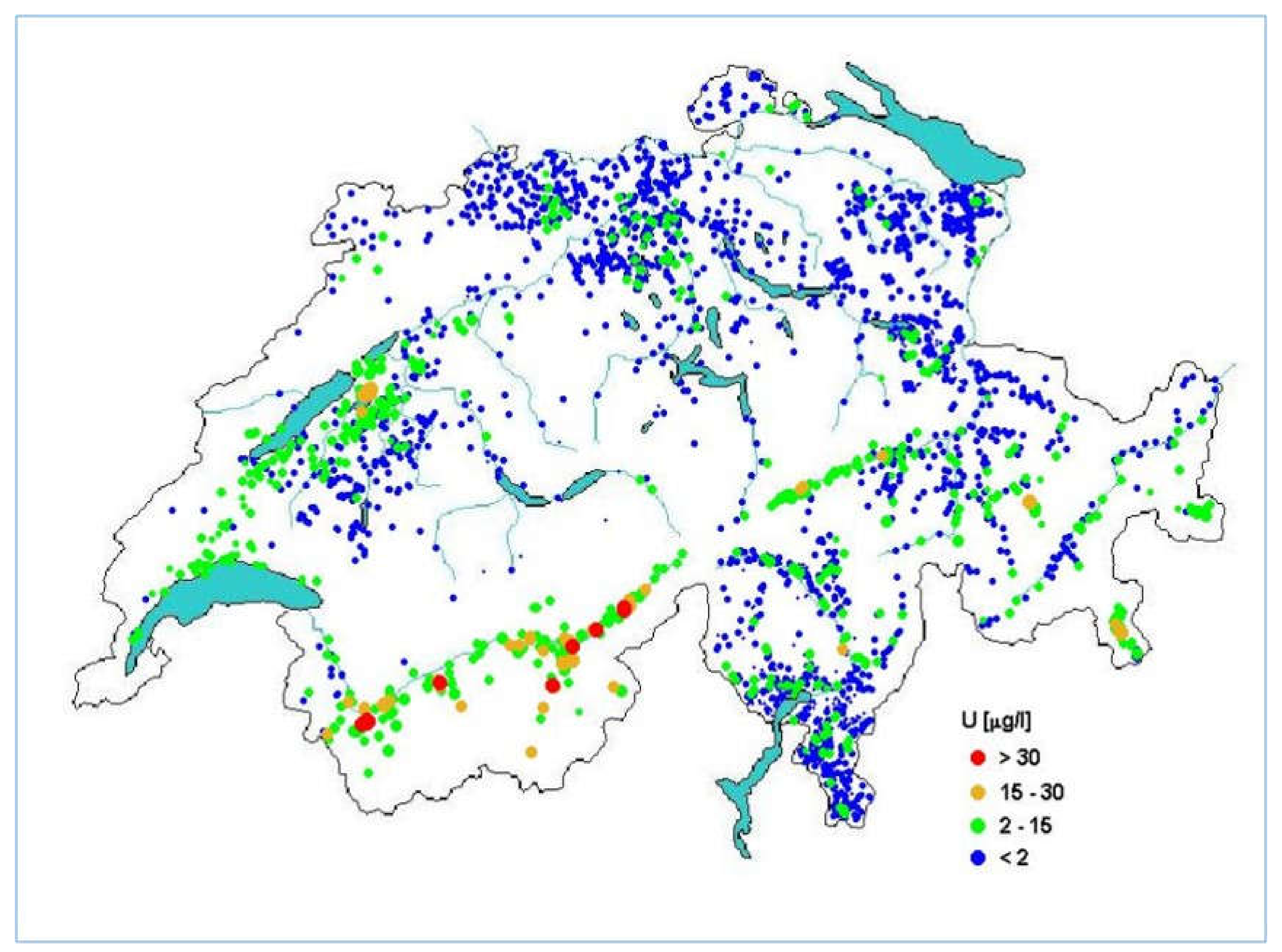
7.3. Uranium Monitoring Campaigns
8. Further Actinides
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICRP. Compendium of Dose Coefficients Based on ICRP Publication 60; ICRP Publication 119, Ann. ICRP 41; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bundesministerium für Umwelt, Naturschutz, Bau und Reaktorsicherheit (BMUB). Leitfaden zur Untersuchung und Bewertung von radioaktiven Stoffen im Trinkwasser bei der Umsetzung der Trinkwas-ser-Verordnung. 2017. Available online: https://www.bmu.de/download/leitfaden-zur-untersuchung-und-bewertung-von-radioaktiven-stoffen-im-trinkwasser-bei-der-umsetzung-d/ (accessed on 2 July 2021).
- Swiss Federal Council. Ordinance on Drinking Water and Water for Public Baths and Shower Facilities (TBDV); Federal Office for Inner Affairs: Bern, Switzerland, 2016; Available online: https://fedlex.data.admin.ch/filestore/fedlex.data.admin.ch/eli/cc/2017/153/20180501/de/pdf-a/fedlex-data-admin-ch-eli-cc-2017-153-20180501-de-pdf-a.pdf (accessed on 2 July 2021).
- Council of the European Union. Council Directive 2013/51/EURATOM of 2nd October 2013 Laying Down Requirements for the Protection of the Health of the General Public with Regard to Radioactive Substances in Water Intended for Human Consumption; European Union: Luxembourg, 22 October 2013. [Google Scholar]
- Swiss Federal Council. Radiological Protection Ordinance. 2017. Available online: https://fedlex.data.admin.ch/filestore/fedlex.data.admin.ch/eli/cc/1994/1947_1947_1947/20140101/en/pdf-a/fedlex-data-admin-ch-eli-cc-1994-1947_1947_1947-20140101-en-pdf-a.pdf (accessed on 2 July 2021).
- The Federal Department of Home Affairs. Ordinance on Contaminants and Constituents in Food; The Federal Department of Home Affairs: Bern, Switzerland, 1995. [Google Scholar]
- Federal Office of Public Health. Environmental Radioactivity and Radiation Doses in Switzerland, Yearly Report; BAG: Bern, Switzerland, 2019; pp. 93–97. Available online: https://www.bag.admin.ch/bag/de/home/service/publikationen/jahresberichte/jahresberichte-umweltradioaktiviaet.html#par_text (accessed on 2 July 2021).
- McDowell, J.W.; McDowell, B.L. Liquid Scintillation Alpha Spectrometry, 1st ed.; CRC Press: Boca Raton, FL, USA, 1994; ISBN 0-8493-5288-6. Available online: https://www.taylorfrancis.com/books/mono/10.1201/9781351074131/liquid-scintilmtion-alpha-spectrometry-jack-mcdowell-mcdowell-betty?refId=169ce9c3-b198-4b2c-919e-0f940d8b7f45 (accessed on 2 July 2021).
- McKlveen, J.W.; McDowell, W. Liquid scintillation alpha spectrometry techniques. Nucl. Instrum. Methods Phys. Res. 1984, 223, 372–376. [Google Scholar] [CrossRef]
- McDowell, W.J. Photon/Electron-Rejecting Alpha Liquid Scintillation (PERALS) Spectrometry: A Review. Radioact. Radiochem. 1992, 3, 26–54. [Google Scholar]
- McDowell, W.J. Recent Applications of PERALS Spectrometry. In Liquid Scintillation Spectrometry 1994; Cook, G.T., Harkness, D.D., MacKenzie, A.B., Miller, B.F., Scott, E.M., Eds.; Radiocarbon: Tucson, AZ, USA, 1996; pp. 327–337. [Google Scholar]
- Kopp, D.M.; Kopp, M.K. PERALS DatamapTM, a New Method for Simultaneous Counting and Separation of Alpha and Beta/Gamma Activity Using PERALSR spectrometry. Radioact. Radiochem. 1991, 2, 24–34. [Google Scholar]
- Cadieux, J.R., Jr.; Reboul, S.H. Separation and Analysis of Actinides by Extraction Chromatography Coupled with Alpha-Particle Liquid Scintillation. Radioact. Radiochem. 1996, 7, 30–34. [Google Scholar]
- Cadieux, J. Evaluation of a photoelectron-rejecting alpha liquid-scintillation (PERALS) spectrometer for the measurement of alpha-emitting radionuclides. Nucl. Instrum. Methods Phys. Res. 1990, 299, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Ensor, D. Separation and Analytical Chemistry of the Actinides. In Separation and Analytical Chemistry of the Actinides; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 1998. [Google Scholar]
- Zehringer, M. Monitoring of Natural Radioactivity in Drinking Water and Food with Emphasis on Alpha-Emitting Radionuclides. In Ionizing and Non-Ionizing Radiation; InTech: London, UK, 2020; pp. 551–561. ISBN 978–1–78984–142–8. [Google Scholar]
- Véronneau, C.; Aupiais, J.; Dacheux, N. Selective determination of polonium by photon electron rejecting alpha liquid scintillation (PERALS® System). Anal. Chim. Acta 2000, 415, 229–238. [Google Scholar] [CrossRef]
- Ordela, Inc. Instruction Manual, Model 8100AB-HV PERALS®-Spectrometer. Available online: https://ordela.com/peralsr-simplifies-environmental-alpha-spectrometry/ (accessed on 2 July 2021).
- Ordela, Inc. PERALS-Brochure. Available online: https://ordelacom.files.wordpress.com/2017/01/peralsc2ae-color-brochure-revised.pdf (accessed on 2 July 2021).
- Zehringer, M.; Abraham, J.; Kammerer, F.; Syla, V.; Wagmann, M. A Rapid Extraction Procedure for the Determination of Strontium-90 in Water Samples. J. Chem. Chem. Eng. 2017, 11, 116–121. [Google Scholar] [CrossRef] [Green Version]
- ITECH Instruments. Available online: https://tik-online.com/interwiner/ (accessed on 2 July 2021).
- The Lund/LBNL Nuclear Data Search. Available online: http://nucleardata.nuclear.lu.se/toi/ (accessed on 2 July 2021).
- LNHB. Laboratoire National Henri Becquerel. Available online: http://www.nucleide.org/DDEP_WG/DDEPdata.htm (accessed on 2 July 2021).
- Xarchoulakos, D.C.; Kehagia, K. A study of various self deposition solutions for 210Po analysis in tap water. J. Radioanal. Nucl. Chem. 2018, 319, 419–424. [Google Scholar] [CrossRef]
- Carvalho, F.; Fowler, S. An experimental study on the bioaccumulation and turnover of polonium-210 and lead-210 in marine shrimp. Mar. Ecol. Progr. Ser. 1993, 102, 125–133. [Google Scholar] [CrossRef]
- Yamamoto, M.; Abe, T.; Kuwabara, J.; Komura, K.; Ueno, K.; Takizawa, Y. Polonium-210 and lead-210 in marine organisms: Intake levels for Japanese. J. Radioanal. Nucl. Chem. 1994, 178, 81–90. [Google Scholar] [CrossRef]
- Clayton, R.; Bradley, E. A cost effective method for the determination of 210Po and 210Pb in environmental materials. Sci. Total Environ. 1995, 173–174, 23–28. [Google Scholar] [CrossRef]
- Case, G.; McDowell, W. An improved sensitive assay for polonium-210 by use of a background-rejecting extractive liquid-scintillation method. Talanta 1982, 29, 845–848. [Google Scholar] [CrossRef]
- Monna, F.; Mathieu, D.; Marques, A.N., Jr.; Lancelot, L.; Bernat, M. A comparison of PERALS to alpha spectrometry and beta counting: A measure of the sedimentation rate in a coastal basin. Anal. Chim. Acta. 1996, 330, 107–115. [Google Scholar] [CrossRef]
- Borysenko, A.; Ostrowski, A.; Bellifemine, D.; Palmer, G.; Haigh, P.; Johnston, A. Application of PERALSTM alpha spectrometry and gamma spectrometry for analysis and investigation of environmental spills at ISL uranium mining projects. J. Radiol. Prot. 2014, 34, 77–87. [Google Scholar] [CrossRef]
- Landstetter, C.; Hiegesberger, B.; Sinojmeri, M.; Katzlberger, C. Determination of 210Pb and 210Po in water using the extractive scintillation cocktail PolexTM. Appl. Radiat. Isotop. 2014, 93, 76–81. [Google Scholar] [CrossRef]
- Katzlberger, C.; Wallner, G.; Irlweck, K. Determination of 210Pb, 210Bi and 210Po in natural drinking water. J. Radioanal. Nucl. Chem. 2001, 249, 191–196. [Google Scholar]
- Landstetter, U.; Katzlberger, C. Rapid Method for Determining Natural Radionuclides in Drinking Water. In Advances in Liquid Scintillation Spectrometry; Chalupnik, S., Schönhofer, F., Noakes, J., Eds.; University of Arizona: Tucson, AZ, USA, 2006; pp. 181–189. ISBN 0963831453. [Google Scholar]
- Zehringer, M. Radonschlussbericht. Ann. Rep. State Lab. Basel City 2005, 150–153. [Google Scholar] [CrossRef]
- Zehringer, M. Artificial and natural radionuclides in drinking water. A monitoring of the tap water in the Cantons Basel-Country and Basel-City. Ann. Rep. State Lab. 2014, 31–37. [Google Scholar]
- Case, G.N.; McDowell, W.J. Separation of Radium and its Determination by Photon/Electron-Rejecting Alpha Liquid Scintillation Spectrometry. Radioact. Radiochem. 1990, 1, 58–68. [Google Scholar]
- Burnett, W.C.; Tai, W.C. Determination of radium in natural waters by alpha liquid scintillation. Anal. Chem. 1992, 64, 1691–1697. [Google Scholar] [CrossRef]
- Escobar, G.V.; Tomé, F.V.; Lozano, J.C. Extractive Procedure for 226Ra Determination in Aqueous Samples by Liquid Scintillation Counting. Radioact. Radiochem. 1999, 10, 17–21. [Google Scholar]
- Aupiais, J. Radium measurement in water samples by α-liquid scintillation counting with α/β discrimination. Anal. Chim. Acta 2005, 532, 199–207. [Google Scholar] [CrossRef]
- Aupiais, J.; Fayolle, C.; Gilbert, P.; Dacheux, N. Determination of 226Ra in mineral drinking waters by a liquid scintillation with rejection of β−γ emitters. Anal. Chem. 1998, 70, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Escobar, V.G.; Tomé, F.V.; Lozano, J.; Sánchez, A.M. Extractive procedure for uranium determination in water samples by liquid scintillation counting. Appl. Radiat. Isot. 1998, 49, 875–883. [Google Scholar] [CrossRef]
- Jia, G.; Jia, J. Determination of radium isotopes in environmental samples by gamma spectrometry, liquid scintillation counting and alpha spectrometry: A review of analytical methodology. J. Environ. Radioact. 2012, 106, 98–119. [Google Scholar] [CrossRef]
- Hashimoto, T.; Sato, K.; Yoneyama, Y.; Fukuyama, N. Simultaneous determination of environmental α-radionuclides using liquid scintillation counting combined with time interval analyses (TIA) and pulse shape discrimination (PSD). J. Radioanal. Nucl. Chem. 1997, 222, 109–116. [Google Scholar] [CrossRef]
- Surbeck, H. Alpha spectrometry sample preparation using selectively adsorbing thin films. Appl. Radiat. Isot. 2000, 53, 97–100. [Google Scholar] [CrossRef]
- Zehringer, M. Radioactivity in Mineral Water. Ann. Rep. State Lab. Basel City 2018, 48–54. [Google Scholar]
- Zehringer, M. The Radiological Investigation of a Portuguese Mineral. Water 2020. [Google Scholar] [CrossRef]
- Ordela, Inc. Radon (222Rn) in Water by PERALS. Application no 7. Available online: https://ordela.com (accessed on 2 July 2021).
- Hamanaka, S.; Shizuma, K.; Wen, X.-Q.; Iwatani, K.; Hasai, H. Radon concentration measurement in water by means of α liquid-scintillation spectrometry with a PERALS spectrometer. Nucl. Instr. Meth. Phys. Res. 1998, A 410, 314–318. [Google Scholar] [CrossRef]
- Feely, H.; Volchok, H.; Hardy, E.P.; Toonkel, L.E. Worldwide deposition of 90Sr through 1976. Environ. Sci. Technol. 1978, 12, 808–809. [Google Scholar] [CrossRef]
- Dietz, M.L.; Horwitz, E.P.; Nelson, D.M.; Wahlgren, M. An Improved Method for Determining 89Sr and 90Sr in Urine. Health Phys. 1991, 61, 871–877. [Google Scholar] [CrossRef]
- McDowell, W.J.; Case, G.N.; Aldrup, D.W. Investigations of ion-Size-Selective Synergism in Solvent Extraction. Sep. Sci. Technol. 1983, 18, 1483–1507. [Google Scholar] [CrossRef]
- McDowell, W.J. Crown Ethers as Solvent Extraction Reagents: Where do We Stand? Sep. Sci. Technol. 1988, 23, 1251–1268. [Google Scholar] [CrossRef]
- McDowell, W.J. Proposed Separation and Analysis Scheme for Strontium; ETRAC Inc.: San Rafael, CA, USA, 1995. [Google Scholar]
- Dacheux, N.; Aupiais, J. Determination of Uranium, Thorium, Plutonium, Americium and Curium Ultra traces by Photon Electron Rejecting a Liquid Scintillation. Anal. Chem. 1997, 69, 2275–2282. [Google Scholar] [CrossRef]
- Wallner, G. Thorium determination by liquid scintillation counting using an extractive cocktail. Environ. Int. 1996, 22, 101–103. [Google Scholar] [CrossRef]
- Ayranov, M.; Wacker, K.; Krähenbühl, U. Determination of uranium and thorium in complex matrices by two solvent extraction separation techniques and photon electron rejecting alpha liquid spectrometry. Radiochim. Acta 2001, 89, 823–830. [Google Scholar] [CrossRef]
- Füeg, B.; Tschachtli, Τ.; Krähenbühl, U. Alpha Liquid-Scintillation Spectrometry used for the Measurement of Uranium/Thorium-Disequilibria in Soil Samples. Radiochim. Acta 1997, 78, 47–52. [Google Scholar] [CrossRef]
- Fourest, B.; Lagarde, G.; Perrone, J.; Brandel, V.; Dacheux, N.; Genet, M. Solubility of thorium phosphate-diphospate. N. J. Chem. 1999, 23, 645–649. [Google Scholar] [CrossRef]
- Leyba, J.D.; Vollmar, H.S.; Fjeld, R.A.; Devol, T.A.; Brown, D.D.; Cadieux, J.R. Evaluation of a direct extraction/liquid scintillation counting technique for the measurement of uranium in water. J. Radioanal. Nucl. Chem. 1995, 194, 337–344. [Google Scholar] [CrossRef]
- Duffey, J.M.; Case, F.I.; Metzger, R.L.; Jessop, B.J.; Schweitzer, G.K. Development of a rapid procedure for the measurement of uranium in drinking water by PERALS® spectrometry. J. Radioanal. Nucl. Chem. 1997, 221, 115–122. [Google Scholar] [CrossRef]
- Aupiais, J. Rapid determination of uranium activity and concentration in water by alpha liquid scintillation with α/β discrimination. Anal. Chim. Acta 2004, 517, 221–228. [Google Scholar] [CrossRef]
- ASTM D6239–09, Standard Test. Method for Uranium in Drinking Water by High-Resolution Alpha-Liquid-Scintillation Spectrometry; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Dacheux, N.; Aupiais, J.; Courson, O.; Aubert, C. Comparison and improvement of the determination of actinides with low activities using several alpha liquid scintillation spectrometers. Anal. Chem. 2000, 72, 3150–3157. [Google Scholar] [CrossRef] [PubMed]
- Hinton, E.R. Development of a Multipurpose Alpha Detection Procedure for Enriched Uranium in Urine. Anal. Lett. 1983, 16, 367–380. [Google Scholar] [CrossRef]
- Miller, T.J. Development of a Rapid, Economical and Sensitive Method for the Routine Determination of Excreted Uranium in Urine. Anal. Lett. 1991, 24, 657–664. [Google Scholar] [CrossRef]
- Dinse, C.; Baglan, N.; Cossonnet, C.; Bouvier, C. New purification protocol for actinide measurement in excreta based on calixarene chemistry. Appl. Radiat. Isot. 2000, 53, 381–386. [Google Scholar] [CrossRef]
- Dacheux, N.; Aupiais, J. Determination of low concentrations of americium and curium by photon/electron rejecting alpha liquid scintillation. Anal. Chim. Acta 1998, 363, 279–294. [Google Scholar] [CrossRef]
- Xia, X.; Idemitsu, K.; Mitsugashira, T.; Arima, T.; Inagaki, Y. Plutonium Determination in Compacted Bentonite by Using PERALS. J. Nucl. Sci. Technol. 2002, 39, 572–575. [Google Scholar] [CrossRef] [Green Version]
- Zapata-Garcia, D.; Garcia-Miranda, M.; Wershofen, H.; Jerome, S.M. Application of PERALS® Spectrometry for the Rapid Measurement of Alpha Emitters. Application PTB Berlin. 2016. Available online: https://www.researchgate.net/publication/304012198_Application_of_PERALSR_spectrometry_for_the_rapid_measurement_of_alpha_emmitters (accessed on 2 July 2021).
- Aupiais, J.; Dacheux, N.; Thomas, A.; Matton, S. Study of neptunium measurement by alpha liquid scintillation with rejection of β−γ−emitters. Anal. Chim. Acta 1999, 398, 205–2018. [Google Scholar] [CrossRef]
- Baglan, N.; Bouvier-Capely, C.; Cossonnet, C. Determination of 237Np at trace level: Evaluation of various analytical procedures. Radiochim. Acta 2002, 90, 267–272. [Google Scholar] [CrossRef]

| Drinking Water | Dose Coefficients for Different Age Groups * | |||||
|---|---|---|---|---|---|---|
| Radionuclide | TBDV [3] | DAL Infant | DAL Adult | Infant (1–2 Years) | Children >10 Years | Adult Person |
| Bq/L | Bq/L | Bq/L | µSv/Bq | µSv/Bq | µSv/Bq | |
| Americium-241 (241Am) | 0.1 | 0.4 | 0.7 | 0.37 | 0.22 | 0.20 |
| Lead-210 (210Pb) | 0.04 | 0.2 | 3.6 | 1.9 | 0.69 | |
| Polonium-210 (210Po) | 0.02 | 0.1 | 8.8 | 2.6 | 1.2 | |
| Plutonium 239 + 240Pu | 0.3 | 0.6 | 0.42 | 0.27 | 0.25 | |
| Radium-224 (224Ra) | 0.2 | 2 | 0.66 | 0.26 | 0.07 | |
| Radium-226 (226Ra) | 0.1 | 0.5 | 0.96 | 0.8 | 0.28 | |
| Radium-228 (228Ra) | 0.1 | 0.2 | 30 | 3.9 | 0.69 | |
| Radon (222Rn) | 100 | 2 | 5 | 0.073 | 0.06 | 0.028 |
| Strontium-90 (90Sr) | 4.9 | 0.4 | 2 | 0.37 | 0.14 | 0.07 |
| Thorium-228 (228Th) | 0.4 | 2 | 0.37 | 0.14 | 0.07 | |
| Thorium-230 (230Th) | 0.3 | 0.6 | 0.41 | 0.29 | 0.23 | |
| Thorium-232 (232Th) | 0.3 | 5 | 0.45 | 0.06 | 0.028 | |
| Uranium-234 (234U) | 1 | 2.8 | 0.13 | 0.074 | 0.049 | |
| Uranium-238 (238U) | 1 | 3 | 0.12 | 0.068 | 0.045 | |
| Cocktail | Scintillator | Extractant | Energy Transfer Agent | Solvent |
|---|---|---|---|---|
| ALPHAEX | PBBO, PPO, NPO | HDEHP | Naphthalene | Toluene |
| POLEX | PBBO | TOPO | Naphthalene | Toluene |
| RADAEX | PBBO | 2-methyl-2-heptyl-nonanoic acid, Dicyclohexano-21-crown-7 | Naphthalene | Toluene |
| RADONS | PBBO | No extractant | Naphthalene | Xylene |
| STRONEX | PBBO | C6-C8-Neocarboxilic acids, Dicyclohexano-18-crown-6 | Naphthalene | Toluene |
| URAEX | PBBO | Tert. Amine (MG > 300) | Naphthalene | Toluene |
| THOREX | PBBO | Primary Amine (MG > 300) | Naphthalene | Toluene |
| Polonium Nuclide | Decay Chain | Decay | Half-Life | Main Alpha Energy Lines |
|---|---|---|---|---|
| 218Po | 238U-series | α | 3.1 m | 6002 (100) |
| 214Po | α | 162 µs | 7687 (100) | |
| 210Po | α | 138.4 d | 5304 (100) | |
| 216Po | 232Th-series | α | 0.15 s | 6778 (100) |
| 212Po | α | 0.3 µs | 8785 (100) | |
| 215Po | 235U-series | α | 1.8 µs | 7386 (100) |
| 211Po | α | 0.52 s | 7450 (99) | |
| 208Po | tracer | α | 2.99 y | 5216 (100) |
| 209Po | tracer | α | 102 y | 4977 (79) 4979 (20) |
| Radium Nuclide | Decay | Half-Life | Main Alpha Energy Lines |
|---|---|---|---|
| 228Ra | β | 5.75 y | |
| 226Ra | α | 1600 y | 4601 (6), 4784 (94) |
| 224Ra | α | 3.63 d | 5448 (5), 5685 (94) |
| 223Ra | α | 11.43 d | 5539 (11), 5607 (26) 5713 (50), 5757 (10) |
| Radon Nuclide | Decay Chain | Decay | Half-Life | Main Alpha Energy Lines |
|---|---|---|---|---|
| 222Rn (“radon“) | 238U-series | α | 3.82 d | 5489 (100) |
| 220Rn (“thoron”) | 232Th-series | α | 55.8 s | 5449 (5) 6288 (95) |
| 219Rn (“actinon”) | 235U-series | α | 3.98 s | 5425 (8), 6553 (13) 6819 (79) |
| Radio Nuclide | Decay | Half-life | Energy Beta Max |
|---|---|---|---|
| 89Sr | β,γ | 50.6 d | 1495 |
| 90Sr | β | 28.8 y | 545.9 |
| 90Y | β | 64.1 h | 2279 |
| Thorium Nuclide | Decay Chain | Decay | Half-Life | Main Alpha Energy Lines |
|---|---|---|---|---|
| 234Th | 238U-series | β | 4.5 109 y | – |
| 230Th | α | 7.5 104 y | 4621 (23.4) 4787 (76.3) | |
| 232Th | 232Th-series | α | 1.4 1010 y | 3949 (21.0) 4011 (78.9) |
| 228Th | α | 1.92 y | 5340 (26.0) 5423 (73.4) | |
| 231Th | 235U-series | β | 25.52 h | – |
| 227Th | α | 18.72 d | 5757 (20.4) 5978 (23.5) 6038 (24.2) | |
| 229Th | tracer | α | 7.9 103 y | 4837 (58.2) 4894 (10.7) 4806 (11.4) |
| Uranium Nuclide | Decay Chain | Decay | Half-Life | Main Alpha Energy Lines |
|---|---|---|---|---|
| 238U | 238U-series | α | 4.5 109 y | 4151 (22.3) 4198 (77.4) |
| 234U | α | 2.5 105 y | 4722 (28.4) 4775 (71.4) | |
| 235U | 235U-series | α | 7.0 108 y | 4366 (18.8) 4398 (57.2) |
| 232U | Tracer | α | 72 y | 5260 (32) 5320 (68) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zehringer, M.; Kammerer, F.; Pregler, A. Applications of PERALS-Alpha Spectrometry for the Investigation of Radionuclides in Water Samples. Materials 2021, 14, 3787. https://doi.org/10.3390/ma14143787
Zehringer M, Kammerer F, Pregler A. Applications of PERALS-Alpha Spectrometry for the Investigation of Radionuclides in Water Samples. Materials. 2021; 14(14):3787. https://doi.org/10.3390/ma14143787
Chicago/Turabian StyleZehringer, Markus, Franziska Kammerer, and Anja Pregler. 2021. "Applications of PERALS-Alpha Spectrometry for the Investigation of Radionuclides in Water Samples" Materials 14, no. 14: 3787. https://doi.org/10.3390/ma14143787





