Study on a Novel Biodegradable and Antibacterial Fe-Based Alloy Prepared by Microwave Sintering
Abstract
:1. Introduction
2. Experiment Methods
2.1. Sample Preparation
2.2. Microstructure and Hardness
2.3. Degradation Behavior
2.4. Antibacterial Properties and Cytocompatibility
3. Results and Discussion
3.1. Microstructures and Hardness
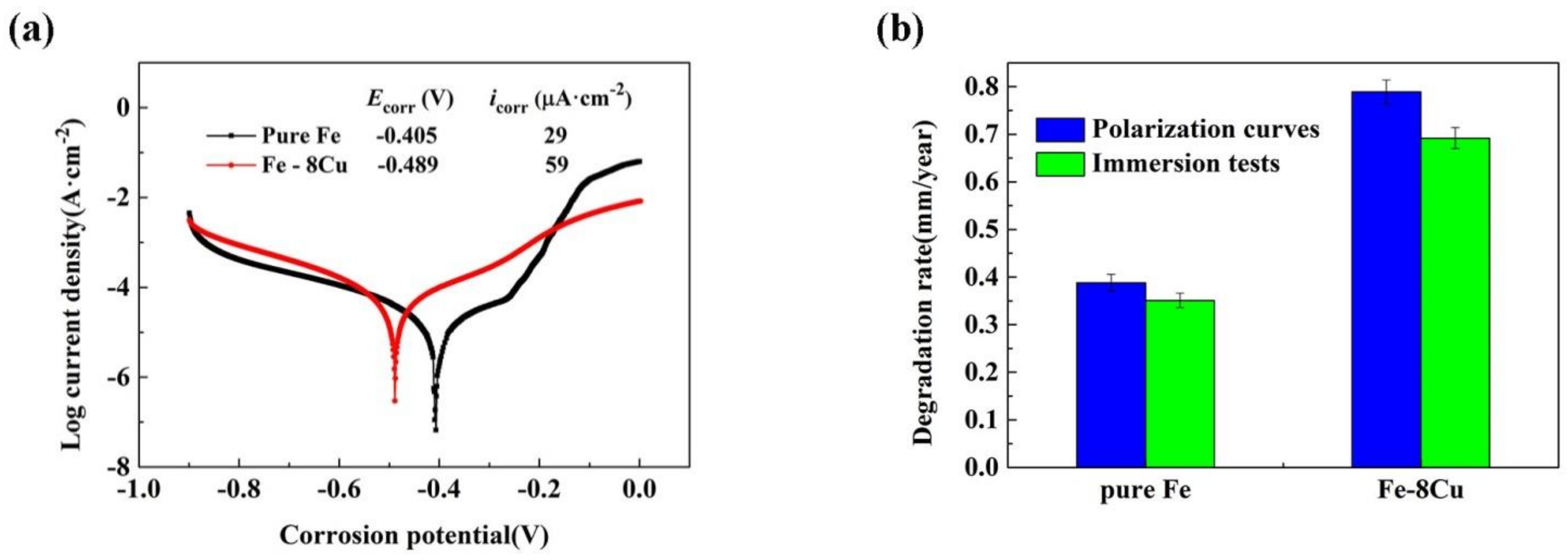
3.2. Degradation Behavior
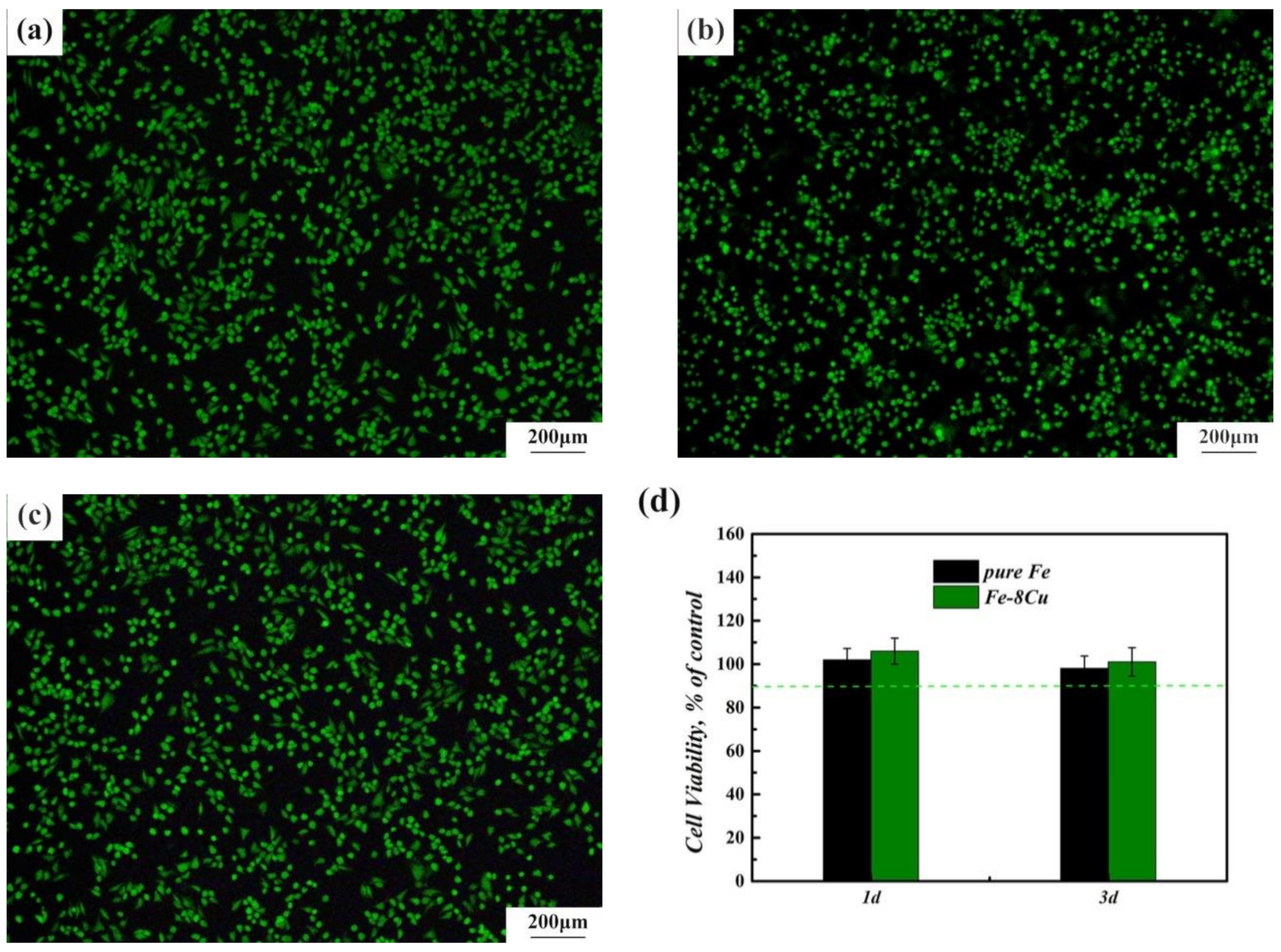
3.3. Antibacterial Properties and Cytocompatibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chou, D.T.; Wells, D.; Hong, D.; Lee, B.; Kuhn, H.; Kumta, P.N. Novel processing of iron-manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 2013, 9, 8593–8603. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, M.; Na, D.; Li, Y.D.; Tan, L.L.; Yang, K. Study on a biodegradable antibacterial Fe-Mn-C-Cu alloy as urinary implant material. Mater. Sci. Eng. C 2019, 103, 109718. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; Zhao, M.; Liu, C.; Liu, L.; Gao, C.; Shuai, C.; Atrens, A. Study on Fe-xGO Composites Prepared by Selective Laser Melting: Microstructure, Hardness, Biodegradation and Cytocompatibility. JOM 2020, 72, 1163–1174. [Google Scholar] [CrossRef]
- Gorejová, R.; Haverová, L.; Oriňaková, R.; Oriňak, A.; Oriňak, M. Recent advancements in Fe-based biodegradable materials for bone repair. J. Mater. Sci. 2019, 54, 1913–1947. [Google Scholar] [CrossRef]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.V. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef]
- Vojtĕch, D.; Kubásek, J.; Čapek, J.; Pospíšilová, I. Comparative mechanical and corrosion studies on magnesium,zinc and iron alloys as biodegradable metals. Mater. Tehnol. 2015, 49, 877–882. [Google Scholar] [CrossRef]
- He, J.; He, F.; Li, D.; Liu, Y.; Liu, Y.; Ye, Y.; Yin, D. Advances in Fe-based biodegradable metallic materials. RSC Adv. 2016, 6, 112819–112838. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M.; et al. Biodegradable Fe-based alloys for use in osteosynthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Purnama, A.; Hermawan, H.; Couet, J.; Mantovani, D. Assessing the biocompatibility of degradable metallic materials: State-of-the-art and focus on the potential of genetic regulation. Acta Biomaterilia 2010, 6, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Ulum, M.F.; Arafat, A.; Noviana, D.; Yusop, A.H.; Nasution, A.K.; Abdul, K.M.R.; Hermawan, H. In vitro and in vivo degradation evaluation of novel iron-bioceramic composites for bone implant applications. Mater. Sci. Eng. C 2014, 36, 336–344. [Google Scholar] [CrossRef]
- Wegener, B.; Sievers, B.; Utzschneider, S.; Müller, P.; Janssona, V.; Rößler, S.; Nies, B.; Stephani, G.; Kieback, B.; Quadbeck, P. Microstructure, cytotoxicity and corrosion of powder-metallurgical iron alloys for biodegradable bone replacement materials. Mater. Sci. Eng. B 2011, 176, 1789–1796. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.F. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef]
- Heiden, M.; Walker, E.; Stanciu, L. Evolution of novel bioresorbable iron-manganese implant surfaces and their degradation behaviors in vitro. J. Biotechnol. Biomater. 2015, 103, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Roberts, S.A.; Fowler, V.G.; Shah, M.A.; Taylor, S.L.; Morris, A.J.; Corey, G.R. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin. Infect. Dis. 2001, 32, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhao, M.; Zhao, Y.; Liu, L.; Liu, C.; Gao, C.; Shuai, C.; Atrens, A. Improved biodegradation resistance by grain refinement of novel antibacterial ZK30-Cu alloys produced via selective laser melting. Mater. Lett. 2019, 237, 253–257. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, L.; Zhao, M.; Feng, P.; Yang, Y.; Guo, W.; Gao, C.; Yuan, F. Microstructure, biodegradation, antibacterial and mechanical properties of ZK60-Cu alloys prepared by selective laser melting technique. J. Mater. Sci. Technol. 2018, 34, 1944–1952. [Google Scholar] [CrossRef]
- Wu, C.T.; Zhou, Y.H.; Xu, M.C.; Han, P.P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Ren, L.; Wong, H.M.; Yan, C.H.; Yeung, K.W.K.; Yang, K. Osteogenic ability of Cu-bearing stainless steel. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1433–1444. [Google Scholar] [CrossRef]
- Burghardt, I.; Lüthen, F.; Prinz, C.; Kreikemeyer, B.; Zietz, C.; Neumann, H.G.; Rychly, J. A dual function of copper in designing regenerative implants. Biomaterials 2015, 44, 36–44. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Ren, G.; Ren, L. Study on behaviour and mechanism of Cu2+ ion release from Cu bearing antibacterial stainless steel. Mater. Technol. 2015, 30, 126–132. [Google Scholar] [CrossRef]
- Guo, Y.X.; Zhao, M.C.; Xie, B.; Zhao, Y.C.; Yin, D.F.; Gao, C.D.; Shuai, C.J.; Atrens, A. In Vitro Corrosion Resistance and Antibacterial Performance of Novel Fe-xCu Biomedical Alloys Prepared by Selective Laser Melting. Adv. Eng. Mater. 2020, 2001000. [Google Scholar]
- Xu, J.L.; Tao, S.C.; Bao, L.Z.; Luo, J.M.; Zheng, Y.F. Effects of Mo contents on the microstructure, properties and cytocompatibility of the microwave sintered porous Ti-Mo alloys. Mater. Sci. Eng. C 2019, 97, 156–165. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, P.M. Morphological and mechanical characterization of topologically ordered open cell porous iron foam fabricated using 3D printing and pressureless microwave sintering. Mater. Des. 2018, 160, 442–454. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloy. Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- ASTM. ASTM B328-96 Standard Test Method for Density, Oil Content, and Interconnected Porosity of Sintered Metal Structural Parts and Oil-Impregnated Bearings; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Tao, J.X.; Zhao, M.C.; Zhao, Y.C.; Yin, D.F.; Liu, L.; Gao, C.D.; Shuai, C.J.; Atrens, A. Influence of graphene oxide (GO) on microstructure and biodegradation of ZK30-xGO composites prepared by selective laser melting. J. Magnes. Alloy. 2020, 8, 952–962. [Google Scholar] [CrossRef]
- Tao, S.C.; Xu, J.L.; Yuan, L.; Luo, J.M.; Zheng, Y.F. Microstructure, mechanical properties and antibacterial properties of the microwave sintered porous Ti-3Cu alloys. J. Alloy. Compd. 2020, 812, 152142. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, M.; Yang, Y.; Tan, L.; Zhao, Y.; Yin, D.; Yang, K.; Atrens, A. Improvement of biodegradable and antibacterial properties by solution treatment and micro-arc oxidation (MAO) of a magnesium alloy with a trace of copper. Corros. Sci. 2019, 156, 125–138. [Google Scholar] [CrossRef]
- Zhao, M.C.; Zhao, Y.C.; Yin, D.F.; Wang, S.; Shangguan, Y.M.; Liu, C.; Tan, L.L.; Shuai, C.J.; Yang, K.; Atrens, A. Biodegradation Behavior of Coated As-Extruded Mg-Sr Alloy in Simulated Body Fluid. Acta Metall. Sin. 2019, 32, 1195–1206. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Agrawal, D.; Cheng, J.P.; Gedevanishvili, S. Full sintering of powdered-metal bodies in a microwave field. Nature 1999, 399, 668–670. [Google Scholar] [CrossRef]
- Annamalai, R.; Upadhyaya, A.; Agrawal, D. An investigation on microwave sintering of Fe, Fe-Cu and Fe-Cu-C alloys. Bull. Mater. Sci. 2013, 36, 447–456. [Google Scholar] [CrossRef]
- Niu, J.; Tang, Z.; Huang, H.; Pei, J.; Zhang, H.; Yuan, G.; Ding, W. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater. Sci. Eng. C 2016, 69, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Xu, X.; Wang, C.; Ma, Y.; Hui, D.; Zhou, Z. Remarkably improvement in antibacterial activity by synergistic effect in n-Cu@T-ZnO nanocomposites, Compos. Part B 2017, 110, 32–38. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Kim, H.Y.; Khil, M. Enhanced bactericidal effect of novel CuO/TiO2 composite nanorods and a mechanism thereof., Compos. Part B 2013, 45, 904–910. [Google Scholar] [CrossRef]
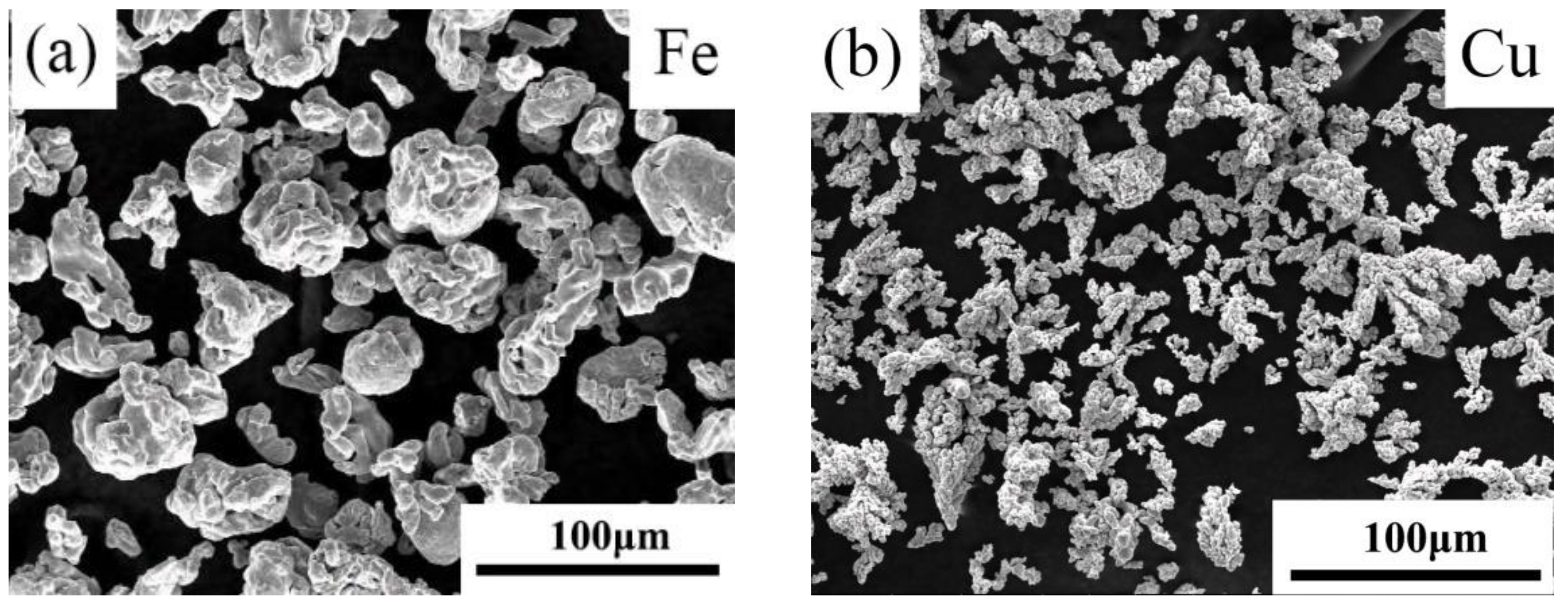
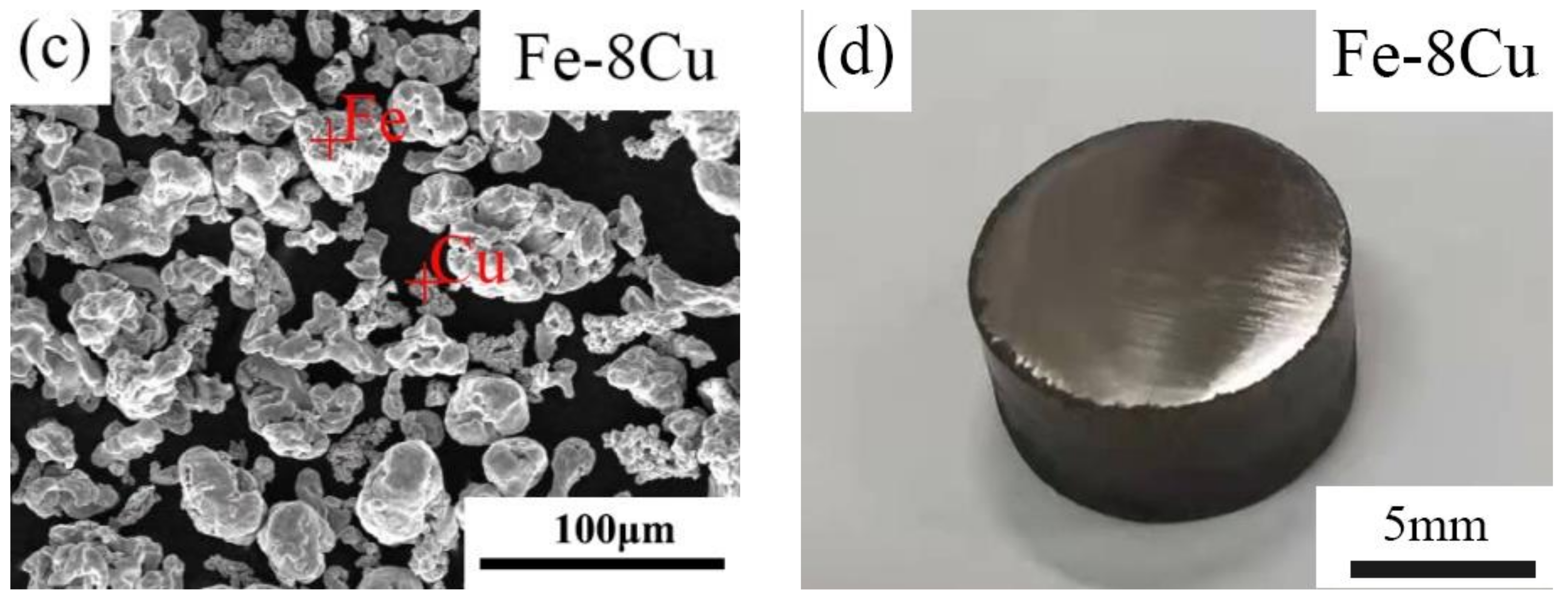
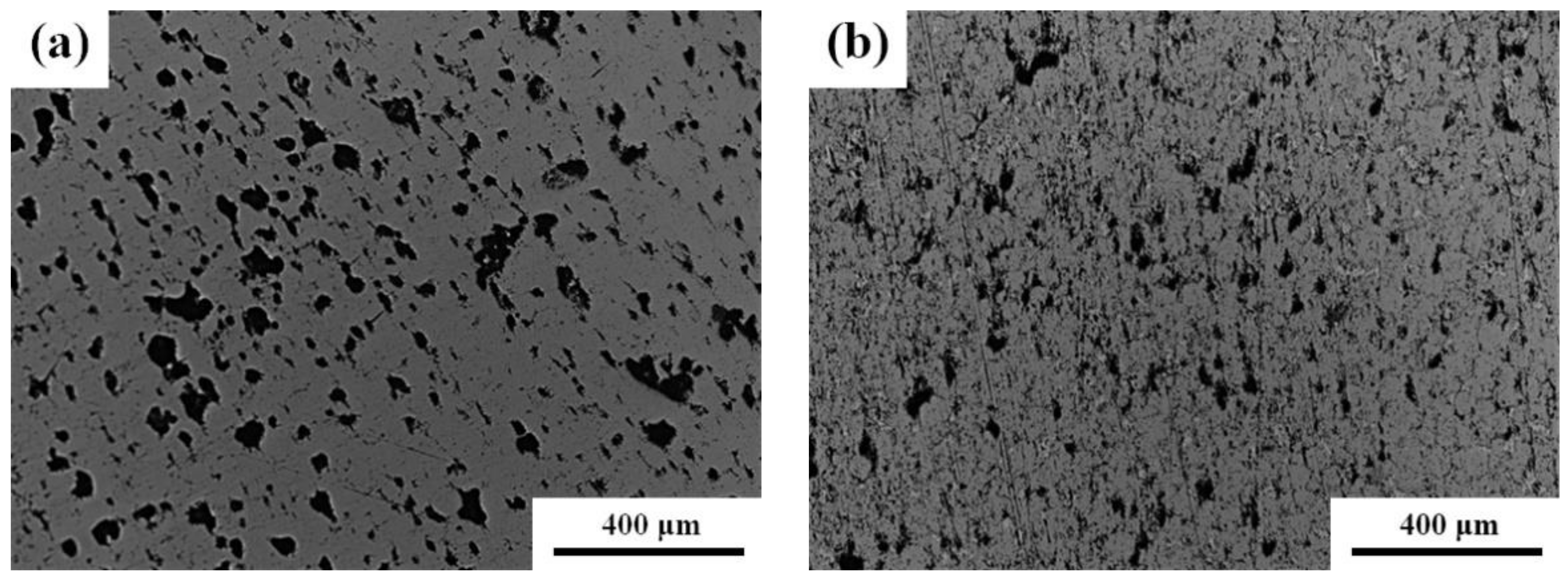
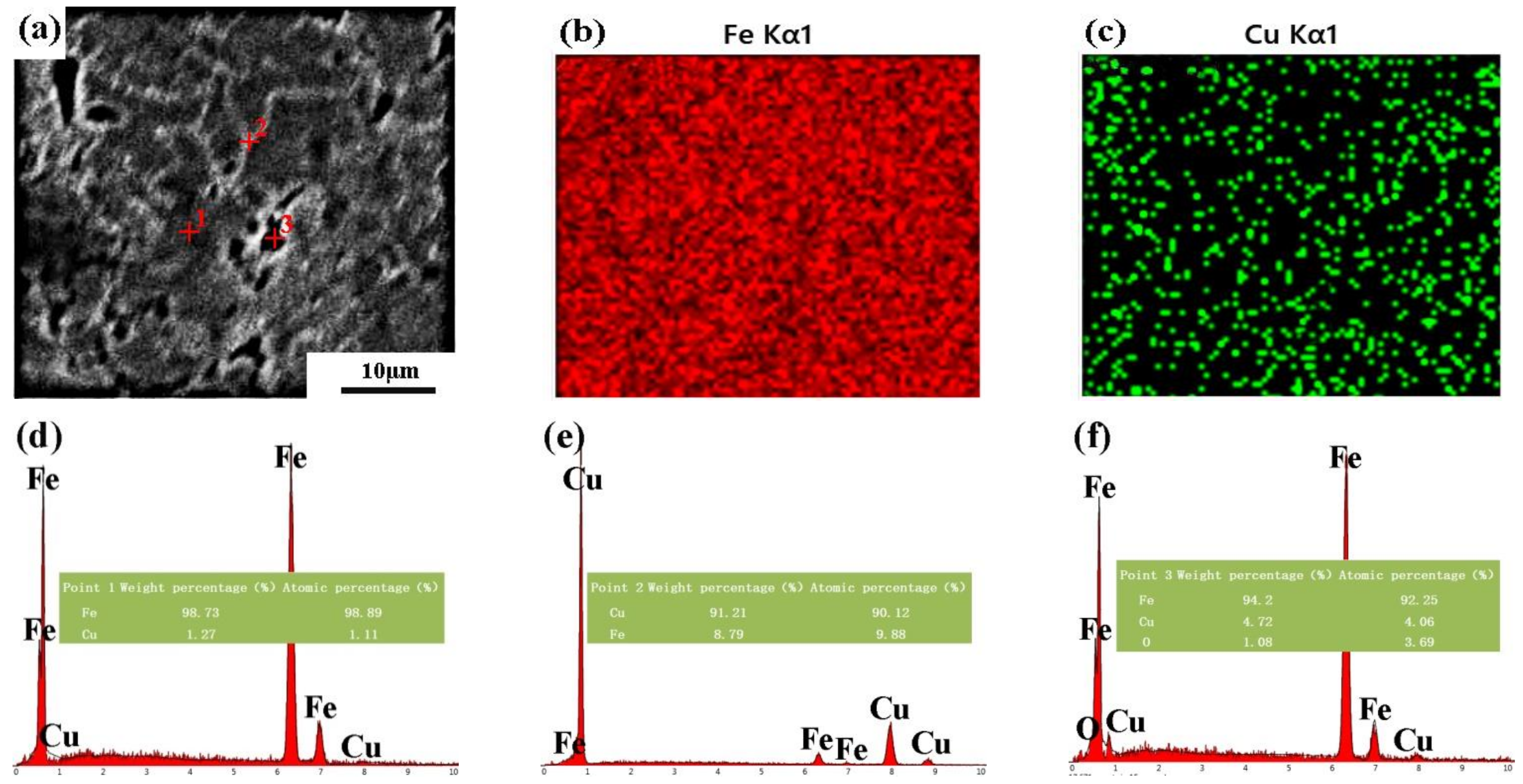

| Pure Fe | Fe-8Cu | |
|---|---|---|
| Density (g/cm3) | 6.85 ± 0.003 | 6.94 ± 0.002 |
| Relative density (%) | 87.04 ± 0.04 | 87.30±0.06 |
| Hardness (HV) | 101 ± 2 | 127 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, B.; Guo, Y.; Zhao, M.-C.; Li, Q.-F.; Ma, B.; Duan, B.; Yin, D.; Atrens, A. Study on a Novel Biodegradable and Antibacterial Fe-Based Alloy Prepared by Microwave Sintering. Materials 2021, 14, 3784. https://doi.org/10.3390/ma14143784
Deng B, Guo Y, Zhao M-C, Li Q-F, Ma B, Duan B, Yin D, Atrens A. Study on a Novel Biodegradable and Antibacterial Fe-Based Alloy Prepared by Microwave Sintering. Materials. 2021; 14(14):3784. https://doi.org/10.3390/ma14143784
Chicago/Turabian StyleDeng, Bin, Yingxue Guo, Ming-Chun Zhao, Qing-Fen Li, Bin Ma, Bohua Duan, Dengfeng Yin, and Andrej Atrens. 2021. "Study on a Novel Biodegradable and Antibacterial Fe-Based Alloy Prepared by Microwave Sintering" Materials 14, no. 14: 3784. https://doi.org/10.3390/ma14143784
APA StyleDeng, B., Guo, Y., Zhao, M.-C., Li, Q.-F., Ma, B., Duan, B., Yin, D., & Atrens, A. (2021). Study on a Novel Biodegradable and Antibacterial Fe-Based Alloy Prepared by Microwave Sintering. Materials, 14(14), 3784. https://doi.org/10.3390/ma14143784








