FAU-Type Zeolite Synthesis from Clays and Its Use for the Simultaneous Adsorption of Five Divalent Metals from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nonlinear Adsorption Isotherms
2.3. FAU Zeolite Preparation and Metal Adsorption
3. Results and Discussion
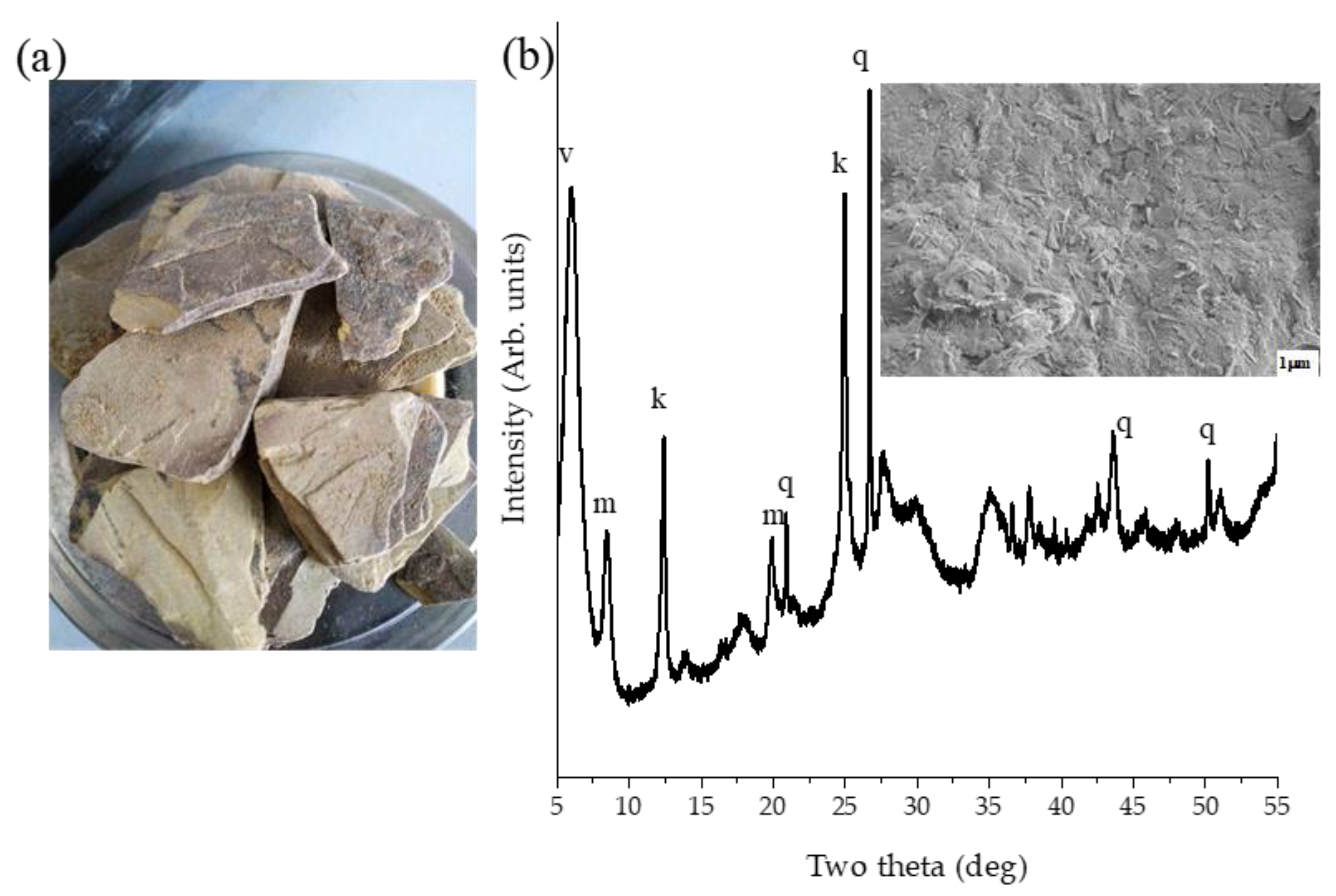
3.1. Clay Characterisation
3.2. VK as Adsorbent
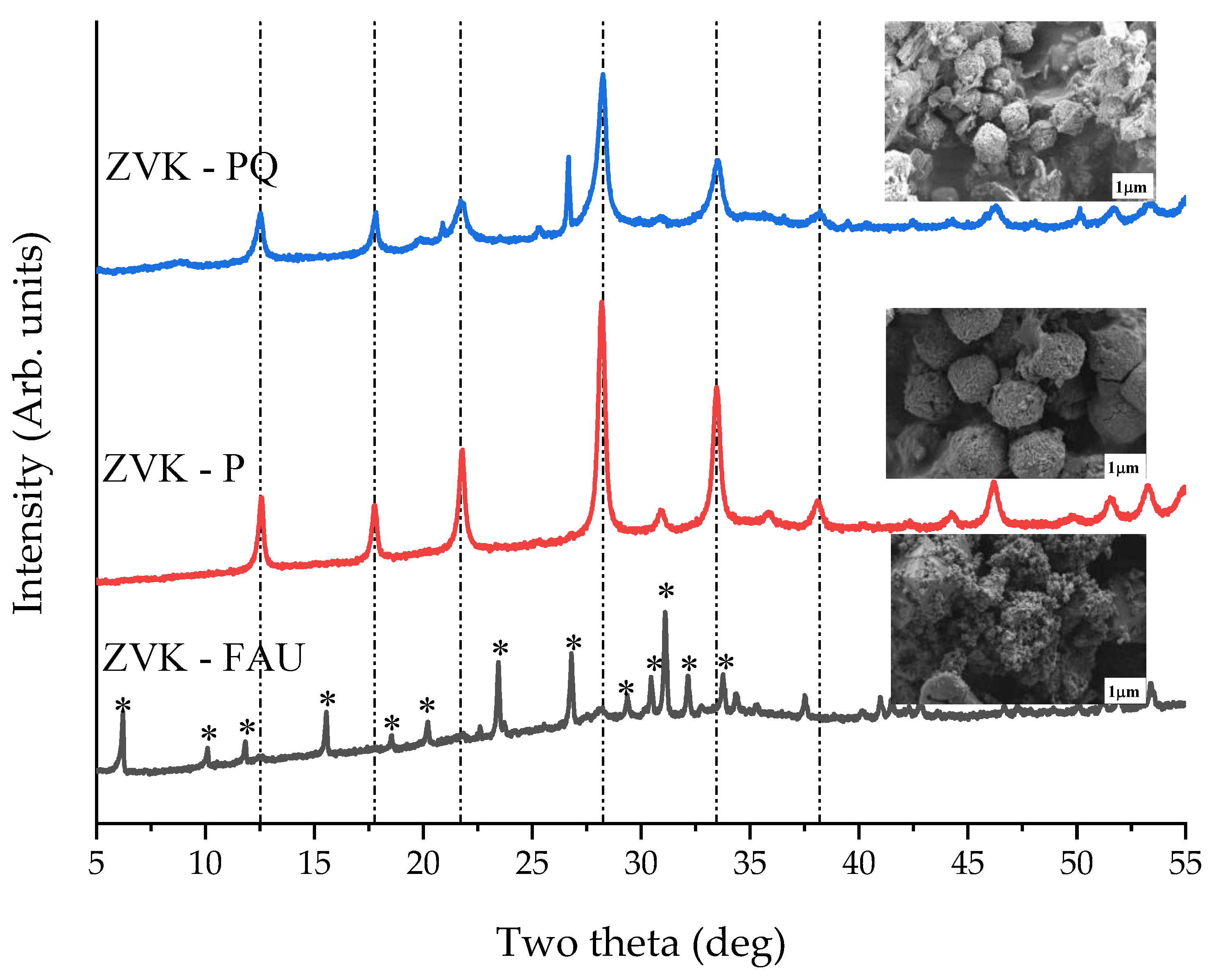
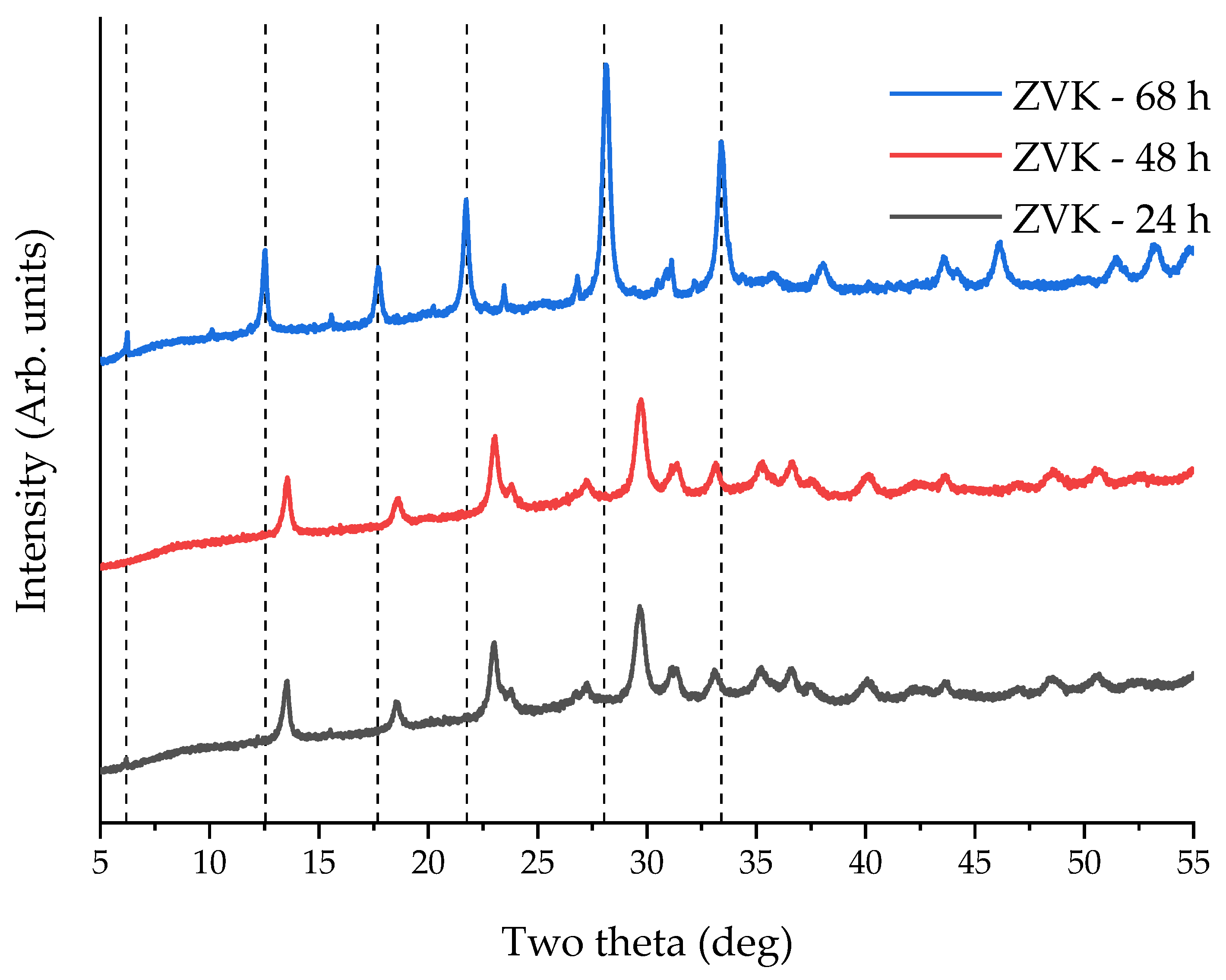
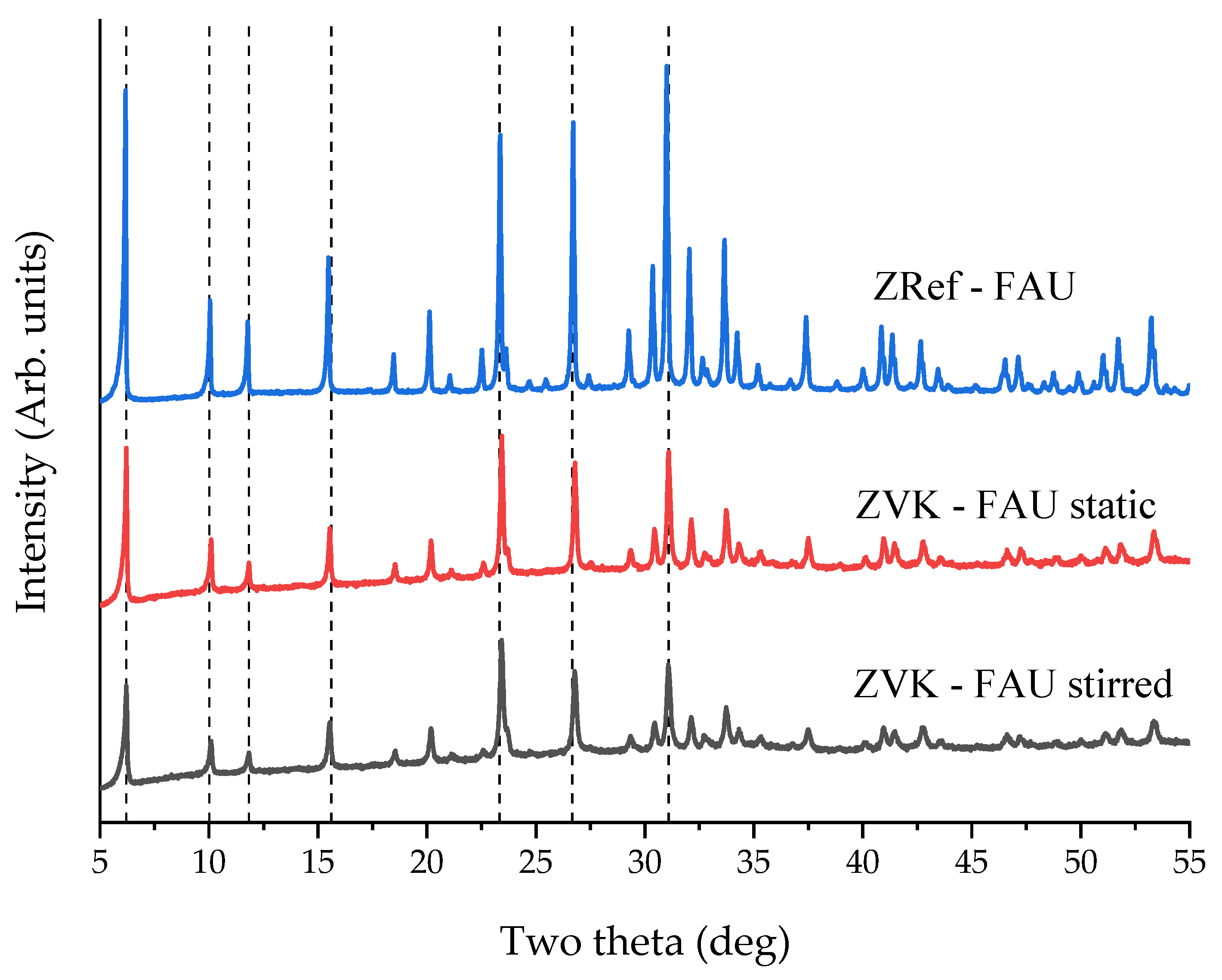
3.3. Zeolite Characterisation
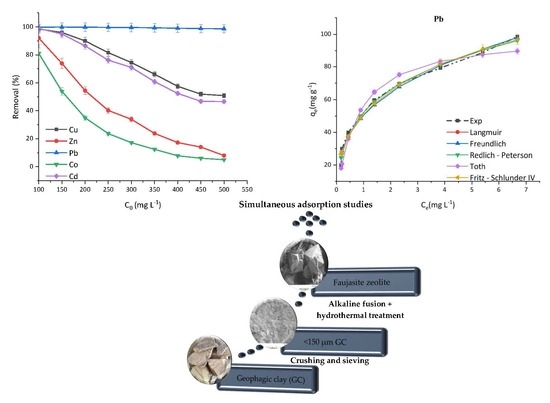
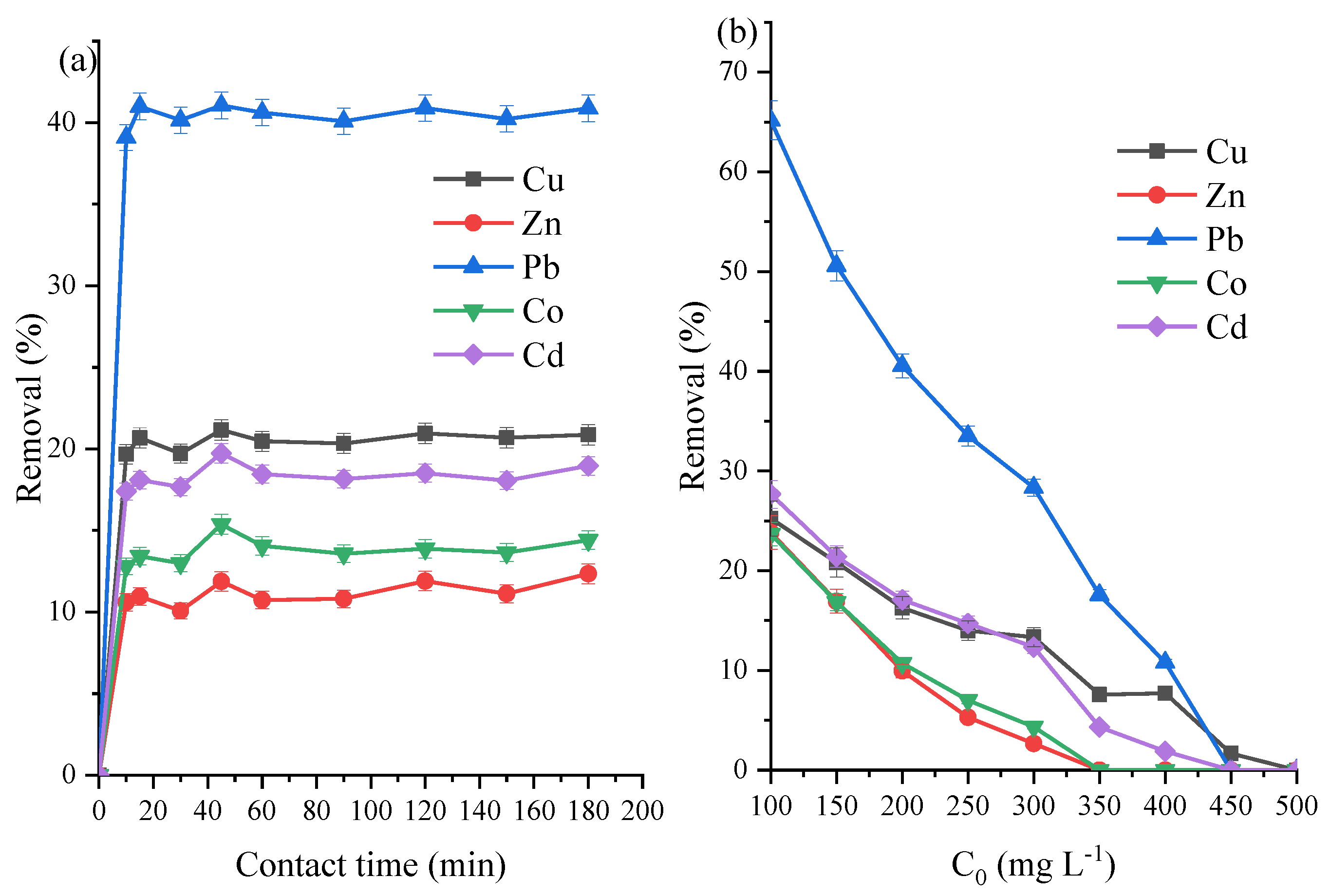
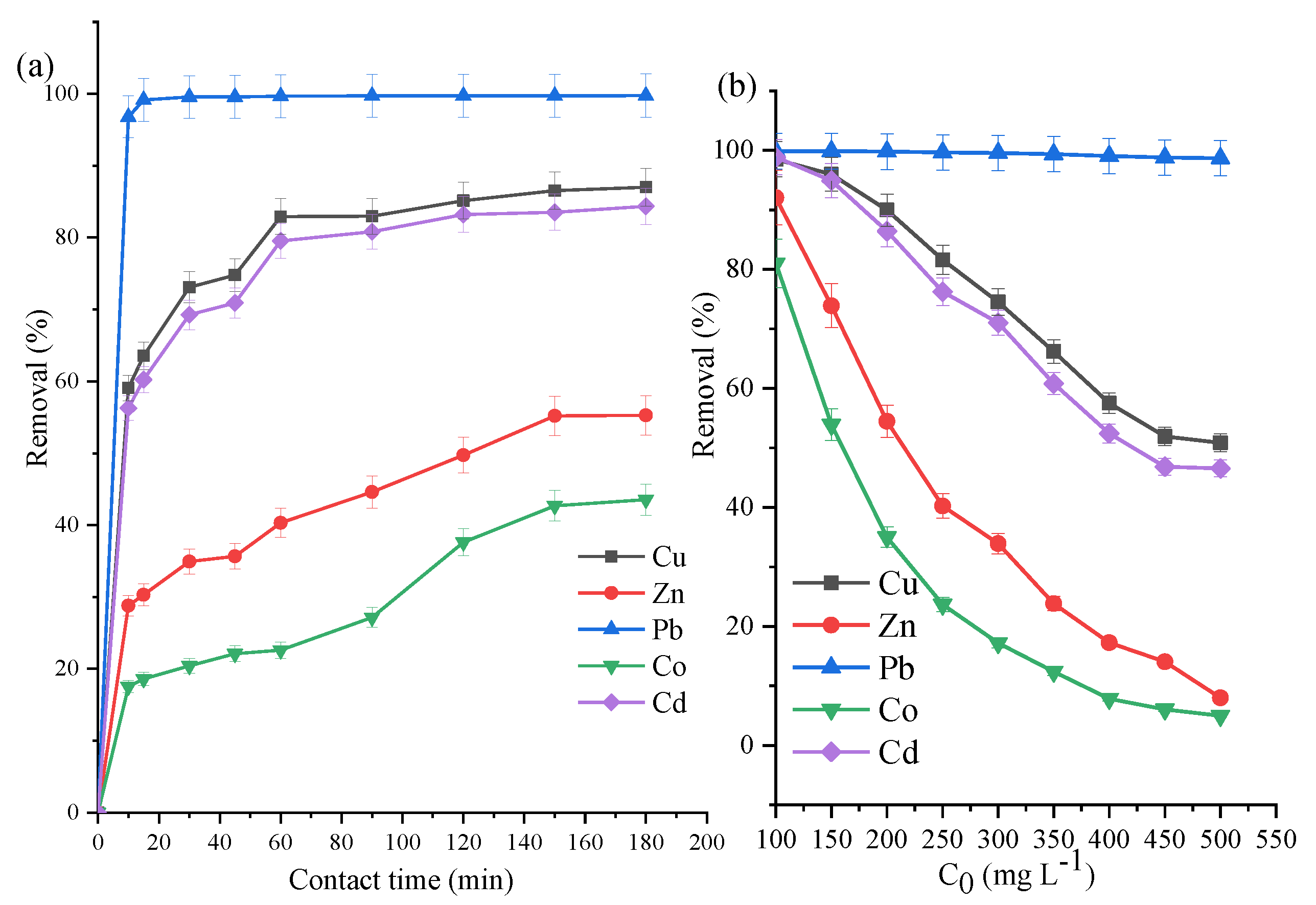
3.4. ZVK-FAU Removal Efficiency
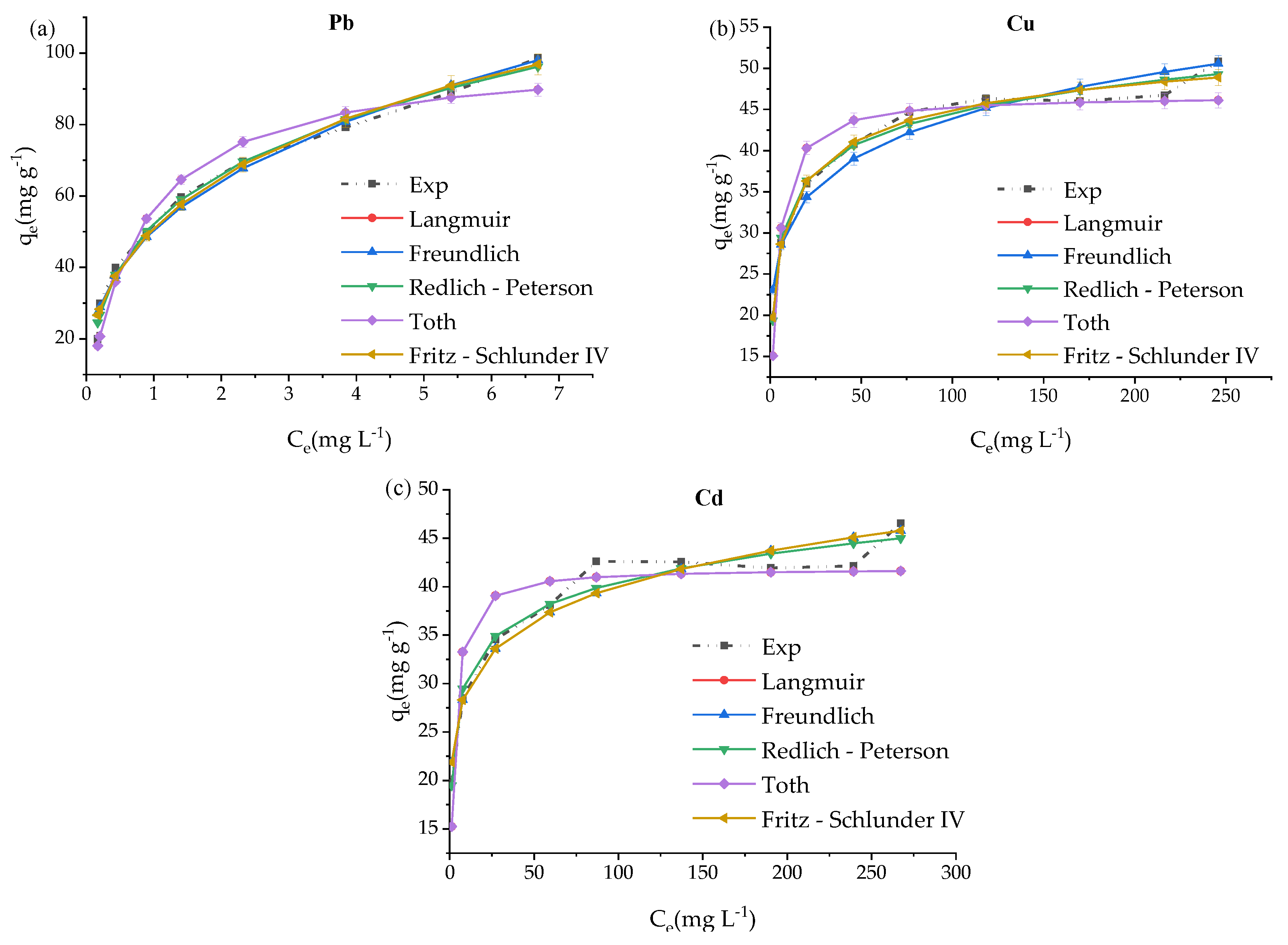
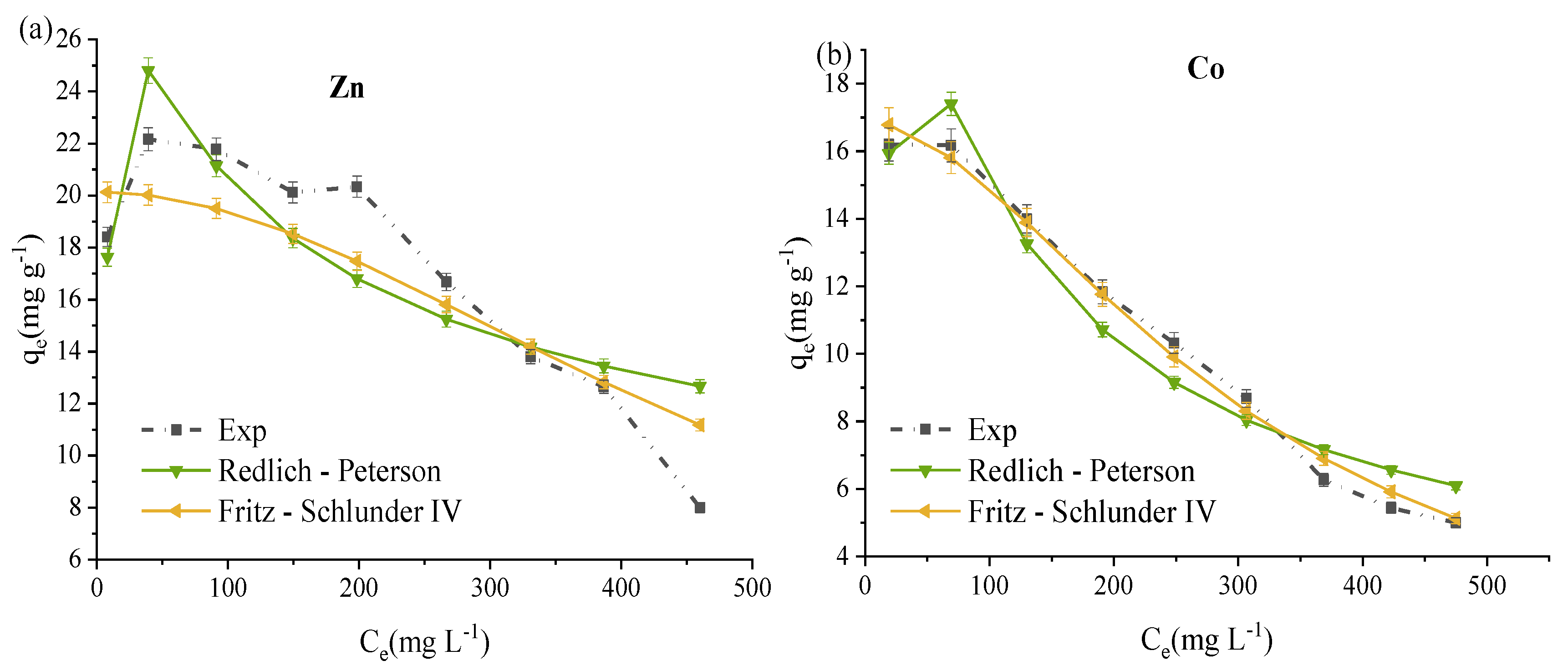
3.5. ZVK-FAU Nonlinear Adsorption Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Abe, S.S.; Masunaga, T.; Honna, T.; Wakatsuki, T. Comprehensive assessment of the clay mineralogical composition of lowland soils in West Africa. Soil Sci. Plant Nutr. 2006, 52, 479–488. [Google Scholar] [CrossRef]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252–253, 428–461. [Google Scholar] [CrossRef]
- Vhahangwele, M.; Mugera, G.W. The potential of ball-milled South African bentonite clay for attenuation of heavy metals from acidic wastewaters: Simultaneous sorption of Co2+, Cu2+, Ni2+, Pb2+, and Zn2+ ions. J. Environ. Chem. Eng. 2015, 3, 2416–2425. [Google Scholar] [CrossRef]
- Raghuvir, P.B.; Prabhu, P.P.; Prabhu, B.; Mathew, T.M. A review on removal of heavy metal ions from waste water using natural/modified bentonite. MATEC Web Conf. 2018, 144, 02021. [Google Scholar]
- Vega, J.L.; Ayala, J.; Loredo, J.; Iglesias, J.G. Bentonites as adsorbents of heavy metals ions from mine waste leachates: Experimental data. In Proceedings of the 9th International Mine Water Congress, Oviedo, Spain, 5–7 September 2005; pp. 603–609. [Google Scholar]
- Sen Gupta, S.; Bhattacharyya, K.G. Immobilization of Pb(II), Cd(Ii) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J. Environ. Manag. 2008, 87, 46–58. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, O.; Altunkaynak, Y.; Guzel, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res. 2003, 37, 948–952. [Google Scholar] [CrossRef]
- de Pablo, L.; Chávez, M.L.; Abatal, M. Adsorption of heavy metals in acid to alkaline environments by montmorillonite and ca-montmorillonite. Chem. Eng. J. 2011, 171, 1276–1286. [Google Scholar] [CrossRef]
- Matusik, J.; Wścisło, A. Enhanced heavy metal adsorption on functionalized nanotubular halloysite interlayer grafted with aminoalcohols. Appl. Clay Sci. 2014, 100, 50–59. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Mittal, A.; Usman, M.; Mittal, J.; Yu, G.; Núñez-Delgado, A.; Kornaros, M. A review on halloysite-based adsorbents to remove pollutants in water and wastewater. J. Mol. Liq. 2018, 269, 855–868. [Google Scholar] [CrossRef]
- Yadav, V.B.; Gadi, R.; Kalra, S. Clay based nanocomposites for removal of heavy metals from water: A review. J. Environ. Manag. 2019, 232, 803–817. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, Y.; Wu, X.; Fan, P.; Song, R. La(III)-bentonite/chitosan composite: A new type adsorbent for rapid removal of phosphate from water bodies. Appl. Clay Sci. 2020, 190, 105547. [Google Scholar] [CrossRef]
- Payra, P.; Dutta, P.K. Zeolites: A primer. ChemInform 2004, 35, 1–19. [Google Scholar] [CrossRef]
- Dyer, A. Ion exchange capacity. In Verified Syntheses of Zeolitic Materials; Robson, H., Lillerud, K.P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 67–68. [Google Scholar]
- Komvokis, V.; Tan, L.X.L.; Clough, M.; Pan, S.S.; Yilmaz, B. Zeolites in fluid catalytic cracking (FCC). In Zeolites in Sustainable Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 271–297. [Google Scholar]
- Asghari, M.; Mohammadi, T.; Aziznia, A.; Danayi, M.R.; Moosavi, S.H.; Alamdari, R.F.; Agand, F. Preparation and characterization of a thin continuous faujasite membrane on tubular porous mullite support. Desalination 2008, 220, 65–71. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef] [PubMed]
- Ltaief, O.O.; Siffert, S.; Poupin, C.; Fourmentin, S.; Benzina, M. Optimal synthesis of faujasite-type zeolites with a hierarchical porosity from natural clay. Eur. J. Inorg. Chem. 2015, 2015, 4658–4665. [Google Scholar] [CrossRef]
- Moneim, M.A.; Ahmed, E.A. Synthesis of faujasite from Egyptian clays: Characterizations and removal of heavy metals. Geomaterials 2015, 5, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Stamires, D.N. Properties of the zeolite, faujasite, substitutional series: A review with new data. Clays Clay Miner. 1973, 21, 379–389. [Google Scholar] [CrossRef]
- Ltaief, O.O.; Siffert, S.; Fourmentin, S.; Benzina, M. Synthesis of faujasite type zeolite from low grade Tunisian clay for the removal of heavy metals from aqueous waste by batch process: Kinetic and equilibrium study. Comptes Rendus Chim. 2015, 18, 1123–1133. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Niceforo, G.; Lettino, A. Sodalite, faujasite and a-type zeolite from 2:1dioctahedral and 2:1:1 trioctahedral clay minerals. A singular review of synthesis methods through laboratory trials at a low incubation temperature. Powder Technol. 2017, 320, 483–497. [Google Scholar] [CrossRef]
- Doyle, A.M.; Albayati, T.M.; Abbas, A.S.; Alismaeel, Z.T. Biodiesel production by esterification of oleic acid over zeolite y prepared from kaolin. Renew. Energy 2016, 97, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Krongkrachang, P.; Thungngern, P.; Asawaworarit, P.; Houngkamhang, N.; Eiad-Ua, A. Synthesis of zeolite Y from kaolin via hydrothermal method. Mater. Today Proc. 2019, 17, 1431–1436. [Google Scholar] [CrossRef]
- Hunter, J.M. Geophagy in Africa and in the united states: A culture-nutrition hypothesis. Geogr. Rev. 1973, 63, 170–195. [Google Scholar] [CrossRef]
- Chen, X. Modeling of experimental adsorption isotherm data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 2002, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Toth, J. Calculation of the bet-compatible surface area from any type I isotherms measured above the critical temperature. J. Colloid Interface Sci. 2000, 225, 378–383. [Google Scholar] [CrossRef]
- Toth, J. State equations of solid-gas interface layers. Acta Chim. Acad. Sci. Hung. 1971, 69, 311–320. [Google Scholar]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Part II. Models with more than two parameters. J. Hazard. Mater. 2007, 147, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Zafar, M.; Prajapati, J.K.; Kumar, S.; Kannepalli, S. Modeling studies on simultaneous adsorption of phenol and resorcinol onto granular activated carbon from simulated aqueous solution. J. Hazard. Mater. 2011, 185, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto, N.; Hayashi, H.; Miyaura, K. Selective formation of na-x zeolite from coal fly ash by fusion with sodium hydroxide prior to hydrothermal reaction. J. Mater. Sci. 1993, 28, 4781–4786. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Valtchev, V.P.; Bozhilov, K.N. Transmission electron microscopy study of the formation of FAU-type zeolite at room temperature. J. Phys. Chem. B 2004, 108, 15587–15598. [Google Scholar] [CrossRef]
- Tosheva, L.; Brockbank, A.; Mihailova, B.; Sutula, J.; Ludwig, J.; Potgieter, H.; Verran, J. Micron- and nanosized FAU-type zeolites from fly ash for antibacterial applications. J. Mater. Chem. 2012, 22, 16897–16905. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Berkgaut, V.; Singer, A. High capacity cation exchanger by hydrothermal zeolitization of coal fly ash. Appl. Clay Sci. 1996, 10, 369–378. [Google Scholar] [CrossRef]
- Mora-Fonz, M.J.; Catlow, C.R.; Lewis, D.W. H-bond interactions between silicates and water during zeolite pre-nucleation. Phys. Chem. Chem. Phys. 2008, 10, 6571–6578. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Meng, X.; Gao, X.; Xiao, F.S. Solvent-free synthesis of zeolites: Mechanism and utility. Acc. Chem. Res. 2018, 51, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Bushuev, Y.G.; Sastre, G.; de Julián-Ortiz, J.V. The structural directing role of water and hydroxyl groups in the synthesis of beta zeolite polymorphs. J. Phys. Chem. C 2009, 114, 345–356. [Google Scholar] [CrossRef]
- Mahamadi, C.; Almomani, F. On the dominance of Pb during competitive biosorption from multi-metal systems: A review. Cogent Environ. Sci. 2019, 5, 1635335. [Google Scholar] [CrossRef]
- Selim, H.M. Competitive Sorption and Transport of Heavy Metals in Soils and Geological Media; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–404. [Google Scholar]
- Nebaghe, K.C.; El Boundati, Y.; Ziat, K.; Naji, A.; Rghioui, L.; Saidi, M. Comparison of linear and non-linear method for determination of optimum equilibrium isotherm for adsorption of copper(II) onto treated Martil sand. Fluid Phase Equilib. 2016, 430, 188–194. [Google Scholar] [CrossRef]
- Zand, A.D.; Abyaneh, M.R. Adsorption of lead, manganese, and copper onto biochar in landfill leachate: Implication of non-linear regression analysis. Sustain. Environ. Res. 2020, 30, 18. [Google Scholar] [CrossRef]
| MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | MnO | Fe2O3 |
|---|---|---|---|---|---|---|---|
| 3.18 | 15.10 | 43.90 | 0.77 | 0.66 | 0.98 | 0.12 | 5.91 |
| VK Load (g L−1) | Cu (%) | Zn (%) | Pb (%) | Co (%) | Cd (%) |
|---|---|---|---|---|---|
| 5.0 | 16.3 | 10.0 | 40.5 | 10.7 | 17.1 |
| 10.0 | 30.9 | 18.1 | 61.2 | 20.2 | 23.9 |
| 15.0 | 43.6 | 28.2 | 75.9 | 29.6 | 32.4 |
| ZVK-FAU Load (g L−1) | Cu (%) | Zn (%) | Pb (%) | Co (%) | Cd (%) |
|---|---|---|---|---|---|
| 2.5 | 37.7 | 12.5 | 98.1 | 6.7 | 35.7 |
| 5.0 | 74.5 | 33.9 | 99.5 | 20.4 | 71.0 |
| 10.0 | 97.3 | 79.7 | 99.9 | 59.6 | 97.2 |
| 15.0 | 99.4 | 97.0 | 99.9 | 90.5 | 99.7 |
| 20.0 | 99.7 | 99.4 | 100.0 | 97.8 | 99.9 |
| Model | Parameter | Pb | Cu | Cd | Zn | Co |
|---|---|---|---|---|---|---|
| Langmuir | qmax (mg g−1) | 100.153 | 46.712 | 41.924 | n/a | n/a |
| KL (L mg−1) | 1.292 | 0.314 | 0.502 | n/a | n/a | |
| R2 | 0.957 | 0.915 | 0.836 | n/a | n/a | |
| Freundlich | 1/n | 0.349 | 0.154 | 0.135 | n/a | n/a |
| KF | 50.521 | 21.615 | 21.515 | n/a | n/a | |
| R2 | 0.986 | 0.956 | 0.948 | n/a | n/a | |
| Redlich-Peterson | KRP | 425.625 | 37.795 | 72.577 | 3.281 | 1.216 |
| aRP | 7.127 | 1.349 | 2.884 | 0.029 | 0.003 | |
| bRP | 0.731 | 0.896 | 0.896 | 1.356 | 1.659 | |
| R2 | 0.992 | 0.986 | 0.967 | 0.736 | 0.946 | |
| Tóth | KT | 6.734 | 4.599 | 15.123 | n/a | n/a |
| αT | 0.774 | 3.182 | 1.993 | n/a | n/a | |
| t | 14.872 | 10.157 | 2.772 | n/a | n/a | |
| R2 | 0.953 | 0.907 | 0.829 | n/a | n/a | |
| Fritz-Schlunder IV | β1 | 0.3687 | 0.5122 | 0.1351 | 0.0005 | 0.0001 |
| β2 | 1.4019 | 0.4816 | 0.0001 | 1.9657 | 1.8211 | |
| α1 | 51.2060 | 27.5655 | 21.5237 | 20.1108 | 16.8901 | |
| α2 | 0.0046 | 0.5964 | 0.0004 | 0.0000 | 0.0000 | |
| R2 | 0.9877 | 0.9878 | 0.9484 | 0.8110 | 0.9907 |
| Model | Parameters | Pb | Cu | Cd | Zn | Co |
|---|---|---|---|---|---|---|
| Langmuir | qmax (mg g−1) | 111.081 | 46.874 | 52.105 | 34.820 | 27.098 |
| KL (L mg−1) | 3.835 | 1.991 | 6.353 | 3.988 | 3.761 | |
| R2 | 0.977 | 0.904 | 0.941 | 0.891 | 0.988 | |
| Freundlich | 1/n | 0.617 | 0.180 | 0.198 | 0.088 | 0.031 |
| KF | 128.232 | 26.921 | 37.056 | 24.687 | 23.048 | |
| R2 | 0.992 | 0.991 | 0.993 | 0.849 | 0.336 | |
| Redlich-Peterson | KRP | 4228.923 | 738.597 | 1587.369 | 206.628 | 53.863 |
| aRP | 32.316 | 25.990 | 40.492 | 6.878 | 1.464 | |
| bRP | 0.406 | 0.837 | 0.831 | 0.960 | 1.073 | |
| R2 | 0.988 | 0.992 | 0.995 | 0.937 | 0.778 | |
| Tóth | KT | 1.151 | 5.303 | 18.625 | 3.911 | 4.891 |
| αT | 0.268 | 0.769 | 47.504 | 0.211 | 0.140 | |
| t | 96.680 | 9.875 | 0.881 | 8.454 | 4.937 | |
| R2 | 0.973 | 0.817 | 0.985 | 0.772 | 0.114 | |
| Fritz-Schlunder IV | β1 | 0.778 | 0.539 | 0.175 | 0.088 | 0.214 |
| β2 | 0.692 | 0.468 | 0.384 | 1.883 | 0.674 | |
| α1 | 291.082 | 91.213 | 37.403 | 24.695 | 21.775 | |
| α2 | 2.337 | 2.247 | 0.026 | 0.000 | 0.052 | |
| R2 | 0.980 | 0.954 | 0.971 | 0.851 | 0.914 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, I.V.; Tosheva, L.; Miller, G.; Doyle, A.M. FAU-Type Zeolite Synthesis from Clays and Its Use for the Simultaneous Adsorption of Five Divalent Metals from Aqueous Solutions. Materials 2021, 14, 3738. https://doi.org/10.3390/ma14133738
Joseph IV, Tosheva L, Miller G, Doyle AM. FAU-Type Zeolite Synthesis from Clays and Its Use for the Simultaneous Adsorption of Five Divalent Metals from Aqueous Solutions. Materials. 2021; 14(13):3738. https://doi.org/10.3390/ma14133738
Chicago/Turabian StyleJoseph, Ifeoma V., Lubomira Tosheva, Gary Miller, and Aidan M. Doyle. 2021. "FAU-Type Zeolite Synthesis from Clays and Its Use for the Simultaneous Adsorption of Five Divalent Metals from Aqueous Solutions" Materials 14, no. 13: 3738. https://doi.org/10.3390/ma14133738
APA StyleJoseph, I. V., Tosheva, L., Miller, G., & Doyle, A. M. (2021). FAU-Type Zeolite Synthesis from Clays and Its Use for the Simultaneous Adsorption of Five Divalent Metals from Aqueous Solutions. Materials, 14(13), 3738. https://doi.org/10.3390/ma14133738