High Pressure Brillouin Spectroscopy and X-ray Diffraction of Cerium Dioxide
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| PTM | Pressure Transmitting Medium |
References
- Whitfield, C.H.; Brody, E.M.; Bassett, W.A. Elastic moduli of NaCl by Brillouin scattering at high pressure in a diamond anvil cell. Rev. Sci. Instrum. 1976, 47, 942–947. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Jasinski, P.; Suzuki, T.; Anderson, H.U. Nanocrystalline undoped ceria oxygen sensor. Sens. Actuators B Chem. 2003, 95, 73–77. [Google Scholar] [CrossRef]
- Kharton, V.; Figueiredo, F.; Navarro, L.; Naumovich, E.; Kovalevsky, A.; Yaremchenko, A.; Viskup, A.; Carneiro, A.; Marques, F.; Frade, J. Ceria-based materials for solid oxide fuel cells. J. Mater. Sci. 2001, 36, 1105–1117. [Google Scholar] [CrossRef]
- Stennett, M.C.; Corkhill, C.L.; Marshall, L.A.; Hyatt, N.C. Preparation, characterisation and dissolution of a CeO2 analogue for UO2 nuclear fuel. J. Nucl. Mater. 2013, 432, 182–188. [Google Scholar] [CrossRef]
- Watkinson, E.J.; Ambrosi, R.M.; Kramer, D.; Williams, H.R.; Reece, M.; Chen, K.; Sarsfield, M.; Barklay, C.; Fenwick, H.; Weston, D.P.; et al. Sintering trials of analogues of americium oxides for radioisotope power systems. J. Nucl. Mater. 2017, 491, 18–30. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Bailey, D.J.; Tocino, F.Y.; Stennett, M.C.; Miller, J.A.; Provis, J.L.; Travis, K.P.; Hyatt, N.C. Role of microstructure and surface defects on the dissolution kinetics of CeO2, a UO2 fuel analogue. ACS Appl. Mater. Interfaces 2016, 8, 10562–10571. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.A.; McKinley, I.G.; Hill, M.D. The Geological Disposal of Nuclear Waste; John Wiley and Sons: Hoboken, NJ, USA, 1987. [Google Scholar]
- Duclos, S.J.; Vohra, Y.K.; Ruoff, A.L.; Jayaraman, A.; Espinosa, G. High-pressure x-ray diffraction study of CeO2 to 70 GPa and pressure-induced phase transformation from the fluorite structure. Phys. Rev. B 1988, 38, 7755. [Google Scholar] [CrossRef]
- Gerward, L.; Olsen, J.S. Powder diffraction analysis of cerium dioxide at high pressure. Powder Diffr. 1993, 8, 127–129. [Google Scholar] [CrossRef]
- Kourouklis, G.; Jayaraman, A.; Espinosa, G. High-pressure Raman study of CeO2 to 35 GPa and pressure-induced phase transformation from the fluorite structure. Phys. Rev. B 1988, 37, 4250. [Google Scholar] [CrossRef]
- Rekhi, S.; Saxena, S.; Lazor, P. High-pressure Raman study on nanocrystalline CeO2. J. Appl. Phys. 2001, 89, 2968–2971. [Google Scholar] [CrossRef]
- Liu, L.; Song, H.X.; Wang, Z.; Geng, H.Y.; Jing, Q.; Zhang, Y.; Liu, S.; Xiang, S.; Bi, Y.; Xu, J.; et al. Strength and equation of state of fluorite phase CeO2 under high pressure. J. Appl. Phys. 2012, 112, 013532. [Google Scholar] [CrossRef]
- Gerward, L.; Olsen, J.S.; Petit, L.; Vaitheeswaran, G.; Kanchana, V.; Svane, A. Bulk modulus of CeO2 and PrO2: An experimental and theoretical study. J. Alloys Compd. 2005, 400, 56–61. [Google Scholar] [CrossRef]
- Singh, A.K. The lattice strains in a specimen (cubic system) compressed nonhydrostatically in an opposed anvil device. J. Appl. Phys. 1993, 73, 4278–4286. [Google Scholar] [CrossRef]
- Jeanloz, R.; Godwal, B. Deriving equations of state from non-hydrostatic data. J. Phys. Conf. Ser. IOP Publ. 2012, 377, 012032. [Google Scholar] [CrossRef]
- Klotz, S.; Chervin, J.; Munsch, P.; Le Marchand, G. Hydrostatic limits of 11 pressure transmitting media. J. Phys. D Appl. Phys. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Wang, Q.; He, D.; Peng, F.; Lei, L.; Liu, P.; Yin, S.; Wang, P.; Xu, C.; Liu, J. Unusual compression behavior of nanocrystalline CeO2. Sci. Rep. 2014, 4, 4441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Schiferl, D.; Zha, C.; Downs, R.T. Pressure induced increase of particle size and resulting weakening of elastic stiffness of CeO2 nanocrystals. Appl. Phys. Lett. 2004, 85, 124–126. [Google Scholar] [CrossRef]
- Ge, M.; Fang, Y.; Wang, H.; Chen, W.; He, Y.; Liu, E.; Su, N.; Stahl, K.; Feng, Y.; Tse, J.; et al. Anomalous compressive behavior in CeO2 nanocubes under high pressure. New J. Phys. 2008, 10, 123016. [Google Scholar] [CrossRef]
- Liu, B.; Yao, M.; Liu, B.; Li, Z.; Liu, R.; Li, Q.; Li, D.; Zou, B.; Cui, T.; Zou, G.; et al. High-pressure studies on CeO2 nano-octahedrons with a (111)-terminated surface. J. Phys. Chem. C 2011, 115, 4546–4551. [Google Scholar] [CrossRef]
- Born, M. On the stability of crystal lattices. I. In Mathematical Proceedings of the Cambridge Philosophical Society; Cambridge University Press: Cambridge, UK, 1940; Volume 36, pp. 160–172. [Google Scholar]
- Nakajima, A.; Yoshihara, A.; Ishigame, M. Defect-induced Raman spectra in doped CeO2. Phys. Rev. B 1994, 50, 13297. [Google Scholar] [CrossRef] [PubMed]
- Dekker, A.J. The Specific Heat of Solids and Lattice Vibrations. In Solid State Physics; Macmillan Education UK: London, UK, 1981; pp. 32–59. [Google Scholar] [CrossRef]
- Gaskell, D.R.; Laughlin, D.E. Introduction to the Thermodynamics of Materials; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Mehrotra, S.; Sharma, P.; Rajagopalan, M.; Bandyopadhyay, A. High pressure phase transition and band structures of different phases in CeO2. Solid State Commun. 2006, 140, 313–317. [Google Scholar] [CrossRef]
- Skorodumova, N.; Ahuja, R.; Simak, S.; Abrikosov, I.; Johansson, B.; Lundqvist, B. Electronic, bonding, and optical properties of CeO2 and Ce2O3 from first principles. Phys. Rev. B 2001, 64, 115108. [Google Scholar] [CrossRef]
- Kanchana, V.; Vaitheeswaran, G.; Svane, A.; Delin, A. First-principles study of elastic properties of CeO2, ThO2 and PoO2. J. Phys. Condens. Matter 2006, 18, 9615. [Google Scholar] [CrossRef]
- Fabris, S.; de Gironcoli, S.; Baroni, S.; Vicario, G.; Balducci, G. Taming multiple valency with density functionals: A case study of defective ceria. Phys. Rev. B 2005, 71, 041102. [Google Scholar] [CrossRef]
- Gürel, T.; Eryiğit, R. Ab initio pressure-dependent vibrational and dielectric properties of CeO2. Phys. Rev. B 2006, 74, 014302. [Google Scholar] [CrossRef]
- Gleason, A.; Marquardt, H.; Chen, B.; Speziale, S.; Wu, J.; Jeanloz, R. Anomalous sound velocities in polycrystalline MgO under non-hydrostatic compression. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef][Green Version]
- Marquardt, H.; Speziale, S.; Marquardt, K.; Reichmann, H.; Konôpková, Z.; Morgenroth, W.; Liermann, H.P. The effect of crystallite size and stress condition on the equation of state of nanocrystalline MgO. J. Appl. Phys. 2011, 110, 113512. [Google Scholar] [CrossRef]
- Jimlim, P.; Bovornratanaraks, T.; Chaimayo, W.; Pratontep, S. Effect of Nano Particle Sizes on High Pressure Raman Scattering in Nanocrystalline Cerium Dioxide. Mod. Phys. Lett. B 2011, 25, 2399–2405. [Google Scholar] [CrossRef]
- Dogra, S.; Sharma, N.D.; Singh, J.; Poswal, H.K.; Sharma, S.M.; Bandyopadhyay, A.K. High pressure behavior of nano-crystalline CeO2 up to 35 GPa: A Raman investigation. High Press. Res. 2011, 31, 292–303. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.; Li, G.; Lu, J.; Wu, Q.; Gu, Y. Stress measurement for nonstoichiometric ceria films based on Raman spectroscopy. J. Alloys Compd. 2016, 682, 132–137. [Google Scholar] [CrossRef]
- Weck, P.F.; Cochrane, K.R.; Root, S.; Lane, J.M.D.; Shulenburger, L.; Carpenter, J.H.; Sjostrom, T.; Mattsson, T.R.; Vogler, T.J. Shock compression of strongly correlated oxides: A liquid-regime equation of state for cerium(IV) oxide. Phys. Rev. B 2018, 97, 125106. [Google Scholar] [CrossRef]
- Lang, J.M., Jr.; Voorhees, T.J.; Steiner, J.W.; Goodbody, A.B.; Bartram, B.D. Elastic limits of near-solid CeO2 to 25 GPa. AIP Conf. Proc. 2020, 2272, 100017. [Google Scholar]
- Frost, M.; Curry, C.; Glenzer, S. Laser cutting apparatus for high energy density and diamond anvil cell science. J. Instrum. 2020, 15, P05004. [Google Scholar] [CrossRef]
- Dewaele, A.; Torrent, M.; Loubeyre, P.; Mezouar, M. Compression curves of transition metals in the Mbar range: Experiments and projector augmented-wave calculations. Phys. Rev. B 2008, 78, 104102. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P.; Mezouar, M. Equations of state of six metals above 94 GPa. Phys. Rev. B 2004, 70, 094112. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Für-Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Sinogeikin, S.; Bass, J.; Prakapenka, V.; Lakshtanov, D.; Shen, G.; Sanchez-Valle, C.; Rivers, M. Brillouin spectrometer interfaced with synchrotron radiation for simultaneous X-ray density and acoustic velocity measurements. Rev. Sci. Instrum. 2006, 77, 103905. [Google Scholar] [CrossRef]
- Chen, B.; Gleason, A.; Yan, J.; Koski, K.; Clark, S.; Jeanloz, R. Elasticity, strength, and refractive index of argon at high pressures. Phys. Rev. B 2010, 81, 144110. [Google Scholar] [CrossRef]
- Rodenbough, P.P.; Song, J.; Walker, D.; Clark, S.M.; Kalkan, B.; Chan, S.W. Size dependent compressibility of nano-ceria: Minimum near 33 nm. Appl. Phys. Lett. 2015, 106, 163101. [Google Scholar] [CrossRef]
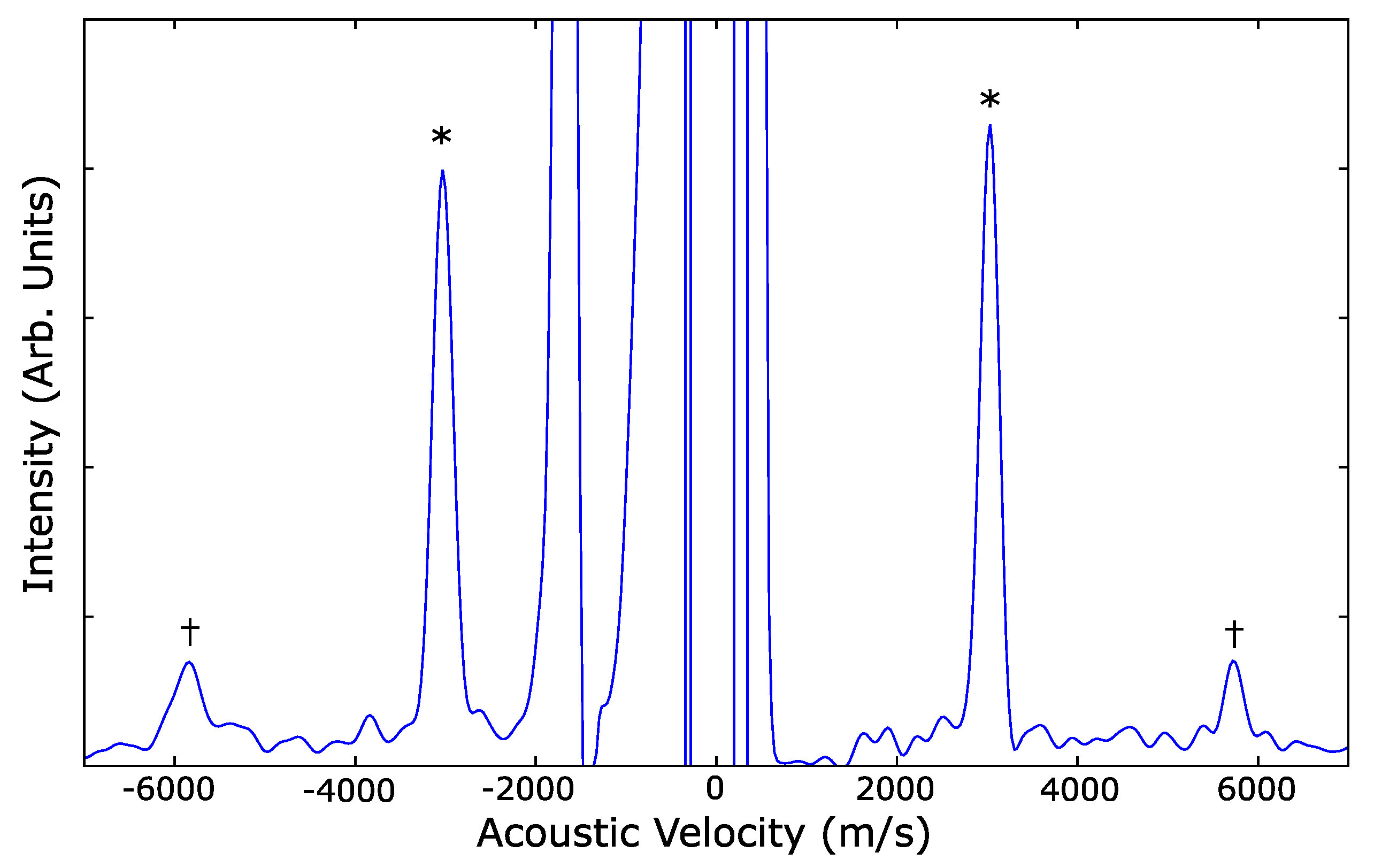
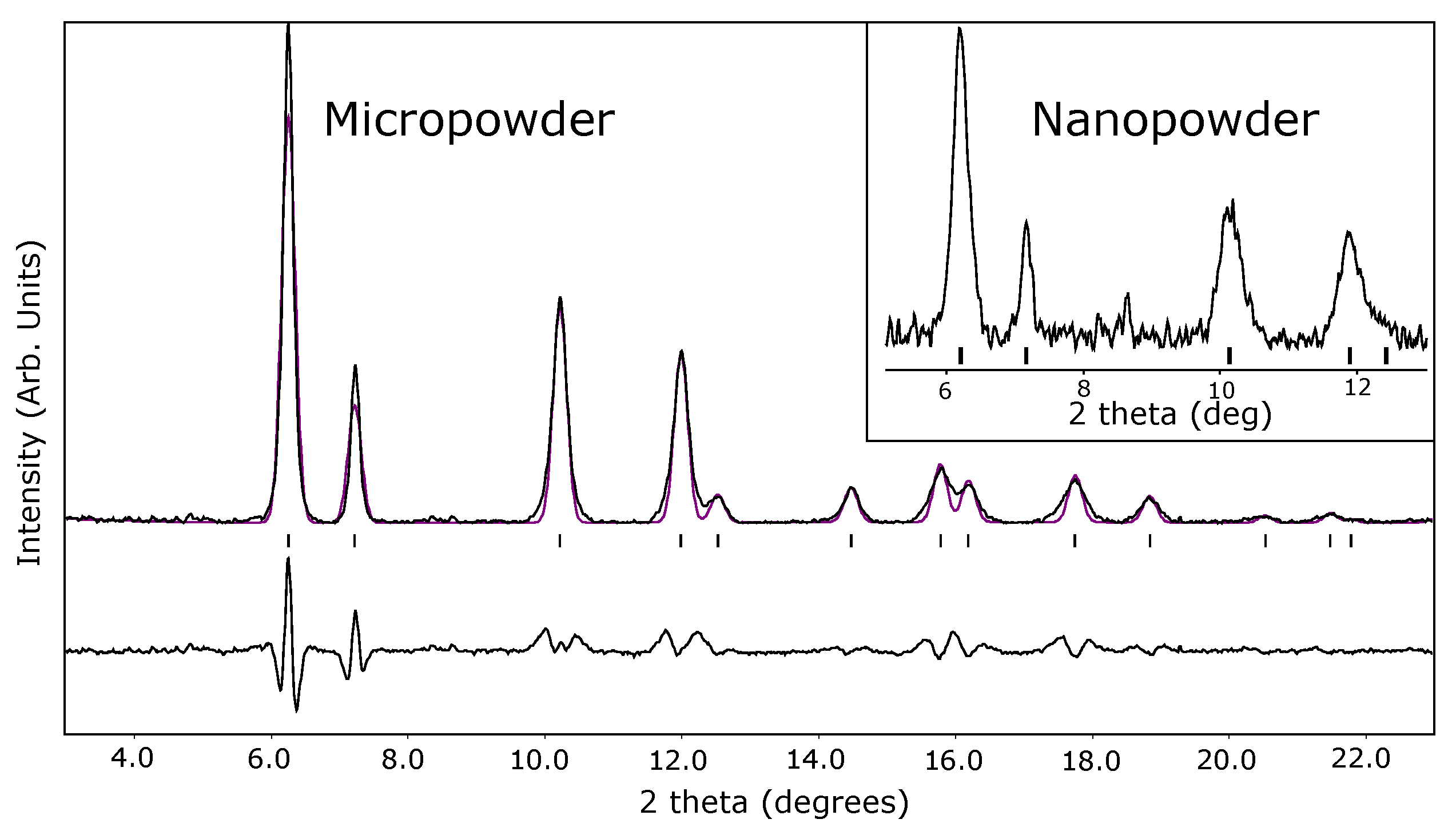
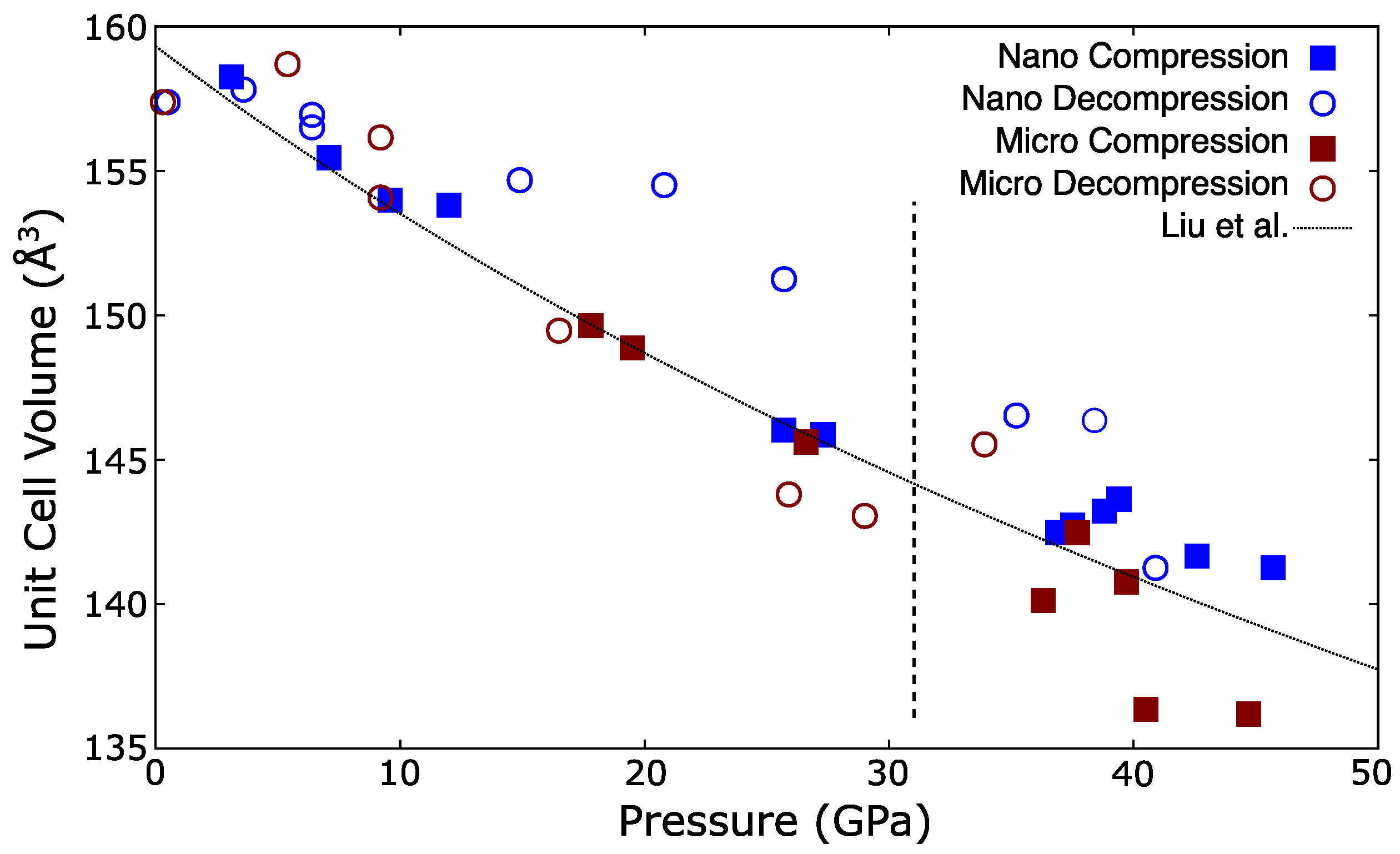

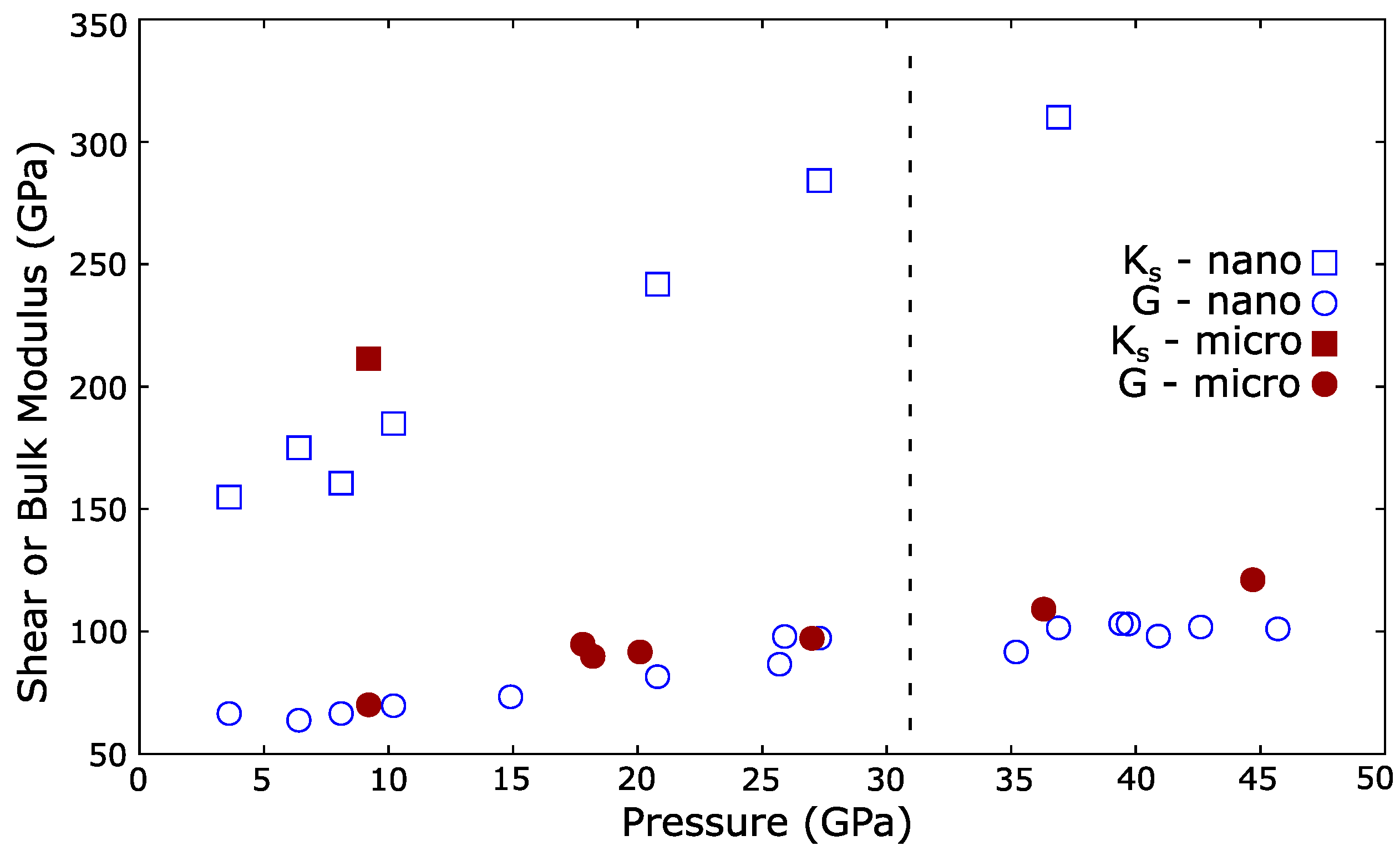
| Ref. | PTM | Grain Size | (GPa) | P (GPa) | EoS Form | |
|---|---|---|---|---|---|---|
| Duclos [9] | None | Not Specified | 230 (10) | 4 (fixed) | 38 | Birch |
| Gerward [14] | 16:3:1 MeOH:EtOH:HO | ‘Finely Ground’ | 220 (9) | 4.4 (4) | 20 | BM |
| Liu 2012 [13] | None (with corrections) | 5 m | 235 (18) | 3.67 | 27 | Vinet |
| Liu 2012 [13] | None (no corrections) | 5 m | 248 (52) | 4.56 | 27 | Vinet |
| Liu 2011 [21] | 4:1 MeOH:EtOH | 150 nm | 260 (10) | 4 (fixed) | 55 | Vinet |
| Wang 2014 [18] | Various | 12 nm | 287 (5) | 4 (fixed) | 16 | BM |
| Wang 2004 [19] | None | 10 nm | 328 (12) | 4 (fixed) | 20 | BM |
| Ge [20] | 16:3:1 MeOH:EtOH:HO | 4.7 nm | 230 | 28 |
| Ref. | Method | (GPa) | EoS Form | |
|---|---|---|---|---|
| Gerward [14] | LDA | 176.9 | BM | |
| Mehrotra [26] | LDA | 236 | Birch | |
| Skorodumova [27] | LDA | 214.7 | ||
| Skorodumova [27] | GGA | 187.7 | ||
| Gurel [30] | LDA | 210.1 | 4.4 | Vinet |
| Kanchana [28] | LDA | 218 | 4.2 | Birch |
| Kanchana [28] | GGA | 184 | 4.2 | Birch |
| Fabris [29] | LDA | 210.7 | ||
| Fabris [29] | GGA | 178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frost, M.; Lazarz, J.D.; Levitan, A.L.; Prakapenka, V.B.; Sun, P.; Tkachev, S.N.; Yang, H.; Glenzer, S.H.; Gleason, A.E. High Pressure Brillouin Spectroscopy and X-ray Diffraction of Cerium Dioxide. Materials 2021, 14, 3683. https://doi.org/10.3390/ma14133683
Frost M, Lazarz JD, Levitan AL, Prakapenka VB, Sun P, Tkachev SN, Yang H, Glenzer SH, Gleason AE. High Pressure Brillouin Spectroscopy and X-ray Diffraction of Cerium Dioxide. Materials. 2021; 14(13):3683. https://doi.org/10.3390/ma14133683
Chicago/Turabian StyleFrost, Mungo, John D. Lazarz, Abraham L. Levitan, Vitali B. Prakapenka, Peihao Sun, Sergey N. Tkachev, Hong Yang, Siegfried H. Glenzer, and Arianna E. Gleason. 2021. "High Pressure Brillouin Spectroscopy and X-ray Diffraction of Cerium Dioxide" Materials 14, no. 13: 3683. https://doi.org/10.3390/ma14133683
APA StyleFrost, M., Lazarz, J. D., Levitan, A. L., Prakapenka, V. B., Sun, P., Tkachev, S. N., Yang, H., Glenzer, S. H., & Gleason, A. E. (2021). High Pressure Brillouin Spectroscopy and X-ray Diffraction of Cerium Dioxide. Materials, 14(13), 3683. https://doi.org/10.3390/ma14133683







