Electrochemical Oscillation during Galvanostatic Charging of LiCrTiO4 in Li-Ion Batteries
Abstract
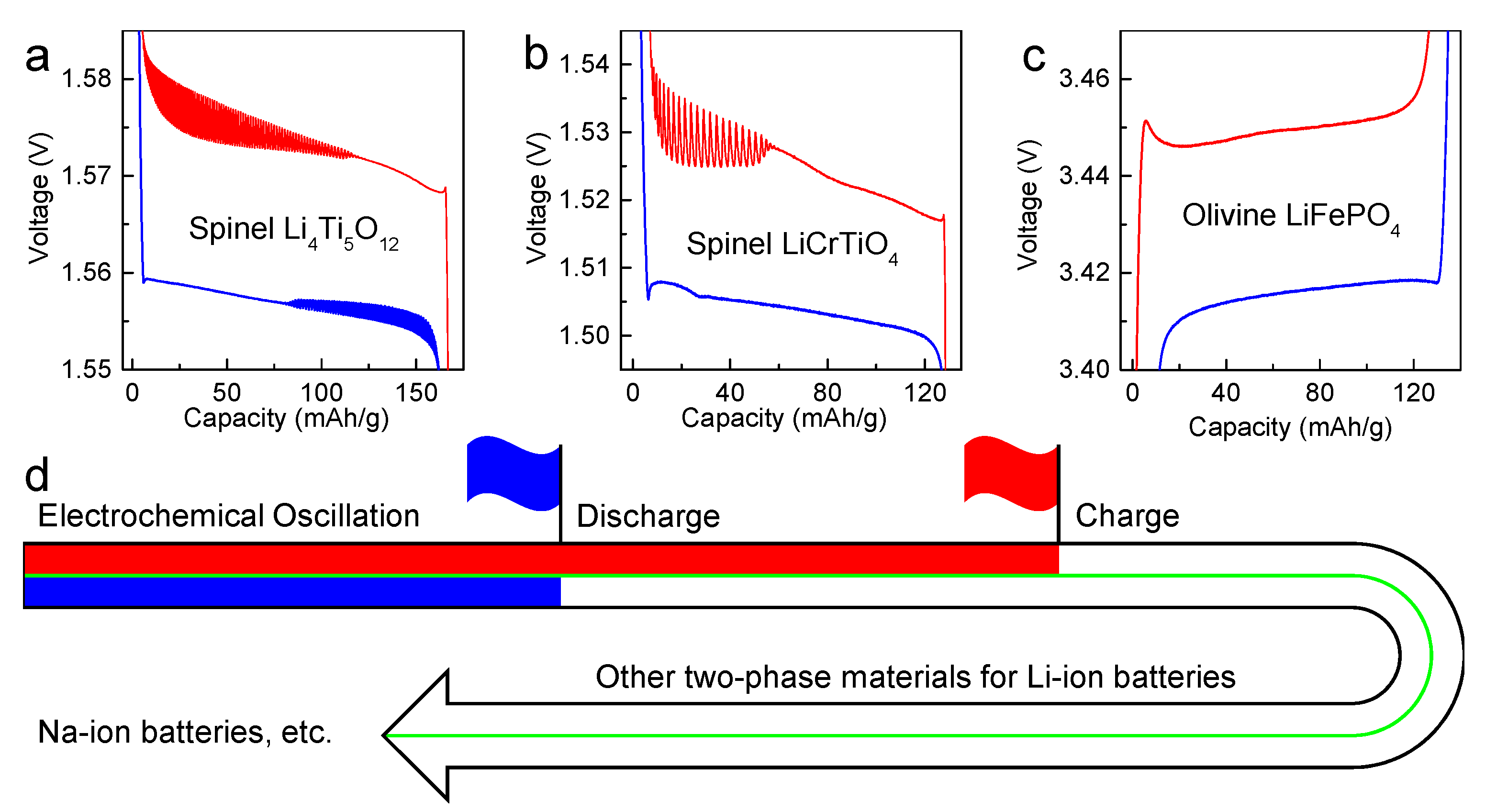
:1. Introduction
2. Experimental Section
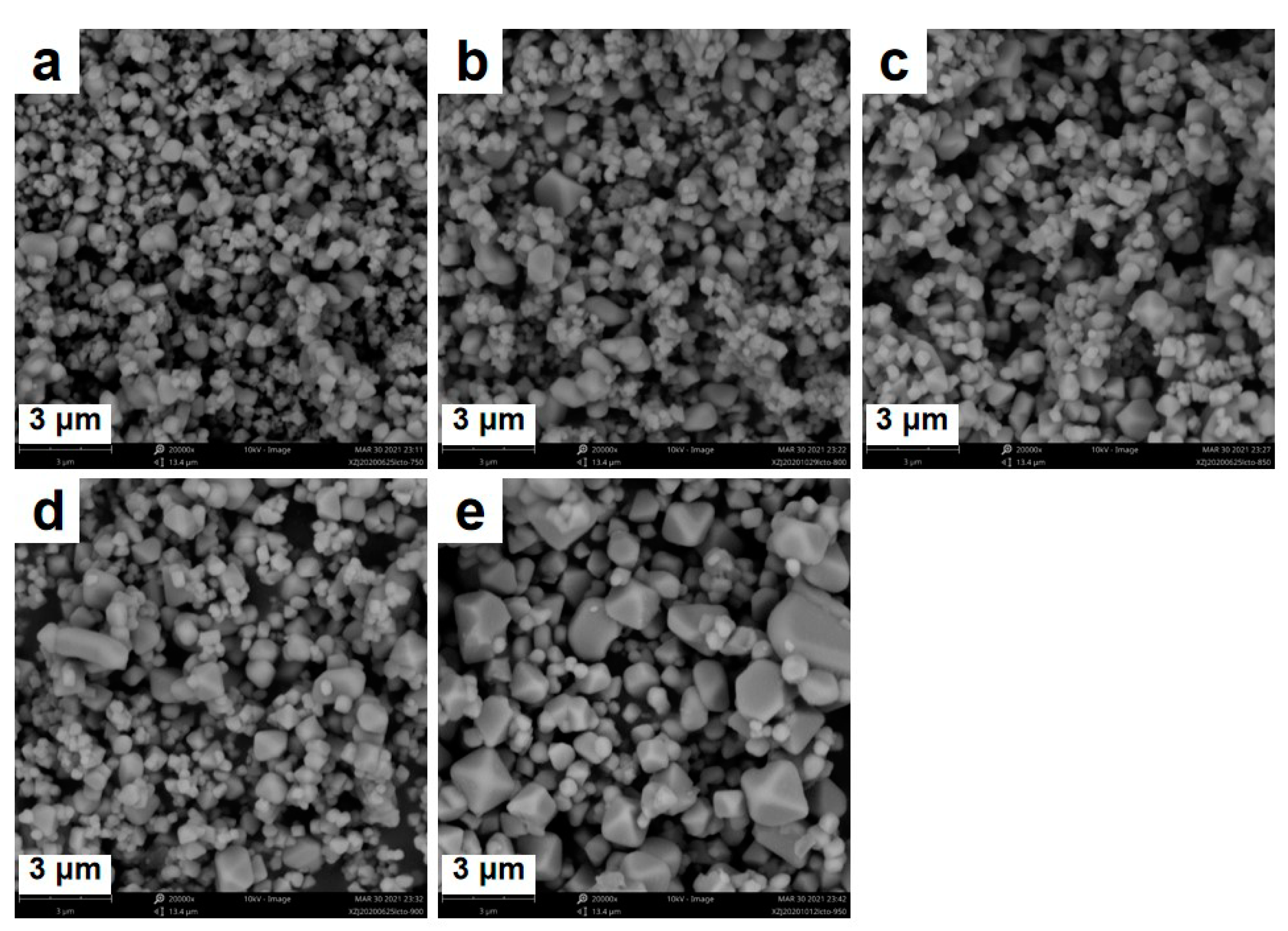
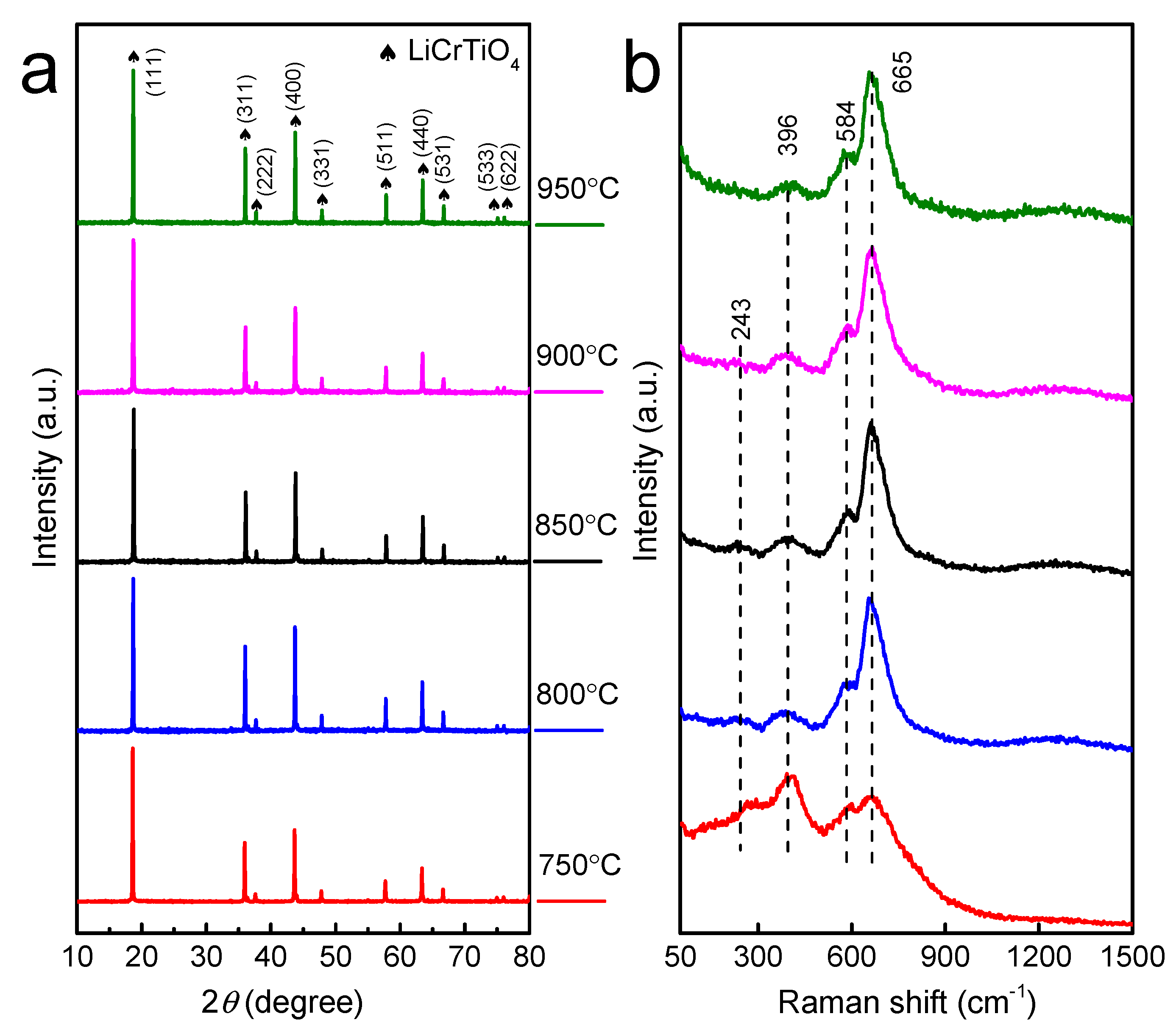
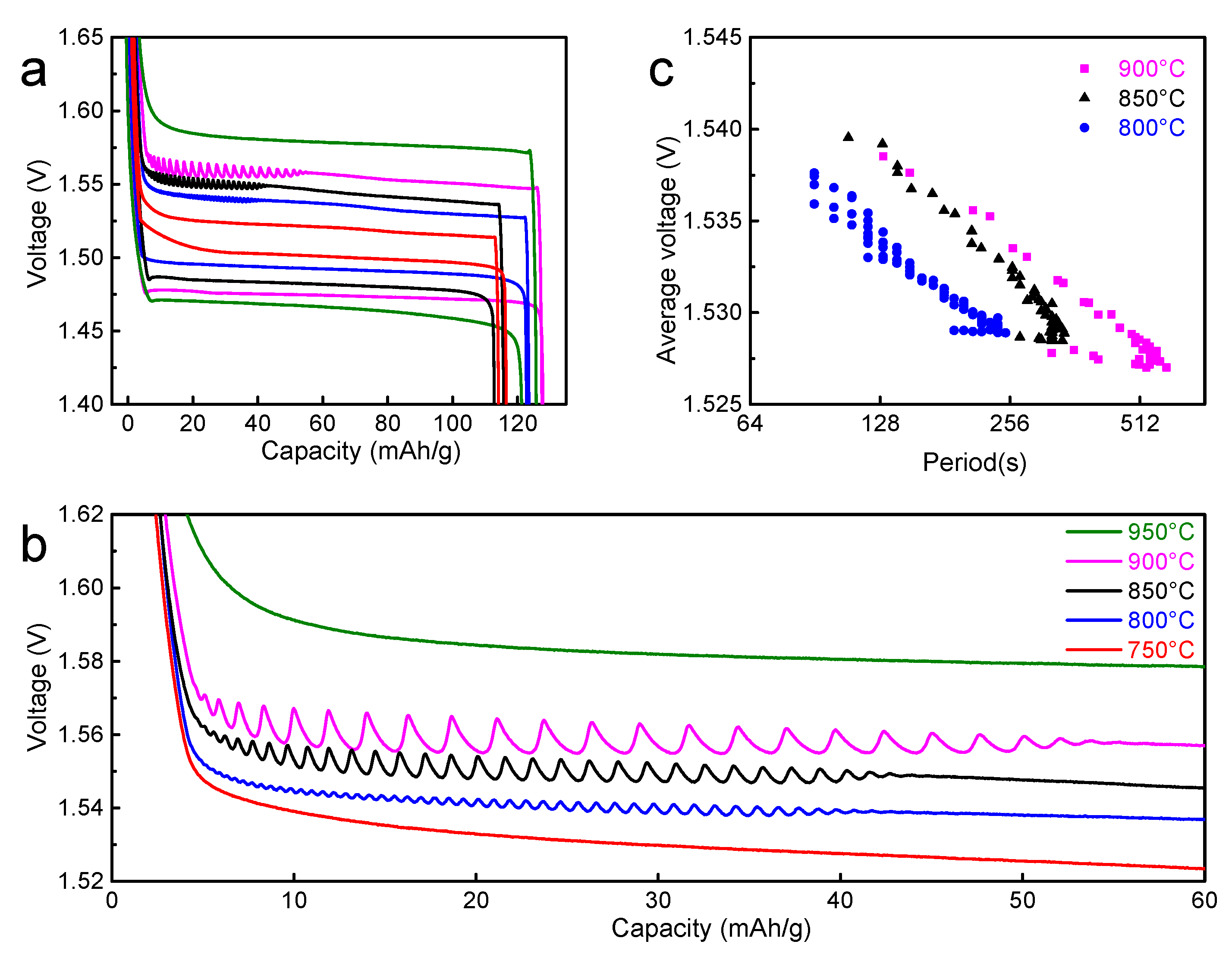
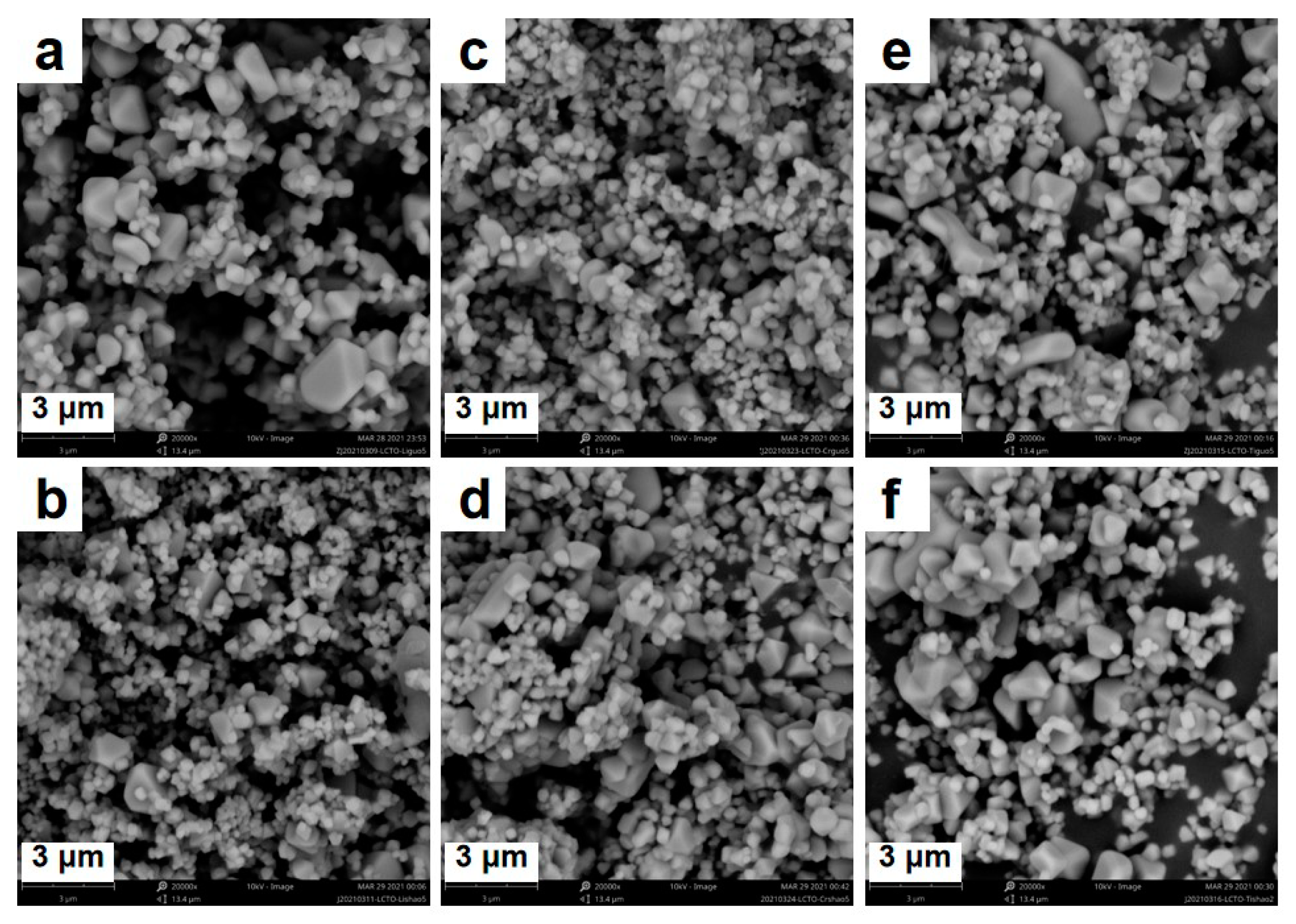
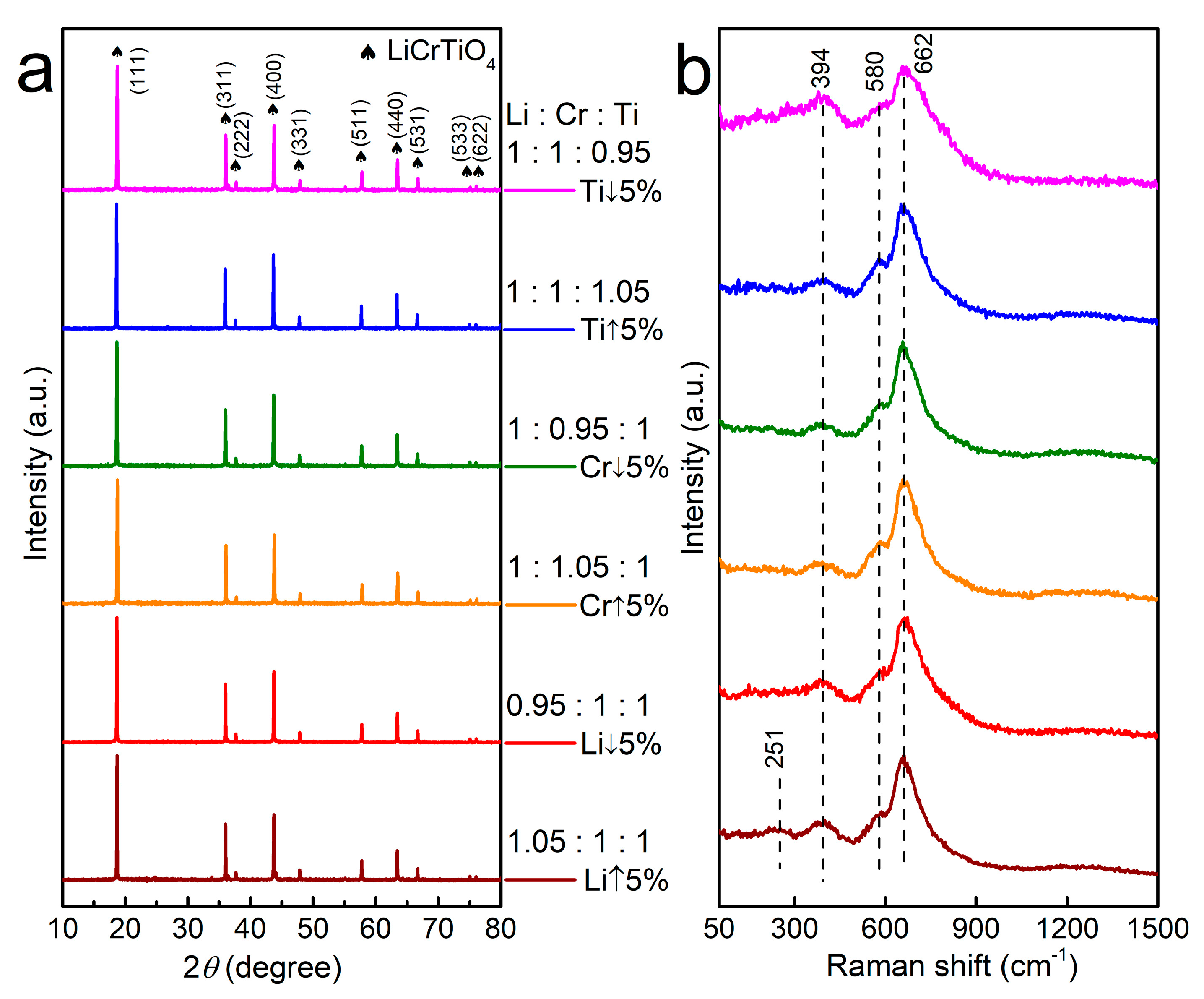
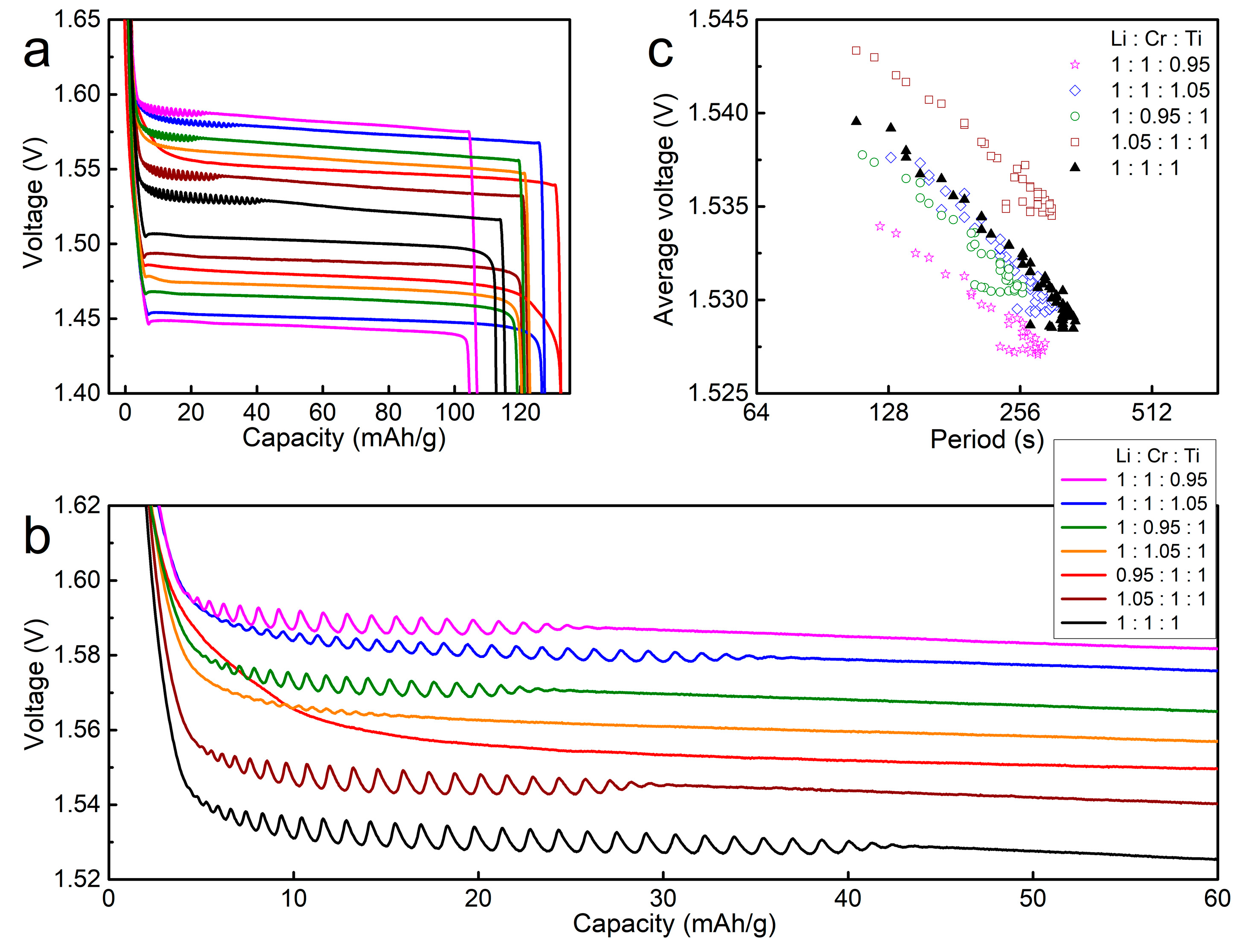
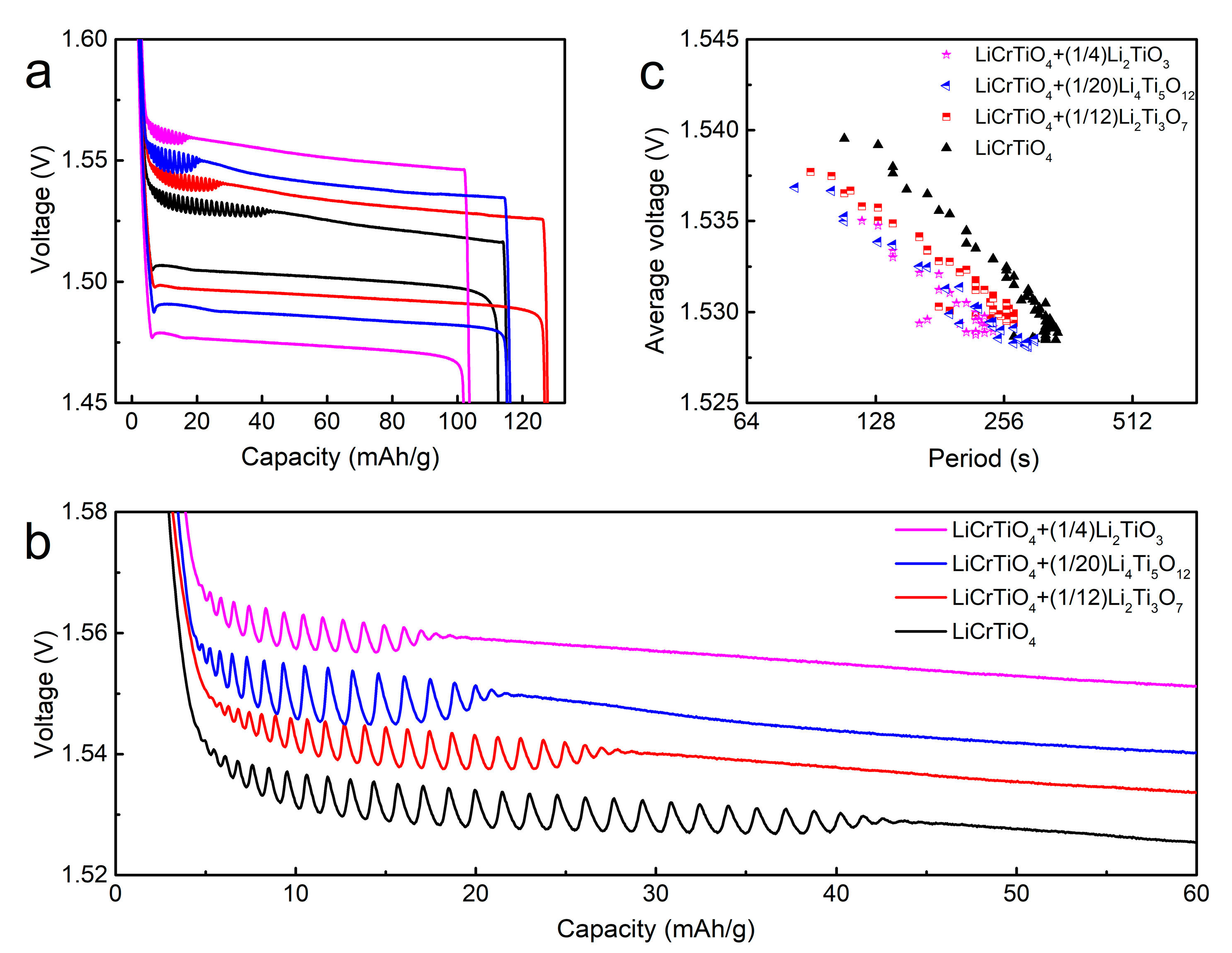
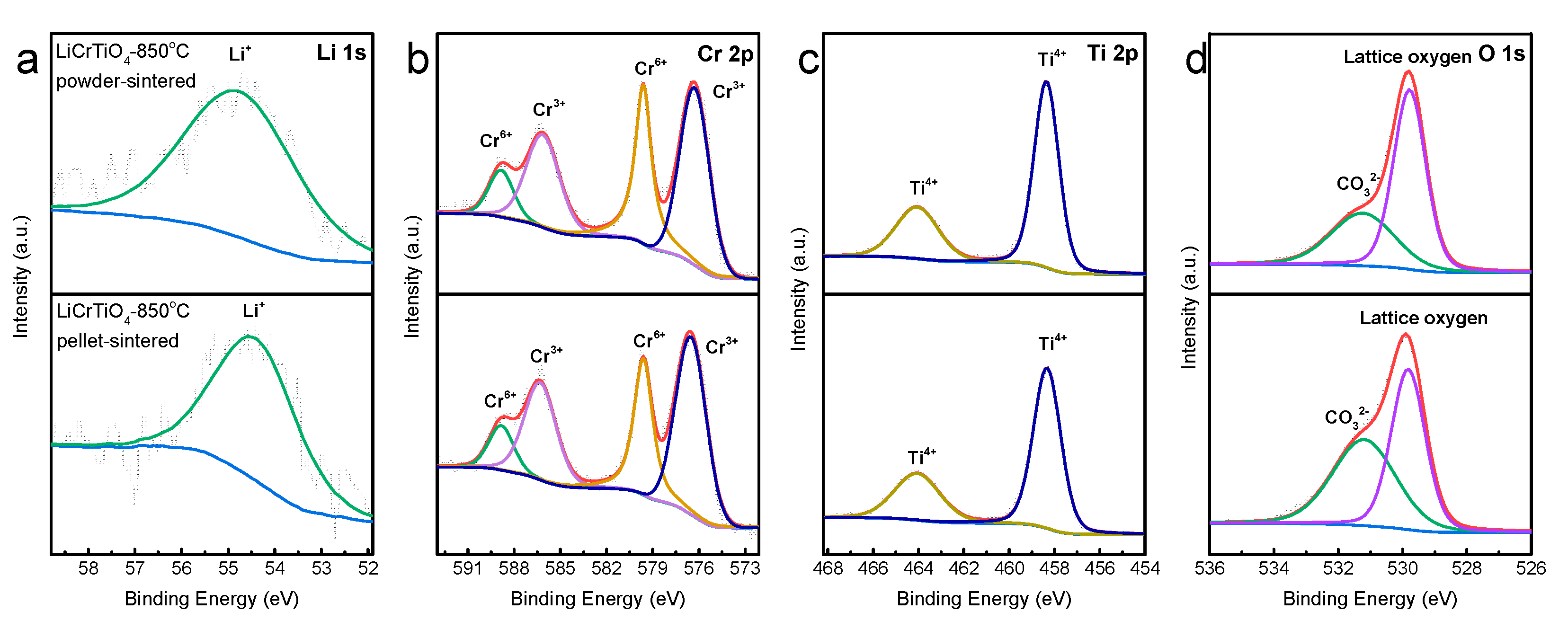
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eddahech, A.; Briat, O.; Vinassa, J.-M. Lithium-ion battery performance improvement based on capacity recovery exploitation. Electrochim. Acta 2013, 114, 750–757. [Google Scholar] [CrossRef]
- Mulder, G.; Omar, N.; Pauwels, S.; Meeus, M.; Leemans, F.; Verbrugge, B.; Nijs, W.D.; Van den Bossche, P.; Six, D.; Van Mierlo, J. Comparison of commercial battery cells in relation to material properties. Electrochim. Acta 2013, 87, 473–488. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y.; Ren, Y.; Lu, W.; Orendorff, C.; Roth, E.P.; Amine, K. Multi-scale study of thermal stability of lithiated graphite. Energy Environ. Sci. 2011, 4, 4023–4030. [Google Scholar] [CrossRef]
- Guo, B.; Wang, X.; Fulvio, P.F.; Chi, M.; Mahurin, S.M.; Sun, X.G.; Dai, S. Soft-templated mesoporous carbon-carbon nanotube composites for high performance lithium-ion batteries. Adv. Mater. 2011, 23, 4661–4666. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Mao, J. Improve the low-temperature electrochemical performance of Li4Ti5O12 anode materials by ion doping. J. Mater. Sci. Mater. Electron. 2020, 31, 21444–21454. [Google Scholar] [CrossRef]
- Jung, H.-G.; Myung, S.-T.; Yoon, C.S.; Son, S.-B.; Oh, K.H.; Amine, K.; Scrosati, B.; Sun, Y.-K. Microscale spherical carbon-coated Li4Ti5O12 as ultra high power anode material for lithium batteries. Energy Environ. Sci. 2011, 4, 1345–1351. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Y.S.; Li, H.; Wang, Z.; Chen, L. Porous Li4Ti5 O12 coated with N-doped carbon from ionic liquids for Li-ion batteries. Adv. Mater. 2011, 23, 1385–1388. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Li, Y.; Sun, S.; Liu, Y.; Luo, J.; Han, J.; Huang, Y. Al doping effects on LiCrTiO4 as an anode for lithium-ion batteries. RSC Adv. 2017, 7, 4791–4797. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.L.; Jow, T.R.; Wolfenstine, J. Low temperature performance of nanophase Li4Ti5O12. J. Power Sources 2006, 159, 1340–1345. [Google Scholar] [CrossRef]
- Arun, N.; Jain, A.; Aravindan, V.; Jayaraman, S.; Chui Ling, W.; Srinivasan, M.P.; Madhavi, S. Nanostructured spinel LiNi0.5 Mn1.5 O4 as new insertion anode for advanced Li-ion capacitors with high power capability. Nano Energy 2015, 12, 69–75. [Google Scholar] [CrossRef]
- Bai, P.; Cogswell, D.A.; Bazant, M.Z. Suppression of phase separation in LiFePO(4) nanoparticles during battery discharge. Nano Lett. 2011, 11, 4890–4896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; He, J.; Song, X.; Huang, X.; Yu, X.; Fang, Y.; Chen, D. Bamboo carbon assisted sol–gel synthesis of Li4Ti5O12 anode material with enhanced electrochemical activity for lithium ion battery. J. Alloys Compd. 2015, 621, 268–273. [Google Scholar] [CrossRef]
- Gurzęda, B.; Krawczyk, P. Potential oscillations affected by the electrochemical overoxidation of graphite in aqueous nitric acid. Electrochim. Acta 2018, 267, 102–109. [Google Scholar] [CrossRef]
- Ozkan, S.; Mazare, A.; Schmuki, P. Self-induced current oscillations during anodization of Ti in LA containing DMSO electrolyte. Electrochem. Commun. 2016, 65, 18–22. [Google Scholar] [CrossRef]
- Spendelow, J.S.; Wieckowski, A. Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media. Phys. Chem. Chem. Phys. 2007, 9, 2654–2675. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Hashimoto, K.; Nakanishi, S. Acceleration effect of adsorbed thiocyanate ions on electrodeposition of CuSCN, causing spontaneous electrochemical oscillation. Chem. Phys. Lett. 2012, 530, 77–80. [Google Scholar] [CrossRef]
- Kuběna, J.; Kuběna, A.; Caha, O.; Mikulík, P. Development of oxide precipitates in silicon: Calculation of the distribution function of the classical theory of nucleation by a nodal-points approximation. J. Phys. Condens. Matter. 2007, 19, 496202. [Google Scholar] [CrossRef]
- Sugiura, H.; Ito, M.; Okuaki, T.; Mori, Y.; Kitahata, H.; Takinoue, M. Pulse-density modulation control of chemical oscillation far from equilibrium in a droplet open-reactor system. Nat. Commun. 2016, 7, 10212. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Liu, Z.; Zhang, X.; Yang, L.; Chang, J.; Tao, C. Electrochemical oscillation on anode regulated by sodium oleate in electrolytic metal manganese. J. Electroanal. Chem. 2019, 845, 13–21. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, L.; Zhao, H.; Gao, S.; Lv, Y.; Kong, L.; Ma, J.; Jia, D. Hybrid porous bamboo-like CNTs embedding ultrasmall LiCrTiO4 nanoparticles as high rate and long life anode materials for lithium ion batteries. Chem. Commun. 2017, 53, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Díaz-Carrasco, P.; Arroyo y de Dompablo, M.E.; García-Alvarado, F. On the synthesis of ramsdellite LiTiMO4 (M = Ti, V, Cr, Mn, Fe): An experimental and computational study of the spinel–ramsdellite transformation. Eur. J. Inorg. Chem. 2007, 2007, 3375–3384. [Google Scholar] [CrossRef]
- Kuhn, A.; Martín, M.; García-Alvarado, F. Synthesis, structure and electrochemical lithium intercalation chemistry of ramsdellite-type LiCrTiO4. Z. Anorg. Allg. Chem. 2008, 634, 880–886. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, Q.; Wu, L.; Lei, G.; Li, Z. Spinel LiCrTiO4 fibers as an advanced anode material in high performance lithium ion batteries. Solid State Ion. 2013, 236, 43–47. [Google Scholar] [CrossRef]
- Yang, J.; Yan, B.; Ye, J.; Li, X.; Liu, Y.; You, H. Carbon-coated LiCrTiO4 electrode material promoting phase transition to reduce asymmetric polarization for lithium-ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 2882–2891. [Google Scholar] [CrossRef]
- Aravindan, V.; Ling, W.C.; Madhavi, S. LiCrTiO(4): A high-performance insertion anode for lithium-ion batteries. ChemPhysChem 2012, 13, 3263–3266. [Google Scholar] [CrossRef]
- Luo, M.; Yu, H.; Cheng, X.; Zhu, H.; Ye, W.; Yan, L.; Qian, S.; Shui, M.; Shu, J. LiCrTiO4 Nanowires with the (111) peak evolution during cycling for high-performance lithium ion battery anodes. ACS Sustain. Chem. Eng. 2017, 5, 10580–10587. [Google Scholar] [CrossRef]
- Feng, X.; Shen, C.; Ding, N.; Chen, C. Lithium chromium oxide modified spinel LiCrTiO4 with improved electrochemical properties. J. Mater. Chem. 2012, 22, 20861–20865. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, L.; Bie, X.; Chen, H.; Wang, C.; Du, F.; Chen, G. Exploration of spinel LiCrTiO4 as cathode material for rechargeable Mg-Li hybrid batteries. Chemistry 2017, 23, 17935–17939. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-N.; Chen, L.; Wang, Y.-G.; Wang, C.-X.; Che, R.-C.; Xia, Y.-Y. Binary Li4Ti5O12-Li2Ti3O7 nanocomposite as an anode material for Li-Ion batteries. Adv. Funct. Mater. 2013, 23, 640–647. [Google Scholar] [CrossRef]
- Charles-Blin, Y.; Flahaut, D.; Ledeuil, J.-B.; Guérin, K.; Dubois, M.; Deschamps, M.; Perbost, A.-M.; Monconduit, L.; Martinez, H.; Louvain, N. Atomic layer fluorination of the Li4Ti5O12 surface: A multiprobing survey. ACS Appl. Energy Mater. 2019, 2, 6681–6692. [Google Scholar] [CrossRef]
- Tian, H. Synthesis and electrochemical properties of spinel LiCrTiO4 and its application in LiFePO4/LiCrTiO4 full cells. Int. J. Electrochem. Sci. 2017, 6980–6989. [Google Scholar] [CrossRef]
- Yu, H.; Qian, S.; Yan, L.; Li, P.; Lin, X.; Luo, M.; Long, N.; Shui, M.; Shu, J. Observation of the lithium storage behavior in LiCrTiO4 via in-situ and ex-situ techniques. Electrochim. Acta 2016, 212, 84–94. [Google Scholar] [CrossRef]
- Zhang, W.; Song, S.; Nath, M.; Xue, Z.; Ma, G.; Li, Y. Inhibition of Cr6+ by the formation of in-situ Cr3+ containing solid-solution in Al2O3–CaO–Cr2O3–SiO2 system. Ceram. Int. 2021, 47, 9578–9584. [Google Scholar] [CrossRef]
- Yuan, L.; Weng, X.; Zhou, M.; Zhang, Q.; Deng, L. Structural and visible-near infrared optical properties of Cr-doped TiO2 for colored cool pigments. Nanoscale Res. Lett. 2017, 12, 597. [Google Scholar] [CrossRef] [Green Version]
- Almaev, A.V.; Kushnarev, B.O.; Chernikov, E.V.; Novikov, V.A.; Korusenko, P.M.; Nesov, S.N. Structural, electrical and gas-sensitive properties of Cr2O3 thin films. Superlattices Microstruct. 2021, 151, 106835. [Google Scholar] [CrossRef]
- Orliukas, A.F.; Fung, K.Z.; Venckutė, V.; Kazlauskienė, V.; Miškinis, J.; Lelis, M. Structure, surface and broadband impedance spectroscopy of Li4Ti5O12 based ceramics with Nb and Ta. Solid State Ion. 2015, 271, 34–41. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, L.; Li, F.; Cheng, H.-M. Nanosized Li4Ti5O12/graphene hybrid materials with low polarization for high rate lithium ion batteries. J. Power Sources 2011, 196, 8610–8617. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Hu, F.; Li, D.; Chen, Y. Electrochemical Oscillation during Galvanostatic Charging of LiCrTiO4 in Li-Ion Batteries. Materials 2021, 14, 3624. https://doi.org/10.3390/ma14133624
Xu Z, Hu F, Li D, Chen Y. Electrochemical Oscillation during Galvanostatic Charging of LiCrTiO4 in Li-Ion Batteries. Materials. 2021; 14(13):3624. https://doi.org/10.3390/ma14133624
Chicago/Turabian StyleXu, Zhijie, Fangxu Hu, De Li, and Yong Chen. 2021. "Electrochemical Oscillation during Galvanostatic Charging of LiCrTiO4 in Li-Ion Batteries" Materials 14, no. 13: 3624. https://doi.org/10.3390/ma14133624
APA StyleXu, Z., Hu, F., Li, D., & Chen, Y. (2021). Electrochemical Oscillation during Galvanostatic Charging of LiCrTiO4 in Li-Ion Batteries. Materials, 14(13), 3624. https://doi.org/10.3390/ma14133624




