Formation and Investigation of Physicochemical, Biological and Bacteriostatic Properties of Nanocomposite Foils Containing Silver Nanoparticles and Graphene Oxide in Hyaluronic Acid Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles Composites
2.2.1. Preparation of Hyal/C Foils
2.2.2. Preparation of Hyal/Ag Composite
2.2.3. Preparation of Hyal/GO Composite
2.2.4. Preparation of Hyal/Ag/GO Composite
2.3. Characterization of Nanoparticle Composites
2.3.1. Determination of Molecular Weight MW and Radii of Gyration Rg of Starch Polysaccharide Molecules
2.3.2. UV-Vis Absorption Spectrophotometry
2.3.3. Scanning and Transmission Electron Microscopy (SEM and TEM)
2.3.4. FTIR-ATR Spectrophotometry
2.3.5. Thickness and Mechanical Properties of Composites
2.3.6. Contact Angle Determination
2.3.7. Bacteriostatic Activity Assay
2.3.8. Cell Culture
2.3.9. Cell Treatment
2.3.10. Cytotoxicity and Viability Assay
2.3.11. Statistical Analysis
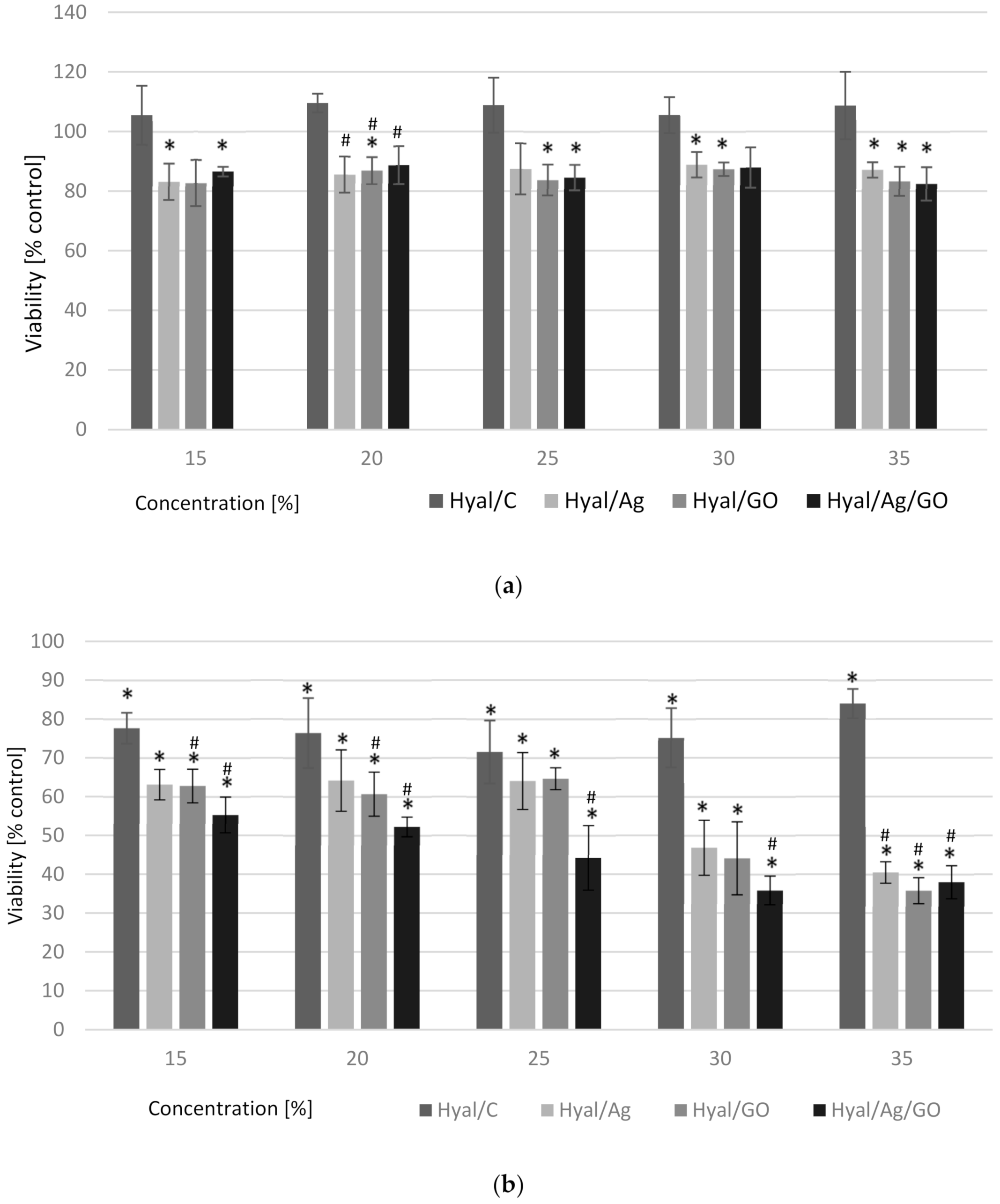
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cumpstey, I. Chemical Modification of Polysaccharides. ISRN Org. Chem. 2013, 2013, 27. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eldeen, A.M.; Ahmed, E.F.; Abo-Zeid, M.A. In vitro cancer chemopreventive properties of polysaccharide extract from the brown alga, Sargassum latifolium. Food Chem. Toxicol. 2009, 47, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zeng, H.; Xu, Z.; Zheng, B.; Lin, Y.; Gan, C.; Lo, Y.M. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus). Carbohydr. Polym. 2012, 88, 522–529. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.M.; Benito, M.; Pérez, E.; María Teijón, J.; Dolores Blanco, M. The Role of Anionic Polysaccharides in the Preparation of Nanomedicines with Anticancer Applications. Curr. Pharm. Des. 2016, 22, 3364–3379. [Google Scholar] [CrossRef] [PubMed]
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A new concept of ecological, bioinspired, and functional hybrid materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Dias, A.M.G.C.; Hussain, A.; Marcos, A.S.; Roque, A.C.A. A biotechnological perspective on the application of iron oxide magnetic colloids modified with polysaccharides. Biotechnol. Adv. 2011, 29, 142–155. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Emam, H.E.; Ahmed, H.B. Polysaccharides templates for assembly of nanosilver. Carbohydr. Polym. 2016, 135, 300–307. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, L.S.; Chen, S.Y.; Huang, M.C.; Li, Y.H.; Lin, Y.C.; Chen, P.F.; Shaw, J.F.; Huang, K.S. Microfluidic assisted synthesis of silver nanoparticle–chitosan composite microparticles for antibacterial applications. Int. J. Pharm. 2016, 510, 493–500. [Google Scholar] [CrossRef]
- Kemp, M.M.; Kumar, A.; Clement, D.; Ajayan, P.; Mousa, S.; Linhardt, R.J. Hyaluronan- and heparin-reduced silver nanoparticles with antimicrobial properties. Nanomedicine 2009, 4, 421–429. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Stobinski, L.; Tomasik, P.; Fiedorowicz, M.; Lin, H.M. CdS and ZnS quantum dots embedded in hyaluronic acid films. J. Alloys Compd. 2009, 481, 402–406. [Google Scholar] [CrossRef]
- Price, R.D.; Berry, M.G.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthetic Surg. 2007, 60, 1110–1119. [Google Scholar] [CrossRef]
- Allison, D.D.; Grande-Allen, K.J. Hyaluronan: A powerful tissue engineering tool. Tissue Eng. 2006, 12, 2131–2140. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Aghayee, S.; Fereydooni, Y.; Talebi, A. The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J. Mater. Chem. 2012, 22, 13773–13781. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef]
- Luo, Y.; Cai, X.; Li, H.; Lin, Y.; Du, D. Hyaluronic Acid-Modified Multifunctional Q-Graphene for Targeted Killing of Drug-Resistant Lung Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 4048–4055. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ge, H.; Zou, S.; Xiao, Y.; Wen, H.; Li, Y.; Feng, H.; Nie, M. Sodium alginate conjugated graphene oxide as a new carrier for drug delivery system. Int. J. Biol. Macromol. 2016, 93, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Lee, M.Y.; Kong, W.H.; Do, I.H.; Hahn, S.K. Nano graphene oxide-hyaluronic acid conjugate for target specific cancer drug delivery. RSC Adv. 2014, 4, 14197–14200. [Google Scholar] [CrossRef]
- Song, E.; Han, W.; Li, C.; Cheng, D.; Li, L.; Liu, L.; Zhu, G.; Song, Y.; Tan, W. Hyaluronic acid-decorated graphene oxide nanohybrids as nanocarriers for targeted and pH-responsive anticancer drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 11882–11890. [Google Scholar] [CrossRef]
- Dantas, P.C.d.L.; Martins-Júnior, P.A.; Coutinho, D.C.O.; Andrade, V.B.; Valverde, T.M.; Ávila, E.d.S.; Almeida, T.C.S.; Queiroz-Junior, C.M.; Sá, M.A.; Góes, A.M.; et al. Nanohybrid composed of graphene oxide functionalized with sodium hyaluronate accelerates bone healing in the tibia of rats. Mater. Sci. Eng. C 2021, 123. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J. Graphene—Metal particle nanocomposites. J. Phys. Chem. C 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Chen, X.; Wu, Y.; Dong, L. Synthesis and characterization of Ag/graphene nano-composite. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2015, 44, 2138–2142. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Li, N.; Yan, B.; Ma, H.; Hu, Y.; Ye, M. Facile synthesis and application of Ag-chemically converted graphene nanocomposite. Nano Res. 2010, 3, 339–349. [Google Scholar] [CrossRef]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M.Q. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomed. 2014, 9, 2399. [Google Scholar]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial Activity of Silver-Nanoparticles Against Staphylococcus aureus and Escherichia coli. Microbiol. Biotechnol. Lett. 2011, 39, 77–85. [Google Scholar]
- Lenart-Boron’1, A.; Boron’1, B.; Lenart-Boroń, A. Antimicrobial resistance and prevalence of extended-spectrum beta-lactamase genes in Escherichia coli from major rivers in Podhale, southern Poland. Int. J. Environ. Sci. Technol. 2017, 14, 241–250. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Nageswara Rao, G.V.S.; Mohan, K.M.; Ramanaviciene, A.; Ramanavicius, A. Antibacterial and antifungal activity of silver nanospheres synthesized by tri-sodium citrate assisted chemical approach. Vacuum 2017, 146, 259–265. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, G.V.S.N.; Mohan, M.K.; Ramanaviciene, A.; Ramanavicius, A. Comparative study of antifungal activity of silver and gold nanoparticles synthesized by facile chemical approach. J. Environ. Chem. Eng. 2018, 6, 5837–5844. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Grzyb, J.; Fiedorowicz, M. Formation and properties of hyaluronan/nano Ag and hyaluronan-lecithin/nano Ag films. Carbohydr. Polym. 2016, 151. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Krzan, M.; Khachatryan, L. Functional properties of composites containing silver nanoparticles embedded in hyaluronan and hyaluronan-lecithin matrix. Int. J. Biol. Macromol. 2020, 149, 417–423. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectros. Relat. Phenomena 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen-Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Nowak, N.; Grzebieniarz, W.; Khachatryan, G.; Khachatryan, K.; Konieczna-Molenda, A.; Krzan, M.; Grzyb, J. Synthesis of Silver and Gold Nanoparticles in Sodium Alginate Matrix Enriched with Graphene Oxide and Investigation of Properties of the Obtained Thin Films. Appl. Sci. 2021, 11, 3857. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, C.; Wang, X.; Xiong, L.; Sun, Q. Physicochemical properties of starch nanocomposite films enhanced by self-assembled potato starch nanoparticles. LWT Food Sci. Technol. 2016, 69, 251–257. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Ciesielski, W.; Buksa, K.; Sikora, M. Preparation and characteristics of mechanical and functional properties of starch/Plantago psyllium seeds mucilage films. Starch/Staerke 2017, 69, 1700014. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, D.; Cui, H.; Lin, L.; He, J.; et al. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol. 2020, 155, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mandal, T.; Goswami, S. Fabrication of cellulose acetate nanocomposite films with lignocelluosic nanofiber filler for superior effect on thermal, mechanical and optical properties. Nano-Struct. Nano-Objects 2021, 25. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Thermomechanical-induced polyelectrolyte complexation between chitosan and carboxymethyl cellulose enabling unexpected hydrolytic stability. Compos. Sci. Technol. 2020, 189. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Graphene oxide enhanced ionic liquid plasticisation of chitosan/alginate bionanocomposites. Carbohydr. Polym. 2021, 253, 117231. [Google Scholar] [CrossRef]
- Czarnecka-Komorowska, D.; Wiszumirska, K.; Garbacz, T. Films Ldpe/Lldpe Made from Post—Consumer Plastics: Processing, Structure, Mechanical Properties. Adv. Sci. Technol. Res. J. 2018, 12, 134–142. [Google Scholar] [CrossRef]
- Prasher, P.; Singh, M.; Mudila, H. Silver nanoparticles as antimicrobial therapeutics: Current perspectives and future challenges. 3 Biotech 2018, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Legrand, C.; Bour, J.M.; Jacob, C.; Capiaumont, J.; Martial, A.; Marc, A.; Wudtke, M.; Kretzmer, G.; Demangel, C.; Duval, D.; et al. Lactate dehydrogenase (LDH) activity of the number of dead cells in the medium of cultured eukaryotic cells as marker. J. Biotechnol. 1992, 25, 231–243. [Google Scholar] [CrossRef]

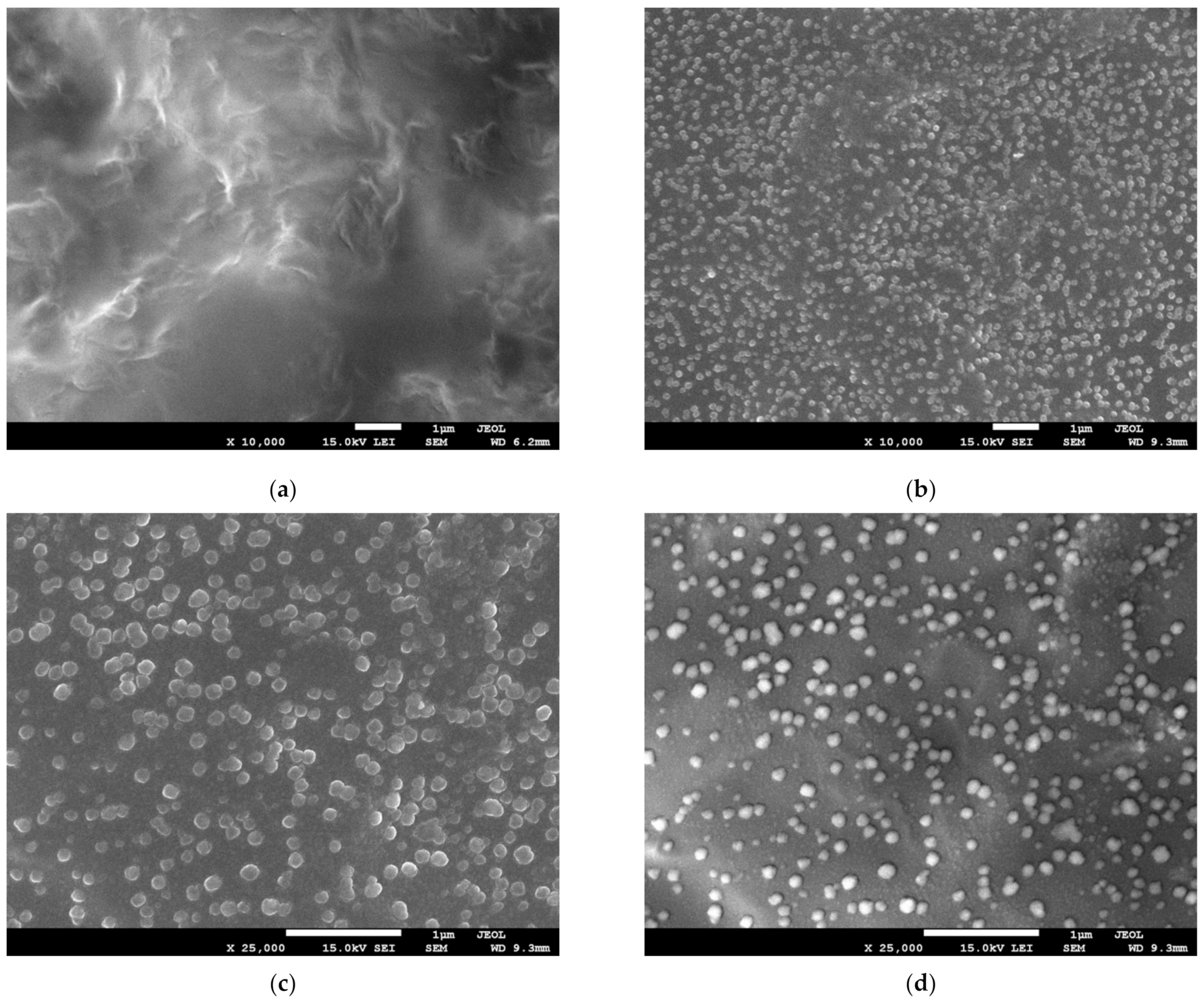
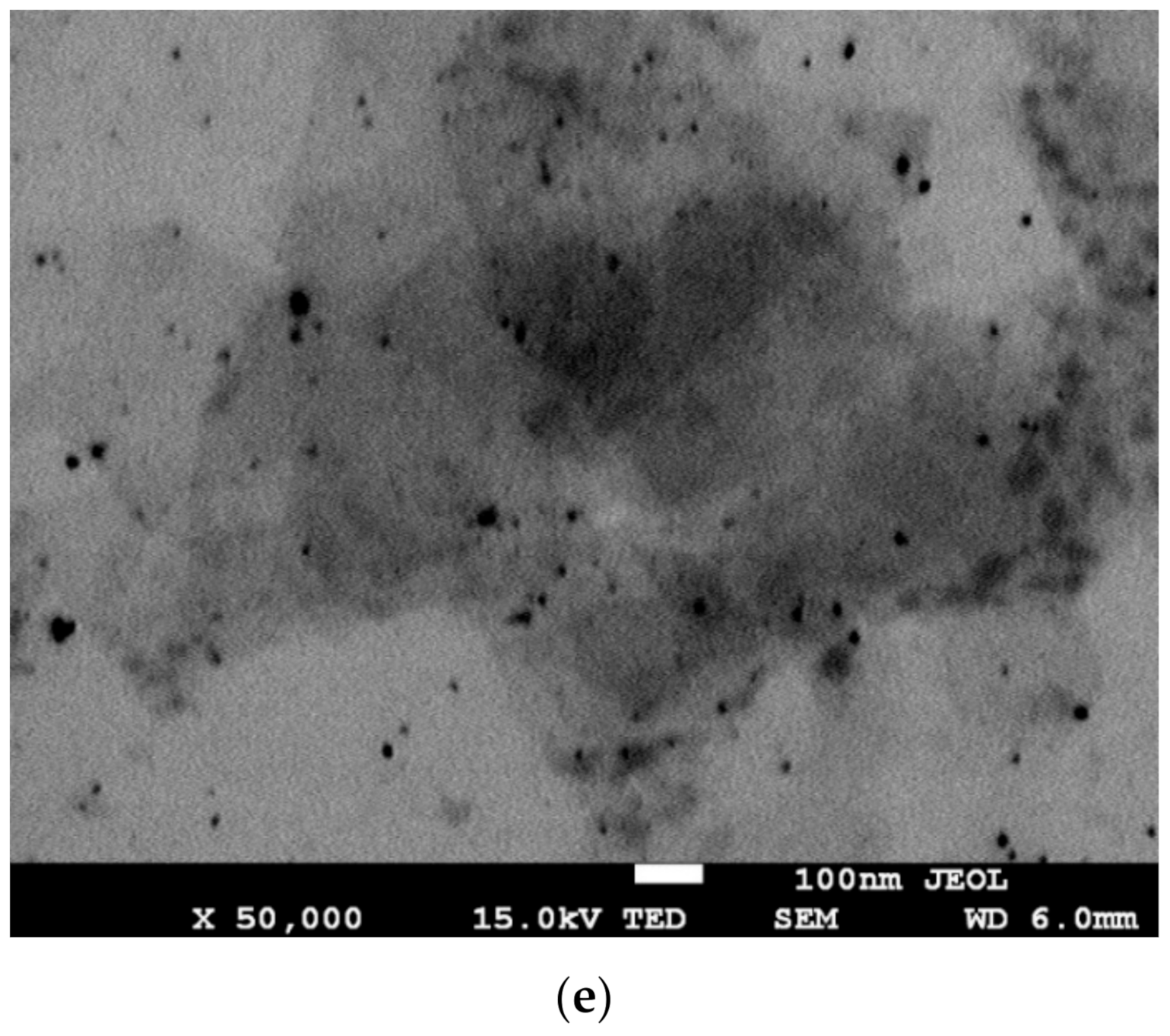
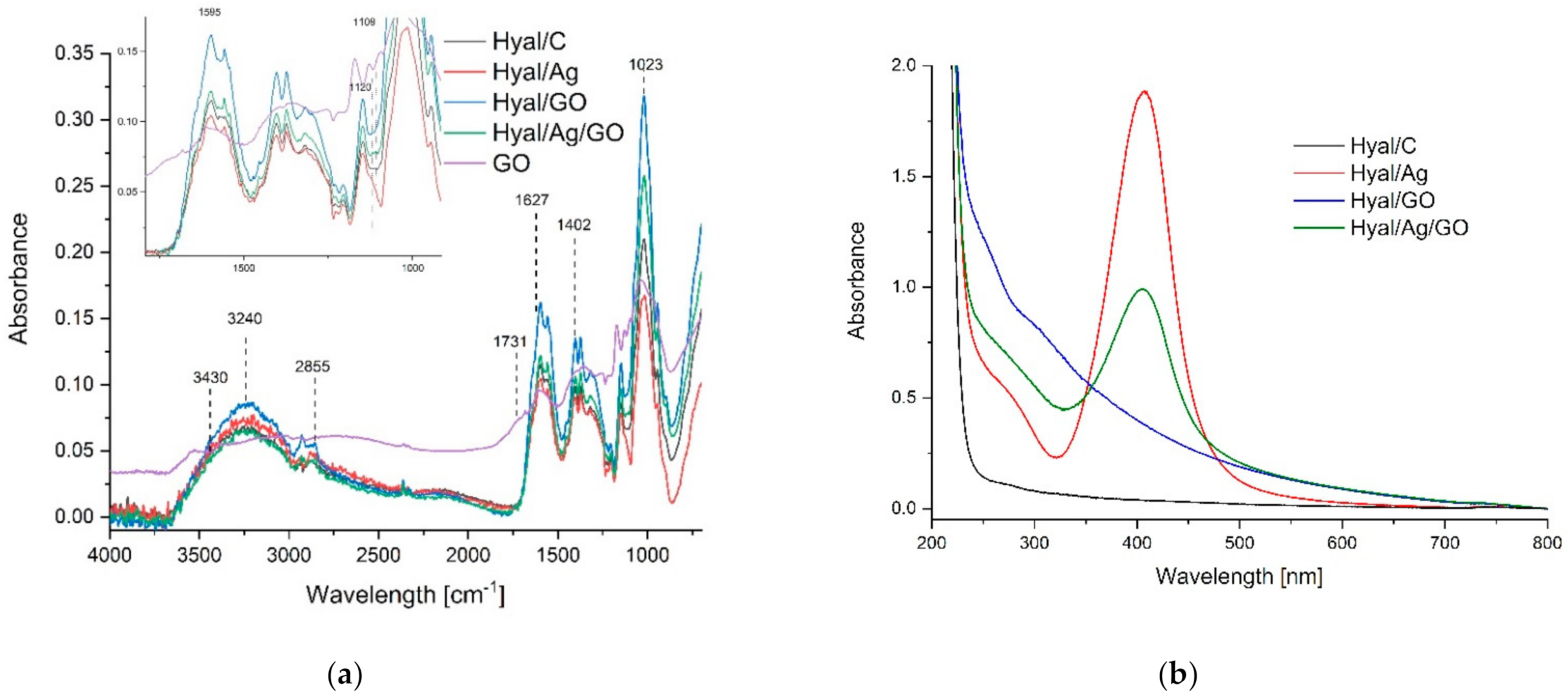
| Sample | Mw (Da) | Rg (nm) |
|---|---|---|
| Hyal/C | 1.865 × 105 | 49.4 |
| Hyal/Ag | 4.090 × 105 | 58.5 |
| Hyal/GO | 4.768 × 105 | 50.5 |
| Hyal/Ag/GO | 5.046 × 105 | 52.8 |
| Sample | Thickness (mm) | TS (MPa) | E (%) |
|---|---|---|---|
| Hyal/C | 0.048 ± 0.011 a | 90.09 ± 9.52 a | 32.51 ± 6.94 a |
| Hyal/Ag | 0.051 ± 0.008 a | 81.27 ± 5.05 a | 14.14 ± 3.75 b |
| Hyal/GO | 0.049 ± 0.001 a | 69.30 ± 3.57 b | 13.99 ± 3.88 b |
| Hyal/Ag/GO | 0.050 ± 0.009 a | 57.80 ± 7.00 c | 5.11 ± 1.54 c |
| Water | DIM | Dispersive Energy (mJ/m2) | Polar Energy (mJ/m2) | Total Surface Free Energy (mJ/m2) | |
|---|---|---|---|---|---|
| Hyal/C | 84.0° | 41.0° | 41.59 | 2 | 43.60 |
| Hyal/Ag | 41.0° | 52.0° | 23.93 | 33.02 | 56.95 |
| Hyal/GO | 79.5° | 55.3° | 30.21 | 6.36 | 36.58 |
| Hyal/Ag/GO | 49.0° | 43.5° | 30.64 | 23.29 | 53.93 |
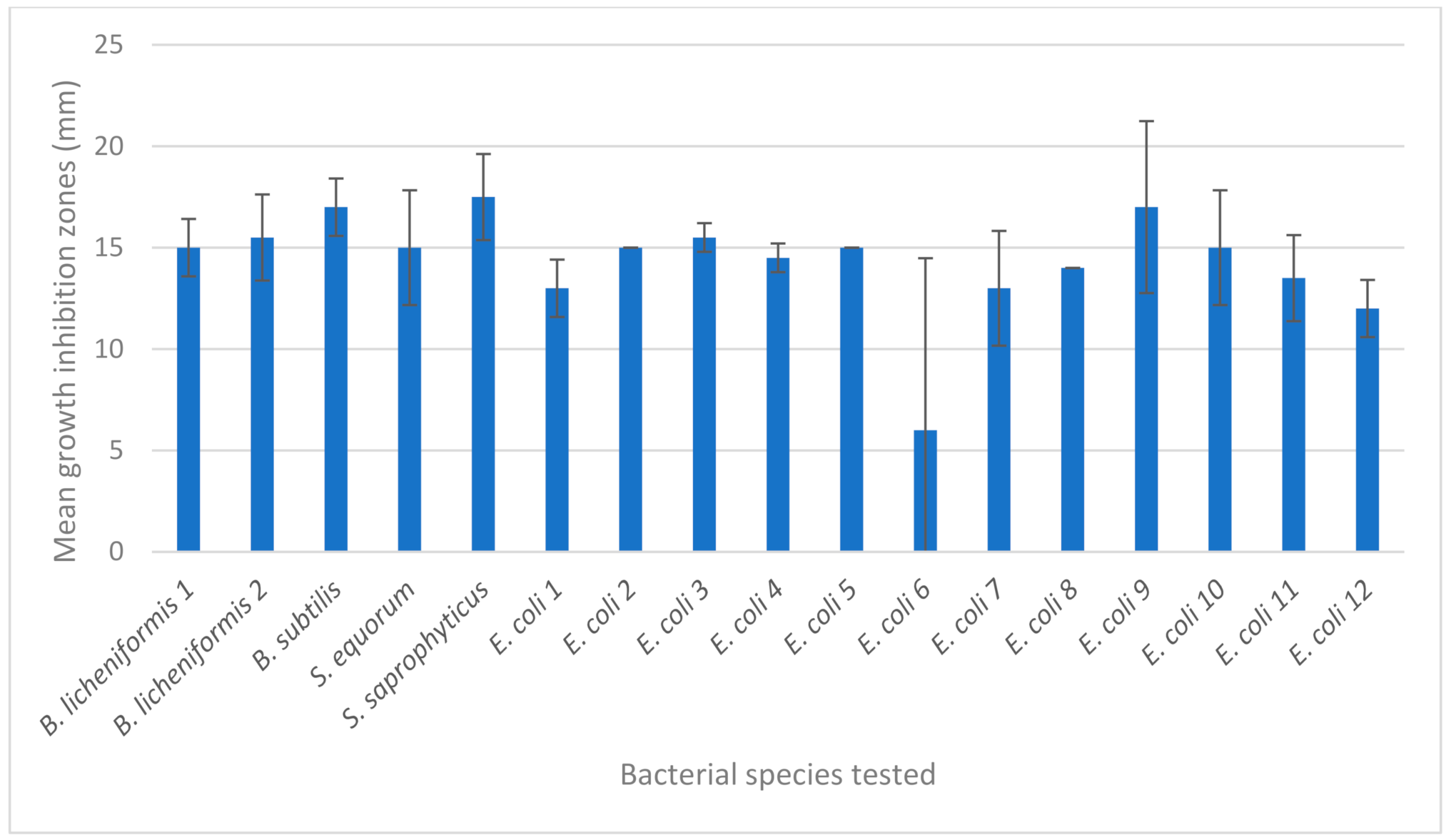
| Bacterial Strain | Nanoparticles | |||
|---|---|---|---|---|
| Hyal/Ag * | Hyal/GO | Hyal/Ag/GO | Hyal/C | |
| Bacillus licheniformis | 14 | 0 | 16 | 0 |
| Bacillus licheniformis | 17 | 0 | 14 | 0 |
| Bacillus subtilis | 18 | 0 | 16 | 0 |
| Staphylococcus equorum | 17 | 0 | 13 | 0 |
| Staphylococcus saprophyticus | 19 | 0 | 16 | 0 |
| Escherichia coli 1 | 14 | 0 | 12 | 0 |
| Escherichia coli 2 | 15 | 0 | 15 | 0 |
| Escherichia coli 3 | 16 | 0 | 15 | 0 |
| Escherichia coli 4 | 14 | 0 | 15 | 0 |
| Escherichia coli 5 | 15 | 0 | 15 | 0 |
| Escherichia coli 6 | 12 | 0 | 0 | 0 |
| Escherichia coli 7 | 11 | 0 | 15 | 0 |
| Escherichia coli 8 | 14 | 0 | 14 | 0 |
| Escherichia coli 9 | 14 | 0 | 20 | 0 |
| Escherichia coli 10 | 13 | 0 | 17 | 0 |
| Escherichia coli 11 | 12 | 0 | 15 | 0 |
| Escherichia coli 12 | 11 | 0 | 13 | 0 |
| mean | 14.47 | 0 | 14.18 | 0 |
| standard deviation | 2.35 | 0 | 4.07 | 0 |
| coefficient of variation (%) | 16.23 | - | 28.68 | - |
| WM-266-4 Cytotoxicity % | ||||
|---|---|---|---|---|
| Concentration | Hyal/C ± SD | Hyal/Ag ± SD | Hyal/GO ± SD | Hyal/Ag/GO ± SD |
| 24 h | ||||
| 15% | 0.35 ± 0.023 | 0.60 ± 0.043 | 2.78 ± 0.023 | −1.34 ± 0.014 |
| 20% | 0.84 ± 0.023 | −3.01 ± 0.049 | 3.96 ± 0.023 | −1.94 ± 0.048 |
| 25% | −3.60 ± 0.033 | −2.70 ± 0.045 | 22.54 ± 0.023 | −2.91 ± 0.044 |
| 30% | −3.59 ± 0.018 | 2.70 ± 0.049 | 12.73 ± 0.023 | −1.12 ± 0.038 |
| 35% | 5.44 ± 0.033 | 4.60 ± 0.025 | 22.50 ± 0.023 | −3.26 ± 0.065 |
| 48 h | ||||
| 15% | −9.39 ± 0.023 | −3.56 ± 0.055 | 6.78 ± 0.033 | −3.53 ± 0.025 |
| 20% | −9.81 ± 0.022 | −3.71 ± 0.029 | 19.45 ± 0.060 | −3.03 ± 0.017 |
| 25% | −10.75 ± 0.019 | −3.99 ± 0.022 | 27.40 ± 0.027 | −1.49 ± 0.069 |
| 30% | −12.03 ± 0.029 | −4.15 ± 0.065 | 25.52 ± 0.015 | −1.75 ± 0.040 |
| 35% | −11.07 ± 0.041 | −4.58 ± 0.070 | 33.81± 0.062 | −2.04 ± 0.074 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khachatryan, K.; Khachatryan, L.; Krzan, M.; Krystyjan, M.; Krzemińska-Fiedorowicz, L.; Lenart-Boroń, A.; Koronowicz, A.; Drozdowska, M.; Khachatryan, G. Formation and Investigation of Physicochemical, Biological and Bacteriostatic Properties of Nanocomposite Foils Containing Silver Nanoparticles and Graphene Oxide in Hyaluronic Acid Matrix. Materials 2021, 14, 3377. https://doi.org/10.3390/ma14123377
Khachatryan K, Khachatryan L, Krzan M, Krystyjan M, Krzemińska-Fiedorowicz L, Lenart-Boroń A, Koronowicz A, Drozdowska M, Khachatryan G. Formation and Investigation of Physicochemical, Biological and Bacteriostatic Properties of Nanocomposite Foils Containing Silver Nanoparticles and Graphene Oxide in Hyaluronic Acid Matrix. Materials. 2021; 14(12):3377. https://doi.org/10.3390/ma14123377
Chicago/Turabian StyleKhachatryan, Karen, Lusine Khachatryan, Marcel Krzan, Magdalena Krystyjan, Lidia Krzemińska-Fiedorowicz, Anna Lenart-Boroń, Aneta Koronowicz, Mariola Drozdowska, and Gohar Khachatryan. 2021. "Formation and Investigation of Physicochemical, Biological and Bacteriostatic Properties of Nanocomposite Foils Containing Silver Nanoparticles and Graphene Oxide in Hyaluronic Acid Matrix" Materials 14, no. 12: 3377. https://doi.org/10.3390/ma14123377
APA StyleKhachatryan, K., Khachatryan, L., Krzan, M., Krystyjan, M., Krzemińska-Fiedorowicz, L., Lenart-Boroń, A., Koronowicz, A., Drozdowska, M., & Khachatryan, G. (2021). Formation and Investigation of Physicochemical, Biological and Bacteriostatic Properties of Nanocomposite Foils Containing Silver Nanoparticles and Graphene Oxide in Hyaluronic Acid Matrix. Materials, 14(12), 3377. https://doi.org/10.3390/ma14123377











