Prussian Blue and Carbon-Dot Hybrids for Enhanced Electrochromic Performance
Abstract
1. Introduction
2. Experiment
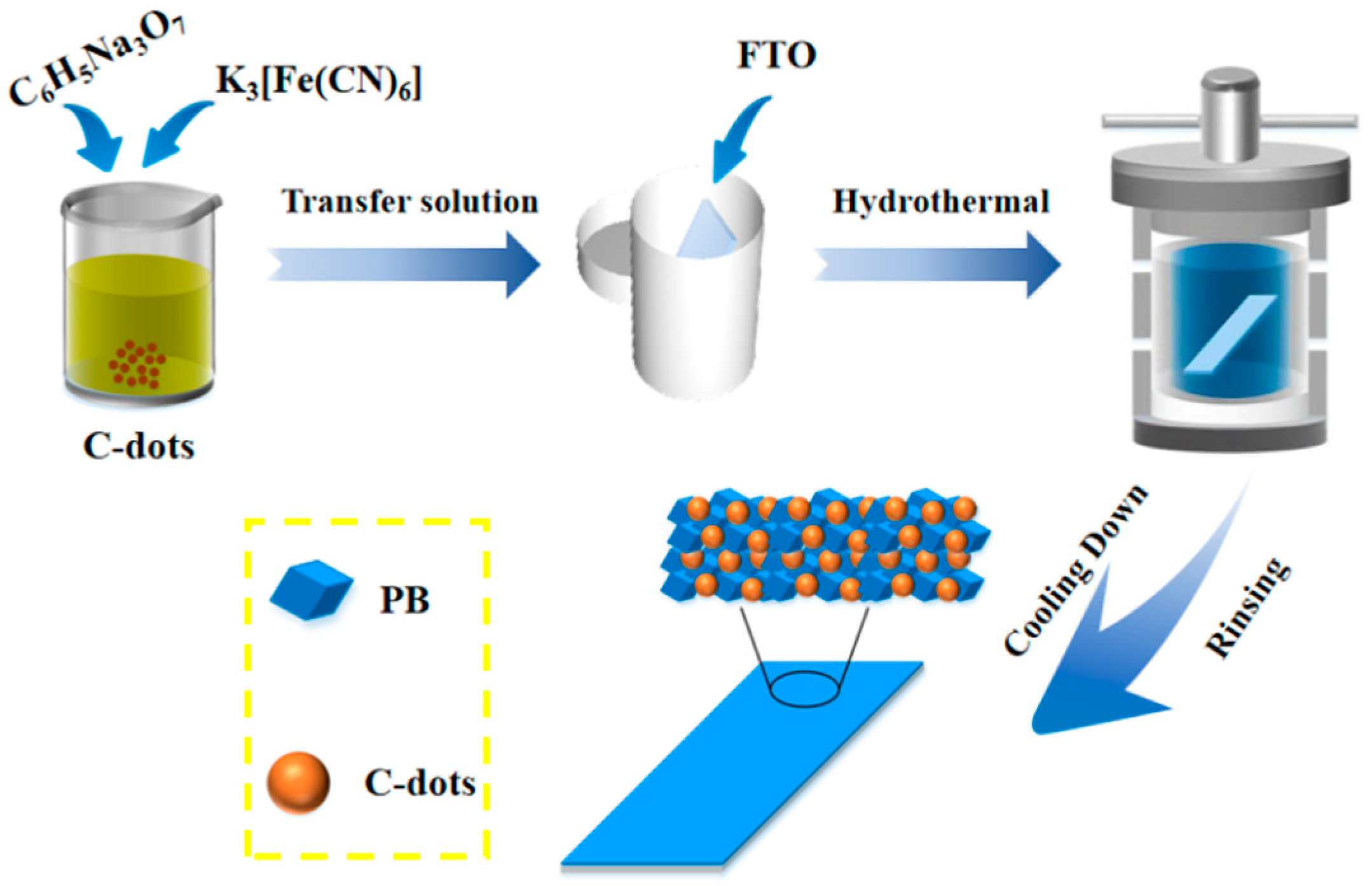
2.1. Synthesis of PB@C-Dot Hybrid
2.2. Characterization
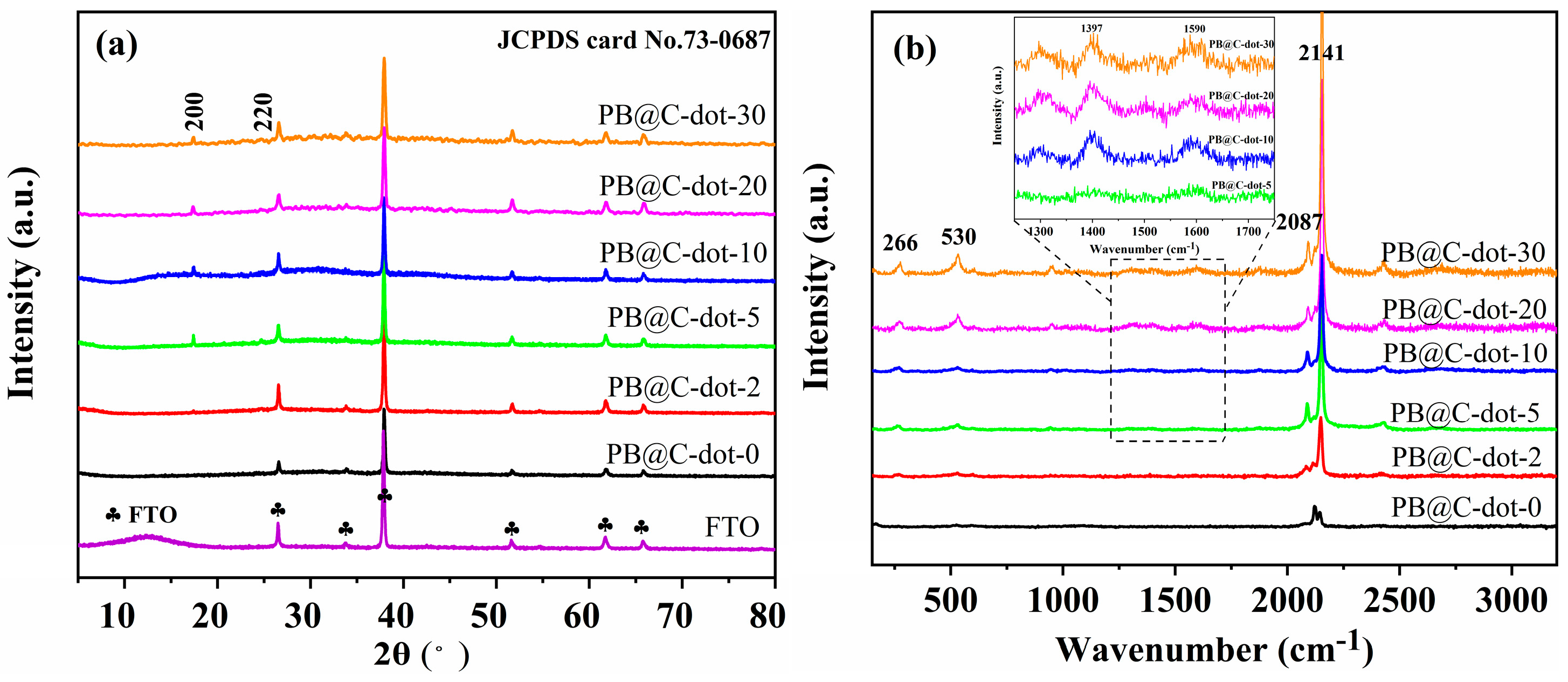
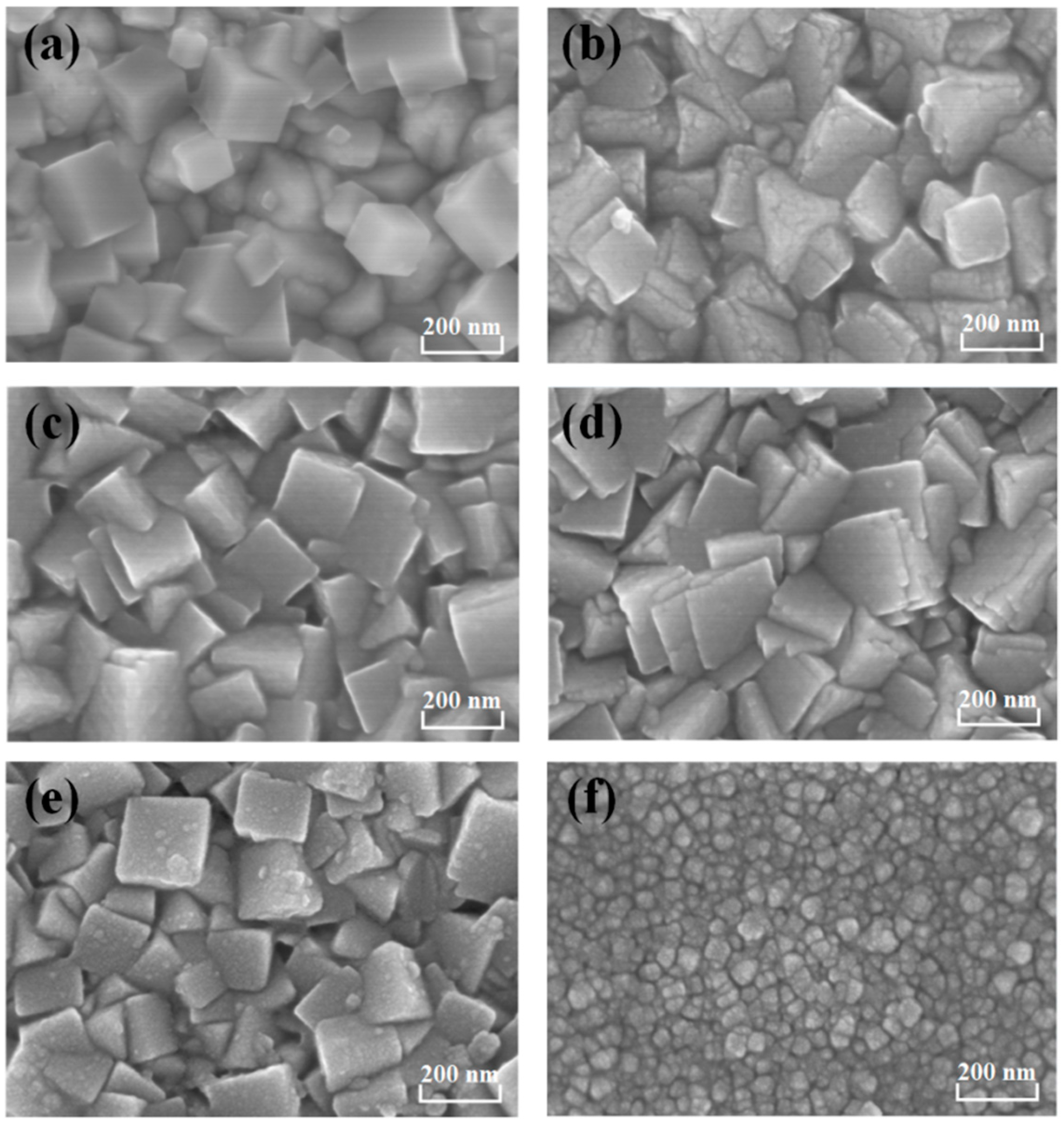
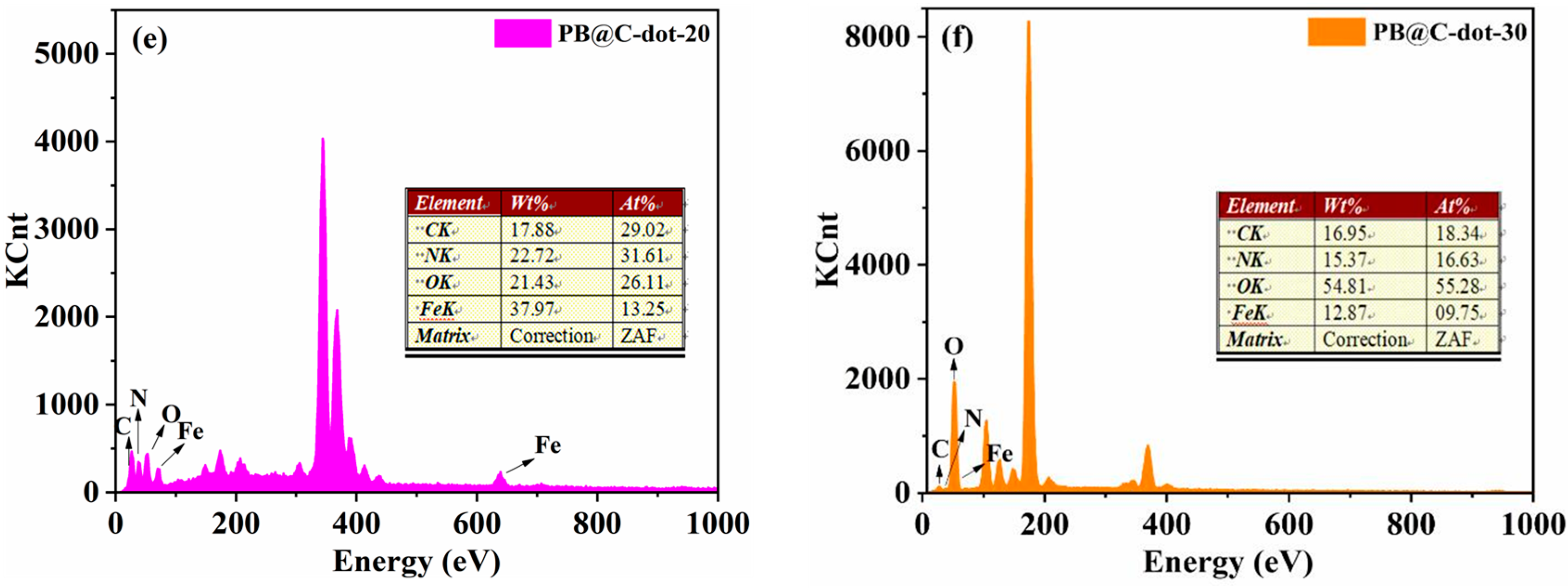
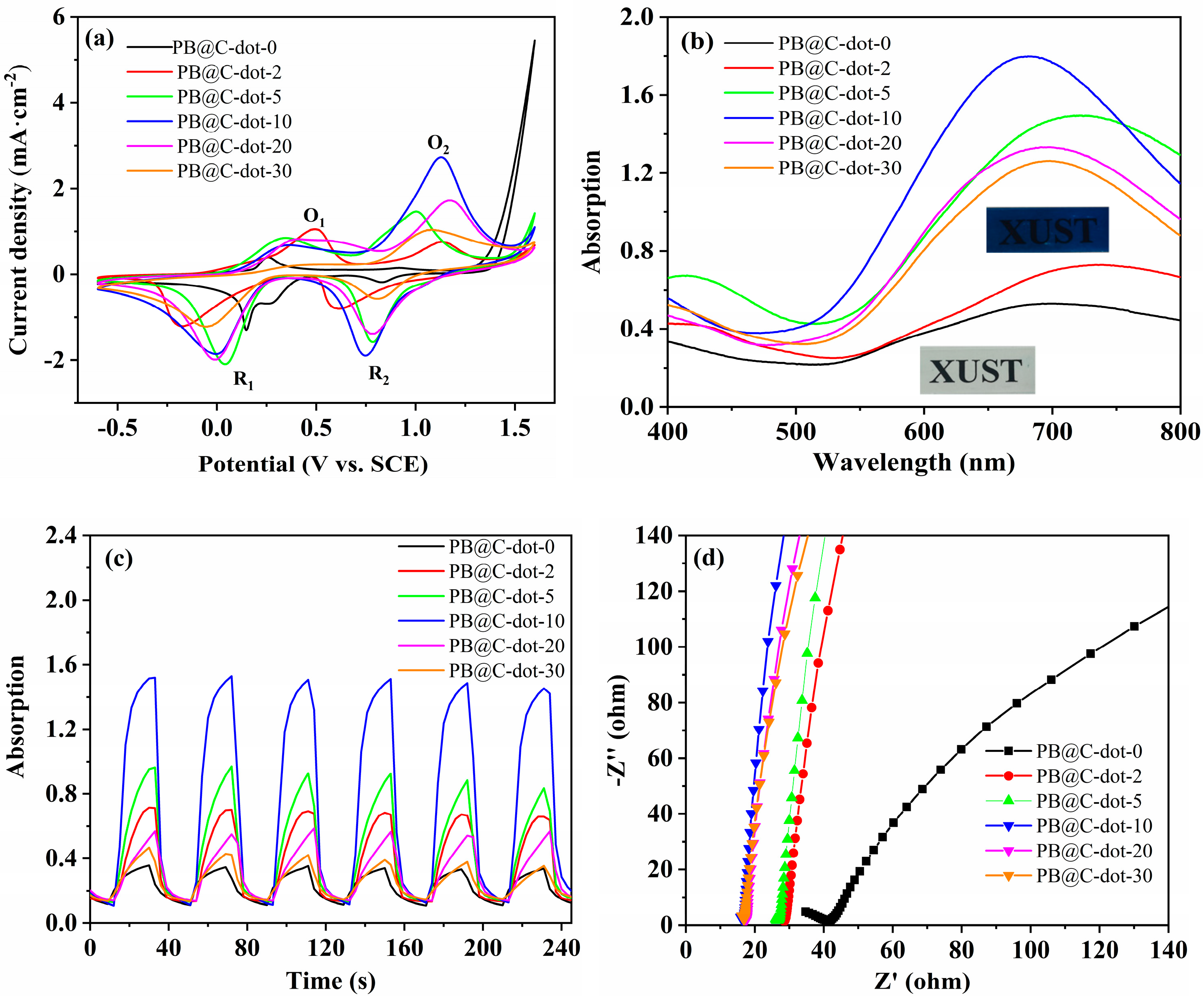
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Films 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, S.; Li, X.; Zhao, J.; Li, Y. Self-supported one-dimensional materials for enhanced electrochromism. Nanoscale Horiz. 2018, 3, 261–292. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Wang, X.; Lan, J.P.; Zhang, R.; Xiong, S.X.; Wu, B.H.; Lu, D.; Chu, J.; Ma, J. Facile fabrication of WO3 crystalline nanoplate on FTO glass and their application in electrochromism. Micro Nano Lett. 2016, 11, 749–752. [Google Scholar]
- Zhou, D.; Che, B.; Kong, J.; Lu, X. A nanocrystalline tungsten oxide electrochromic coating with excellent cycling stability prepared via a complexation-assisted sol–gel method. J. Mater. Chem. C 2016, 4, 8041–8051. [Google Scholar] [CrossRef]
- Jin, L.; Fang, Y.; Shang, L.; Liu, Y.; Li, J.; Wang, L.; Hu, P.; Dong, S. Gold nanocluster-based electrochemically controlled fluorescence switch surface with prussian blue as the electrical signal receptor. Chem. Commun. 2013, 49, 243–245. [Google Scholar] [CrossRef]
- Lin, C.L.; Liao, L.C. Preparation and characterization of micropatterned prussian blue thin films with enhanced electrochromic properties. Surf. Coat. Technol. 2014, 259, 330–334. [Google Scholar] [CrossRef]
- Li, Z.T.; Tang, Y.H.; Zhou, K.L.; Wang, H.; Yan, H. Improving Electrochromic Cycle Life of Prussian Blue by Acid Addition to the Electrolyte. Materials 2018, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chu, J.; Cheng, Y.P.; Yang, F.; Xiong, S.X. A novel PANI@Carbon dot hybrid with enhanced electrochemical and electrochromic properties. Mater. Lett. 2020, 275, 128081. [Google Scholar] [CrossRef]
- Husmann, S.; Orth, E.S.; Zarbin, A.J.G. A multi-technique approach towards the mechanistic investigation of the electrodeposition of Prussian blue over carbon nanotubes film. Electrochim. Acta 2019, 312, 380–391. [Google Scholar] [CrossRef]
- Liu, X.W.; Yao, Z.J.; Wang, Y.F.; Wei, X.W. Graphene oxide sheet–prussian blue nanocomposites: Green synthesis and their extraordinary electrochemical properties. Colloids Surf. B 2010, 81, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Rethinasabapathy, M.; Kang, S.M.; Lee, I.; Lee, G.W.; Lee, S.; Roh, C.; Huh, Y.S. Highly stable Prussian blue nanoparticles containing graphene oxide–chitosan matrix for selective radioactive cesium removal. Mater. Lett. 2019, 241, 194–197. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, X.; Xu, Y.; Xiong, J.; Wang, J. Bio-safety assessment of carbon quantum dots, N-doped and folic acid modified carbon quantum dots: A systemic comparison. Chin. Chem Lett. 2020, 31, 1654–1659. [Google Scholar] [CrossRef]
- Uriel, S.; Patricia, Á.; Clara, B.; Marcos, G.; Ricardo, S.; Rosa, M. New alternatives to graphite for producing graphene materials. Carbon 2015, 93, 812–818. [Google Scholar]
- Kamedulski, P.; Zielinski, W.; Nowak, P.; Lukaszewicz, J.P.; Ilnicka, A. 3D hierarchical porous hybrid nanostructure of carbon nanotubes and N-doped activated carbon. Sci. Rep. 2020, 10, 18793. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Q.; Mao, Y.P.; Lu, S.W.; Cui, B.L.; Yu, L. Seed-Assisted Synthesis of Graphene Films on Insulating Substrate. Materials 2019, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Padigi, P.; Thiebes, J.; Swan, M.; Goncher, G.; Evans, D.; Solanki, R. Prussian Green: A High Rate Capacity Cathode for Potassium Ion Batteries. Electrochim. Acta 2015, 166, 32–39. [Google Scholar] [CrossRef]
- Kuwabara, K.; Nunome, J.; Sugiyama, K. Rechargeability of solid-state copper cells utilizing cathodes of Prussian blue and Berlin green. Solid State Ion. 1991, 48, 303–308. [Google Scholar] [CrossRef]
- Li, F.; Ma, D.Y.; Qian, J.H.; Yang, B.; Xu, Z.P.; Li, D.; Wang, J.M. One-step hydrothermal growth and electrochromic properties of highly stable prussian green film and device. Sol. Energy Mater. Sol. Cells 2019, 192, 103–108. [Google Scholar] [CrossRef]
- Li, X.; Chu, J.; Cheng, Y.P.; Yang, F.; Xiong, S.X. Novel prussian blue@Carbon-dots hybrid thin film: The impact of carbon-dots on material structure and electrochromic performance. Electrochim. Acta 2020, 355, 136659. [Google Scholar] [CrossRef]
- Mai, X.D.; Thi Kim Chi, T.; Nguyen, T.C.; Ta, V.T. Scalable synthesis of highly photoluminescence carbon quantum dots. Mater. Lett. 2020, 268, 127595. [Google Scholar] [CrossRef]
- Shen, K.; Luo, G.; Liu, J.; Zheng, J.; Xu, C. Highly transparent photoelectrochromic device based on carbon quantum dots sensitized photoanode. Sol. Energy Mater Sol. Cells 2019, 193, 372–378. [Google Scholar] [CrossRef]
- Tina, N.; Mojgan, A.; Hamed, G.; Iman, S.; Mohammad, A. Carbon quantum dots originated from chitin nanofibers as a fluorescent chemoprobe for drug sensing. J. Ind. Eng. Chem. 2017, 52, 162–167. [Google Scholar]
- Costa, R.S.D.; Cunha, W.F.D.; Pereira, N.S.; Ceschin, A.M. An Alternative Route to Obtain Carbon Quantum Dots from Photoluminescent Materials in Peat. Materials 2018, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Janus, U.; Radwan-Pragowska, J.; Piatkowski, M.; Bogdal, D. Facile Synthesis of Surface-Modified Carbon Quantum Dots (CQDs) for Biosensing and Bioimaging. Materials 2020, 13, 3313. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jia, X.H.; Yin, X.B.; He, X.W.; Zhang, Y.K. Carbon quantum dot stabilized gadolinium nanoprobe prepared via a one-pot hydrothermal approach for magnetic resonance and fluorescence dual-modality bioimaging. Anal. Chem. 2014, 86, 12122–12129. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ma, D.; Xu, Z.; Li, D.; Wang, J. Electrochromic properties of hydrothermally grown Prussian blue fifilm and device. Sol. Energy Mater. Sol. Cells 2018, 177, 9–14. [Google Scholar] [CrossRef]
- Gao, M.; Miao, P.; Han, X.; Sun, C.; Ma, Y.; Gao, Y.; Xu, P. Hollow transition metal hydroxide octahedral microcages for single particle surface-enhanced Raman spectroscopy. Inorg. Chem. Front. 2019, 6, 2318–2324. [Google Scholar] [CrossRef]
- Díaz, P.; Gonzalez, Z.; Santamaría, R.; Granda, M.; Menendez, R.; Blanco, C. Enhancing energy density of carbon-based supercapacitors using Prussian Blue modifified positive electrodes. Electrochim. Acta 2016, 212, 848–855. [Google Scholar] [CrossRef]
- Chen, Y.B.; Bi, Z.; Li, X. High-Coloration Efficiency Electrochromic Device Based on Novel Porous TiO2@Prussian Blue Core-Shell Nanostructures. Electrochim. Acta 2017, 224, 534–540. [Google Scholar] [CrossRef]
- Rong, K.; Zhang, H.; Hu, Y.Y.; Fang, Y.X.; Dong, S. Deep Eutectic Solvent with Prussian Blue and Tungsten Oxide for Green and Low-Cost Electrochromic Devices. ACS Appl. Electron. Mater. 2019, 1, 1038–1045. [Google Scholar] [CrossRef]
- Hegner, F.S.; Galan-Mascaros, J.R.; Lopez, N. A database of the structural and electronic properties of prussian blue, prussian white, and berlin green compounds through density functional theory. Inorg. Chem. 2016, 55, 12851–12862. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Watanabe, H.; Nishino, M.; Kawamoto, T. Green fabrication of a complementary electrochromic device using water-based ink containing nanoparticles of WO3 and Prussian blue. RSC Adv. 2020, 10, 2562–2565. [Google Scholar] [CrossRef]
- Sanetuntikul, J.; Shanmugam, S. Prussian blue-carbon hybrid as a non-precious electrocatalyst for the oxygen reduction reaction in alkaline medium. Electrochim. Acta 2014, 119, 92–98. [Google Scholar] [CrossRef]
- Que, Q.H.; Xing, Y.L.; He, Z.L.; Yang, Y.W. Bi2O3/Carbon quantum dots heterostructured photocatalysts with enhanced photocatalytic activity. Mater. Lett. 2017, 209, 220–223. [Google Scholar] [CrossRef]
- Zhu, Y.; Otley, M.T.; Alamer, F.A.; Kumar, A.; Zhang, X.; Mamangun, D.; Li, M.; Arden, B.G.; Sotzing, G.A. Electrochromic properties as a function of electrolyte on the performance of electrochromic devices consisting of a single-layer polymer. Org. Electron. 2014, 15, 1378–1386. [Google Scholar] [CrossRef]
- Zhang, S.H.; Chen, S.; Cao, Y.; Yang, F.; Peng, H.C.; Yan, B.; Jiang, H.; Gu, Y.C. Polyaniline nanoparticle coated graphene oxide composite nanofakes for bifunctional multicolor electrochromic and supercapacitor applications. J. Mater. Sci.-Mater. Electron. 2019, 30, 13497–13508. [Google Scholar] [CrossRef]

| Samples | Coloration Time (tc/s) | Bleaching Time (tb/s) |
|---|---|---|
| PB@C-dot-0 | 15 | 12 |
| PB@C-dot-2 | 14 | 7 |
| PB@C-dot-5 | 12 | 6 |
| PB@C-dot-10 | 10 | 3 |
| PB@C-dot-20 | 14 | 9 |
| PB@C-dot-30 | 15 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, J.; Cheng, Y.; Li, X.; Yang, F.; Xiong, S.; Zhang, Z. Prussian Blue and Carbon-Dot Hybrids for Enhanced Electrochromic Performance. Materials 2021, 14, 3166. https://doi.org/10.3390/ma14123166
Chu J, Cheng Y, Li X, Yang F, Xiong S, Zhang Z. Prussian Blue and Carbon-Dot Hybrids for Enhanced Electrochromic Performance. Materials. 2021; 14(12):3166. https://doi.org/10.3390/ma14123166
Chicago/Turabian StyleChu, Jia, Yaping Cheng, Xue Li, Fan Yang, Shanxin Xiong, and Zhao Zhang. 2021. "Prussian Blue and Carbon-Dot Hybrids for Enhanced Electrochromic Performance" Materials 14, no. 12: 3166. https://doi.org/10.3390/ma14123166
APA StyleChu, J., Cheng, Y., Li, X., Yang, F., Xiong, S., & Zhang, Z. (2021). Prussian Blue and Carbon-Dot Hybrids for Enhanced Electrochromic Performance. Materials, 14(12), 3166. https://doi.org/10.3390/ma14123166





