On the Rate of Interaction of Sodium Borohydride with Platinum (IV) Chloride Complexes in Alkaline Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Methods
2.3. Dynamic Light Scattering (DLS)
3. Results
3.1. Experimental Conditions
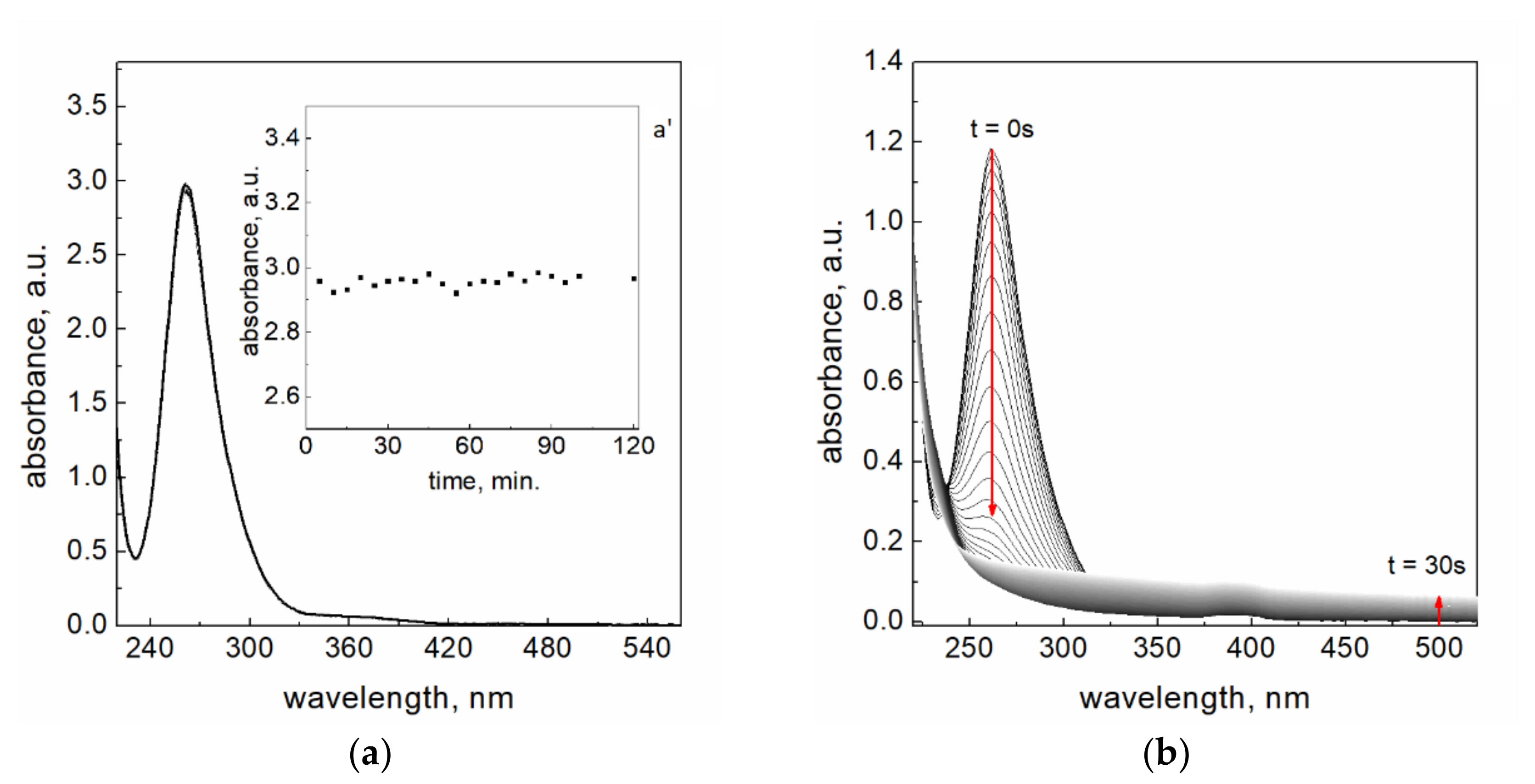
3.2. Spectra of Reagents and Pt(IV) Complex Ions Stability
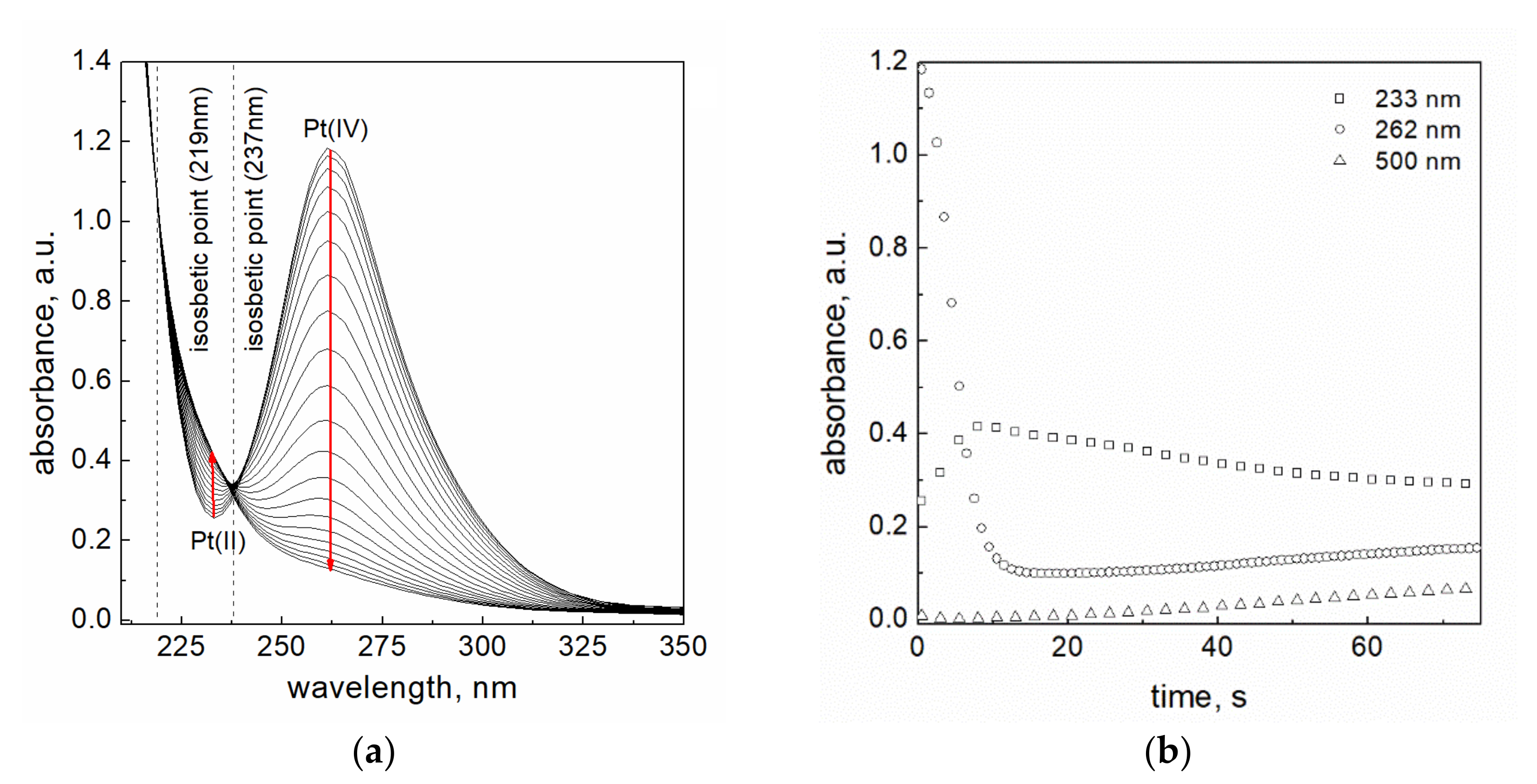
3.3. The Stoichiometry of Reduction Reaction of Pt(IV) Using Sodium Borohydride
3.4. Determination of the Rate Constants
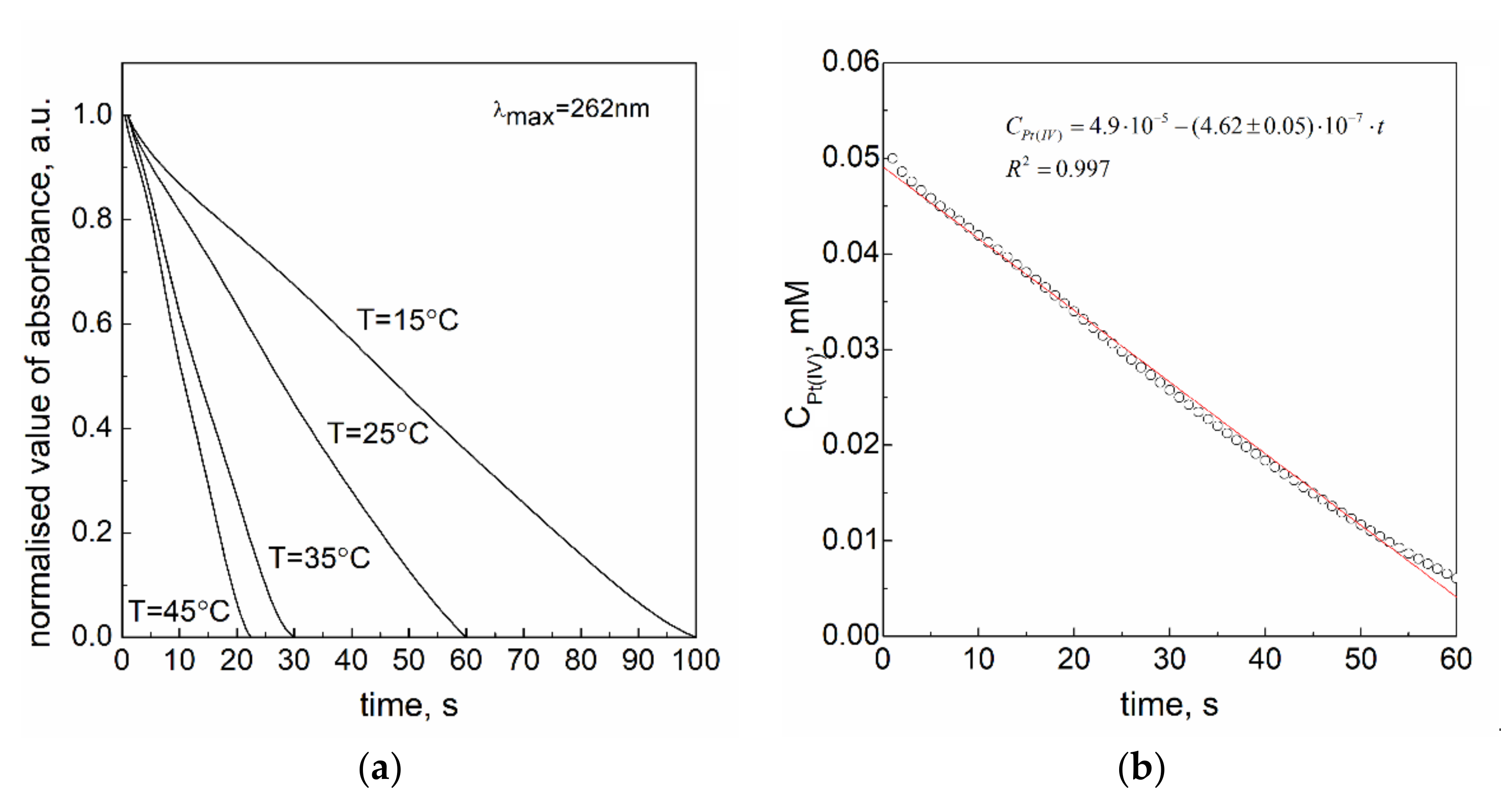
3.5. Effect of Temperature
3.6. Effect of Chloride Ions
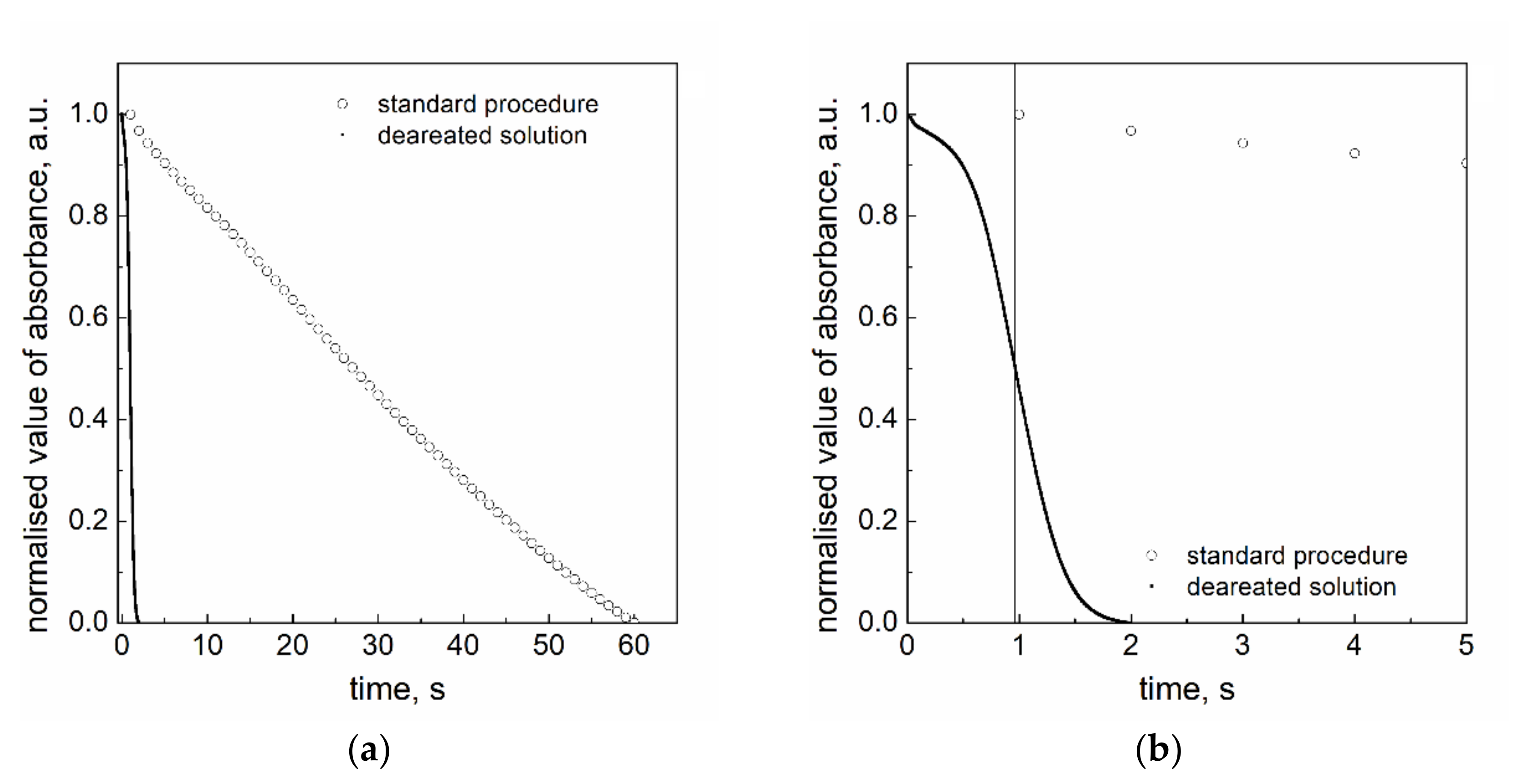
3.7. The Role of Oxygen
4. Discussion
5. Conclusions
- The reduction reaction between NaBH4 and [PtCl5(OH)]2− complex ions can be imagined as a three-stage process:
- Spectrophotometric experiments gave the rate constants of the first stage yielding values of the pseudo-zero order and zero-order rate constants as a function of temperature.
- The obtained values of the enthalpy and entropy of activation are 29.6 ± 2.6 kJ/mol and −131 J/mol·K, respectively.
- Sodium chloride addition (an increase of Cl¯ ions concentration) enhances the rate of reaction.
- The reduction process is much faster in the deaerated solution. It means that the dissolved oxygen acts as the inhibitor of redox reaction.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kettler, P.B. Platinum Group Metals in Catalysis: Fabrication of Catalysts and Catalyst Precursors. Org. Process Res. Dev. 2003, 7, 342–354. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Rane, M.V. PGM ore processing: LIX reagents for palladium extraction & platinum stripping from Alamine 336 using NaOH-NaCl. Miner. Eng. 2019, 138, 119–124. [Google Scholar]
- Jha, M.; Lee, J.-C.; Kim, M.-S.; Jeong, J.; Kim, B.-S.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Wojnicki, M.; Socha, R.P.; Luty-Błocho, M.; Fitzner, K. Kinetic studies of the removal of Pt(IV) chloride complex ions from acidic aqueous solutions using activated carbon. React. Kinet. Mech. Catal. 2017, 120, 715–734. [Google Scholar] [CrossRef]
- Schreier, G.; Edtmaier, C. Separation of Ir, Pd and Rh from secondary Pt scrap by precipitation and calcination. Hydrometallurgy 2003, 68, 69–75. [Google Scholar] [CrossRef]
- Hedrich, S.; Kraemer, D.; Junge, M.; Marbler, H.; Bau, M.; Schippers, A. Bioprocessing of oxidized platinum group element (PGE) ores as pre-treatment for efficient chemical extraction of PGE. Hydrometallurgy 2020, 196, 105419. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of boric acid (E 284) and sodium tetraborate (borax) (E 285) as food additives. EFSA J. 2013, 11, 3407. [Google Scholar]
- Kankala, R.K.; Liu, C.-G.; Yang, D.-Y.; Wang, S.-B.; Chen, A.-Z. Ultrasmall platinum nanoparticles enable deep tumor penetration and synergistic therapeutic abilities through free radical species-assisted catalysis to combat cancer multidrug resistance. Chem. Eng. J. 2020, 383, 123138. [Google Scholar] [CrossRef]
- Lim, G.-H.; Yu, T.; Koh, T.; Lee, J.H.; Jeong, U.; Lim, B. Reduction by water for eco-friendly, capping agent-free synthesis of ultrasmall platinum nanocrystals. Chem. Phys. Lett. 2014, 595–596, 77–82. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhao, Z.L.; Li, C.M. Formic acid-reduced ultrasmall Pd nanocrystals on graphene to provide superior electocatalytic activity and stability toward formic acid oxidation. Nano Energy 2015, 11, 71–77. [Google Scholar] [CrossRef]
- James, B.D.; Wallbridge, M.G.H. Metal Tetrahydroborates. In Progress in Inorganic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1970; pp. 99–231. [Google Scholar]
- Andrieux, J.; Demirci, U.B.; Hannauer, J.; Gervais, C.; Goutaudier, C.; Miele, P. Spontaneous hydrolysis of sodium borohydride in harsh conditions. Int. J. Hydrog. Energy 2011, 36, 224–233. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, C.; Fang, Y.; Zhu, F.; Liu, H.; Ge, H. Hydrogen generation mechanism of BH4− spontaneous hydrolysis: A sight from ab initio calculation. Int. J. Hydrog. Energy 2016, 41, 22668–22676. [Google Scholar] [CrossRef]
- Fawzy, A. Influence of copper(II) catalyst on the oxidation of L-histidine by platinum(IV) in alkaline medium: A kinetic and mechanistic study. Transit. Met. Chem. 2014, 39, 567–576. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Sun, X.; Ortega, J. Kinetics of Catalytic Hydrolysis of Stabilized Sodium Borohydride Solutions. Ind. Eng. Chem. Res. 2007, 46, 1120–1124. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, R. Hydrogen Storage via the Hydrolysis of NaBH4 Basic Solution: Optimization of NaBH4 Concentration. Energy Fuels 2006, 20, 2142–2148. [Google Scholar] [CrossRef]
- Murray, P.; Koch, K.R.; van Eldik, R. Mechanism of tetrachloroplatinate(II) oxidation by hydrogen peroxide in hydrochloric acid solution. Dalton Trans. 2014, 43, 6308–6314. [Google Scholar] [CrossRef]
- Cox, L.E.; Peters, D.G.; Wehry, E.L. Photoaquation of hexachloroplatinate (IV). J. Inorg. Nucl. Chem. 1972, 34, 297–305. [Google Scholar] [CrossRef]
- Gamons, C.H. Experimental investigations of the hydrothermal geochemistry of platinum and palladium: V. Equilibria between platinum metal, Pt(II), and Pt (IV) chloride complexes at 25 to 300 °C. Geochim. Cosmochim. Acta 1996, 60, 1683–1694. [Google Scholar] [CrossRef]
- Finney, E.E.; Finke, R.G. Nanocluster nucleation and growth kinetic and mechanistic studies: A review emphasizing transition-metal nanoclusters. J. Colloid Interface Sci. 2008, 317, 351–374. [Google Scholar] [CrossRef]
- Finney, E.E.; Finke, R.G. Fitting and interpreting transition-metal nanocluster formation and other sigmoidal-appearing kinetic data: A more thorough testing of dispersive kinetic vs chemical-mechanism-based equations ans treatments for 4-step type kinetic data. Chem. Mater. 2009, 21, 4468–4479. [Google Scholar] [CrossRef]
- Kytsya, A.; Bazylyak, L.; Hrynda, Y.; Horechyy, A.; Medvedevdkikh, Y. The Kinetic Rate Law for the Autocatalytic Growth of Citrate-Stabilized Silver Nanoparticles. Int. J. Chem. Kinet. 2015, 47, 351–360. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Watzky, M.A.; Finke, R.G. Transition Metal Nanocluster Formation Kinetic and Mechanistic Studies. A New Mechanism When Hydrogen Is the Reductant: Slow, Continuous Nucleation and Fast Autocatalytic Surface Growth. J. Am. Chem. Soc. 1997, 119, 10382–10400. [Google Scholar] [CrossRef]
- Watzky, M.A.; Finney, E.E.; Finke, R.G. Transition-Metal Nanocluster Size vs Formation Time and the Catalytically Effective Nucleus Number: A Mechanism-Based Treatment. J. Am. Chem. Soc. 2008, 130, 11959–11969. [Google Scholar] [CrossRef] [PubMed]
- Wojnicki, M.; Fitzner, K.; Luty-Błocho, M. Kinetic studies of nucleation and growth of palladium nanoparticles. J. Colloid Interface Sci. 2016, 465, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Lente, G.; Fabian, I.; Poe, A.J. A common misconception about the Eyring equation. New J. Chem. 2005, 29, 759–760. [Google Scholar] [CrossRef]
- Gupta, K.K.S.; Begum, B.A.; Pal, B. Kinetic behaviour and relative reactivities of some aldoses, amino sugars, and methylated sugars towards platinum(IV) in alkaline medium. Carbohydr. Res. 1998, 309, 303–310. [Google Scholar] [CrossRef]
- Smirnov, S.; Vlassiouk, I.; Kutzki, O.; Wedel, M.; Montforts, F.-P. Unusual Role of Oxygen in Electron-Transfer Processes. J. Am. Chem. Soc. 2002, 124, 4212–4213. [Google Scholar] [CrossRef]
- Pourzahedi, L.; Eckelman, M.J. Comparative life cycle assessment of silver nanoparticle synthesis routes. Environ. Sci. Nano 2015, 2, 361–369. [Google Scholar] [CrossRef]
- Schweitzer, G.K.; Pesterfield, L.L. The Aqueous Chemistry of the Elements; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Barin, I.; Knacke, O.; Kubaschewski, O. Tables. In Thermochemical Properties of Inorganic Substances: Supplement; Barin, I., Knacke, O., Kubaschewski, O., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1977; pp. 1–861. [Google Scholar]


| Initial Concentration of Reagents, M | Temperature, K | NaCl Addition, M |
|---|---|---|
| The stoichiometry of reaction | ||
| C0, Pt(IV):C0,NaBH4 | – | – |
| 1:2 | 298 | – |
| 1:1 | – | – |
| 2:1 | – | – |
| 3:1 | – | – |
| – | Effect of temperature | – |
| 0.05 3.0 | 288 | – |
| – | 298 | – |
| – | 308 | – |
| – | 318 | – |
| Effect of Cl¯ concentration (at constant value of ionic strength I = 0.4 M and [Na+] = 0.4 M) | ||
| 0.05 3.0 | 298 | 0.05 |
| – | – | 0.10 |
| – | – | 0.20 |
| – | – | 0.30 |
| – | – | 0.40 |
| Temperature, K | k1,obs 103 Ms−1 | k1.10 s−1 |
|---|---|---|
| 288 | 10.82 ± 0.18 | 36.08 ± 0.59 |
| 298 | 13.55 ± 0.07 | 45.17 ± 0.22 |
| 308 | 28.24 ± 0.24 | 94.13 ± 0.79 |
| 318 | 37.36 ± 0.22 | 124.54 ± 0.74 |
| [Cl¯], M | k1,obs 102, Ms−1 | k1, s−1 |
|---|---|---|
| 0.00 | 14.36 ± 0.73 | 47.88 ± 2.43 |
| 0.05 | 16.12 ± 0.89 | 53.74 ± 2.96 |
| 0.15 | 20.30 ± 1.35 | 67.69 ± 4.50 |
| 0.25 | 28.39 ± 0.96 | 94.64 ± 3.21 |
| 0.35 | 31.78 ± 1.17 | 105.93 ± 3.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luty-Błocho, M.; Wojnicki, M.; Csapo, E.; Fitzner, K. On the Rate of Interaction of Sodium Borohydride with Platinum (IV) Chloride Complexes in Alkaline Media. Materials 2021, 14, 3137. https://doi.org/10.3390/ma14113137
Luty-Błocho M, Wojnicki M, Csapo E, Fitzner K. On the Rate of Interaction of Sodium Borohydride with Platinum (IV) Chloride Complexes in Alkaline Media. Materials. 2021; 14(11):3137. https://doi.org/10.3390/ma14113137
Chicago/Turabian StyleLuty-Błocho, Magdalena, Marek Wojnicki, Edit Csapo, and Krzysztof Fitzner. 2021. "On the Rate of Interaction of Sodium Borohydride with Platinum (IV) Chloride Complexes in Alkaline Media" Materials 14, no. 11: 3137. https://doi.org/10.3390/ma14113137
APA StyleLuty-Błocho, M., Wojnicki, M., Csapo, E., & Fitzner, K. (2021). On the Rate of Interaction of Sodium Borohydride with Platinum (IV) Chloride Complexes in Alkaline Media. Materials, 14(11), 3137. https://doi.org/10.3390/ma14113137









