Advanced Biofuels Based on Fischer–Tropsch Synthesis for Applications in Diesel Engines
Abstract
1. Introduction
- High quality and higher environmental aspects than the existing first generation biofuels;
- Usage in existing engines without any modifications;
- Usage should not affect the engine performance;
- Guaranteed miscibility with petroleum fuels in a defined proportion;
- Easy integration into the current fuel market;
- Production from waste sources is preferred.
1.1. First-Generation Biofuels
1.2. Second-Generation Biofuels
1.3. Third-Generation Biofuels
1.4. Fischer–Tropsch Synthesis
- GTL—natural gas;
- BTL—gasified biomass;
- CTL—coal dust.
2. Materials and Methods
- 100 vol. % pure fossil diesel (Diesel).
- 7 vol. % FT-diesel distillation fraction and 93 vol. % fossil diesel (FT7).
- 15 vol. % FT-diesel distillation fraction and 85 vol. % fossil diesel (FT15).
- 30 vol. % FT-diesel distillation fraction and 70 vol. % fossil diesel (FT30).
- 50 vol. % FT-diesel distillation fraction and 50 vol. % fossil diesel (FT50).
- 70 vol. % FT-diesel distillation fraction and 30 vol. % fossil diesel (FT70).
- 100 vol. % pure FT-diesel distillation fraction (FT100).
3. Results
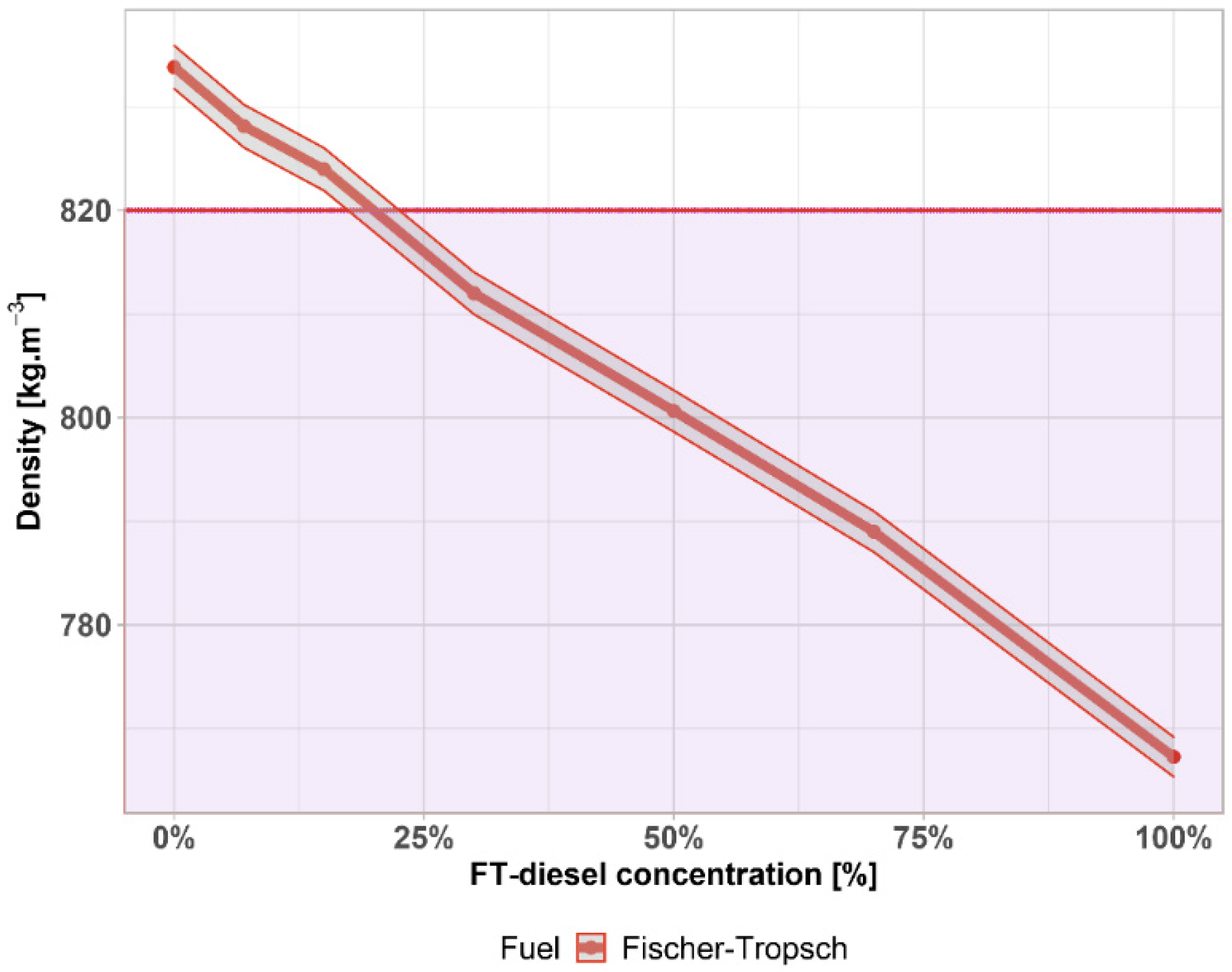
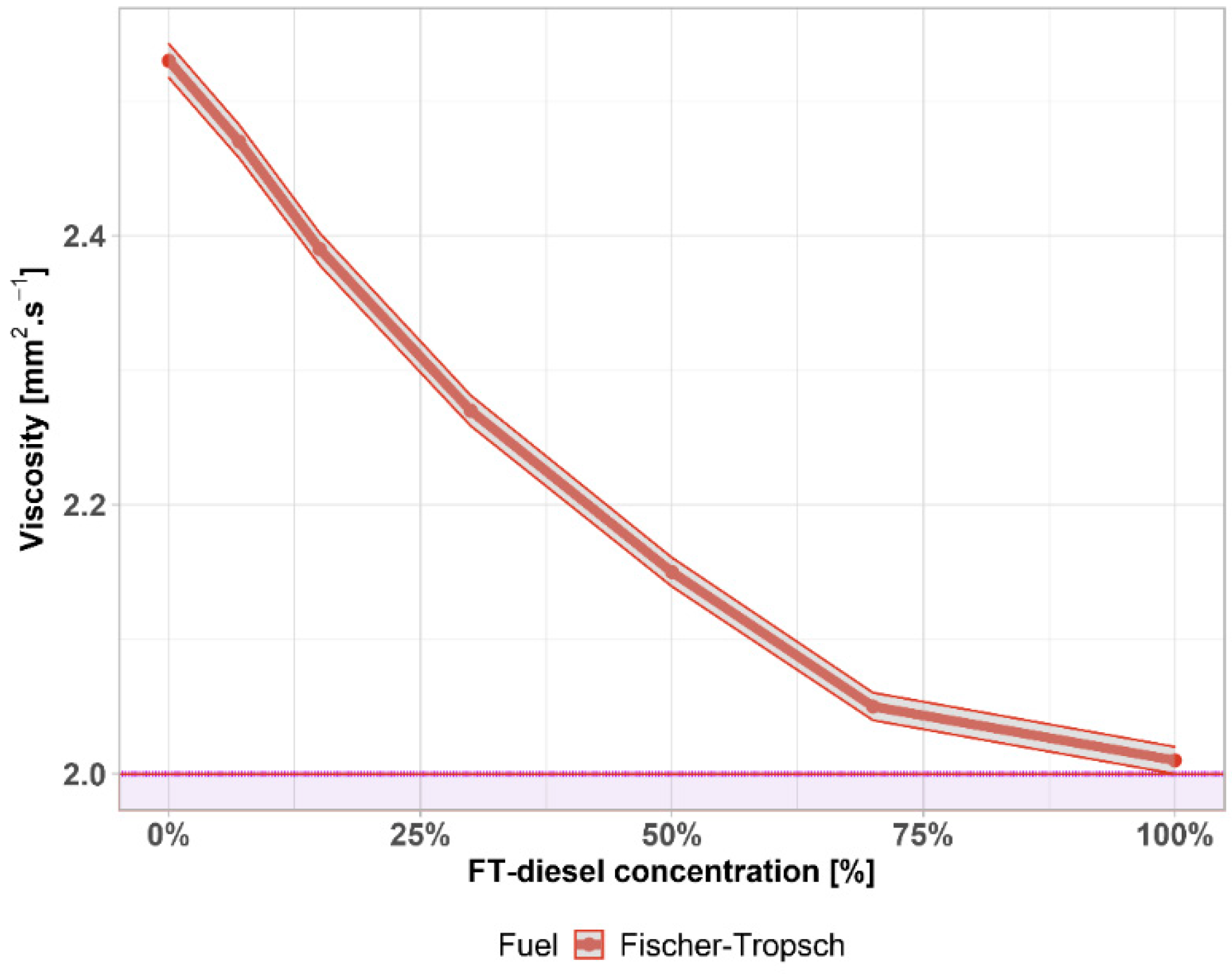
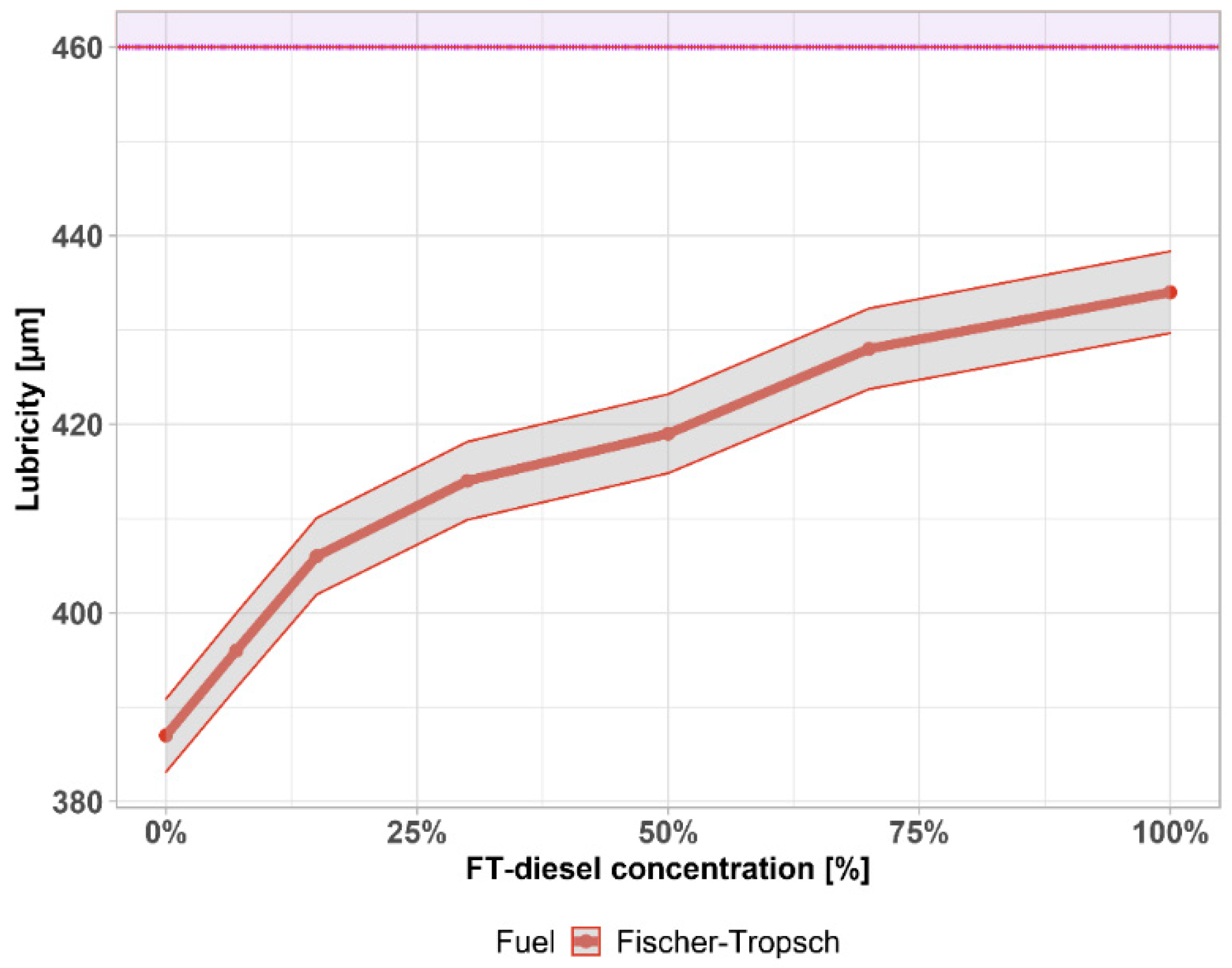
3.1. Fuel Parameters
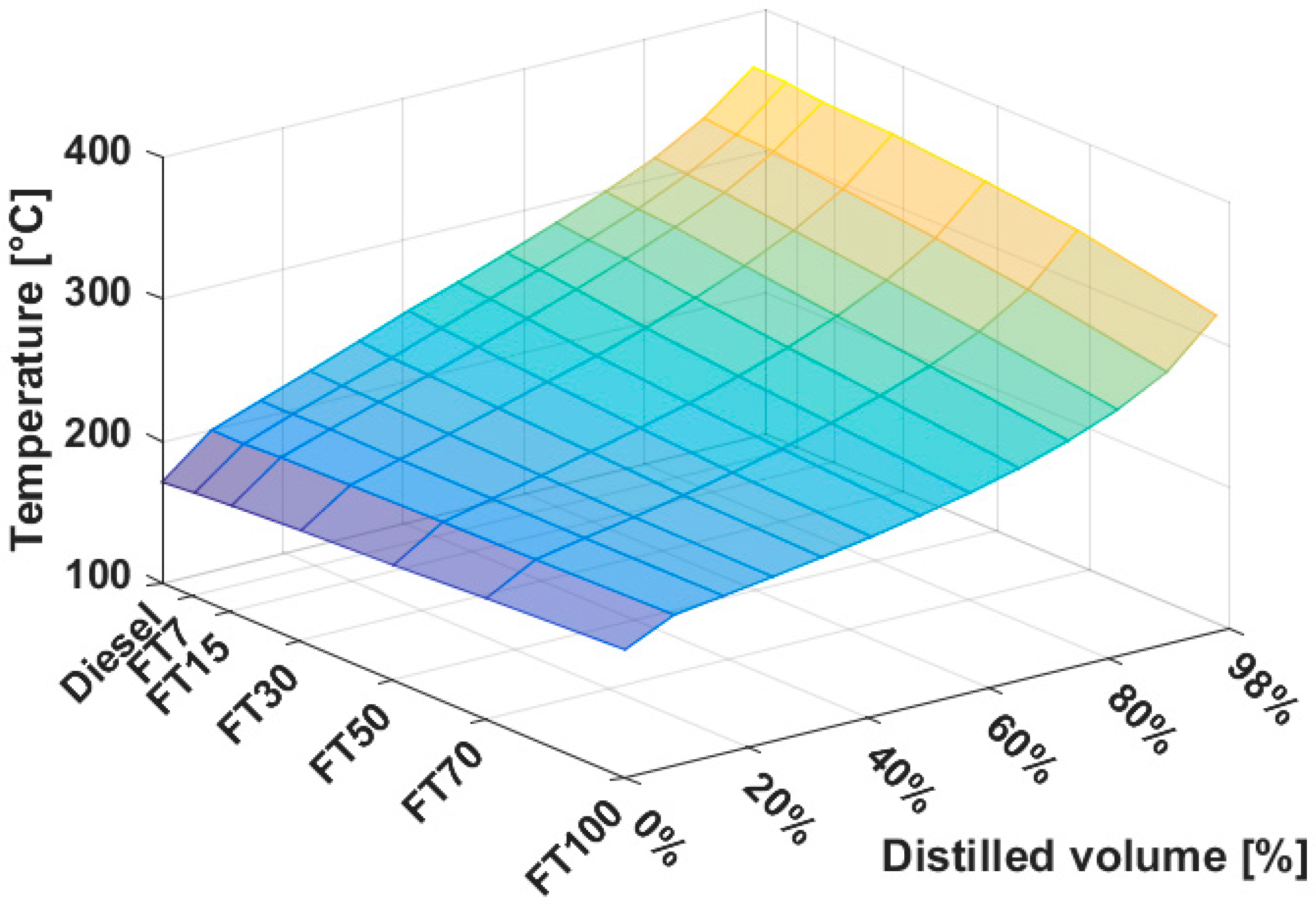
3.2. Distillation Properties
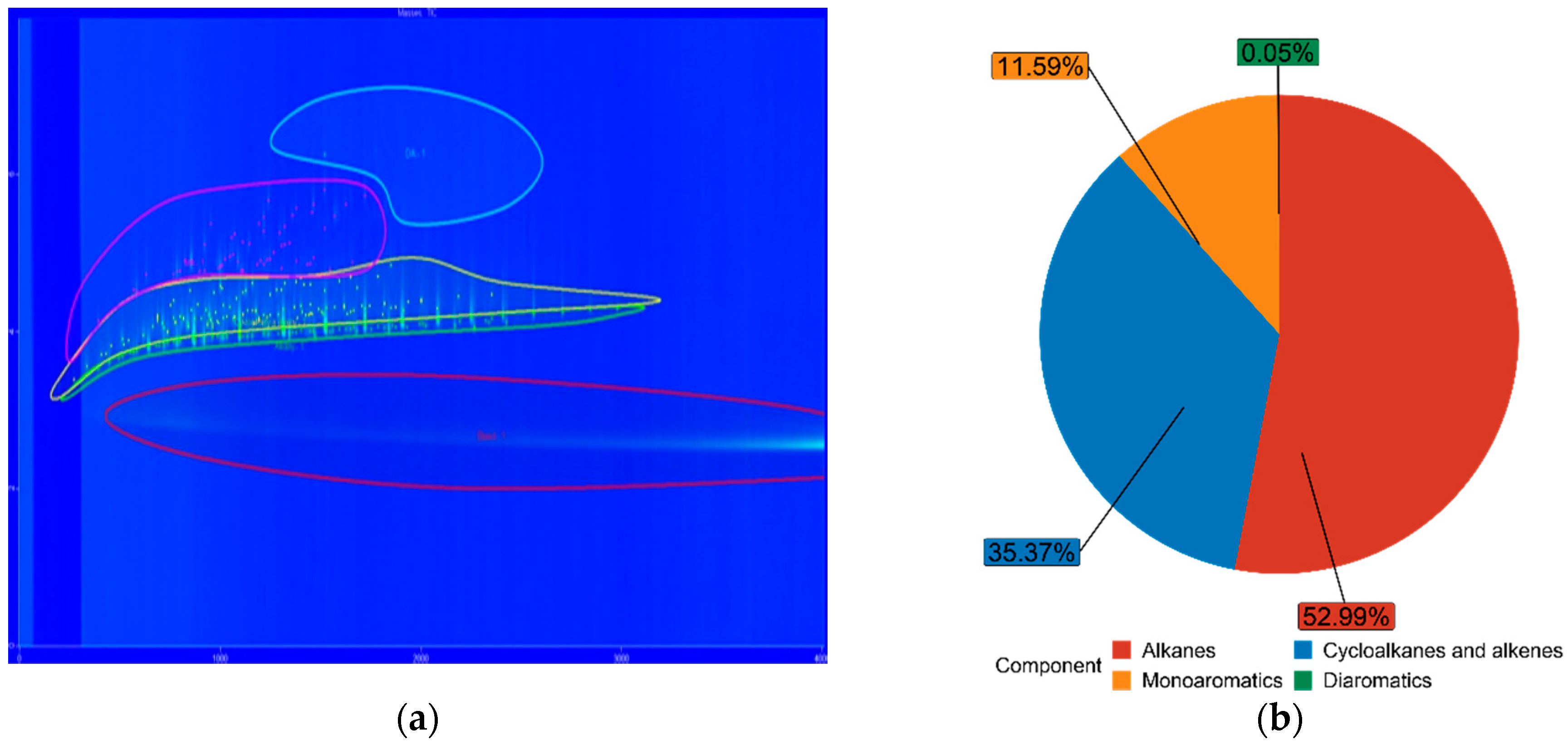
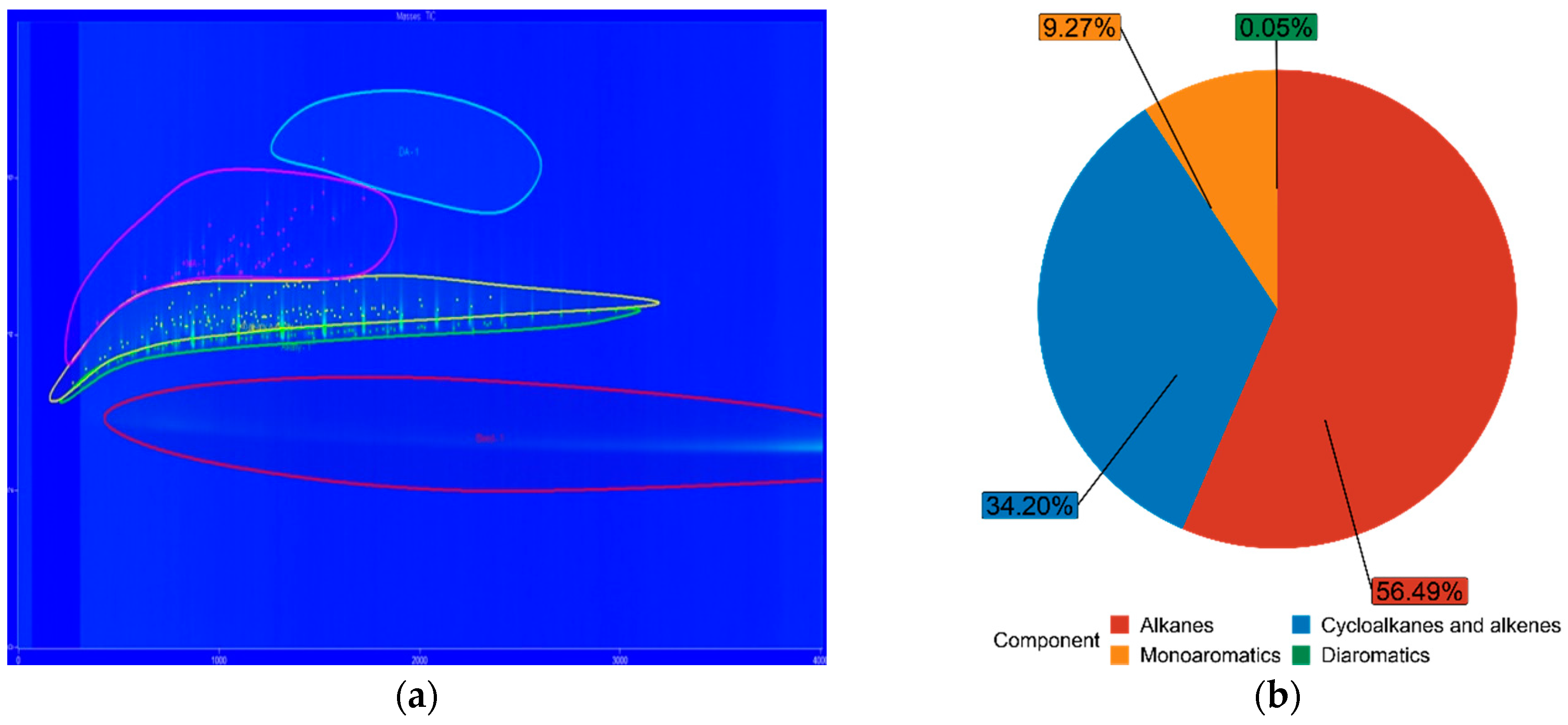
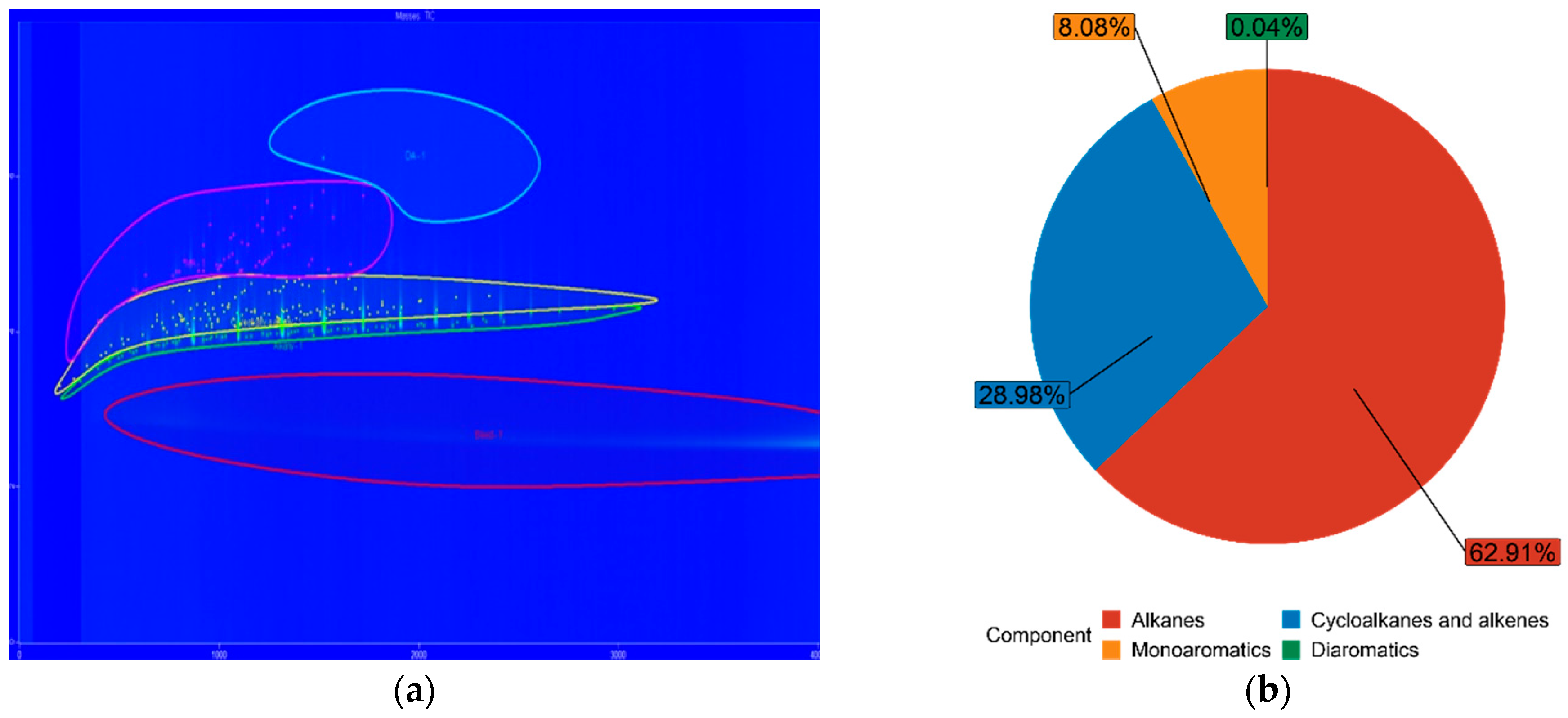
3.3. Fuel Composition Characteristics
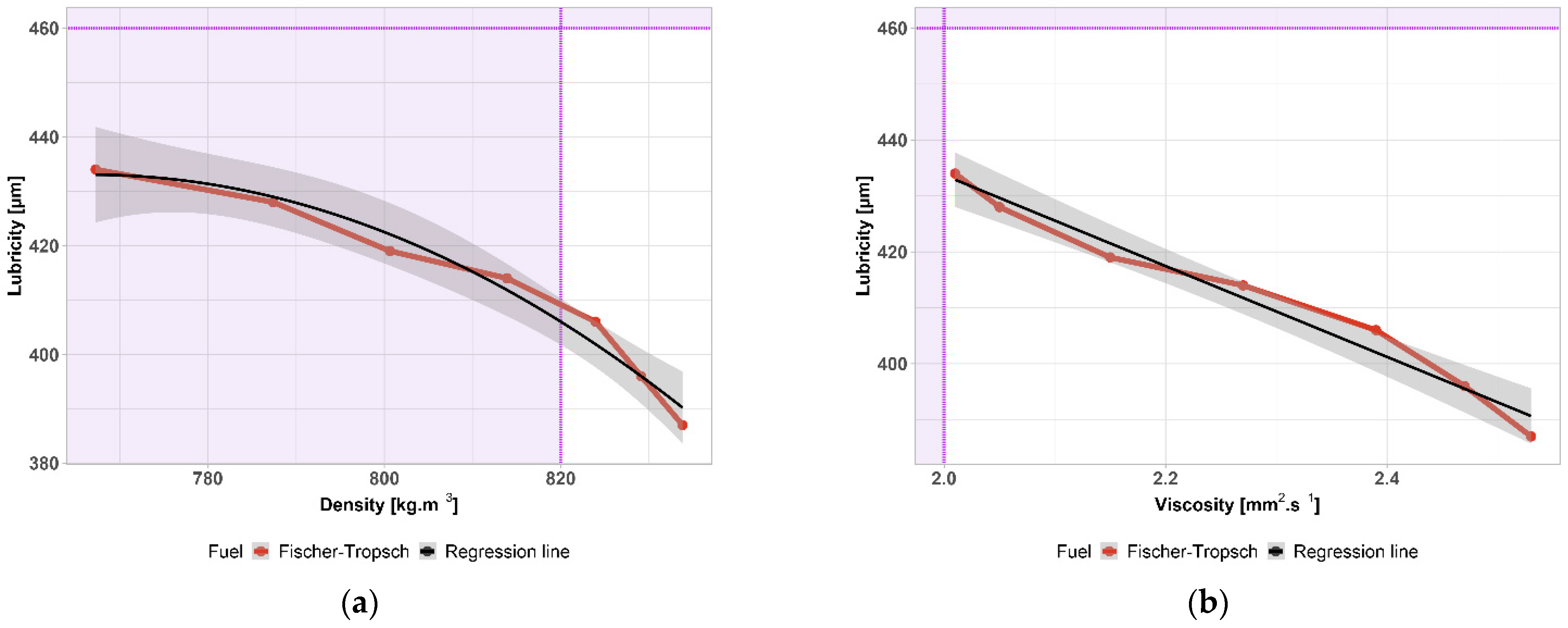
4. Discussion
5. Conclusions
- The physicochemical properties of Fischer–Tropsch diesel are almost comparable to conventional diesel. In properties such as cetane number/index and heat of combustion, synthetic fuels are even better;
- FT-diesel is an advanced biofuel which can be produced from renewable energy sources and waste materials;
- The fuel meets the sustainability criteria;
- The fuel is without the negative effects of biodiesel in the form of FAME. It is a pure hydrocarbon fuel without sulfur or polyaromatic hydrocarbons. Emissions are cleaner and lower.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Office of Energy Efficiency & Renewable Energy: Biofuel Basics. 2021. Available online: https://www.energy.gov/eere/office-energy-efficiency-renewable-energy (accessed on 15 May 2021).
- DIRECTIVE (EU) 2018/2001 of the European Parliament and of the COUNCIL of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018L2001 (accessed on 16 May 2021).
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental Sustainability of Biofuels: A Review. Proc. R. Soc. A 2020, 476, 20200351. [Google Scholar] [CrossRef]
- Laverdura, U.P.; Rossi, L.; Ferella, F.; Courson, C.; Zarli, A.; Alhajyoussef, R.; Gallucci, K. Selective Catalytic Hydrogenation of Vegetable Oils on Lindlar Catalyst. ACS Omega 2020, 5, 22901–22913. [Google Scholar] [CrossRef] [PubMed]
- Kazimierski, P.; Hercel, P.; Januszewicz, K.; Kardaś, D. Pre-Treatment of Furniture Waste for Smokeless Charcoal Production. Materials 2020, 13, 3188. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Techno-Economic Assessment of a Scaled-Up Meat Waste Biorefinery System: A Simulation Study. Materials 2019, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Pulka, J.; Manczarski, P.; Stępień, P.; Styczyńska, M.; Koziel, J.A.; Białowiec, A. Waste-to-Carbon: Is the Torrefied Sewage Sludge with High Ash Content a Better Fuel or Fertilizer? Materials 2020, 13, 954. [Google Scholar] [CrossRef] [PubMed]
- Matas Güell, B.; Sandquist, J.; Sørum, L. Gasification of Biomass to Second Generation Biofuels: A Review. J. Energy Resour. Technol. 2013, 135, 014001. [Google Scholar] [CrossRef]
- Moazeni, F.; Chen, Y.-C.; Zhang, G. Enzymatic Transesterification for Biodiesel Production from Used Cooking Oil, a Review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Avagyan, A.B.; Singh, B. Biodiesel from Plant Oil and Waste Cooking Oil. In Biodiesel: Feedstocks, Technologies, Economics and Barriers; Springer: Singapore, 2019; pp. 15–75. ISBN 9789811357459. [Google Scholar]
- Smoliński, A.; Karwot, J.; Bondaruk, J.; Bąk, A. The Bioconversion of Sewage Sludge to Bio-Fuel: The Environmental and Economic Benefits. Materials 2019, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Dębek, C. Modification of Pyrolytic Oil from Waste Tyres as a Promising Method for Light Fuel Production. Materials 2019, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- Świechowski, K.; Stępień, P.; Syguła, E.; Koziel, J.A.; Białowiec, A. Lab-Scale Study of Temperature and Duration Effects on Carbonized Solid Fuels Properties Produced from Municipal Solid Waste Components. Materials 2021, 14, 1191. [Google Scholar] [CrossRef] [PubMed]
- Kosakowski, W.; Bryszewska, M.A.; Dziugan, P. Biochars from Post-Production Biomass and Waste from Wood Management: Analysis of Carbonization Products. Materials 2020, 13, 4971. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, H.; Duan, C.; Xu, R.; Zhang, Z.; She, D.; Zheng, J. The Eco-Friendly Biochar and Valuable Bio-Oil from Caragana Korshinskii: Pyrolysis Preparation, Characterization, and Adsorption Applications. Materials 2020, 13, 3391. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, O.; Vrtiška, D.; Straka, P.; Vráblík, A.; Jenčík, J.; Šimáček, P. Hydrocracking of a Heavy Vacuum Gas Oil with Fischer–Tropsch Wax. Energies 2020, 13, 5497. [Google Scholar] [CrossRef]
- Kröger, M.; Müller-Langer, F. Review on Possible Algal-Biofuel Production Processes. Biofuels 2012, 3, 333–349. [Google Scholar] [CrossRef]
- Urtubia, H.O.; Betanzo, L.B.; Vásquez, M. Microalgae and Cyanobacteria as Green Molecular Factories: Tools and Perspectives. In Algae-Organisms for Imminent Biotechnology; Thajuddin, N., Dhanasekaran, D., Eds.; IntechOpen Limited: London, UK, 2016; ISBN 978-953-51-2431-3. [Google Scholar]
- Carneiro, M.L.N.M.; Pradelle, F.; Braga, S.L.; Gomes, M.S.P.; Martins, A.R.F.A.; Turkovics, F.; Pradelle, R.N.C. Potential of Biofuels from Algae: Comparison with Fossil Fuels, Ethanol and Biodiesel in Europe and Brazil through Life Cycle Assessment (LCA). Renew. Sustain. Energy Rev. 2017, 73, 632–653. [Google Scholar] [CrossRef]
- Hsu, C.S.; Robinson, P.R. Springer Handbook of Petroleum Technology, 2nd ed.; Springer: New York, NY, USA, 2017; ISBN 978-3-319-49345-9. [Google Scholar]
- Choi, Y.H.; Jang, Y.J.; Park, H.; Kim, W.Y.; Lee, Y.H.; Choi, S.H.; Lee, J.S. Carbon Dioxide Fischer–Tropsch Synthesis: A New Path to Carbon-Neutral Fuels. Appl. Catal. B Environ. 2017, 202, 605–610. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Mahmoudi, M.; Doustdar, O.; Jahangiri, H.; Tsolakis, A.; Gu, S.; LechWyszynski, M. A Review of Fischer Tropsch Synthesis Process, Mechanism, Surface Chemistry and Catalyst Formulation. Biofuels Eng. 2017, 2, 11–31. [Google Scholar] [CrossRef]
- Albuquerque, J.S.; Costa, F.O.; Barbosa, B.V.S. Fischer–Tropsch Synthesis: Analysis of Products by Anderson–Schulz–Flory Distribution Using Promoted Cobalt Catalyst. Catal. Lett. 2019, 149, 831–839. [Google Scholar] [CrossRef]
- Chen, W.; Lin, T.; Dai, Y.; An, Y.; Yu, F.; Zhong, L.; Li, S.; Sun, Y. Recent Advances in the Investigation of Nanoeffects of Fischer–Tropsch Catalysts. Catal. Today 2018, 311, 8–22. [Google Scholar] [CrossRef]
- Shafer, W.D.; Gnanamani, M.K.; Graham, U.M.; Yang, J.; Masuku, C.M.; Jacobs, G.; Davis, B.H. Fischer–Tropsch: Product Selectivity–The Fingerprint of Synthetic Fuels. Catalysts 2019, 9, 259. [Google Scholar] [CrossRef]
- Speight, J.G. The Chemistry and Technology of Petroleum, 5th ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4398-7389-2. [Google Scholar]
- Kaiser, M.J.; de Klerk, A.; Gary, J.H.; Handwerk, G.E. Petroleum Refining: Technology, Economics and Markets; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-1-4665-6301-8. [Google Scholar]
- Ancheyta Juárez, J. Modeling and Simulation of Catalytic Reactors for Petroleum Refining; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-0-470-18530-8. [Google Scholar]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A Review of the Catalytic Hydrogenation of Carbon Dioxide into Value-Added Hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Marion, M.-C.; Bertoncini, F.; Hugues, F.; Forestiere, A. DGMK/SCI-Conference, Synthesis Gas Chemistry. In Comprehensive Characterisation of Products from Cobalt Catalysed FischerTropsch Reaction; Ernst, S., Erdöl, D.W.G.F., Kohle, E.U., Eds.; DGMK-Tagungsbericht: Dresden, Germany, 2006; ISBN 978-3-936418-57-6. [Google Scholar]
- De Klerk, A. Fischer–Tropsch Refining, 1st ed.; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-63560-3. [Google Scholar]
- Warheit, D.B. Hazard and Risk Assessment Strategies for Nanoparticle Exposures: How Far Have We Come in the Past 10 Years? F1000Research 2018, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Morgeneyer, M.; Aguerre-Chariol, O.; Bressot, C. STEM Imaging to Characterize Nanoparticle Emissions and Help to Design Nanosafer Paints. Chem. Eng. Res. Des. 2018, 136, 663–674. [Google Scholar] [CrossRef]
- Bressot, C.; Aubry, A.; Pagnoux, C.; Aguerre-Chariol, O.; Morgeneyer, M. Assessment of Functional Nanomaterials in Medical Applications: Can Time Mend Public and Occupational Health Risks Related to the Products’ Fate? J. Toxicol. Environ. Health Part A 2018, 81, 957–973. [Google Scholar] [CrossRef]
- Bressot, C.; Shandilya, N.; Jayabalan, T.; Fayet, G.; Voetz, M.; Meunier, L.; Le Bihan, O.; Aguerre-Chariol, O.; Morgeneyer, M. Exposure Assessment of Nanomaterials at Production Sites by a Short Time Sampling (STS) Approach Strategy and First Results of Measurement Campaigns. Process Saf. Environ. Prot. 2018, 116, 324–332. [Google Scholar] [CrossRef]
- Morgeneyer, M.; Shandilya, N.; Chen, Y.M.; Le Bihan, O. Use of a Modified Taber Abrasion Apparatus for Investigating the Complete Stress State during Abrasion and In-Process Wear Particle Aerosol Generation. Chem. Eng. Res. Des. 2015, 93, 251. [Google Scholar] [CrossRef]
- Paulick, M.; Morgeneyer, M.; Kwade, A. A New Method for the Determination of Particle Contact Stiffness. Granul. Matter 2015, 17, 83–93. [Google Scholar] [CrossRef]
- Röck, M.; Morgeneyer, M.; Schwedes, J.; Kadau, D.; Brendel, L.; Wolf, D.E. Steady State Flow of Cohesive and Non-Cohesive Powders. Granul. Matter 2008, 10, 285–293. [Google Scholar] [CrossRef]
- Saleh, K.; Jaoude, M.-T.M.A.; Morgeneyer, M.; Lefrancois, E.; Le Bihan, O.; Bouillard, J. Dust generation from powders: A characterization test based on stirred fluidization. Powder Technol. 2014, 255, 141–148. [Google Scholar] [CrossRef]
- Dancuart, L.P.; de Haan, R.; de Klerk, A. Chapter 6-Processing of Primary Fischer–Tropsch Products. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; pp. 482–532. [Google Scholar]
- Akhmedov, V.M.; Al-Khowaiter, S.H.; Akhmedov, E.; Sadikhov, A. Low Temperature Hydrocracking of Hydrocarbons on Ni-Supported Catalysts. Appl. Catal. A Gen. 1999, 181, 51–61. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Kaka khel, T.; Azkaar, M.; Engblom, S.; Murzin, D. Catalytic Hydroisomerization of Long-Chain Hydrocarbons for the Production of Fuels. Catalysts 2018, 8, 534. [Google Scholar] [CrossRef]
- Bressanin, J.M.; Klein, B.C.; Chagas, M.F.; Watanabe, M.D.B.; de Mesquita Sampaio, I.L.; Bonomi, A.; de Morais, E.R.; Cavalett, O. Techno-Economic and Environmental Assessment of Biomass Gasification and Fischer–Tropsch Synthesis Integrated to Sugarcane Biorefineries. Energies 2020, 13, 4576. [Google Scholar] [CrossRef]
- Frilund, C.; Simell, P.; Kurkela, E.; Eskelinen, P. Experimental Bench-Scale Study of Residual Biomass Syngas Desulfurization Using ZnO-Based Adsorbents. Energy Fuels 2020, 34, 3326–3335. [Google Scholar] [CrossRef] [PubMed]
- Ra, H.W.; Mun, T.-Y.; Hong, S.J.; Chun, D.H.; Lee, H.T.; Yoon, S.M.; Moon, J.H.; Park, S.J.; Lee, S.H.; Yang, J.H.; et al. Indirect Coal Liquefaction by Integrated Entrained Flow Gasification and Rectisol/Fischer–Tropsch Processes for Producing Automobile Diesel Substitutes. Energy 2021, 219, 119597. [Google Scholar] [CrossRef]
- Sajjad, H.; Masjuki, H.H.; Varman, M.; Kalam, M.A.; Arbab, M.I.; Imtenan, S.; Ashraful, A.M. Influence of Gas-to-Liquid (GTL) Fuel in the Blends of Calophyllum Inophyllum Biodiesel and Diesel: An Analysis of Combustion–Performance–Emission Characteristics. Energy Convers. Manag. 2015, 97, 42–52. [Google Scholar] [CrossRef]
- Du, J.; Sun, W.; Wang, X.; Li, G.; Tan, M.; Fan, L. Experimental Study on Combustion and Particle Size Distribution of a Common Rail Diesel Engine Fueled with GTL/Diesel Blends. Appl. Therm. Eng. 2014, 70, 430–440. [Google Scholar] [CrossRef]
- Parravicini, M.; Barro, C.; Boulouchos, K. Experimental Characterization of GTL, HVO, and OME Based Alternative Fuels for Diesel Engines. Fuel 2021, 292, 120177. [Google Scholar] [CrossRef]
- Sadeq, A.M.; Ahmed, S.F.; Sleiti, A.K. Transient 3D Simulations of Turbulent Premixed Flames of Gas-to-Liquid (GTL) Fuel in a Fan-Stirred Combustion Vessel. Fuel 2021, 291, 120184. [Google Scholar] [CrossRef]
- Schaberg, P.; Botha, J.; Schnell, M.; Hermann, H.-O.; Pelz, N.; Maly, R. Emissions Performance of GTL Diesel Fuel and Blends with Optimized Engine Calibrations. SAE Trans. 2005, 114, 1074–1087. [Google Scholar]
- Kuszewski, H.; Jaworski, A.; Mądziel, M. Lubricity of Ethanol–Diesel Fuel Blends—Study with the Four-Ball Machine Method. Materials 2021, 14, 2492. [Google Scholar] [CrossRef] [PubMed]
- Gough, R.V.; Bruno, T.J. Composition-Explicit Distillation Curves of Alternative Turbine Fuels. Energy Fuels 2013, 27, 294–302. [Google Scholar] [CrossRef]
- Lissitsyna, K.; Huertas, S.; Quintero, L.C.; Polo, L.M. PIONA Analysis of Kerosene by Comprehensive Two-Dimensional Gas Chromatography Coupled to Time of Flight Mass Spectrometry. Fuel 2014, 116, 716–722. [Google Scholar] [CrossRef]
- Van der Westhuizen, R.; Crous, R.; de Villiers, A.; Sandra, P. Comprehensive Two-Dimensional Gas Chromatography for the Analysis of Fischer–Tropsch Oil Products. J. Chromatogr. A 2010, 1217, 8334–8339. [Google Scholar] [CrossRef]
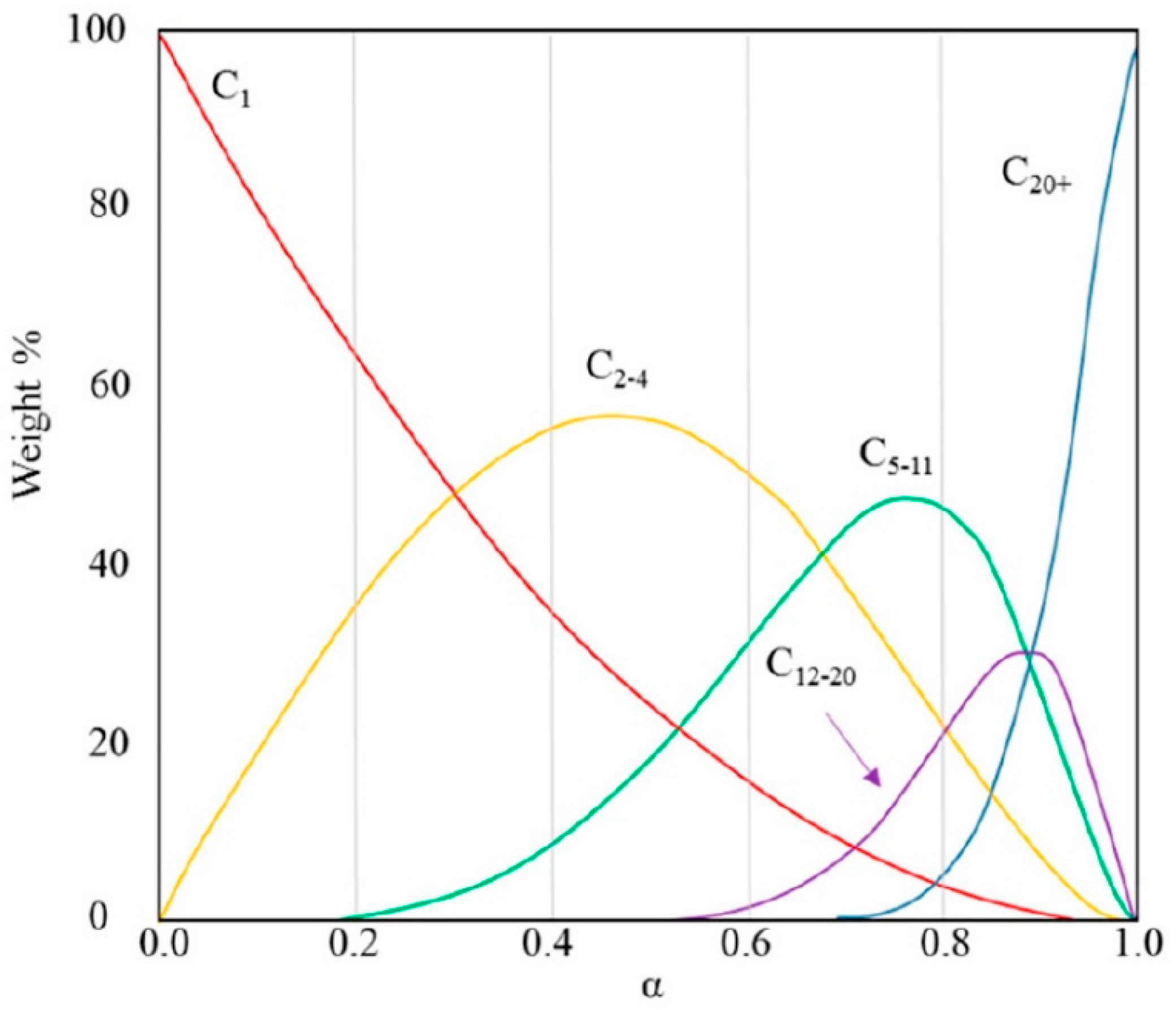
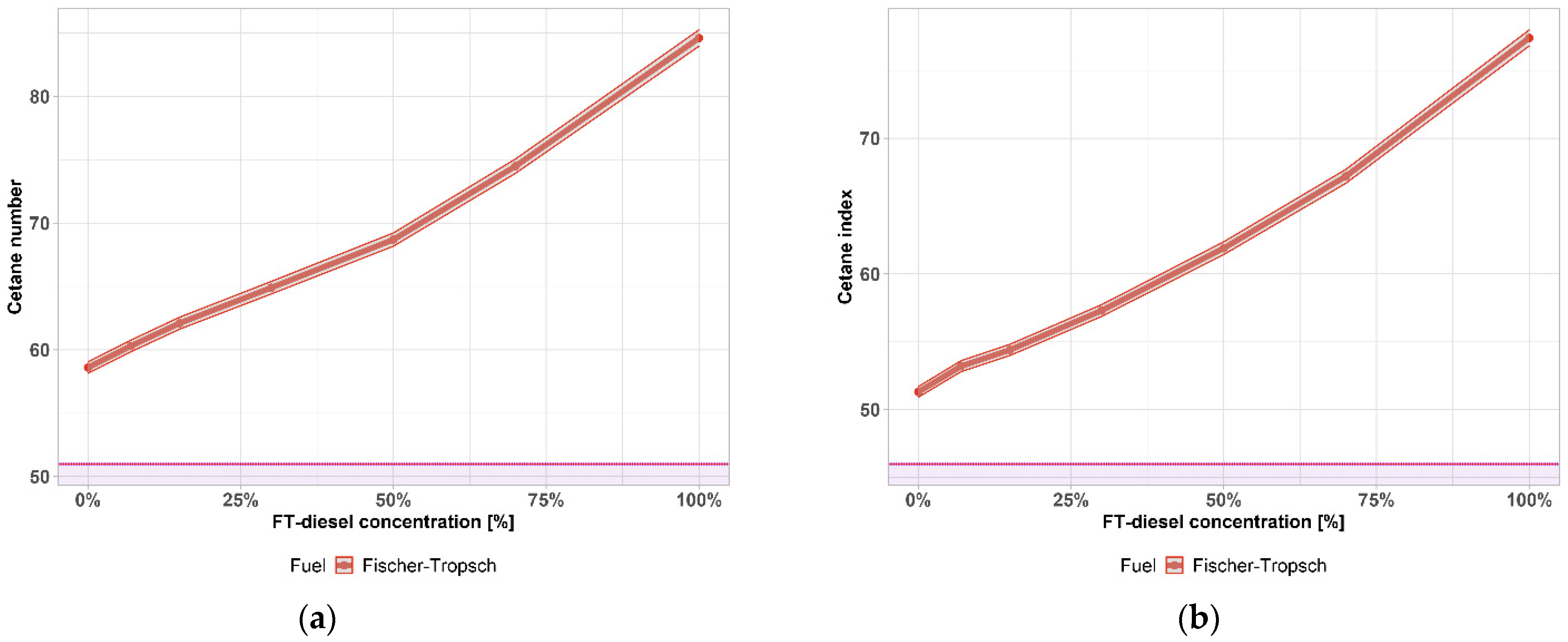
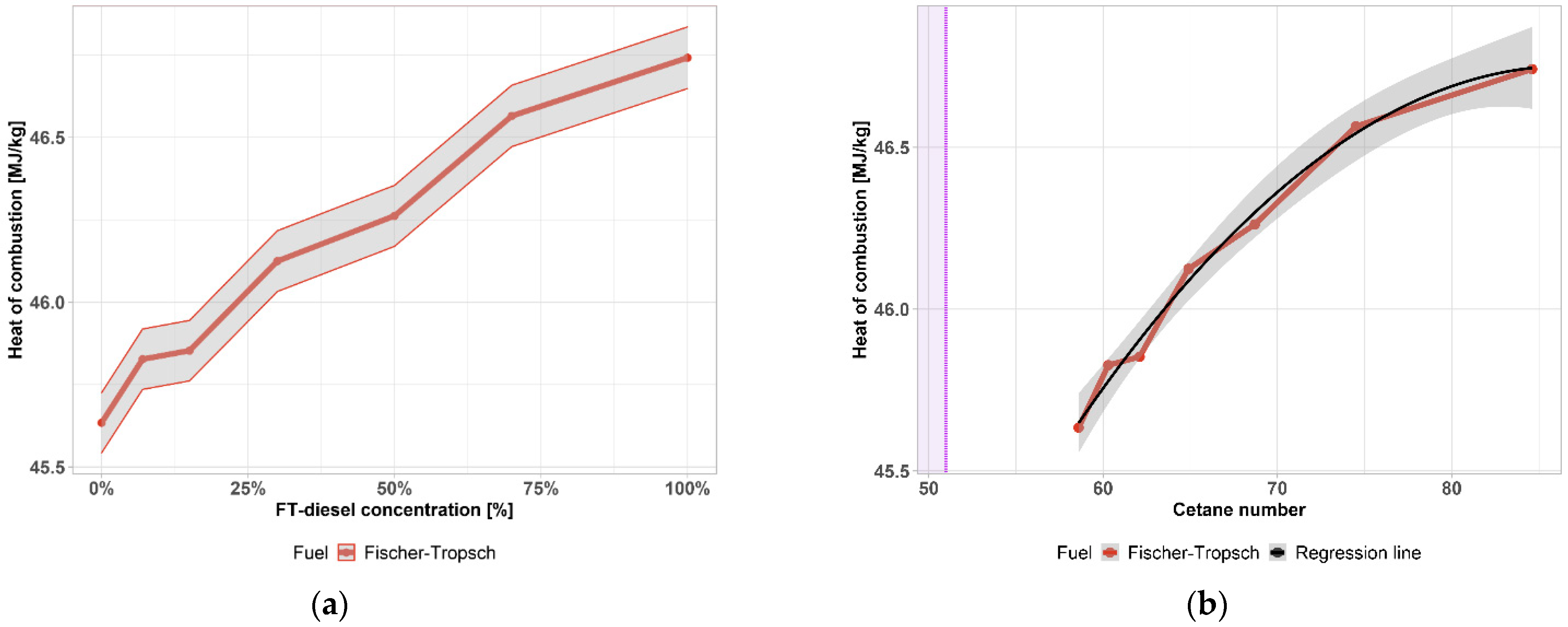

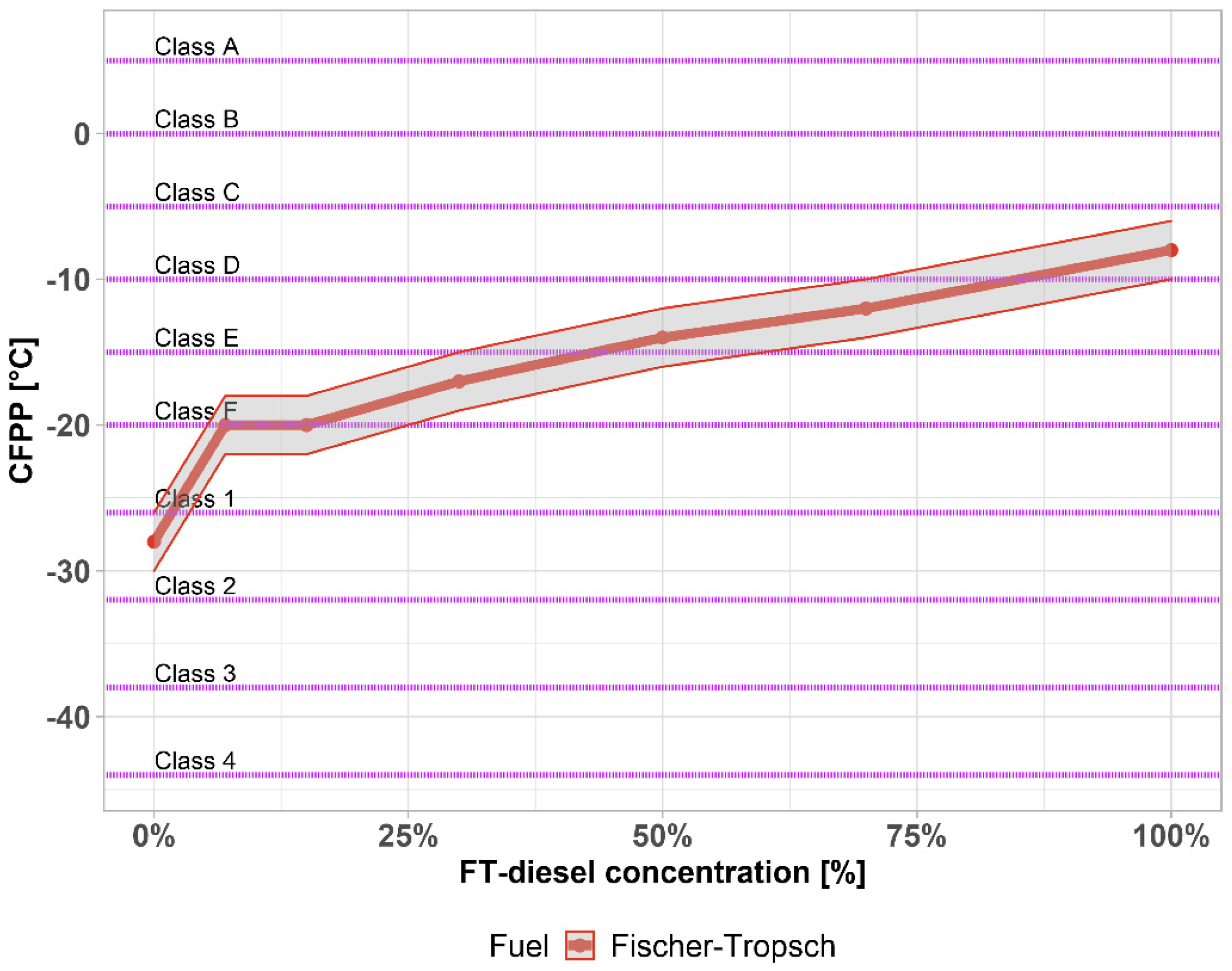
| Components | Fe [wt. %] | Co [wt. %] |
|---|---|---|
| Methane | 4.3 | 5.6 |
| Ethylene | 1 | 0.1 |
| Ethane | 1.0 | 1.0 |
| C3–C4 alkenes | 6.0 | 3.4 |
| C3–C4 alkanes | 1.8 | 1.8 |
| C5–C10 alkenes | 7.7 | 7.8 |
| C5–C10 alkanes | 3.3 | 12.0 |
| C5–C10 oxygenated compounds | 1.3 | 0.2 |
| C11–C22 alkenes | 5.7 | 1.1 |
| C11–C22 alkanes | 13.5 | 20.8 |
| C11–C22 oxygenated compounds | 0.3 | 0.0 |
| C22+ alkenes | 0.7 | 0.0 |
| C22+ alkanes | 49.2 | 44.6 |
| Alcohols (dissolved in water) | 3.9 | 1.4 |
| Carboxylic acid (in water) | 0.3 | 0.2 |
| Distillation Range [°C] | Oil [vol. %] | Wax [vol. %] |
|---|---|---|
| 30−160 °C | 44 | 3 |
| 160−270 °C | 43 | 4 |
| 270−370 °C | 13 | 25 |
| 370−500 °C | 0 | 40 |
| >500 °C | 0 | 28 |
| Machine Assembly | LECO PEGASUS® BT 4D GC × GC-TOFMS |
|---|---|
| Primary column | Rxi-5SilMS, 30 m × 0.25 mm, d = 0.25 µm |
| Secondary column | Rxi-17SilMS, 1.3 m × 0.15 mm, d = 0.15 µm, |
| Carrier gas | He, 1 mL/min |
| Temperature program | 40 °C (1.5 min), 40–300 °C (4°C/min), 300 °C (0.5 min) |
| Injector | 20–320 °C, 720 °C/min, Split 500:1 |
| Sample volume | 0.2 µL |
| Ion Source | 250 °C |
| Modulation period | 8 s |
| Property | A | B | C | p-Value | |
|---|---|---|---|---|---|
| Density | 412.000 | −40.809 | −10.661 | 0.9619 | 6.442 × 10−4 |
| Viscosity | 596.180 | −81.239 | 0 * | 0.9687 | 3.767 × 10−5 |
| Mixture | Alkanes | Cycloalkanes and Alkenes | Monoaromatics | Diaromatics |
|---|---|---|---|---|
| Diesel | 52.99% | 35.37% | 11.59% | 0.05% |
| FT7 | 56.49% | 34.20% | 9.27% | 0.05% |
| FT15 | 62.91% | 28.98% | 8.08% | 0.04% |
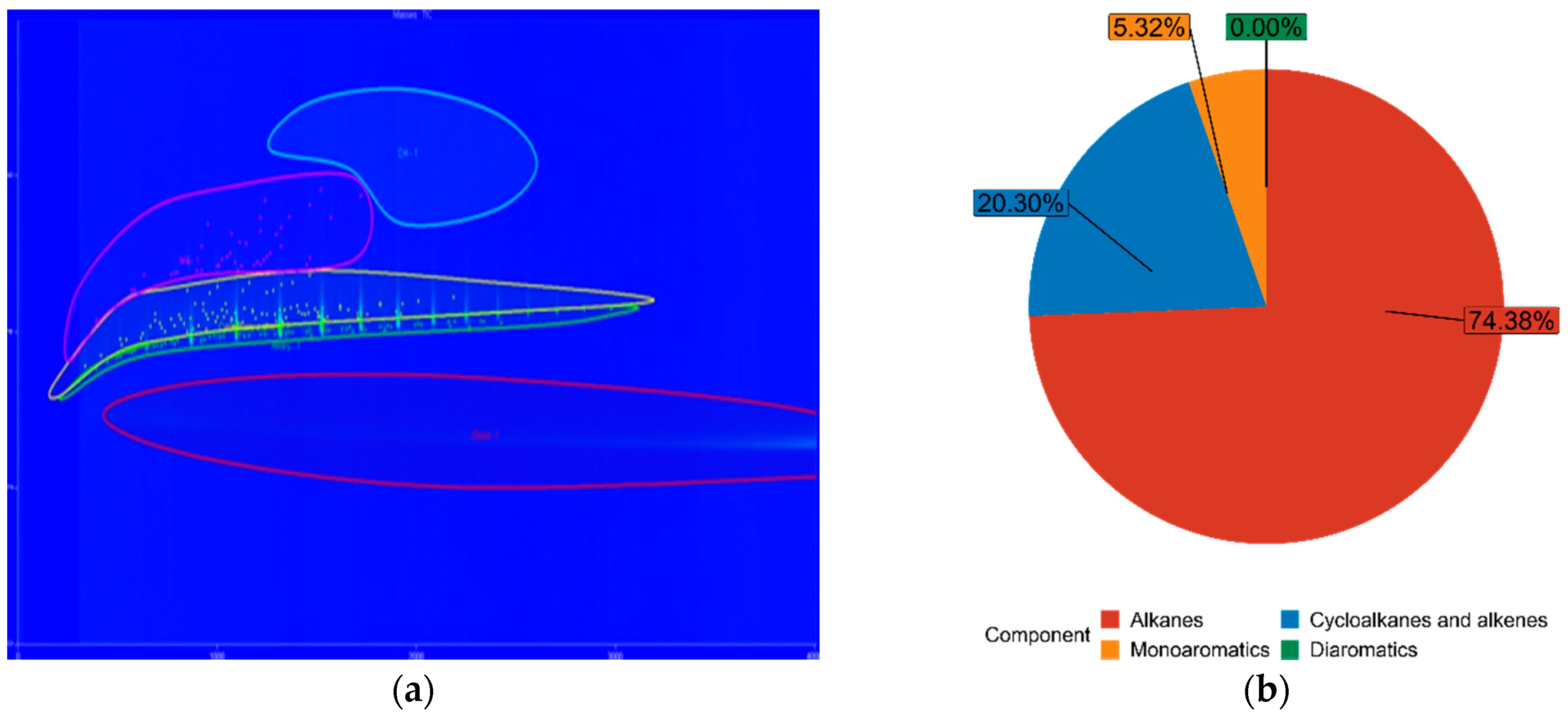
| FT30 | 74.38% | 20.30% | 5.32% | 0.00% |
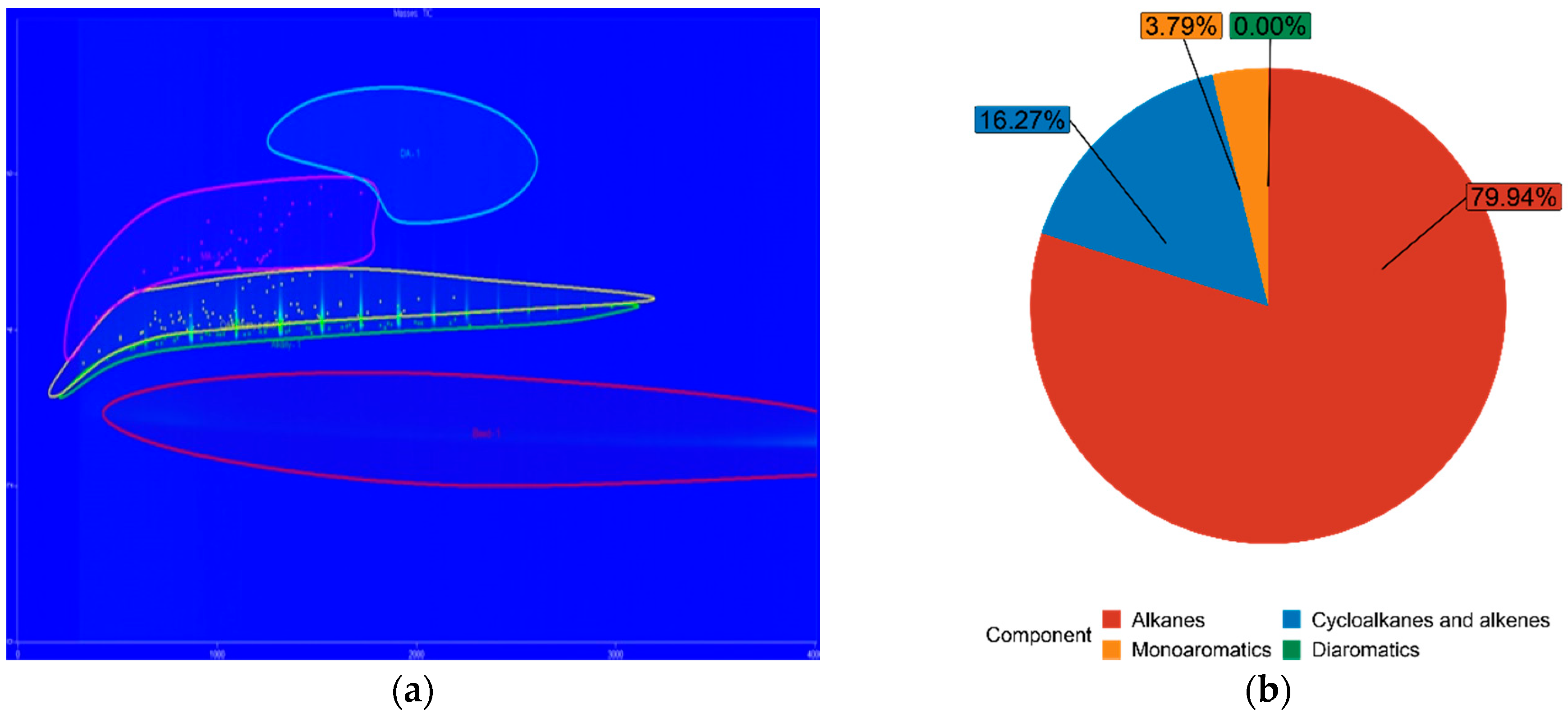
| FT50 | 79.94% | 16.27% | 3.79% | 0.00% |
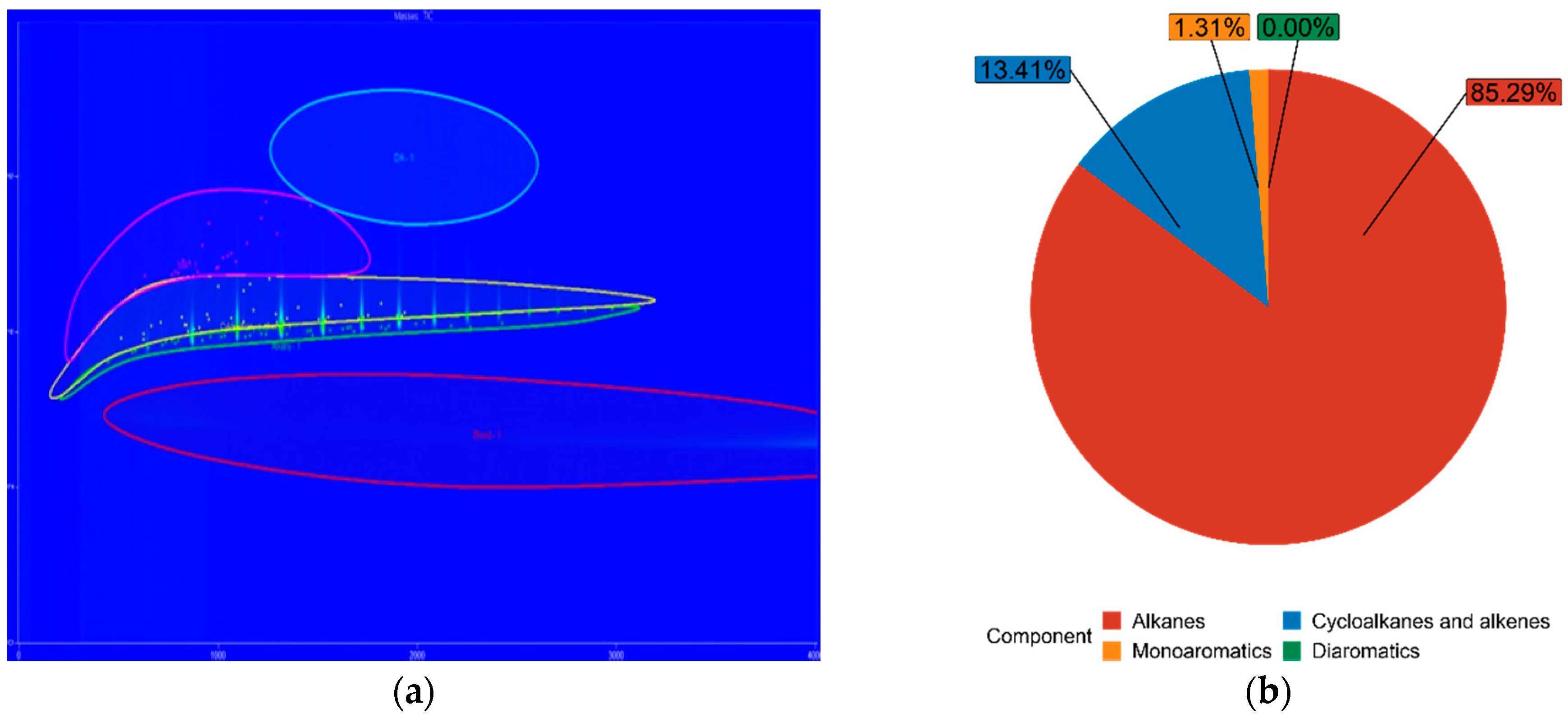
| FT70 | 85.29% | 13.41% | 1.31% | 0.00% |
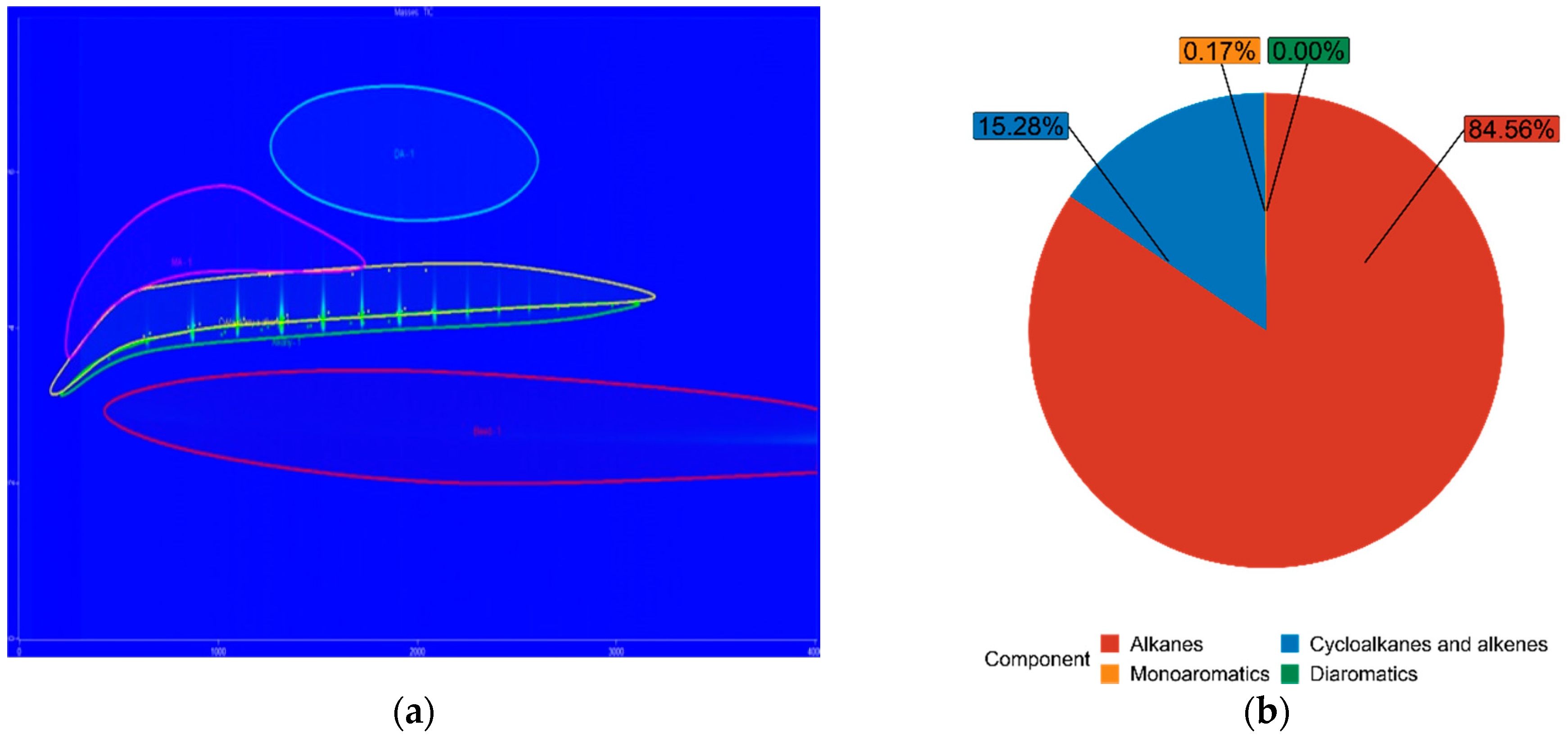
| FT100 | 84.56% | 15.28% | 0.17% | 0.00% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenčík, J.; Hönig, V.; Obergruber, M.; Hájek, J.; Vráblík, A.; Černý, R.; Schlehöfer, D.; Herink, T. Advanced Biofuels Based on Fischer–Tropsch Synthesis for Applications in Diesel Engines. Materials 2021, 14, 3077. https://doi.org/10.3390/ma14113077
Jenčík J, Hönig V, Obergruber M, Hájek J, Vráblík A, Černý R, Schlehöfer D, Herink T. Advanced Biofuels Based on Fischer–Tropsch Synthesis for Applications in Diesel Engines. Materials. 2021; 14(11):3077. https://doi.org/10.3390/ma14113077
Chicago/Turabian StyleJenčík, Jan, Vladimír Hönig, Michal Obergruber, Jiří Hájek, Aleš Vráblík, Radek Černý, Dominik Schlehöfer, and Tomáš Herink. 2021. "Advanced Biofuels Based on Fischer–Tropsch Synthesis for Applications in Diesel Engines" Materials 14, no. 11: 3077. https://doi.org/10.3390/ma14113077
APA StyleJenčík, J., Hönig, V., Obergruber, M., Hájek, J., Vráblík, A., Černý, R., Schlehöfer, D., & Herink, T. (2021). Advanced Biofuels Based on Fischer–Tropsch Synthesis for Applications in Diesel Engines. Materials, 14(11), 3077. https://doi.org/10.3390/ma14113077







