Abstract
In this research work, the quantitative characterization of a binary blend comprised of two pozzolans (sugar cane straw (SCSA)–sugar cane bagasse ashes (SCBA), bamboo leaf ash (BLAsh)–SCBA and paper sludge (PS)–fly ash (FA)) taking into account the calculated values of the kinetic parameters of the reaction in the pozzolan/calcium hydroxide system is shown. The paper shows the most significant and important results obtained by the authors in the quantitative assessment (calculation of kinetic parameters) of the pozzolanic reaction of different mixtures of pozzolanic materials that are residues from agriculture or industrial processes. This allows a direct and rigorous comparison of the pozzolanic activity of the binary combinations of materials. The values of the kinetic parameters (reaction rate constant or activation free energy) constitute a very precise quantitative index of the pozzolanic activity of the binary combinations of materials, which is very useful for its employment in the elaboration of ternary cements. This paper shows that the binary blends 1SCBA60Blash40, 1SCBA50Blash50, 1SCBA70Blash30 have a very high pozzolanic reactivity followed by PSLSFA, 2SCBA50SCSA50, PSISFA and SCWI.
1. Introduction
The use of agro-industrial waste is currently a topic of great interest, the subject of many research works. Most of these materials contain high amounts of silica and/or alumina which allow them to be used as pozzolanic additions in commercial Portland cement. The hydrated phases that are formed during the pozzolanic reaction can greatly improve the performance of concrete [1,2,3,4,5,6,7,8].
The use of agricultural and industrial residues for the production of concrete is a widespread practice worldwide. The permanent and large-scale generation of waste materials has become a serious environmental problem due to the pollution they produce and the space they need for their disposal. This represents a serious problem for agricultural and industrial companies. On the other hand, the evaluation of the pozzolanic reactivity of pozzolanic materials for use as an additive material to cement has become increasingly important because it represents greater sustainability in cement production.
The vast majority of agro-industrial waste is placed in landfills and open spaces, causing a negative effect on the environment. The use of waste as pozzolanic additions to cement makes it possible to reduce these landfills; in addition, the consumption of clinker per ton of cement decreases with the incorporation of additions, which represents great energy saving as well as a decrease in the emission of CO2 to the atmosphere.
During the last decades, there has been a change in the research related to cement matrices. In general terms, it can be said that during the 20th century, most scientific-technical studies were focused on binary cements (with one added additive). However, during the last decade, research developments are focusing on innovative cements made with more than one admixture. This is demonstrated by the large number of scientific contributions that have appeared in the international literature in recent years. These contributions show the combination of three mostly standard products: fly ash–slag, silica fume–fly ash and silica fume–slag [9,10,11,12,13,14,15,16]. In all cases, better results are obtained with ternary cements compared to binary cements (type II CEM), both in pastes and mortars as in concrete. This scientific interest in ternary cements confirms the growing trend of their production in different countries. Given the current economic situation and the serious environmental problems in which we are involved, it is conceivable that this type of cement, with more than one pozzolan, will take the lead in future commercial cement manufacturing due to its lower production cost.
The possibility of the development and production of ternary cements in developing countries is limited, taking into account the limitation of large-scale use of standardized systems: fly ash–slag, silica fume–fly ash and silica fume–slag.
For this reason, in addition to the pozzolans traditionally used in international standards (fly ash, silica fume, natural and calcined pozzolan), there is also research aimed at finding new materials that may be included in these standards in the near future. For this, it is necessary to carry out pre-normative studies that establish the technical basis for the use of these new products and agro-industrial waste as materials with cementitious properties [17,18,19,20]. In the case of calcined agricultural residues, the research works that report their characterization and use in ternary cement matrices are scarce [17,18,19,20,21,22].
Also, the increasing interest in the utilization of ternary cements is a direct result of their excellent performance, which has been frequently attributed to the synergistic effects taking place between the pozzolanic materials in the binary blend. The methods used in most of these investigations to evaluate the pozzolanic activity of the materials and their binary combinations are essentially directed to the qualitative aspect of the behavior of the binary mixtures of materials, not the quantitative aspect of the pozzolanic reaction in the binary mixture/cal system, which focuses on calculating the kinetic parameters of this reaction. There are some methodologies for the calculation of the parameters in different systems. In our research work, we use the application of a mathematical model based on well-founded physical hypotheses and its fitting to the experimental data by non-linear methods, which allows us to determine the kinetic parameters in the fitting process of the model, other common methodologies for parameter estimation and identifiability analysis are employed, among which is the Bayesian approach. The use of the Bayesian method is also of great interest and acceptance in different areas, including the engineering field [23].
In our case, the experimental monitoring of the pozzolanic reaction kinetics allows knowing the constants that characterize the reaction rate. Knowing these constants, it is possible to evaluate the different types of pozzolanic materials for their effective application as additions in mortars and concretes; it is also possible to compare their properties.
Currently, the acceptance by the international scientific community of the use of kinetic coefficients as a rigorous criterion for evaluating the pozzolanic reactivity of materials is already a fact. With this objective, traditional mathematical models have been developed and applied to study the pozzolanic reaction kinetics [24,25,26,27,28,29,30,31], which describe the experimental results with greater or lesser precision. Other researchers [32,33,34,35] have continued the development of new models that allow the quantitative characterization of the reaction kinetics in pozzolan/CH and cement/CH systems. Recently, Villar-Cociña et al. [36,37,38] developed a new kinetic-diffusive model that allows calculating the kinetic parameters of the pozzolanic reaction in the sugarcane/CH waste system and quantitatively characterizing the pozzolanic activity of these materials for all reaction ages. A good correlation between the experimental data and the theoretical model was obtained, which motivated the application of the model to evaluate different pozzolans.
This paper shows important results obtained by the authors in the study and quantitative characterization of several binary pozzolanic systems: sugar cane bagasse ash (SCBA) and sugar cane straw ash (SCSA), SCBA and bamboo leaf ash (BLAsh), calcined paper sludge (CPS) and Fly Ash (FA) applying the kinetic-diffusive model, which allows the calculation of the kinetic parameters of the pozzolanic reaction.
This allowed the rigorous characterization (obtaining the kinetic parameters) of these binary mixtures of materials, as well as a direct comparison of the pozzolanic behavior of the binary systems, the dosages of its components and their origins (industrial or laboratory).
The above is very useful for the selection of one or another binary pozzolanic system, which will depend on the characteristics needed for the building site. For elaborating on ternary cements, which is of great importance in the actuality due to the present world-wide economic crisis, the knowledge of the kinetic parameters is a fundamental tool for quantifying the reactivity of the pozzolanic materials that will be added to the cement. Other complementary experimental techniques were also used in the research including scanning electron microscopy (SEM), X-ray diffraction (XRD), porosimetry, which are not shown in the article for reasons of space, this would lead to the article being too long and would not contribute to the essence of the subject.
2. Materials and Methods
2.1. Materials
The materials analyzed involve both artificial pozzolan (thermally activated waste) and natural pozzolans that are readily available in industrial and agricultural economies. For the selection of the calcining temperature necessary for the activation of the materials, the best results (high reactivity) from some research works were taken into account [17,18,39,40].
The samples and their designations are shown in Table 1.

Table 1.
Designations of the samples used in the present work.
2.1.1. Binary System SCBA and SCSA
Two different agricultural ashes were employed: (i) an industrial bottom ash, a blend of SCBA and SCSA (SCWI), from a Brazilian sugar cane mill, used as biomass for cogeneration (around 40–50% of the mix, reaching 700–800 °C in the burning process); and (ii) ashes produced in the laboratory (2SCBA50SCSA50) resulting from a 1:1 ratio (by weight) of sugar cane bagasse and sugar cane leaf ashes, separately burned in an electric furnace (heating ramp of 10 °C/min up to 700 °C for 90 min).
Both ashes were ground and sized down to below 90 μm, similar to the commercial Portland cement [41].
2.1.2. Binary System SCBA and BLAsh
The raw materials were collected in the Pirassununga region, state of Sao Paulo, Brazil: bamboo leaves from the University of Sao Paulo Campus and sugar cane bagasse furnished by the Abengoa industry.
The raw materials were burned in a muffle furnace (JUNG 10010, Brazil) at 400 °C for 60 min and then, calcined at 700 °C for 60 min for sugar cane bagasse [42] and 600 °C for 60 min for bamboo leaves [21,43] (10 °C/min of heating ramp). The pozzolanic activity of the resulting ashes is the highest, for those specific temperatures. Fast cooling from the muffle furnace to room temperature was adopted to obtain an amorphous structure. The ashes were then ground and sieved until below 90 µm [9].
2.1.3. Binary System PS and FA
Two paper sludges were used: (i) a Spanish paper sludge activated at 700 °C for 2 h on laboratory scale (LS), according to [39,44]; (ii) a paper sludge waste activated in a fluidized bed system for the generation of electric energy. The industrial process operates in the range of 720–740 °C and the ash particles are collected by a filtering system (IS). The patented pozzolanic ashes were marketed as ‘‘Topcrete’’, CDEM [45].
Fly ash (FA) from Spain was utilized as a traditional pozzolan for the Portland cement industry [46]. At the first step, each pozzolan was ground and sieved at 90 μm. After, the mixes LS/FA and IS/FA were prepared at proportions of 50/50 (by weight) [47].
2.1.4. Lime
Calcium hydroxide (CH) (95% of minimum purity, 4.8% of maximum magnesium and alkaline salts content and 0.5% of maximum insoluble substance content) was used in the pozzolans/lime suspension.
To differentiate the pozzolans in the binary mixture that are the most abundant (1SCBA, 2SCBA and PS), we will call them base pozzolans. Those that are less abundant and complement the binary mixture will be called complementary pozzolans.
2.2. Test Methodologies
2.2.1. Pozzolanic Activity Methods
To carry out a qualitative or quantitative determination of pozzolanic activity many experimental methodologies have been developed [48,49,50].
In this research, as in other studies carried out by the authors [39,40,51,52], two different pozzolanic activity methods were applied. Both methods are based on the monitoring of the lime consumption with the reaction time and they can be used indistinctly.
It is important to note that the application of the kinetic-diffusive model used in this research to calculate the kinetic parameters of the pozzolanic reaction is independent of the method used for the evaluation of the pozzolanic activity, these have been shown elsewhere [51].
(a) Conductometric method
This method follows the conductivity of the pozzolan/calcium hydroxide solution with reaction time.
A saturated solution of calcium hydroxide (CH) was prepared, for which deionized water and CH were used. Both were mixed and the solution was stirred for 2 h, then the solution was kept at rest for 24 h, it was subsequently filtered and evaluated with hydrochloric acid.
Each 100 mL of saturated Ca(OH)2 solution was mixed with 2.10 g of pozzolanic material (which is the proportion commonly found in the literature for similar experiments) and magnetically stirred. Immediately after the pozzolan was mixed with the CH solution, the conductivity measurements began.
The measurements of conductivity were made at (40 ± 1) °C at different times. To correlate the CH concentration with the conductivity of the CH solution, a calibration curve that demonstrates a linear dependence was applied [19,36,53].
(b) Accelerated chemical method
This method also follows the pozzolan-calcium hydroxide reaction over time. The test consisted of putting the pozzolanic material (1 g) in contact with a saturated lime solution (75 mL) at (40 ± 1) °C for 1, 7, 28 and 90 days. At the end of each period, the CaO (quicklime) concentration in the solution was analyzed. Fixed calcium hydroxide (mmol/L) was obtained as the difference between the original concentration of a solution of saturated calcium hydroxide and the CaO found in the solution in contact with the sample, at the end of a given period [52].
2.2.2. Mathematical Model
It is known that the pozzolanic reaction in CH/pozzolan systems is heterogeneous and it can be considered a solid-solution type reaction:
A(L) + bB(S) → F(S) + E(L)
For elaborating on the model, we initially used the Decreasing Nucleus Model (DNM). In accordance with this model, when solution A reacts on the surface of reactant B (stoichiometry coefficient b) a reaction product layer F is formed around the unreacted nucleus of reactant B which gradually reduces. The diffusion of A through layer F (in the case this is porous) occurs until it reaches the interface between F and the unreacted nucleus. E(L) refers to the fluid products of the solid-solution reaction.
The following assumptions were made:
- −
- The spherical form of the granule is retained, and the densities of F and B are the same. Consequently, the total radius of the granule rs (considering the reaction product layer and the nucleus without reacting) does not change with time and an intermediate region does not exist between the nucleus and the layer of product [54].
- −
- The movement rate of the reaction interface, drc/dt, is small in comparison to the diffusion speed of A through the product layer (pseudo-stable state) [55]. This is valid when the density of the fluid in the pores of F is smaller than the density of the solid reactant, which is certain in general.
- −
- Taking into account the pseudo-stable conditions, where the speed equations, expressed as a mole of solution A (CH solution) that disappears per unit of time per particle, are identical, the rate equation is obtained by handling these equations and considering well-founded physical hypotheses. It is determined by the control regime or by the rate-limiting step which can be [56,57]: (i) diffusive control and (ii) kinetic control.
- −
- In accordance with all the above, Villar-Cociña et al. [36,37] proposed a kinetic-diffusive model that allows the characterization of the pozzolanic activity in the sugar cane straw-clay ash/CH solution. Subsequently, the model was perfected in the characterization of the reaction kinetics in the sugar cane straw ash/CH and sugar cane bagasse ash/CH systems, where a correction term Ccorr was incorporated. This term is related to the remaining concentration of CH, which in some systems is not consumed totally. The corrected model is [38]:
The dimensionless magnitude ξ = (C0 − Ct)/C0 represents the relative loss of lime concentration and Ct represents the absolute loss of lime concentration with time for the pozzolan/CH system.
It is well known that the pozzolanic reaction develops in stages. Therefore it may happen that the rate-limiting stage corresponds with the diffusion through the layer of reacting product or with the chemical reaction at the surface of the unreacted nucleus.
Consequently, it is possible to have different behavior: diffusive (described by the second term of Equation (2)), kinetic (third term) and kinetic-diffusive (second and third terms). Further explanations of the model can be found elsewhere [36,37].
The fitting process of this model allows the computing of the kinetic parameters (effective diffusion coefficient and reaction rate constant) and, therefore, a rigorous characterization of the process.
3. Results
3.1. Chemical Characterization
The chemical compositions of the samples were determined by an X-ray fluorescence (XRF) technique (Table 2).

Table 2.
Chemical composition of the binary pozzolanic systems.
3.1.1. SCBA/BLAsh System
Silica (SiO2) is the major constituent followed by K2O, Al2O3, Fe2O3, CaO, P2O5, MgO, SO3 and TiO2 in the sugar cane bagasse ashes (1SCBA). On the other hand, the major component is SiO2 followed by CaO, K2O, SO3, MgO and P2O5 in bamboo leaf ashes (BLAsh).
The presence of amorphous silica is due to the absorption of the silicic acid from the soil followed by the deposition in the different parts of the plant [58]. The sums of SiO2 + Al2O3 + Fe2O3 for 1SCBA and BLAsh are 57% and 72%, respectively. The chemical composition of 1SCBA and BLAsh differs from each other due to the grass precursors, the plant maturity and the soil where they are grown. 1SCBA presented the highest content of K2O (13% by mass); K2O is associated mainly with the fertilizers utilized in the sugarcane fields. Some authors demonstrated the use of sugarcane bagasse ashes with high K2O content (9 wt%) as mineral addition in cement-based binders [59,60].
The account SiO2 + Al2O3 + Fe2O3 for the two ashes is higher than 50% (minimum amount recommended by ASTM C618 [61]), being considered as a pozzolanic material for partial cement replacement. The loss on ignition (LOI) of all ashes is below 10 wt%, indicating that the calcination was efficient to remove organic and volatile compounds.
3.1.2. SCBA/SCSA System
Table 2 shows the chemical composition of the binary mix 2SCBA50SCSA50 and SCWI. These two ashes are similar but with different amounts of the main oxides. The laboratory ashes (2SCBA50SCSA50) showed a silico-calcium nature and the industrial ashes (SCWI) presented a silico-alumina-ferrous nature. The chemical composition revealed low silica content (50–60%) in comparison to other bagasse ashes (65–80%) [62], although the corresponding values were similar for sugar cane ash from the cogeneration process (58%) available in the international literature [63].
In both ashes, K2O was in the range between 6.0 and 8.5%, similar to other agro-industrial ashes reported in the literature [64]. LOI values were below 10%. According to Chusilp et al. [65], the referred composition will generate a very reactive pozzolan.
The compounds of the mixes: The main oxide present in SCSA and 2SCBA ashes is SiO2, followed by CaO and Al2O3 respectively. Other oxides such as MgO, SO3, K2O and P2O5 are present in smaller amounts. The LOI of the ashes is under 10% (by mass), indicating that the burning conditions were enough for the removal of the organic and volatile compounds.
3.1.3. Paper Sludge/FA System
The chemical results (by XRF) of the three pozzolans follow in Table 2. Based on the analytical data, it is possible to observe that the two starting sludges were formed by SiO2, Al2O3 and CaO, accounting for more than 69% of the total mass. For FA, the sum of SiO2, Al2O3 and Fe2O3 represents 85% of the total mass. The activated paper sludges showed LOI above 20%, which can be understood to be due to the calcite present in the starting materials, as detected by the XRD patterns [47].
3.2. Pozzolanic Activity
The results obtained for pozzolanic activity are shown in Figure 1, Figure 2 and Figure 3. In Figure 1 the conductivity variations (milliSiemens per centimeter) versus time (in hours) for the pozzolans/CH suspensions are shown for the binary mixtures of pozzolans 1SCSA50Blash50, 1SCBA60BLAsh40, 1SCBA70BLAsh30 and the pozzolans 1SCBA100 and BLAsh100; while Figure 2; Figure 3 show the results of accelerated pozzolanic tests for SCWI, 2SCBA50SCSA50CSCA, 2SCSA100 and PSISFA, PSLSFA, FA, LS respectively, ones up to 120 days of reaction. In the present study, there was no influence of the pozzolanic activity methods on the computing of the kinetic parameters [51].
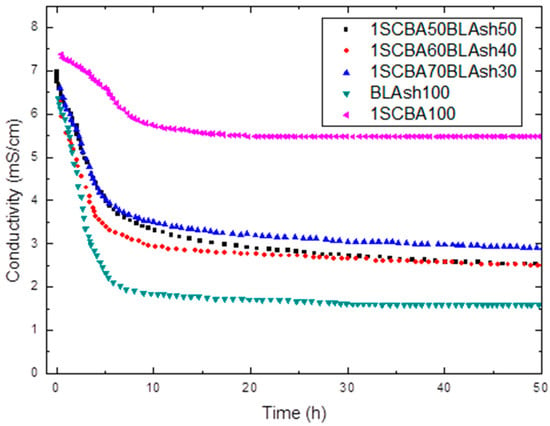
Figure 1.
Variation of electrical conductivity with reaction time for binary system 1SCBA + BLAsh.
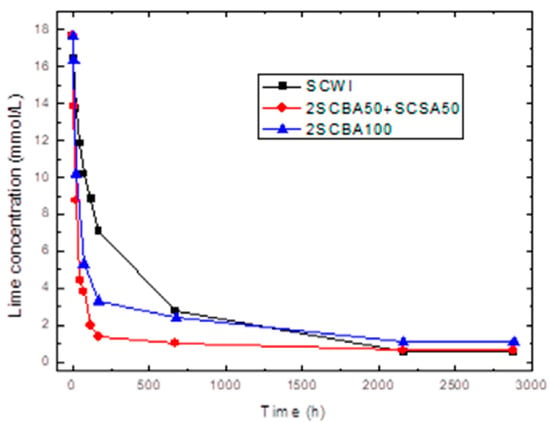
Figure 2.
Variation of electrical conductivity with reaction time for the binary system 2SCBA + SCSA.
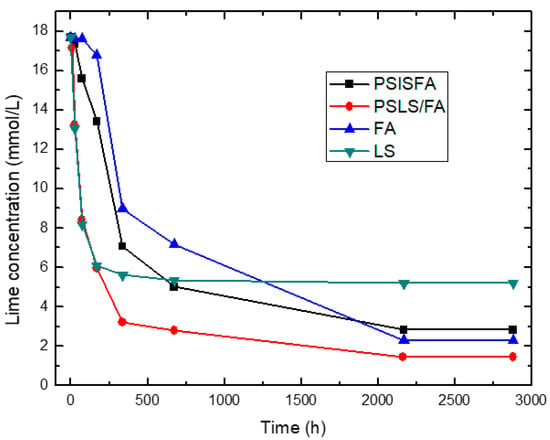
Figure 3.
Variation of electrical conductivity with reaction time for the binary system PSIS + FA, PSLS + FA.
In the conductometric test (Figure 1) a decrease in the electrical conductivity of the solution is obtained. The formation of hydrated phases with the corresponding decrease of the CH concentration in the solution (which leads to a decrease in conductivity) can be the cause of this behavior.
A considerable loss of conductivity in early ages is obtained; is evident that BLAsh consumes more CH than 1SCBA. From the qualitative point of view, the high reactivity of BLAsh, which is comparable to silica fume (SF) [66] (a highly expensive pozzolan) is appreciated, followed by 1SCBA60BLAsh40, 1SCSA50Blash50 and 1SCBA70BLAsh30. The 1SCBA shows the lowest consumption of CH (minor reactivity).
The stabilization of the curve is more rapidly reached for BLAsh and the binary mixtures in comparison with 1SCBA. The stabilization of the curve indicates the moment when the reaction has finished practically.
However, it is very important to take into account another interesting factor when an evaluation of the pozzolanic reactivity is carried out. This is the difference between the initial and final conductivities (stabilized conductivity) in the different pozzolan/CH solutions. To a greater difference, the reactivity of the material will be higher [67]. This could be related to calcium hydroxide consumption, a greater difference corresponds to greater consumption of CH in comparison with a minor difference.
For example, for the binary pozzolanic mixture 1SCBA + BLAsh, a qualitative analysis shows a greater reactivity for 1SCBA60BLA40 followed by 1SCBA50BLA50 and 1SCBA70BLA30. The 1SCBA100 shows the lowest reactivity. The sample BLAsh100 has a much higher fixation of calcium hydroxide than 1SCBA100.
The result of the binary mixtures is very important in comparison with the 1SCBA100 sample, this implies that with fewer amounts of BLAsh (which are abundant in a smaller quantity), higher reactivities can be obtained than with 1SCBA100 which is very abundant, that is, the combination of both offers better qualitative results than if we use bagasse alone, a good synergy effect.
Theoretically, the higher content of silica is, the higher the fixation of calcium hydroxide. However, 1SCBA60BLAsh40 has a higher fixation of calcium hydroxide than 1SCBA50BLAsh50. This result can be explained by the fact that SCBA contains the finest particles compared to BLAsh, which causes an increase in the fixation of calcium hydroxide.
However, for the case of the materials shown in Figure 1, it is evident that 1SCBA60BLAsh50 consumes more CH than the 1SCBA100 but does not provide information on the rapidity of the reaction. The difficulties of the qualitative analyses are evident, for this reason, it is very important to evaluate from the quantitative point of view of the pozzolanic reactivity. This involves all these aspects and allows the computation of the kinetic parameters.
On the other hand, the results of accelerated pozzolanic tests for reaction times of up to 90 days are shown in Figure 2 and Figure 3. The absolute loss of lime concentration (CH) plotted against the reaction time for the pozzolans/CH samples is shown.
According to Figure 2, a considerable loss of lime concentration in early ages is obtained for the samples 2SCBA50SCSA50 and 2SCBA100. The stabilization of the curve was reached after a long period of time and it depends on the analyzed sample. A qualitative analysis shows a greater reactivity for 2SCBA50SCSA50 followed by 2SCBA100 and SCWI. The result of the binary mixture 2SCBA50SCSA50 in comparison with the sample 2SCBA100 is very important; this implies that with fewer amounts of SCSA (which are abundant in a lower quantity than sugar cane bagasse) higher reactivities can be obtained. The combination of both offers better qualitative results than if we used bagasse alone, a good synergy effect. According to Figure 3, a considerable loss of lime concentration in early ages is obtained for the samples PSLSFA and LS. The stabilization of the curve was reached after a long period of time and it depends on the analyzed sample. A qualitative analysis shows a greater reactivity for the mixture PSFSFA followed by LS. The mixture PSISFA and FA show a minor reactivity.
We must point out the result of the binary mixture PSLSFA in comparison with the samples LS and FA, it implies that the combination of both offers better qualitative results than if we use FA or LS only.
3.3. Application of the Mathematical Model and Determination of the Kinetic Parameters
As mentioned above, the knowledge of the kinetic parameters of the pozzolanic reaction is a good criterion for evaluating quantitatively the pozzolanic activity of the materials and their combinations. In the international bibliography, the values reported of kinetic parameters for pozzolanic binary mixes/CH solution are very scarce. We have not encountered in the literature at our disposal any attempt reporting on the kinetic parameters for the combinations of pozzolanic materials that allow the quantitative comparison of their reactivity.
With the aim of computing the kinetic parameters, the kinetic-diffusive model (Equation (2)) was applied for all samples.
Figure 4, Figure 5 and Figure 6 illustrate the relative loss of lime concentration plotted against reaction time for binary systems 1SCBA + BLAsh, 2SCBA + SCSA and PS + FA. Figure 7 shows the samples LS, FA and SCSA that are used as complementary components in the binary mixtures. The solid and dash lines represent the curves of the fitted model.
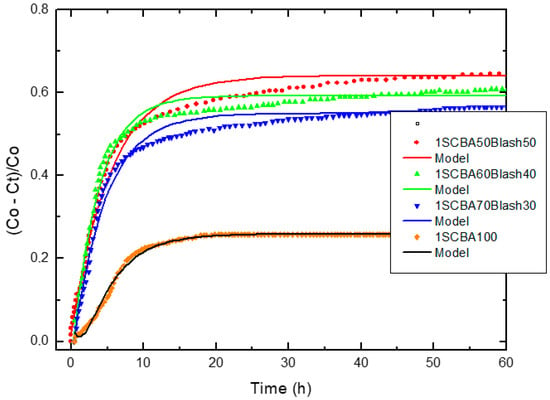
Figure 4.
Relative loss of lime concentration versus reaction times for 1SCBA50BLAsh50, 1SCBA60BLAsh40, 1SCBA70BLAsh30 and 1SCBA100. (Note: Black circle (experimental), solid line (model)).
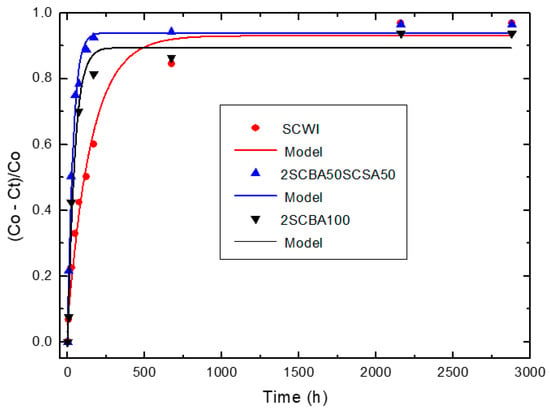
Figure 5.
Relative loss of lime concentration versus reaction times for SCWI, 2SCBA50SCSA50 and 2SCBA100 (Note: Black circle (experimental), solid line (model)).
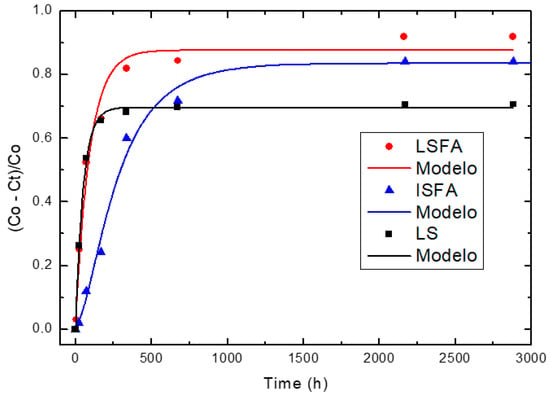
Figure 6.
Relative loss of lime concentration versus reaction times for PSLSFA, PSISFA and LS (Note: Black circle (experimental), solid line (model)).
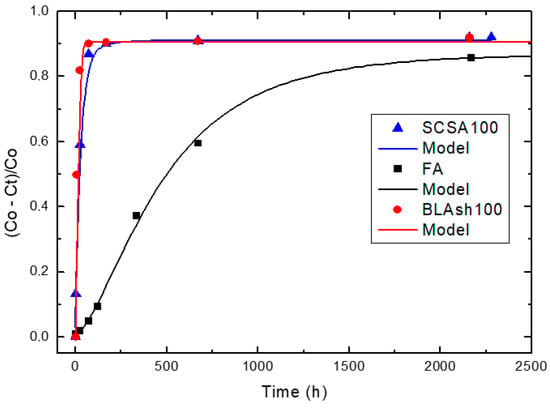
Figure 7.
Relative loss of lime concentration versus reaction times for complementary pozzolans SCSA100, FA and BLAsh100 (Note: Black circle (experimental), solid line (model)).
Fitting the relative loss of lime concentration versus reaction time successively to the model in its different variants (kinetic control, diffusive control and a mixed (kinetic-diffusive control) and analyzing important parameters, such as coefficient of multiple determination (R2), residual sum of squares (RSS), 95% confidence intervals, residual scatter, residual probability and variance analysis, it can be stated that:
For the case of SCWI, 2SCBA50SCSA50, SCSA100, 2SCBA100, 1SCBA50BLAsh50, 1SCBA60BLAsh40, 1SCBA70BLAsh30, BLAsh100, PSLSFA and LS the best correspondence with the experimental data is showed by the kinetic control model. This implies that the chemical reaction speed on the surface of the nucleus of the pozzolan particle is slower than the diffusion speed of CH through the reaction product layer formed around the nucleus. This is due to a quick diffusion process, which is facilitated by the high porosity of the reaction product layer in these materials [22].
For the samples of 1SCBA100, PSISFA and FA, the best correspondence with the experimental data is showed by the kinetic-diffusive control model. The regime that predominates is kinetic-diffusive. This means that the diffusion speed of the reactant through the reaction product layer around the nucleus and the chemical interaction speed on the surface of the nucleus of the pozzolan particle are comparable. Therefore, both processes determine the general speed of the whole process.
The values of the parameters (τ, K and Ccorr) are given in Table 3.

Table 3.
Reaction rate constant K, τ parameter, Ccorr. parameter and statistical parameters for the pozzolanic materials.
Taking into account the values of the kinetic parameters, it is possible to conclude that: For the binary system 2SCBA + SCSA, the binary mixture 2SCBA50SCSA50 has the highest reactivity (order of 10−2 h−1). This reactivity is greater than that of the base pozzolan 2SCBA100 (order of 10−3 h−1) (the one with the highest availability or most abundance) and less than the complementary pozzolan SCSA100 (order of 10−1 h−1) (with the lowest availability or minor abundance). The synergy that arises between 2SCBA and SCSA is appreciated because the pozzolanic binary mixture exceeds 2SCBA100. This reactivity was in the same order as the rice husk ashes and sugar cane straw ashes, one order lower than the bamboo leaf ashes, materials considered in the technical literature to be high pozzolanic reactivity ashes [68,69]
The binary mixture SWI showed a minor reactivity (in one order near to 10−3 h−1).
For the binary system 1SCBA + BLAsh, 1SCBA60BLAsh40 has the highest reactivity (order of 10−1 h−1). This reactivity is much higher than that of the pozzolan base 1SCBA100 (in an order near to 10−3 h−1). It is the one with the highest availability or most abundance. This reactivity is also less than the complementary pozzolan BLAsh100 (with the lowest availability or less abundance). The synergy that arises between 1SCBA and BLAsh is appreciated because the reactivity of the pozzolanic binary mixture is more than 1SCBA.
For the binary system PS + FA, PSLSFA has the highest reactivity of the binary system with a very high reactivity (order of 10−2 h−1). This reactivity is greater than that of the base pozzolan LS (order of 10−3 h−1) (which exists with greater availability) and also greater than the complementary pozzolan FA (order of 10−3 h−1) (with less availability). The good synergy that arises between LS and FA is appreciated when the pozzolanic reactivity of the binary mixture is more than its LS and FA components.
Analyzing the three binary systems, we can point out that the 1SCBA + BLAsh system is in general the one that shows the greatest pozzolanic reactivity, showing the highest values of the reaction rate constant K (order of 10−1 h−1) for all binary mixtures. The highest pozzolanic reactivity is shown by 1SCBA60BLAsh40 (the same order as the silica fume) [69], followed by the PS + FA system, specifically the PSLSFA binary mixture. In the 2SCBA + SCSA system, the 2SCBA50SCSA50 binary mixture shows the highest reactivity.
These results agree with the qualitative analysis carried out previously in Section 2.2 above.
4. Conclusions
The conclusions drawn from the present study are listed below:
- –
- Chemically, all the binary samples are formed by the same oxides but with different contents. The main oxides are silica and alumina (although PSLSFA and POISFA shows a 47% and 54% of CaO respectively), whose contents are: 71.6% (SCSA), 70.5% (BLAsh), 69.79% (2SCBA), 60.10% (SCWI), 55.70% (FA), 75.39% (LS), 72.13% (SCSA), 49.79% (2SCBA50SCSA50) and 36.20% (1SCBA).
- –
- The values of the reaction rate constant, obtained in the fitting process of the kinetic-diffusive model, show that the binary blends 1SCBA60BLAsh40 (6.18 · 10−1 h−1), 1SCBA50BLAsh50 (1.72 · 10−2 h−1), 1SCBA70BLAsh30 (2.89 · 10−1 h−1) have a very high pozzolanic reactivity followed by PSLSFA (6.26 · 10−2 h−1), 2SCBA50SCSA50 (1.72 · 10−2 h−1), PSISFA (5.51 · 10−3 h−1) and SCWI (9.32 · 10−4 h−1).
- –
- Analyzing the three binary systems we can point out that the 1SCBA + BLAsh system is, in general, the one that shows the greatest pozzolanic reactivity, showing the highest values of the reaction rate constant K (order of 10−1 h−1) for all the binary mixtures, the highest being 1SCBA60BLAsh40 (the same order of silica fume), followed by the PS + FA system, specifically the PSLSFA binary mixture, and by the 2SCBA + SCSA system, specifically the 2SCBA50SCSA50 binary mixture. In all these cases, there is a good synergy between the basic and complementary pozzolans, and the mentioned binary mixtures have greater reactivity than the basic pozzolans (1SCBA, 2SCBA and LS).
- –
- The availability of pozzolanic blends with different pozzolanic reaction rates can become an important technological advantage in the manufacturing of new ternary cements that include pozzolanic binary blends. The selection of one binary system or another as the one preferred will depend on the characteristics needed for the building site.
Author Contributions
Conceptualization, E.V.-C., M.F., H.S., L.R., M.I.S.d.R., I.F.S.d.B. and C.M.; methodology, E.V.-C., H.S. and C.M.; formal analysis, E.V.-C., L.R. and I.F.S.d.B.; investigation, E.V.-C., M.F., H.S., L.R., M.I.S.d.R., I.F.S.d.B. and C.M.; writing—original draft preparation, E.V.-C., L.R. and I.F.S.d.B.; writing—review and editing, E.V.-C., M.F., H.S., I.F.S.d.B. and C.M.; supervision, H.S. and C.M.; project administration, E.V.-C., H.S. and C.M.; funding acquisition, H.S. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank FAPESP (Brazil) (process 2011/12691-2) and CNPq (Brazil) (401704/2013-0 and 306386/2013-5) for the financial support to carry out and to conclude this research work. Also, the authors would like to thank the FAPESP (process n. 2011/16842-5) and the CSIC-FAPESP program (i-Link+2013, project ref: i-link0675, 2013/50790-8) for their financial support and the Framework Agreement of Collaboration between IETcc/CSIC (Spain) and FZEA/USP (Brazil) (ref: BDC 20195707 ACAM 2019040029).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Massazza, F. Chemistry of pozzolanic additions and mixed cement. Cemento 1976, 73, 3–39. [Google Scholar]
- Metha, P.K. Role of cementitious material in sustainable development of the concrete industry. In Proceeding of the Sixth CANMET/ACI International Conference on the Fly Ash, Silica Fume, Slag and Natural Pozzolans in Concrete; Malhotra, V.M., Ed.; ACI International: Bangkok, Thailand, 1998; Volume 1, pp. 1–20. [Google Scholar]
- Swathi, V.; Asadi, S.S. An influence of pozzolanic materials with hybrid fibers on structural performance of concrete: A review. Mater. Today Proc. 2021, 43, 1956–1959. [Google Scholar] [CrossRef]
- AlBiajawi, M.I.; Embong, R.; Muthusamy, K. An overview of the utilization and method for improving pozzolanic performance of agricultural and industrial wastes in concrete. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Pachideh, G.; Gholhaki, M. Effect of pozzolanic materials on mechanical properties and water absorption of autoclaved aerated concrete. J. Build. Eng. 2019, 26, 100856. [Google Scholar] [CrossRef]
- Yin, K.; Ahamed, A.; Lisak, G. Environmental perspectives of recycling various combustion ashes in cement production—A review. Waste Manag. 2018, 78, 401–416. [Google Scholar] [CrossRef]
- Kumar, V.P.; Vasugi, V. Bamboo materials in cement, geopolymer and reinforced concrete as sustainable solutions for better to-morrow. IOP Conf. Ser. Earth Environ. Sci. 2020, 573, 012036. [Google Scholar] [CrossRef]
- Aprianti, E.; Shafigh, P.; Bahri, S.; Farahani, J.N. Supplementary cementitious materials origin from agricultural wastes—A review. Constr. Build. Mater. 2015, 74, 176–187. [Google Scholar] [CrossRef]
- Rodier, L.; Villar-Cociña, E.; Mejia Ballesteros, J.; Savastano, H., Jr. Potential use of sugarcane bagasse and bam-boo leaf ashes for elaboration of green cementitious materials. J. Clean. Prod. 2019, 231, 54–63. [Google Scholar] [CrossRef]
- Hale, W.M.; Freyne, S.F.; Bush, T.D.; Russell, B.W. Properties of concrete mixtures containing slag cement and fly ash for use in transportation structures. Constr. Build. Mater. 2008, 22, 1990–2000. [Google Scholar] [CrossRef]
- Sisomphon, K.; Franke, L. Carbonation rates of concretes containing high volume of pozzolanic materials. Cem. Concr. Res. 2007, 37, 1647–1653. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Kayali, O.; Anderson, W. Chloride penetration in binary and ternary blended cement concretes as measured by two different rapid methods. Cem. Concr. Compos. 2008, 30, 576–582. [Google Scholar] [CrossRef]
- Garas, V.Y.; Kurtis, K. Assessment of methods for optimising ternary blended concrete containing metakaolin. Mag. Concr. Res. 2008, 60, 499–510. [Google Scholar] [CrossRef]
- Moser, R.D.; Jayapalan, A.R.; Garas, V.Y.; Kurtis, K.E. Assessment of binary and ternary blends of metakaolin and Class C fly ash for alkali-silica reaction mitigation in concrete. Cem. Concr. Res. 2010, 40, 1664–1672. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, N.; Singh, S.K.; Singh, N. Tertiary biocomposite cement and its hydration. Constr. Build. Mater. 2012, 29, 1–6. [Google Scholar] [CrossRef]
- Frías, M.; Villar-Cociña, E.; Valencia-Morales, E. Characterization of sugar cane straw waste as pozzolanic material for construction: Calcining temperature and kinetic parameters. Waste Manag. 2007, 27, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Frías, M.; Villar-Cociña, E. Influence of calcining temperature on the activation of sugar-cane bagasse: Kinetic parameters. Adv. Cem. Res. 2007, 19, 109–115. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Frias, M.; Valencia Morales, E.; Savastano, H., Jr. Study of the pozzolanic reaction kinetics in sugar cane bagasse–clay ash/calcium hydroxide system: Kinetic parameters and pozzolanic activity. Adv. Cem. Res. 2009, 21, 23–30. [Google Scholar] [CrossRef]
- Nguyen Van Tuan, G.Y.; van Breugel, K.; Alex, L.A.F.; Bui, D.D. The study of using rice husk ash to produce ultra high performance concrete. Constr. Build. Mater. 2011, 25, 2030–2035. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Morales, E.V.; Santos, S.F.; Savastano, H.; Frías, M. Pozzolanic behavior of bamboo leaf ash: Characterization and determination of the kinetic parameters. Cem. Concr. Compos. 2011, 33, 68–73. [Google Scholar] [CrossRef]
- Morales, E.V.; Villar-Cociña, E.; Frías, M.; Santos, S.F.; Savastano, H. Effects of calcining conditions on the mi-crostructure of sugar cane waste ashes (SCWA): Influence in the pozzolanic activation. Cem. Concr. Compos. 2009, 31, 22–28. [Google Scholar] [CrossRef]
- Khodadadian, A.; Noii, N.; Parvizi, M.; Abbaszadeh, M.; Wick, T.; Heitzinger, C. A Bayesian estimation method for variational phase-field fracture problems. Comput. Mech. 2020, 66, 827–849. [Google Scholar] [CrossRef]
- Khangaonkar, P.; Rahmat, A.; Kutty, K.J. Kinetic study of the hydrothermal reaction between lime and rice-husk-ash silica. Cem. Concr. Res. 1992, 22, 577–588. [Google Scholar] [CrossRef]
- Frías, M.; Cabrera, J. The effect of temperature on the hydration rate and stability of the hydration phases of metakaolin-lime-water systems. Cem. Concr. Res. 2002, 32, 133–138. [Google Scholar]
- Jander, W. Reaktionen im festen zustande bei hoheren temperature. Z. Anorg. Allg. Chem. 1927, 163, 1–30. [Google Scholar] [CrossRef]
- Kondo, R.; Lee, K.; Diamond, M. Kinetics and mechanism of hydrothermal reaction in lime–quartz–water systems. J. Ceram. Soc. Jpn. 1976, 84, 573–578. [Google Scholar]
- Brouwers, H.J.H.; Van Eijk, R.J. Fly ash reactivity: Extension and application of a shrinking core model and thermodynamic approach. J. Mater. Sci. 2002, 37, 2129–2141. [Google Scholar] [CrossRef]
- Ninov, J.; Dimova, I.D.L.; Petrov, B.; Brakalov, L. On the kinetics of pozzolanic reaction in metakaolin–lime–water system. J. Therm. Anal. Calorim. 2011, 105, 245–250. [Google Scholar] [CrossRef]
- Rao, S.M.; Asha, K. Activation of Fly Ash–Lime Reactions: Kinetic Approach. J. Mater. Civ. Eng. 2012, 24, 1110–1117. [Google Scholar] [CrossRef]
- Wang, X.-Y. Evaluation of compressive strength of hardening silica fume blended concrete. J. Mater. Sci. 2013, 48, 5953–5961. [Google Scholar] [CrossRef]
- Glosser, D.; Suraneni, P.; Burkan, O.; Isgor, W.; Weiss, J. Estimating reaction kinetics of cementitious pastes containing fly ash. Cem. Concr. Compos. 2020, 112, 10365. [Google Scholar] [CrossRef]
- Valentini, L.; Favero, M.; Dalconi, M.C.; Russo, V.; Ferrari, G.; Artioli, G. Kinetic Model of Calcium-Silicate Hydrate Nucleation and Growth in the Presence of PCE Superplasticizers. Cryst. Growth Des. 2016, 16, 646–654. [Google Scholar] [CrossRef]
- Frías, M.; Vigil de la Villa, R.; Martínez-Ramírez, S.; Fernández-Carrasco, L.; Villar-Cociña, E.; García-Giménez, R. Mul-ti-Technique Characterization of a Fine Fraction of CDW and Assessment of Reactivity in a CDW/Lime System. Minerals 2020, 10, 590. [Google Scholar] [CrossRef]
- Honorio, T.; Bary, B.; Benboudjema, F.; Poyet, S. Modeling hydration kinetics based on boundary nucleation and space-filling growth in a fixed confined zone. Cem. Concr. Res. 2016, 83, 31–44. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Valencia-Morales, E.; Gonzalez-Rodriguez, R.; Hernandez-Ruiz, J. Kinetics of the pozzolanic re-action between lime and sugar cane straw ash by electrical conductivity measurement: A kinetic-diffusive model. Cem. Concr. Res. 2003, 33, 517–524. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Frías, M.; Valencia-Morales, E.; De Rojas, M.I.S. An evaluation of different kinetic models for determining the kinetic coefficients in sugar cane straw–clay ash/lime system. Adv. Cem. Res. 2006, 18, 17–26. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Frías, M.; Valencia-Morales, E. Sugar cane wastes as pozzolanic materials: Application of mathematical model. ACI Mater. J. 2008, 105, 258–264. [Google Scholar]
- Frías, M.; Rodriguez, O.; Nebreda, B.; Garcia, R.; Villar-Cociña, E. Influence of activation temperature of kaolinite-based clay wastes on pozzolanic activity and kinetic parameters. Adv. Cem. Res. 2010, 22, 135–142. [Google Scholar] [CrossRef]
- Frías, M.; García, R.; Vigil, R.; Ferreiro, S. Calcination of art paper sludge waste for the use as a supplementary cementing material. Appl. Clay Sci. 2008, 42, 189–193. [Google Scholar] [CrossRef]
- Frías, M.; Rodríguez, O.; Sanchez de Rojas, M.I.; Villar-Cociña, E.S.; Rodrigues, M.; Savastano, H., Jr. Advances on the development of ternary cements elaborated with biomass ashes coming from different activation process. Con. Build. Mat. 2017, 136, 73–80. [Google Scholar] [CrossRef]
- Cordeiro, G.; Filho, R.T.; Tavares, L.; Fairbairn, E. Pozzolanic activity and filler effect of sugar cane bagasse ash in Portland cement and lime mortars. Cem. Concr. Compos. 2008, 30, 410–418. [Google Scholar] [CrossRef]
- Dwivedi, V.N.; Singh, N.P.; Dasa, S.S.; Singh, N.B. A new pozzolanic material for cement industry: Bamboo leaf ash. Int. J. Phys. Sci. 2006, 1, 106–111. [Google Scholar]
- De La Villa, R.V.; Rodriguez, O.; Garcia, R.; Frías, M. Mineral phases in an activated kaolinitic waste blended cement system. Appl. Clay Sci. 2010, 50, 137–142. [Google Scholar] [CrossRef]
- International Patent. A method of preparing a pozzolanic material form paper residue and a method for the manufacture of cement from said material.
- European Standard EN197-1. Cement-Part 1: Composition, Specifications and Conformity Criteria for Common Cements. Available online: https://www.aenor.com/normas-y-libros/buscador-de-normas/une/?c=N0048623 (accessed on 1 April 2021).
- Frías, M.; Garcia, R.; De La Villa, R.V.; Villar, E. The effect of binary pozzolan mix on the mineralogical changes in the ternary activated paper sludge–fly ash–Ca(OH)2 system. Constr. Build. Mater. 2013, 38, 48–53. [Google Scholar] [CrossRef]
- British Standard BS EN 196 Methods of Testing Cement. Available online: https://www.aenor.com/normas-y-libros/buscador-de-normas (accessed on 1 April 2021).
- American Society for Testing and Materials (ASTM) C-311. Available online: https://www.astm.org/Standards/C311.htm (accessed on 1 April 2021).
- Donatello, S.; Tyrer, M.; Cheeseman, C. Comparison of test methods to assess pozzolanic activity. Cem. Concr. Compos. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- Frías, M.; Villar-Cociña, E.; Sánchez de Rojas, M.I.; Valencia-Morales, E. The effect of different pozzolanic activity methods on the kinetics constants of the pozzolanic reaction: Application of a kinetic-diffusive model. Cem. Concr. Res. 2005, 35, 2137–2142. [Google Scholar] [CrossRef]
- Rodríguez, O.; Frías, M.; Sánchez de Rojas, M.I. Influence of the calcined paper sludge on the development of hydration heat in blended cement mortars. J. Therm. Anal. Cal. 2008, 92, 865–871. [Google Scholar] [CrossRef]
- Quintana-Crespo, E.; Villar-Cociña, E. A kinetic study about the pozzolanic reactivity of loessic soils by con-ductometric methods: Kinetic parameters. Adv. Cem. Res. 2011, 23, 3–10. [Google Scholar] [CrossRef]
- Yagi, S.; Kunii, D. Studies on Fluidized-Solids Reactors for Particles with Decreasing Diameters. Chem. Eng. 1955, 19, 500–506. [Google Scholar] [CrossRef]
- Bischoff, K. Accuracy of the pseudo steady state approximation for moving boundary diffusion problems. Chem. Eng. Sci. 1963, 18, 711–713. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering. Ind. Eng. Chem. Res. 1999, 38, 4140–4143. [Google Scholar] [CrossRef]
- Renedo, M.J.; Fernández, J. Kinetic modeling of the hydrothermal reaction of fly ash, Ca(OH)2 and CaSO4 in the preparation of desulphurant sorbents. Fuel 2004, 83, 525–532. [Google Scholar] [CrossRef]
- Norsuraya, S.; Fazlena, H.; Norhasyimi, R. Sugarcane Bagasse as a Renewable Source of Silica to Synthesize Santa Barbara Amorphous-15 (SBA-15). Procedia Eng. 2016, 148, 839–846. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Toledo Filho, R.D.; Fairbairn, E.D.M.R. Caracterizaç~ao de cinza do bagaço de cana-de-açúcar para emprego como pozolana em materiais cimentícios Characterization of sugar cane bagasse ash for use as pozzolan in cementitious materials. Quím. Nova 2009, 32, 82–86. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Filho, R.D.T.; Tavares, L.M.; Fairbairn, E.D.M.R. Ultrafine grinding of sugar cane bagasse ash for application as pozzolanic admixture in concrete. Cem. Concr. Res. 2009, 39, 110–115. [Google Scholar] [CrossRef]
- ASTM C618. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; Annual Book of ASTM Standards, 04.01; American Society for Testing and Materials: Philadelphia, PA, USA, 2012. [Google Scholar]
- Demis, S.; Tapali, J.; Papadakis, V. An investigation of the effectiveness of the utilization of biomass ashes as pozzolanic materials. Constr. Build. Mater. 2014, 68, 291–300. [Google Scholar] [CrossRef]
- Bahurudeen, A.; Marckson, A.; Kishore, A.; Santhanam, M. Development of sugarcane bagasse ash based Portland pozzolana cement and evaluation of compatibility with superplasticizers. Constr. Build. Mater. 2014, 68, 465–475. [Google Scholar] [CrossRef]
- Nakanishi, E.Y.; Frías, M.; Martínez-Ramírez, S.; Santos, S.F.; Rodrigues, M.S.; Rodríguez, O.; Savastano, H. Characterization and properties of elephant grass ashes as supplementary cementing material in pozzolan/Ca(OH)2 pastes. Constr. Build. Mater. 2014, 73, 391–398. [Google Scholar] [CrossRef]
- Chusilp, N.; Jaturapitakkul, C.; Kiattikomol, K. Effects of LOI of ground bagasse ash on the compressive strength and sulfate resistance of mortars. Constr. Build. Mater. 2009, 23, 3523–3531. [Google Scholar] [CrossRef]
- Villar-Cocina, E.; Rodier, L.; Savastano, H.; Lefran, M.; Frias, M. A Comparative Study on the Pozzolanic Activity Between Bamboo Leaves Ash and Silica Fume: Kinetic Parameters. Waste Biomass Valorization 2020, 11, 1627–1634. [Google Scholar] [CrossRef]
- Sinthaworn, S.; Nimityongskul, P. Quick monitoring of pozzolanic reactivity of waste ashes. Waste Manag. 2009, 29, 1526–1531. [Google Scholar] [CrossRef]
- Rahman, A.; Rasul, M.; Khan, M.; Sharma, S. Recent development on the uses of alternative fuels in cement manufacturing process. Fuel 2015, 145, 84–99. [Google Scholar] [CrossRef]
- Frías, M.; Savastano, H.; Villar, E.; de Rojas, M.I.S.; Santos, S. Characterization and properties of blended cement matrices containing activated bamboo leaf wastes. Cem. Concr. Compos. 2012, 34, 1019–1023. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).