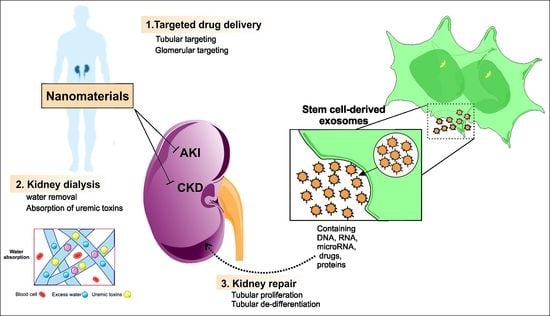
Application of Advanced Nanomaterials for Kidney Failure Treatment and Regeneration
Abstract
1. Introduction
2. Kidney-Targeted Delivery Systems
Kidney-Targeted Drug Delivery Systems Based on Nanoparticles
3. Nanoparticles and Nanomaterials for Kidney Regeneration
3.1. Carbon-Based Nanomaterials
3.2. Endagenous Nanosized Structures
3.3. Biomimicking Nanostructures
3.4. Nanofibrous Membranes for Wearable Blood Purification Systems
3.5. Nanomaterial-Based Adsorbents for Artificial Kidney
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murugan, R.; Ramakrishna, S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007, 13, 1845–1866. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly (lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.G.; Jungvid, H.; Kumar, A. Skin tissue engineering for tissue repair and regeneration. Tissue Eng. Part B Rev. 2008, 14, 105–118. [Google Scholar] [CrossRef]
- Ghavimi, M.A.; Shahabadi, A.B.; Jarolmasjed, S.; Memar, M.Y.; Dizaj, S.M.; Sharifi, S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Sharifi, S.; Jahangiri, A. Electrospun nanofibers as versatile platform in antimicrobial delivery: Current state and perspectives. Pharm. Dev. Technol. 2019, 24, 1187–1199. [Google Scholar] [CrossRef]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vacanti, J.P.; Atala, A. Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Eftekhari, A.; Maleki Dizaj, S.; Sharifi, S.; Salatin, S.; Rahbar Saadat, Y.; Zununi Vahed, S.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E. The use of nanomaterials in tissue engineering for cartilage regeneration; current approaches and future perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef]
- Parveen, S.; Krishnakumar, K.; Sahoo, S. New era in health care: Tissue engineering. J. Stem Cells Regen. Med. 2006, 1, 8. [Google Scholar] [PubMed]
- Sharma, C.; Dinda, A.K.; Mishra, N.C. Synthesis and characterization of glycine modified chitosan-gelatin-alginate composite scaffold for tissue engineering applications. J. Biomater. Tissue Eng. 2012, 2, 133–142. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Czernuszka, J. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Eaglstein, W.H.; Falanga, V. Tissue engineering and the development of Apligraf®, a human skin equivalent. Clin. Ther. 1997, 19, 894–905. [Google Scholar] [CrossRef]
- Chandler, L.A.; Ma, C.; Gonzalez, A.M.; Doukas, J.; Nguyen, T.; Pierce, G.F.; Phillips, M.L. Matrix-enabled gene transfer for cutaneous wound repair. Wound Repair Regen. 2000, 8, 473–479. [Google Scholar] [CrossRef]
- Xiao, Y.; Qian, H.; Young, W.G.; Bartold, P.M. Tissue engineering for bone regeneration using differentiated alveolar bone cells in collagen scaffolds. Tissue Eng. 2003, 9, 1167–1177. [Google Scholar] [CrossRef]
- Lee, S.J.; Wang, H.J.; Kim, T.H.; Choi, J.S.; Kulkarni, G.; Jackson, J.D.; Atala, A.; Yoo, J.J. In situ tissue regeneration of renal tissue induced by collagen hydrogel injection. Stem Cells Transl. Med. 2018, 7, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Vashi, A.V.; Abberton, K.M.; Thomas, G.P.; Morrison, W.A.; O’connor, A.J.; Cooper-White, J.J.; Thompson, E.W. Adipose tissue engineering based on the controlled release of fibroblast growth factor-2 in a collagen matrix. Tissue Eng. 2006, 12, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Nam, S.-H.; Im, S.-Y.; Park, Y.-J.; Lee, Y.-M.; Seol, Y.-J.; Chung, C.-P.; Lee, S.-J. Enhanced bone formation by controlled growth factor delivery from chitosan-based biomaterials. J. Control. Release 2002, 78, 187–197. [Google Scholar] [CrossRef]
- Ghosh, S.; Jassal, M. Use of polysaccharide fibres for modern wound dressings. Indian J. Fibre Text. Res. 2002, 27, 434–450. [Google Scholar]
- Cai, S.; Liu, Y.; Shu, X.Z.; Prestwich, G.D. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 2005, 26, 6054–6067. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Long, D.A.; Norman, J.T.; Fine, L.G. Restoring the renal microvasculature to treat chronic kidney disease. Nat. Rev. Nephrol. 2012, 8, 244. [Google Scholar] [CrossRef]
- Uzarski, J.S.; Xia, Y.; Belmonte, J.C.; Wertheim, J.A. New strategies in kidney regeneration and tissue engineering. Curr. Opin. Nephrol. Hypertens. 2014, 23, 399–405. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Dalir, E.; Vahedi, P.; Hasanzadeh, A.; Samiei, M. The potential applications of hyaluronic acid hydrogels in biomedicine. Drug Res. 2020, 70, 6–11. [Google Scholar] [CrossRef]
- Ahmadian, E.; Dizaj, S.M.; Rahimpour, E.; Hasanzadeh, A.; Eftekhari, A.; Halajzadeh, J.; Ahmadian, H. Effect of silver nanoparticles in the induction of apoptosis on human hepatocellular carcinoma (HepG2) cell line. Mater. Sci. Eng. C 2018, 93, 465–471. [Google Scholar] [CrossRef]
- Dizaj, S.; Adibkia, K. A Short Overview on the Nanoparticle-based smart Drug Delivery Systems. J. Pharm. Pharm. 2015, 2, 1–2. [Google Scholar] [CrossRef]
- Dizaj, S.M. Can nanotechnology present new strategies to overcome COVID-19? J. Adv. Chem. Pharm. Mater. 2020, 3, 258–259. [Google Scholar]
- Fujisato, T.; Sajiki, T.; Liu, Q.; Ikada, Y. Effect of basic fibroblast growth factor on cartilage regeneration in chondrocyte-seeded collagen sponge scaffold. Biomaterials 1996, 17, 155–162. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Uyama, S.; Kaufmann, P.M.; Kneser, U.; Fiegel, H.C.; Pollok, J.M.; Kluth, D.; Vacanti, J.P.; Rogiers, X. Hepatocyte transplantation using biodegradable matrices in ascorbic acid-deficient rats: Comparison with heterotopically transplanted liver grafts1. Transplantation 2001, 71, 1226–1231. [Google Scholar] [CrossRef][Green Version]
- Gomes, S.R.; Rodrigues, G.; Martins, G.G.; Roberto, M.A.; Mafra, M.; Henriques, C.; Silva, J.C. In vitro and in vivo evaluation of electrospun nanofibers of PCL, chitosan and gelatin: A comparative study. Mater. Sci. Eng. C 2015, 46, 348–358. [Google Scholar] [CrossRef]
- Hasirci, V.; Berthiaume, F.; Bondre, S.; Gresser, J.; Trantolo, D.; Toner, M.; Wise, D. Expression of liver-specific functions by rat hepatocytes seeded in treated poly (lactic-co-glycolic) acid biodegradable foams. Tissue Eng. 2001, 7, 385–394. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.-H.; Ramakrishna, S. Electrospun poly (ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: Comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019, 14, 6615. [Google Scholar] [CrossRef]
- Wu, L.; Zang, J.; Lee, L.A.; Niu, Z.; Horvatha, G.C.; Braxtona, V.; Wibowo, A.C.; Bruckman, M.A.; Ghoshroy, S.; zur Loye, H.-C. Electrospinning fabrication, structural and mechanical characterization of rod-like virus-based composite nanofibers. J. Mater. Chem. 2011, 21, 8550–8557. [Google Scholar] [CrossRef]
- Zhang, Y.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials 2008, 29, 4314–4322. [Google Scholar] [CrossRef]
- Ngiam, M.; Liao, S.; Patil, A.J.; Cheng, Z.; Chan, C.K.; Ramakrishna, S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone 2009, 45, 4–16. [Google Scholar] [CrossRef]
- Stupp, S.I. Biomaterials for regenerative medicine. Mrs Bull. 2005, 30, 546–553. [Google Scholar] [CrossRef]
- Anandhan, S.; Bandyopadhyay, S. Polymer nanocomposites: From synthesis to applications. Nanocomposites Polym. Anal. Methods 2011, 1, 1–28. [Google Scholar]
- Cozad, M.J.; Bachman, S.L.; Grant, S.A. Assessment of decellularized porcine diaphragm conjugated with gold nanomaterials as a tissue scaffold for wound healing. J. Biomed. Mater. Res. Part A 2011, 99, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Rieder, E.; Kasimir, M.-T.; Silberhumer, G.; Seebacher, G.; Wolner, E.; Simon, P.; Weigel, G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J. Thorac. Cardiovasc. Surg. 2004, 127, 399–405. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Stolz, D.B.; Biancaniello, F.; Simmons-Byrd, A.; Badylak, S.F. Production and characterization of ECM powder: Implications for tissue engineering applications. Biomaterials 2005, 26, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef]
- Choi, C.H.J.; Zuckerman, J.E.; Webster, P.; Davis, M.E.J. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661. [Google Scholar] [CrossRef]
- Williams, R.M.; Shah, J.; Ng, B.D.; Minton, D.R.; Gudas, L.J.; Park, C.Y.; Heller, D.A. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 2015, 15, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; He, J.C.; Ausiello, D.A.; Farokhzad, O.C.J.N.R.N. Nanomedicines for renal disease: Current status and future applications. Nat. Rev. Nephrol. 2016, 12, 738–753. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R.J. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Miner, J.H. The glomerular basement membrane as a barrier to albumin. Nat. Rev. Nephrol. 2013, 9, 470. [Google Scholar] [CrossRef]
- Kanwar, Y.S.; Farquhar, M.G. Presence of heparan sulfate in the glomerular basement membrane. Proc. Natl. Acad. Sci. USA 1979, 76, 1303–1307. [Google Scholar] [CrossRef]
- Lahdenkari, A.-T.; Lounatmaa, K.; Patrakka, J.; Holmberg, C.; Wartiovaara, J.; Kestilä, M.; Koskimies, O.; Jalanko, H. Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J. Am. Soc. Nephrol. 2004, 15, 2611–2618. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Wang, L.; Wang, W.; Liu, B.; Liu, J.; Chen, M.; He, Q.; Liao, Y.; Yu, X. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 2012, 379, 815–822. [Google Scholar] [CrossRef]
- Watanabe, Y.; Suzuki, H.; Suzuki, K.; Ando, T.; Nakabayashi, S.; Sugiyama, Y. Detection of the membrane protein recognized by the kidney-specific alkylglucoside vector. Pharm. Res. 2000, 17, 49–54. [Google Scholar] [CrossRef]
- Liu, K.-X.; Kato, Y.; Kino, I.; Nakamura, T.; Sugiyama, Y. Ligand-induced downregulation of receptor-mediated clearance of hepatocyte growth factor in rats. Am. J. Physiol. Endocrinol. Metab. 1998, 275, E835–E842. [Google Scholar] [CrossRef] [PubMed]
- Manil, L.; Davin, J.-C.; Duchenne, C.; Kubiak, C.; Foidart, J.; Couvreur, P.; Mahieu, P. Uptake of nanoparticles by rat glomerular mesangial cells in vivo and in vitro. Pharm. Res. 1994, 11, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cai, F.; Li, Y.; Chen, J.; Han, F.; Lin, W. A review of the application of nanoparticles in the diagnosis and treatment of chronic kidney disease. Bioact. Mater. 2020, 5, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Fissell, W.H.; Fleischman, A.J.; Humes, H.D.; Roy, S. Development of continuous implantable renal replacement: Past and future. Transl. Res. 2007, 150, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Patial, V. Nanotechnological interventions for the treatment of renal diseases: Current scenario and future prospects. J. Drug Deliv. Sci. Technol. 2020, 59, 101917. [Google Scholar] [CrossRef]
- Singh, M.; Ghose, T.; Faulkner, G.; Kralovec, J.; Mezei, M. Targeting of methotrexate-containing liposomes with a monoclonal antibody against human renal cancer. Cancer Res. 1989, 49, 3976–3984. [Google Scholar]
- Tuffin, G.; Waelti, E.; Huwyler, J.; Hammer, C.; Marti, H.-P. Immunoliposome targeting to mesangial cells: A promising strategy for specific drug delivery to the kidney. J. Am. Soc. Nephrol. 2005, 16, 3295–3305. [Google Scholar] [CrossRef]
- Kim, C.K.; Kim, T.; Choi, I.Y.; Soh, M.; Kim, D.; Kim, Y.J.; Jang, H.; Yang, H.S.; Kim, J.Y.; Park, H.K. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. Int. Ed. 2012, 51, 11039–11043. [Google Scholar] [CrossRef]
- Soh, M.; Kang, D.W.; Jeong, H.G.; Kim, D.; Kim, D.Y.; Yang, W.; Song, C.; Baik, S.; Choi, I.Y.; Ki, S.K. Ceria–Zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem. Int. Ed. 2017, 56, 11399–11403. [Google Scholar] [CrossRef]
- Saifi, M.A.; Sangomla, S.; Khurana, A.; Godugu, C. Protective effect of nanoceria on cisplatin-induced nephrotoxicity by amelioration of oxidative stress and pro-inflammatory mechanisms. Biol. Trace Elem. Res. 2019, 189, 145–156. [Google Scholar] [CrossRef]
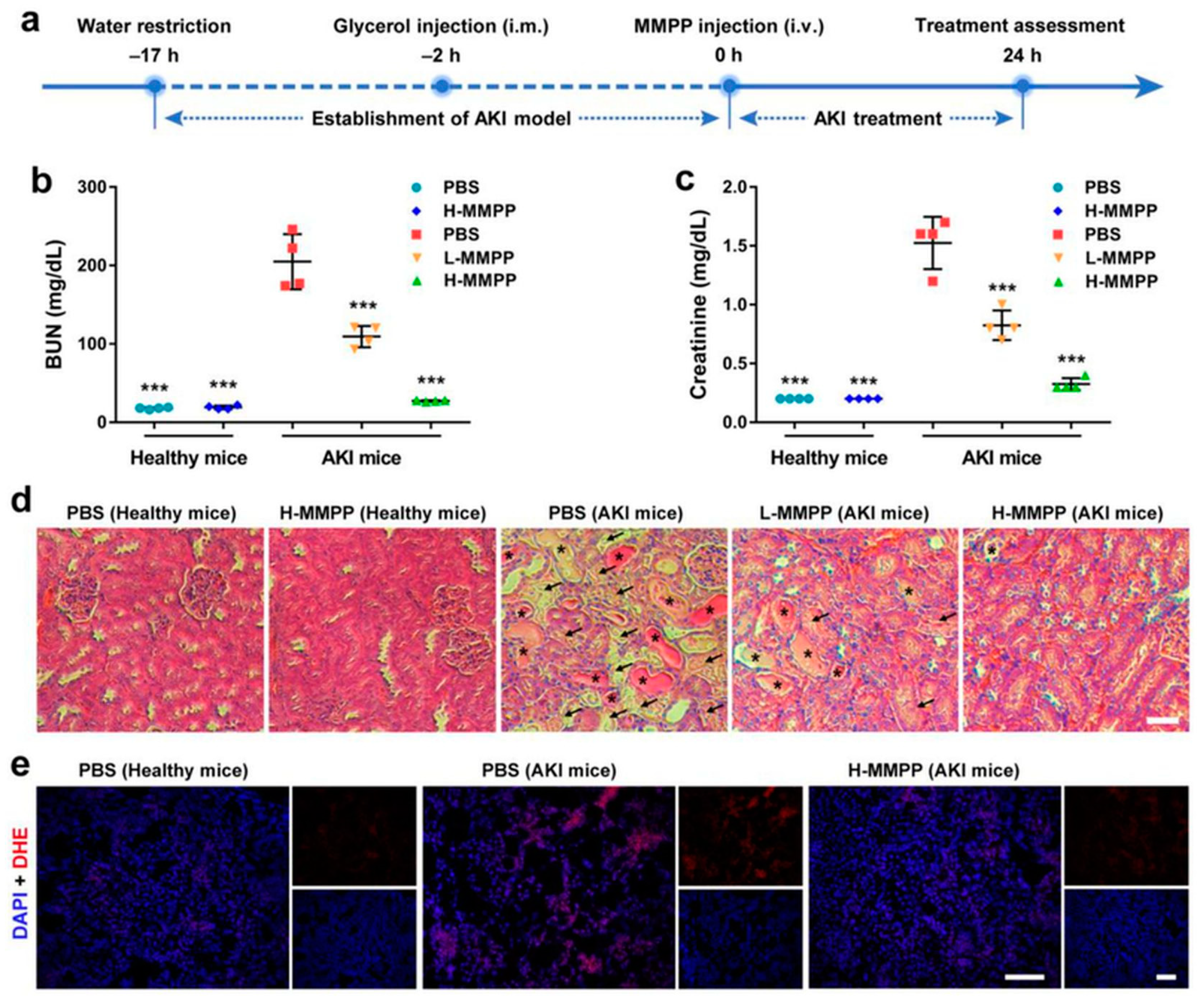
- Ni, D.; Jiang, D.; Kutyreff, C.J.; Lai, J.; Yan, Y.; Barnhart, T.E.; Yu, B.; Im, H.-J.; Kang, L.; Cho, S.Y. Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- AlBasher, G.; Alfarraj, S.; Alarifi, S.; Alkhtani, S.; Almeer, R.; Alsultan, N.; Alharthi, M.; Alotibi, N.; Al-Dbass, A.; Moneim, A.E.A. Nephroprotective role of selenium nanoparticles against glycerol-induced acute kidney injury in rats. Biol. Trace Elem. Res. 2020, 194, 444–454. [Google Scholar] [CrossRef]
- Zheng, Z.; Deng, G.; Qi, C.; Xu, Y.; Liu, X.; Zhao, Z.; Zhang, Z.; Chu, Y.; Wu, H.; Liu, J. Porous Se@ SiO2 nanospheres attenuate ischemia/reperfusion (I/R)-induced acute kidney injury (AKI) and inflammation by antioxidative stress. Int. J. Nanomed. 2019, 14, 215. [Google Scholar] [CrossRef]
- Yu, H.; Lin, T.; Chen, W.; Cao, W.; Zhang, C.; Wang, T.; Ding, M.; Zhao, S.; Wei, H.; Guo, H. Size and temporal-dependent efficacy of oltipraz-loaded PLGA nanoparticles for treatment of acute kidney injury and fibrosis. Biomaterials 2019, 219, 119368. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Jiang, D.; Rosenkrans, Z.T.; Ehlerding, E.B.; Ni, D.; Qi, C.; Kutyreff, C.J.; Barnhart, T.E.; Engle, J.W.; Huang, P.J. A Melanin-Based Natural Antioxidant Defense Nanosystem for Theranostic Application in Acute Kidney Injury. Adv. Funct. Mater. 2019, 29, 1904833. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19. [Google Scholar]
- Bianco, A.; Kostarelos, K.; Partidos, C.D.; Prato, M. Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 2005, 5, 571–577. [Google Scholar] [CrossRef]
- Bussy, C.; Methven, L.; Kostarelos, K. Hemotoxicity of carbon nanotubes. Adv. Drug Deliv. Rev. 2013, 65, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Malhotra, M.; Shao, W.; Tomaro-Duchesneau, C.; Abbasi, S. Polymeric nanohybrids and functionalized carbon nanotubes as drug delivery carriers for cancer therapy. Adv. Drug Deliv. Rev. 2011, 63, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Zhang, J.; Sun, W.; Zhang, R.; Gu, H. Fabrication of a novel polymer-free nanostructured drug-eluting coating for cardiovascular stents. ACS Appl. Mater. Interfaces 2013, 5, 10337–10345. [Google Scholar] [CrossRef]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef]
- Sharifi, S.; Vahed, S.Z.; Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Khalilov, R.; Ahmadi, M.; Hamidi-Asl, E.; Labib, M. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens. Bioelectron. 2020, 150, 111933. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, Y.; Shan, Y.; Ju, H.; Xue, X. Preparation and characterization of grafted collagen-multiwalled carbon nanotubes composites. J. Nanosci. Nanotechnol. 2007, 7, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Twomey, J.; Guo, D.; Madhavan, K.; Li, M. Evaluation of nanostructural, mechanical, and biological properties of collagen–nanotube composites. IEEE Trans. Nanobiosci. 2010, 9, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Kandadai, M.A.; Cech, J.; Roth, S.; Curran, S.A. Poly (L-lactide)(PLLA)/multiwalled carbon nanotube (MWCNT) composite: Characterization and biocompatibility evaluation. J. Phys. Chem. B 2006, 110, 12910–12915. [Google Scholar] [CrossRef]
- McCullen, S.D.; Stevens, D.R.; Roberts, W.A.; Clarke, L.I.; Bernacki, S.H.; Gorga, R.E.; Loboa, E.G. Characterization of electrospun nanocomposite scaffolds and biocompatibility with adipose-derived human mesenchymal stem cells. Int. J. Nanomed. 2007, 2, 253. [Google Scholar]
- Grasa, L.; Ansón-Casaos, A.; Martínez, M.T.; Albendea, R.; De Martino, A.; Gonzalo, S.; Murillo, M.D. Single-Walled Carbon Nanotubes (SWCNTs) Enhance KCl-, Acetylcholine-, and Serotonin-Induced Contractions and Evoke Oxidative Stress on Rabbit Ileum. J. Biomed. Nanotechnol. 2014, 10, 529–542. [Google Scholar] [CrossRef]
- Firme, C.P., III; Bandaru, P.R. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 245–256. [Google Scholar] [CrossRef]
- Endo, M.; Strano, M.S.; Ajayan, P.M. Potential applications of carbon nanotubes. In Carbon Nanotubes; Springer: Berlin, Germany, 2007; pp. 13–62. [Google Scholar]
- Murugesan, S.; Mousa, S.; Vijayaraghavan, A.; Ajayan, P.M.; Linhardt, R.J. Ionic liquid-derived blood-compatible composite membranes for kidney dialysis. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 79, 298–304. [Google Scholar] [CrossRef][Green Version]
- Reddy, A.R.N.; Reddy, Y.N.; Krishna, D.R.; Himabindu, V. Multi wall carbon nanotubes induce oxidative stress and cytotoxicity in human embryonic kidney (HEK293) cells. Toxicology 2010, 272, 11–16. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Gherardini, L.; Bardi, G.; Nunes, A.; Guo, C.; Bussy, C.; Herrero, M.A.; Bianco, A.; Prato, M.; Kostarelos, K.J.; et al. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc. Natl. Acad. Sci. USA 2011, 108, 10952–10957. [Google Scholar] [CrossRef]
- Scheinberg, D.A.; Villa, C.H.; Escorcia, F.E.; McDevitt, M.R. Conscripts of the infinite armada: Systemic cancer therapy using nanomaterials. Nat. Rev. Clin. Oncol. 2010, 7, 266–276. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Chattopadhyay, D.; Kappel, B.J.; Jaggi, J.S.; Schiffman, S.R.; Antczak, C.; Njardarson, J.T.; Brentjens, R.; Scheinberg, D.A. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J. Nucl. Med. 2007, 48, 1180–1189. [Google Scholar] [CrossRef]
- Alidori, S.; Akhavein, N.; Thorek, D.L.; Behling, K.; Romin, Y.; Queen, D.; Beattie, B.J.; Manova-Todorova, K.; Bergkvist, M.; Scheinberg, D.A. Targeted fibrillar nanocarbon RNAi treatment of acute kidney injury. Sci. Transl. Med. 2016, 8, 331ra39. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yamashita, Y.; Akatsuka, S.; Ishihara, T.; Yamashita, K.; Yoshikawa, Y.; Yasui, H. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, E1330–E1338. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.C.; Weston, P.; Yan, A.; Hurt, R.H.; Kane, A.B. A 3-dimensional in vitro model of epithelioid granulomas induced by high aspect ratio nanomaterials. Part. Fibre Toxicol. 2011, 8, 1–19. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Famian, M.H.; Saheb, S.M.; Montaseri, A. Conditioned medium of wharton’s jelly derived stem cells can enhance the cartilage specific genes expression by chondrocytes in monolayer and mass culture systems. Adv. Pharm. Bull. 2017, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.; Hong, S.; Park, J.H.; Park, H.H. Stem Cell-Derived Exosomes and Nanovesicles: Promotion of Cell Proliferation, Migration, and Anti-Senescence for Treatment of Wound Damage and Skin Ageing. Pharmaceutics 2020, 12, 1135. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019, 54, 789–792. [Google Scholar] [CrossRef]
- Bi, B.; Schmitt, R.; Israilova, M.; Nishio, H.; Cantley, L.G. Stromal cells protect against acute tubular injury via an endocrine effect. J. Am. Soc. Nephrol. 2007, 18, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; De Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic potential of the MSC exosome proteome: Implications for an exosome-mediated delivery of therapeutic proteasome. Int. J. Proteom. 2012, 2012. [Google Scholar] [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Kooijmans, S.A.; Schiffelers, R.M.; Zarovni, N.; Vago, R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol. Res. 2016, 111, 487–500. [Google Scholar] [CrossRef]
- Bruno, S.; Chiabotto, G.; Favaro, E.; Deregibus, M.C.; Camussi, G. Role of extracellular vesicles in stem cell biology. Am. J. Physiol. Cell Physiol. 2019, 317, C303–C313. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Falda, M.; Bussolati, B.; Tetta, C. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, C.; Zhang, P.; Jiang, H.; Chen, J. Genetic communication by extracellular vesicles is an important mechanism underlying stem cell-based therapy-mediated protection against acute kidney injury. Stem Cell Res. Ther. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Collino, F.; Bruno, S.; Incarnato, D.; Dettori, D.; Neri, F.; Provero, P.; Pomatto, M.; Oliviero, S.; Tetta, C.; Quesenberry, P.J. AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNAs. J. Am. Soc. Nephrol. 2015, 26, 2349–2360. [Google Scholar] [CrossRef]
- Tomasoni, S.; Longaretti, L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013, 22, 772–780. [Google Scholar] [CrossRef]
- Sanchez, M.B.H.; Bruno, S.; Grange, C.; Tapparo, M.; Cantaluppi, V.; Tetta, C.; Camussi, G. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res. Ther. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Tapparo, M.; Collino, F.; Chiabotto, G.; Deregibus, M.C.; Soares Lindoso, R.; Neri, F.; Kholia, S.; Giunti, S.; Wen, S. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng. Part A 2017, 23, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Deregibus, M.-C.; Bruno, S.; Grange, C.; Fonsato, V.; Tetta, C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am. J. Cancer Res. 2011, 1, 98. [Google Scholar] [PubMed]
- Cantaluppi, V.; Medica, D.; Mannari, C.; Stiaccini, G.; Figliolini, F.; Dellepiane, S.; Quercia, A.D.; Migliori, M.; Panichi, V.; Giovannini, L.; et al. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1. 1 glomerulonephritis. Nephrol. Dial. Transplant. 2015, 30, 410–422. [Google Scholar] [CrossRef]
- Vincent, J.F.; Bogatyreva, O.A.; Bogatyrev, N.R.; Bowyer, A.; Pahl, A.-K. Biomimetics: Its practice and theory. J. R. Soc. Interface 2006, 3, 471–482. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhang, S.; Qin, J.; Wang, J. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B. 2018, 8, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Papachristopoulou, K.; Vainos, N.A. Systolic Nanofabrication of Super-Resolved Photonics and Biomimetics. Nanomaterials 2020, 10, 2418. [Google Scholar] [CrossRef]
- Butt, H. Imaging surfaces with scanning tunnelling and scanning force microscopes. In X-Ray Optics and Microanalysis 1992, Proceedings of the 13th INT Conference, Manchester, UK, 31 August–4 September 1992; CRC Press: Boca Raton, FL, USA, 2020; p. 339. [Google Scholar]
- Schröder, B.; Bunjes, O.; Wimmer, L.; Kaiser, K.; Traeger, G.A.; Kotzott, T.; Ropers, C.; Wenderoth, M. Controlling photocurrent channels in scanning tunneling microscopy. New J. Phys. 2020, 22, 033047. [Google Scholar] [CrossRef]
- Adibkia, K.; Yaqoubi, S.; Dizaj, S.M. Pharmaceutical and Medical Applications of Nanofibers. In Novel Approaches for Drug Delivery; IGI Global: Hershey, PA, USA, 2017; pp. 338–363. [Google Scholar]
- Ghavimi, M.A.; Negahdari, R.; Bani Shahabadi, A.; Sharifi, S.; Kazeminejad, E.; Shahi, S.; Maleki Dizaj, S. Preparation and study of starch/collagen/polycaprolactone nanofiber scaffolds for bone tissue engineering using electrospinning technique. Eurasian Chem. Commun. 2020, 2, 122–127. [Google Scholar]
- Sharifi, S.; Mokhtarpour, M.; Jahangiri, A.; Dehghanzadeh, S.; Maleki-Dizaj, S.; Shahi, S. Hydroxyapatite nanofibers as beneficial nanomaterial in dental sciences. Biointerface Res. Appl. Chem. 2018, 8, 3695–3699. [Google Scholar]
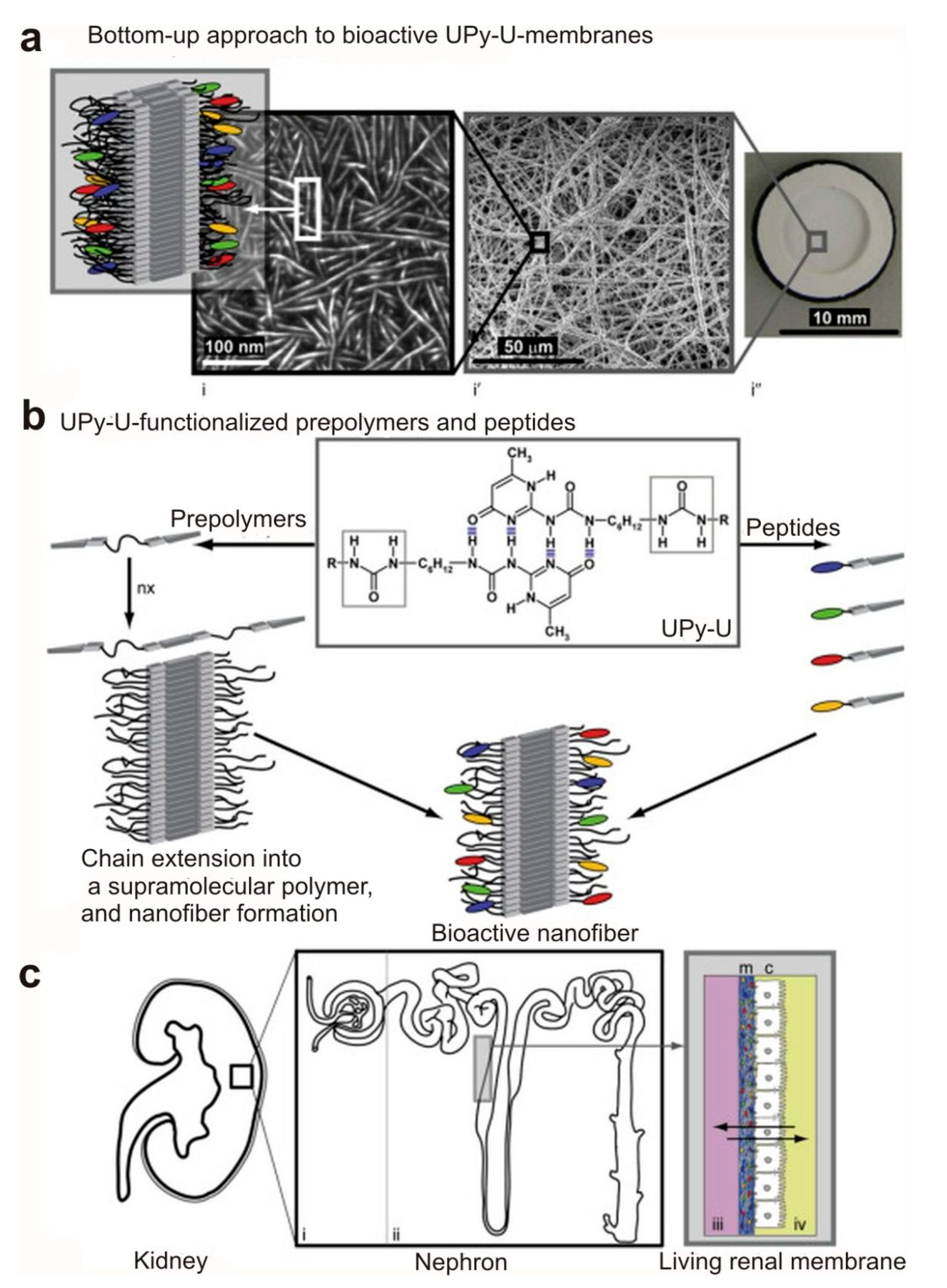
- Dankers, P.Y.; Boomker, J.M.; der Vlag, A.H.V.; Smedts, F.M.; Harmsen, M.C.; van Luyn, M.J. The use of fibrous, supramolecular membranes and human tubular cells for renal epithelial tissue engineering: Towards a suitable membrane for a bioartificial kidney. Macromol. Biosci. 2010, 10, 1345–1354. [Google Scholar] [CrossRef]
- Mollet, B.B.; Bogaerts, I.L.; van Almen, G.C.; Dankers, P.Y. A bioartificial environment for kidney epithelial cells based on a supramolecular polymer basement membrane mimic and an organotypical culture system. J. Tissue Eng. Regen. Med. 2017, 11, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.-S.; Bäckhed, F.; von Euler, A.; Richter-Dahlfors, A.; Sutherland, D.; Kasemo, B. Nanoscale features influence epithelial cell morphology and cytokine production. Biomaterials 2003, 24, 3427–3436. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, D.J.; Min, B.G.; Lee, S.H. Nano web based novel microchip for artificial kidney. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Seoul, Korea, 27 August–1 September 2006; Springer: Berlin/Heidelberg, Germany, 2007; pp. 279–282. [Google Scholar]
- Pechar, T.W.; Kim, S.; Vaughan, B.; Marand, E.; Tsapatsis, M.; Jeong, H.K.; Cornelius, C.J. Fabrication and characterization of polyimide–zeolite L mixed matrix membranes for gas separations. J. Membr. Sci. 2006, 277, 195–202. [Google Scholar] [CrossRef]
- Malamis, S.; Katsou, E.; Stylianou, M.; Haralambous, K.; Loizidou, M. Copper removal from sludge permeate with ultrafiltration membranes using zeolite, bentonite and vermiculite as adsorbents. Water Sci. Technol. 2010, 61, 581–589. [Google Scholar] [CrossRef]
- Dankers, P.Y.; Boomker, J.M.; Huizinga-van der Vlag, A.; Wisse, E.; Appel, W.P.; Smedts, F.M.; Harmsen, M.C.; Bosman, A.W.; Meijer, W.; van Luyn, M.J. Bioengineering of living renal membranes consisting of hierarchical, bioactive supramolecular meshes and human tubular cells. Biomaterials 2011, 32, 723–733. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef]
- Lei, K.F. Materials and Fabrication Techniques for Nano- and Microfluidic Devices. In Microfluidics in Detection Science Lab-on-a-chip Technologies; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Smeby, L.C.; Widerøe, T.-E.; Balstad, T.; Jørstad, S. Biocompatibility aspects of cellophane, cellulose acetate, polyacrylonitrile, polysulfone and polycarbonate hemodialyzers. Blood Purif. 1986, 4, 93–101. [Google Scholar] [CrossRef]
- Lu, L.; Samarasekera, C.; Yeow, J.T. Creatinine adsorption capacity of electrospun polyacrylonitrile (PAN)-zeolite nanofiber membranes for potential artificial kidney applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Tsuge, M.; Takahashi, K.; Kurimoto, R.; Fulati, A.; Uto, K.; Kikuchi, A.; Ebara, M. Fabrication of water absorbing nanofiber meshes toward an efficient removal of excess water from kidney failure patients. Fibers 2019, 7, 39. [Google Scholar] [CrossRef]
- Namekawa, K.; Schreiber, M.T.; Aoyagi, T.; Ebara, M. Fabrication of zeolite–polymer composite nanofibers for removal of uremic toxins from kidney failure patients. Biomater. Sci. 2014, 2, 674–679. [Google Scholar] [CrossRef]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Organ printing: From bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 2011, 22, 667–673. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eftekhari, A.; Maleki Dizaj, S.; Ahmadian, E.; Przekora, A.; Hosseiniyan Khatibi, S.M.; Ardalan, M.; Zununi Vahed, S.; Valiyeva, M.; Mehraliyeva, S.; Khalilov, R.; et al. Application of Advanced Nanomaterials for Kidney Failure Treatment and Regeneration. Materials 2021, 14, 2939. https://doi.org/10.3390/ma14112939
Eftekhari A, Maleki Dizaj S, Ahmadian E, Przekora A, Hosseiniyan Khatibi SM, Ardalan M, Zununi Vahed S, Valiyeva M, Mehraliyeva S, Khalilov R, et al. Application of Advanced Nanomaterials for Kidney Failure Treatment and Regeneration. Materials. 2021; 14(11):2939. https://doi.org/10.3390/ma14112939
Chicago/Turabian StyleEftekhari, Aziz, Solmaz Maleki Dizaj, Elham Ahmadian, Agata Przekora, Seyed Mahdi Hosseiniyan Khatibi, Mohammadreza Ardalan, Sepideh Zununi Vahed, Mahbuba Valiyeva, Sevil Mehraliyeva, Rovshan Khalilov, and et al. 2021. "Application of Advanced Nanomaterials for Kidney Failure Treatment and Regeneration" Materials 14, no. 11: 2939. https://doi.org/10.3390/ma14112939
APA StyleEftekhari, A., Maleki Dizaj, S., Ahmadian, E., Przekora, A., Hosseiniyan Khatibi, S. M., Ardalan, M., Zununi Vahed, S., Valiyeva, M., Mehraliyeva, S., Khalilov, R., & Hasanzadeh, M. (2021). Application of Advanced Nanomaterials for Kidney Failure Treatment and Regeneration. Materials, 14(11), 2939. https://doi.org/10.3390/ma14112939