Advanced Simulation of Removing Chromium from a Synthetic Wastewater by Rhamnolipidic Bioflotation Using Hybrid Neural Networks with Metaheuristic Algorithms
Abstract
1. Introduction
2. Materials and Methods
2.1. Biosurfactant Production and Reagents
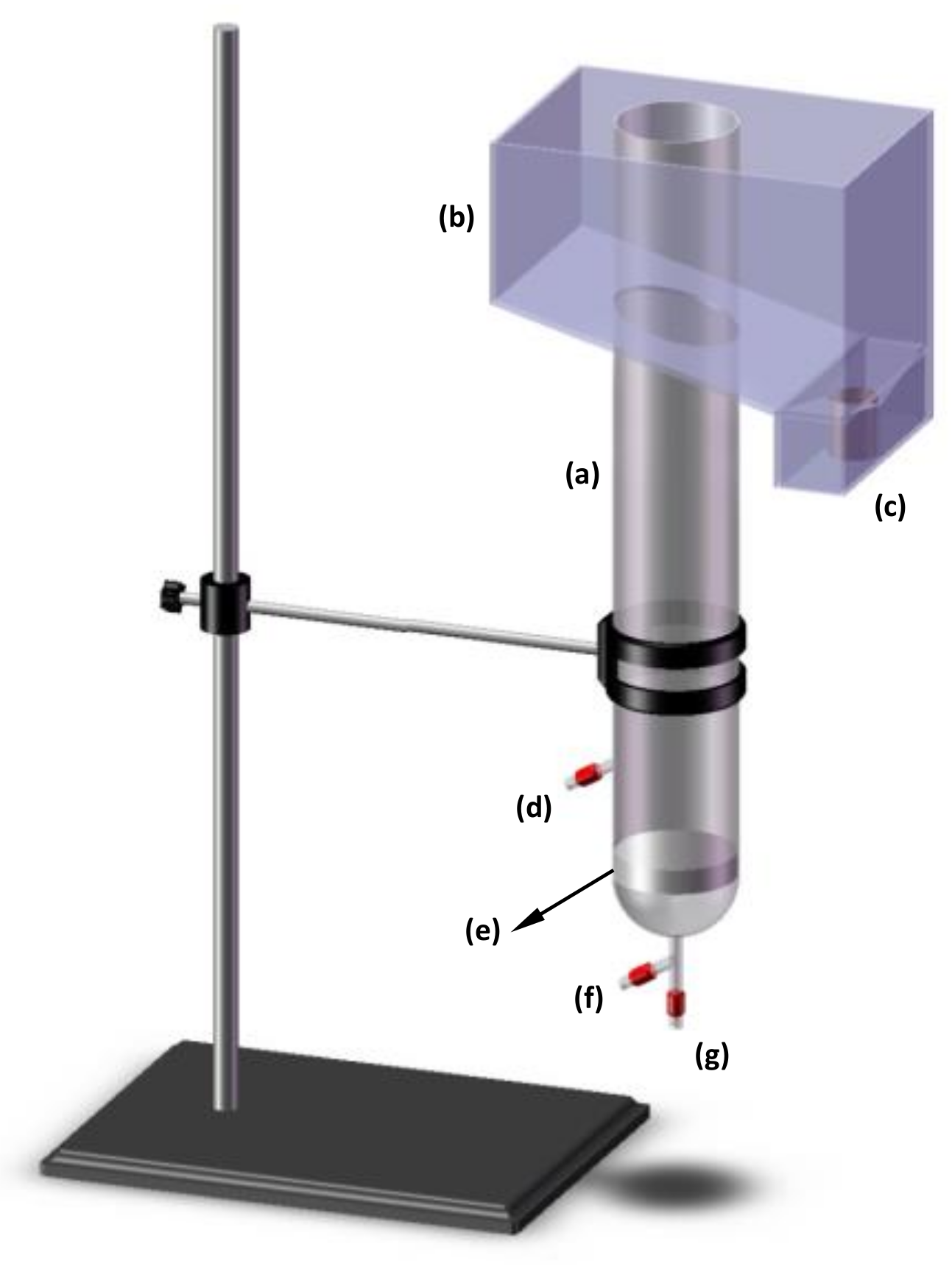
2.2. Bioflotation Variables and Experiment
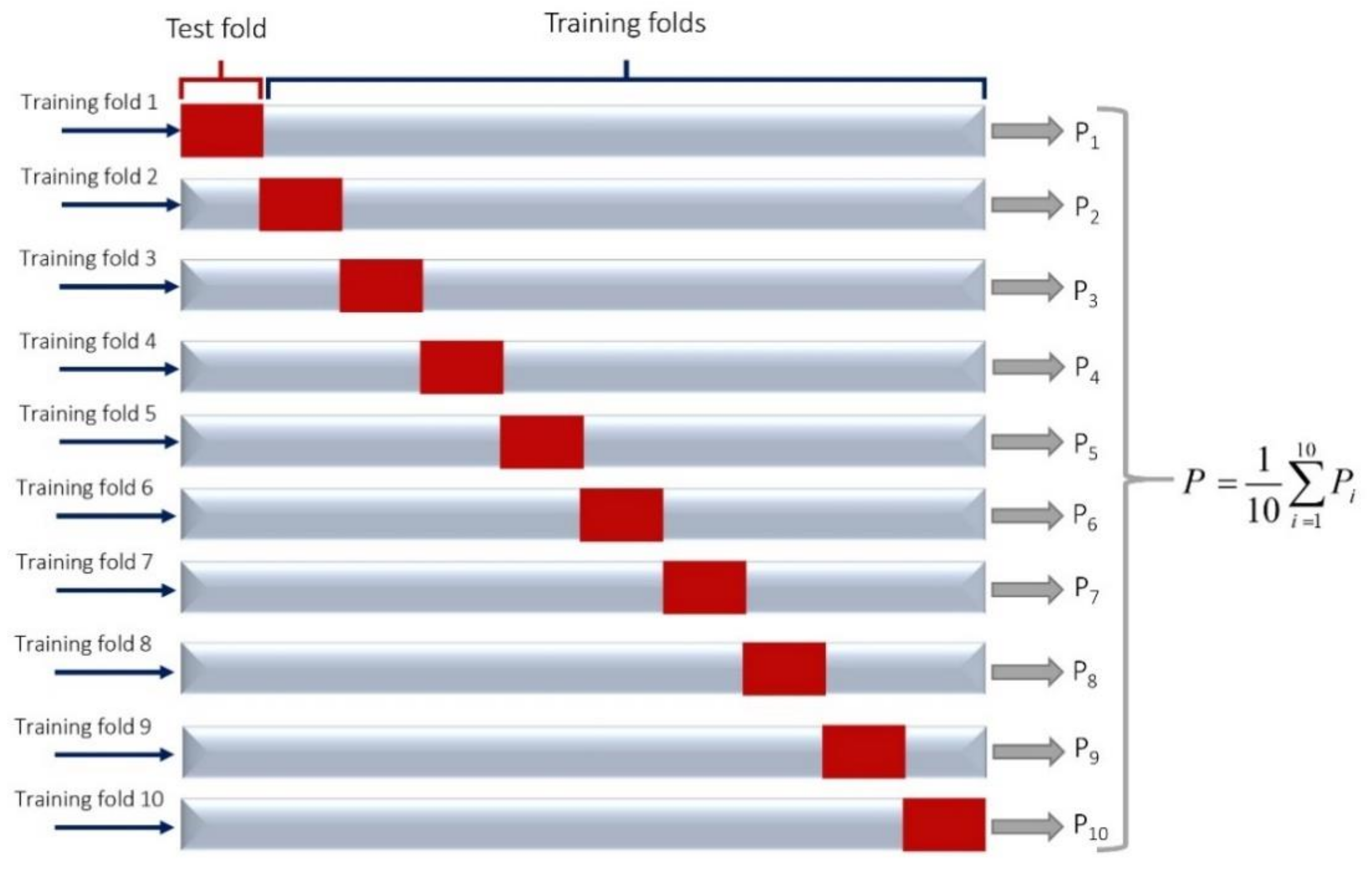
2.3. ANN Simulation
- Data selection and preprocessing
- Divide the samples into two sets: training and test
- Select modeling method, geometry, and optimization algorithms
- Develop appropriate ANN model and verification of results
2.3.1. Optimization Algorithms Applied
- (a)
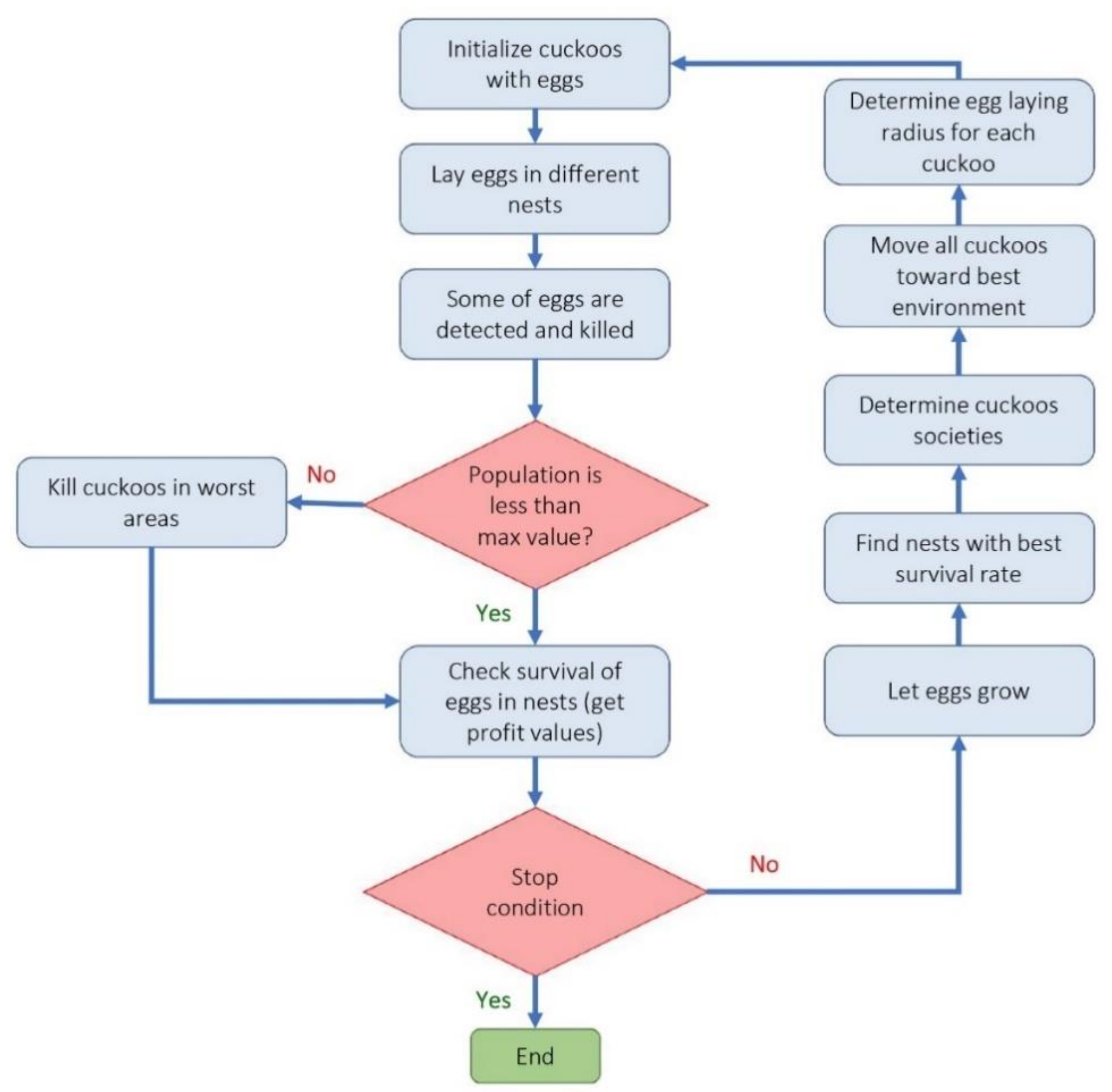
- Cuckoo optimization algorithm (COA): Cuckoo optimization algorithm developed to solve nonlinear and/or continuous processes [39]. This algorithm is driven from the lives of a family of birds called cuckoo and is based on the optimal lifestyle and exciting features of this species, such as spawning and reproduction. Adult cuckoos and cuckoo eggs make up the initial population of the cuckoo optimization algorithm. Adult cuckoos lay their eggs in other birds’ nests. If the cuckoo eggs are not detected and destroyed by the host birds, they will grow into adult cuckoos [40]. Adult cuckoos migrate en masse under the influence of environmental characteristics and hope to find an optimal environment for life and reproduction. In this algorithm, the optimal environment will be the global optimum in the optimization problem’s objective function. This algorithm has so far performed well in various optimization scenarios and real-world applications [41,42]. Figure 2 illustrates the general route used for a COA development.
- (b)
- Genetic optimization algorithm (GA): The genetic algorithm is a subset of computational models inspired by the concept of evolution. This algorithm encodes potential or candidate solutions for a particular problem in a chromosome-like data structure [43]. Implementation of a genetic algorithm usually begins with producing a population of chromosomes (the initial population of chromosomes in genetic algorithms is usually randomly generated and bound to the upper and lower limits of the problem variables). Next, the generated data structures (chromosomes) are evaluated, and chromosomes that better represent the problem’s optimal solution have a favorable reproduction chance than other chromosomes. In general, the goodness of an answer is usually measured concerning the population of obtained answers. Nowadays, due to this algorithm’s capabilities, especially in solving regression problems, it has a good position among other optimization methods [44,45]. The steps used to develop a genetic optimization code are shown in Figure 3.
- (c)
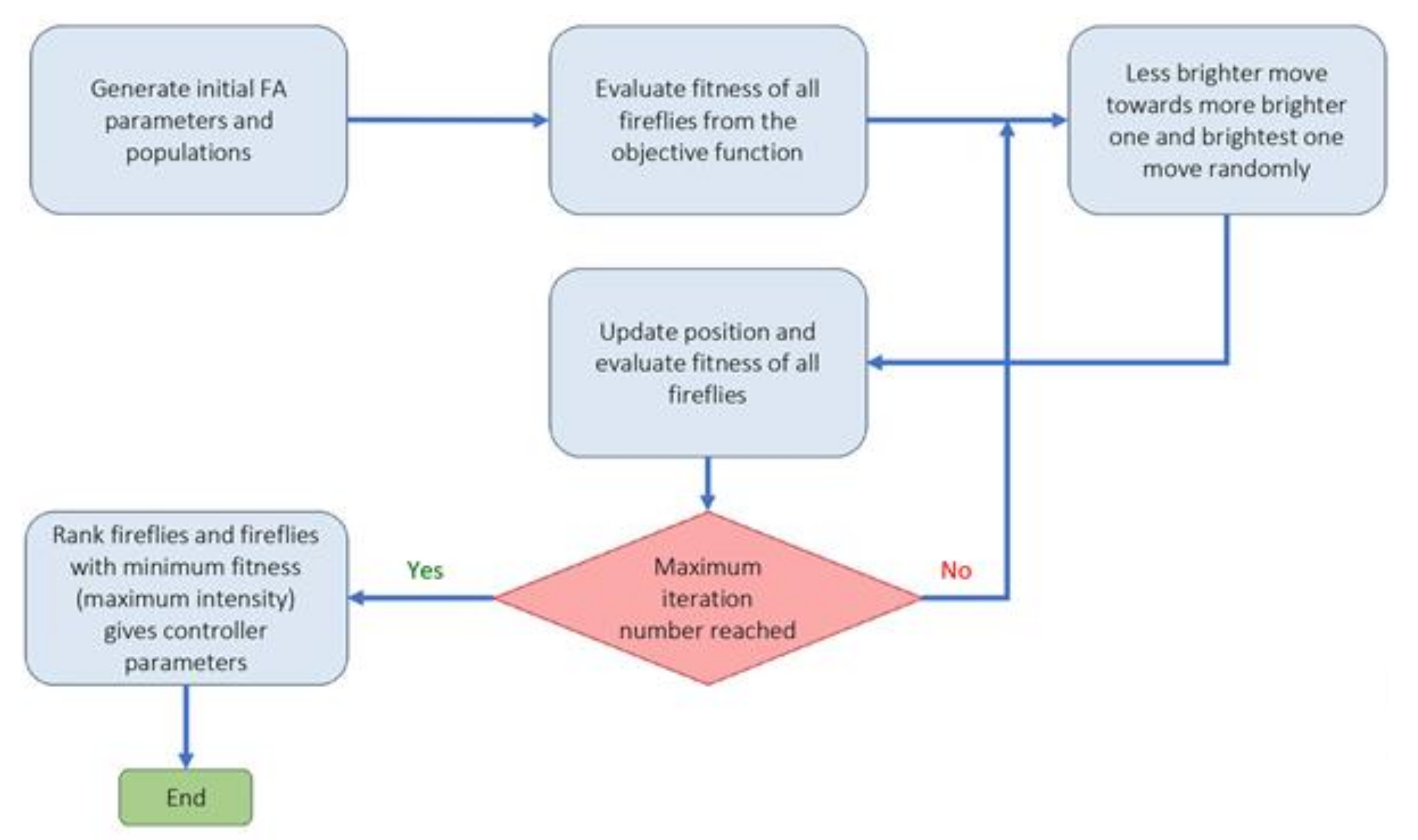
- Firefly optimization algorithm (FFA): The firefly optimization algorithm is inspired by firefly behavior—they live together in large collections—and is one of the most efficient algorithms when solving hybrid optimization problems [46]. The firefly algorithm is a good example of collective intelligence in which agents that do not have very high abilities on their own can achieve great results by working together. The main assumptions of this algorithm are as follows [47,48]: (i) Fireflies are attracted to each other regardless of gender. (ii) The factor of attraction is proportional to their brightness; the brighter Firefly absorbs the lighter firefly. However, as the distance between the two fireflies increases, the attractiveness decreases. (iii) The fireflies with the same brightness move randomly. New pathways are created arbitrarily and generally lead to bright fireflies. Based on the flashing behavior of fireflies and the characteristics of their biological connections, Yang modeled firefly behaviors and developed this algorithm in 2010 [49]. Figure 4 illustrates a simple structure to optimize problems based on FFA.
- (d)
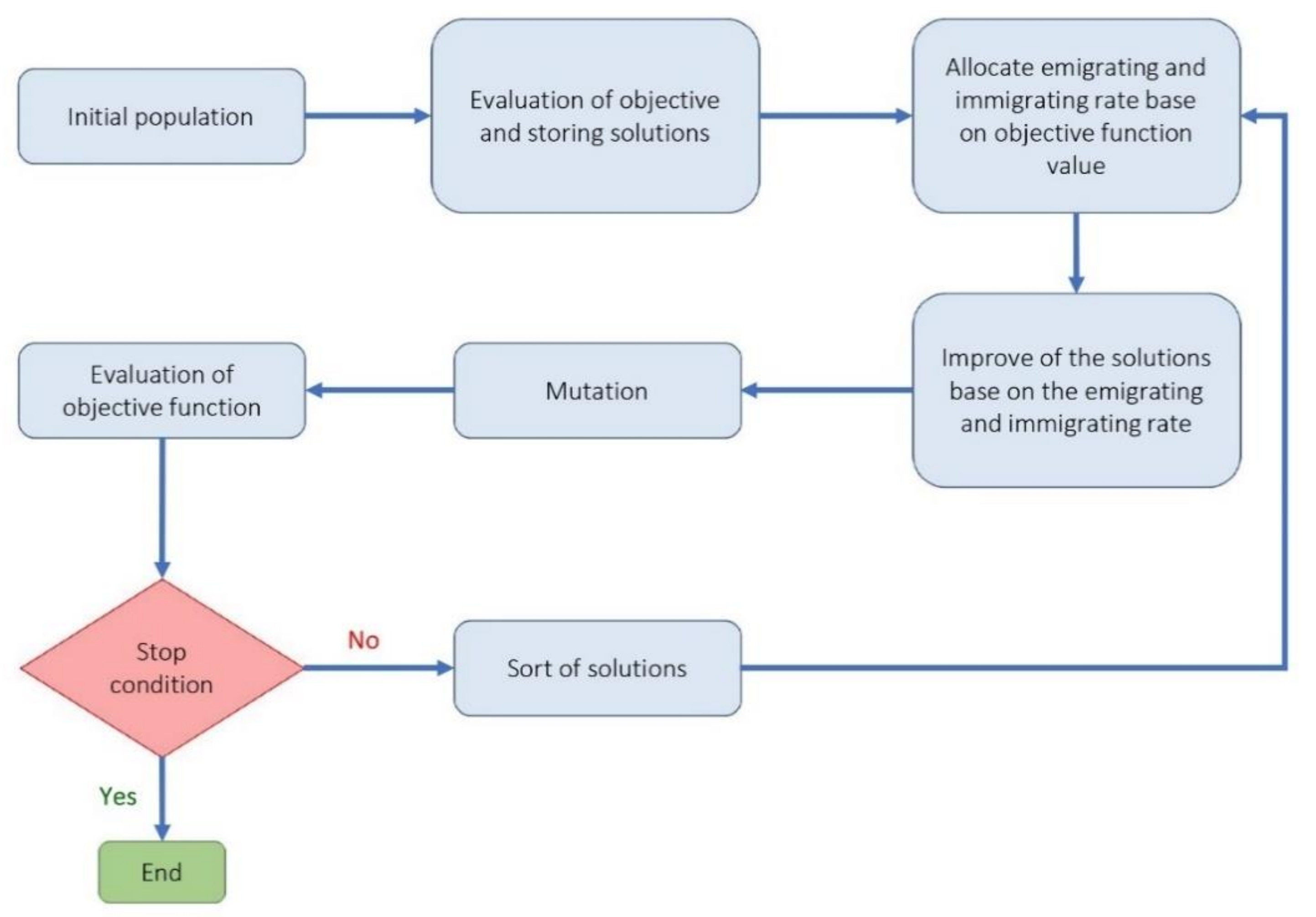
- Biogeography-based optimization algorithm (BBO): The BBO algorithm, like the genetic and the firefly algorithm, is one of the collective intelligence algorithms. It is a nature-based method that uses the principles of biogeography to find the answer. In general, biogeography, as a sub-branch of biological science, studies different species’ behavior in different times and places [50]. In the BBO algorithm, each biological zone is recognized as a single member and has its habitat suitability index (HSI). In this algorithm, the answer or biological region with higher HSI indicates a better answer [51]. In BBO, properties are usually migrating from regions with higher HSI to regions with lower HIS. In other words, regions with low HSI take properties from regions with higher HSI. Each region’s variables are called suitability index variables (SIV), which express each region’s properties and are used in migrations. The BBO algorithm is developed by Simon to solve optimization problems and generate responses that maximize HIS [52]. The main steps to develop a genetic optimization code are shown in Figure 5.
2.3.2. Data Preparation and Pre-Processing
3. Results and Discussions
3.1. Statistical Analysis of Experimental Results
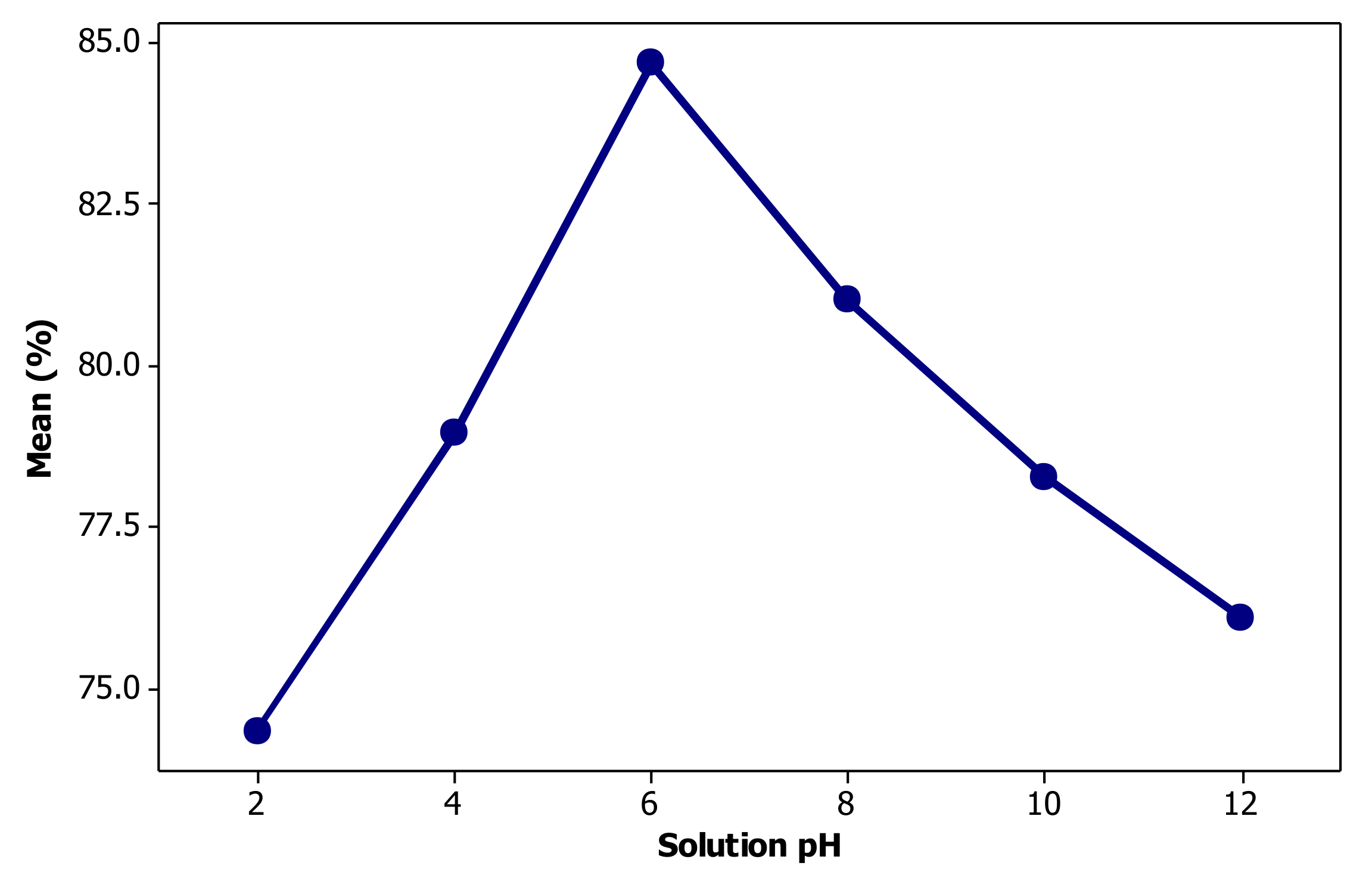
3.2. Effect of Solution pH
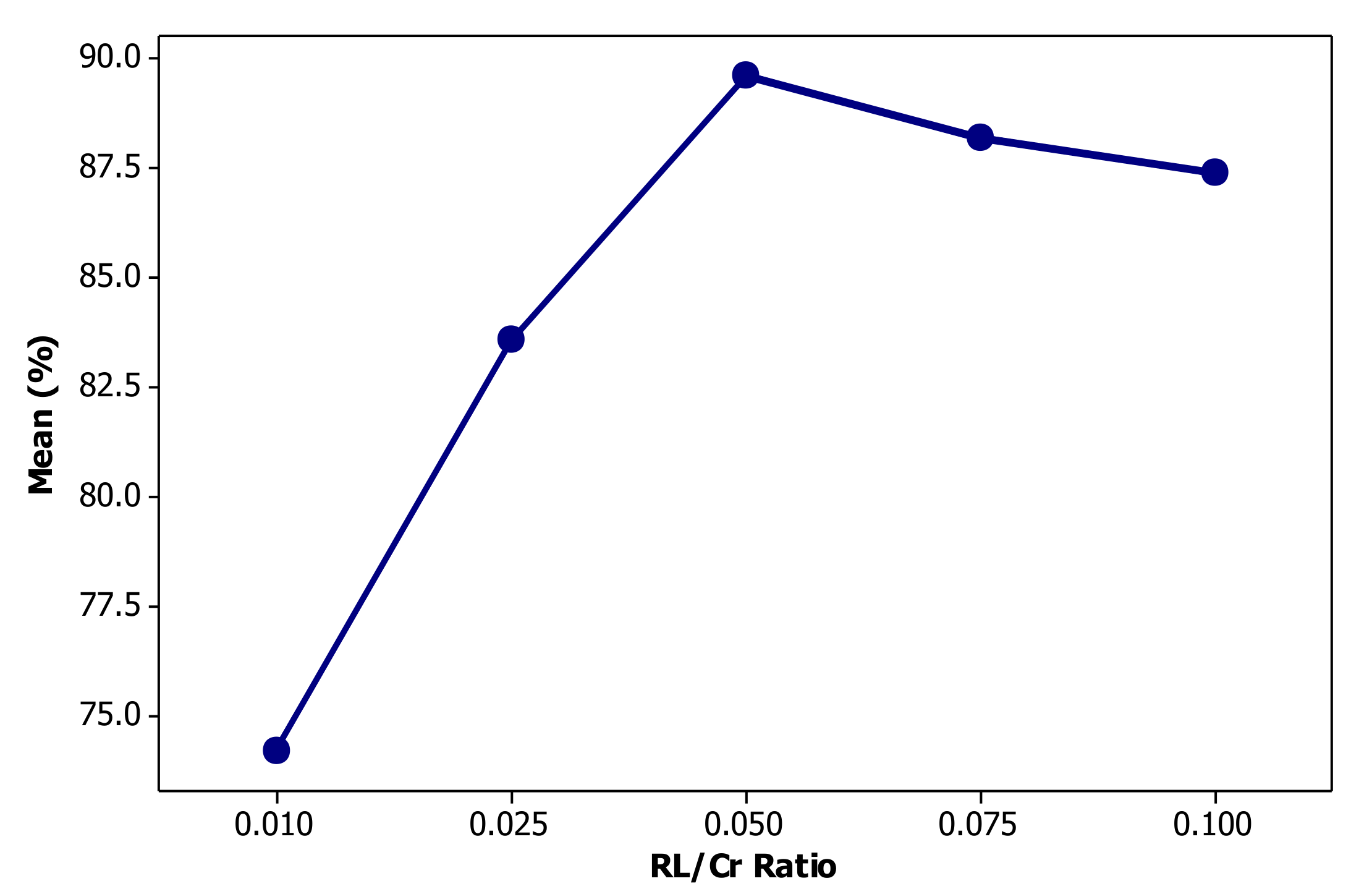
3.3. Effect of Rhamnolipid Concentration
3.4. Effect of Aeration Rate
3.5. Effect of Reductant Concentration
3.6. Interaction Effects and Process Optimization
3.7. Kinetics of Chromium Bioflotation
4. Simulation Results
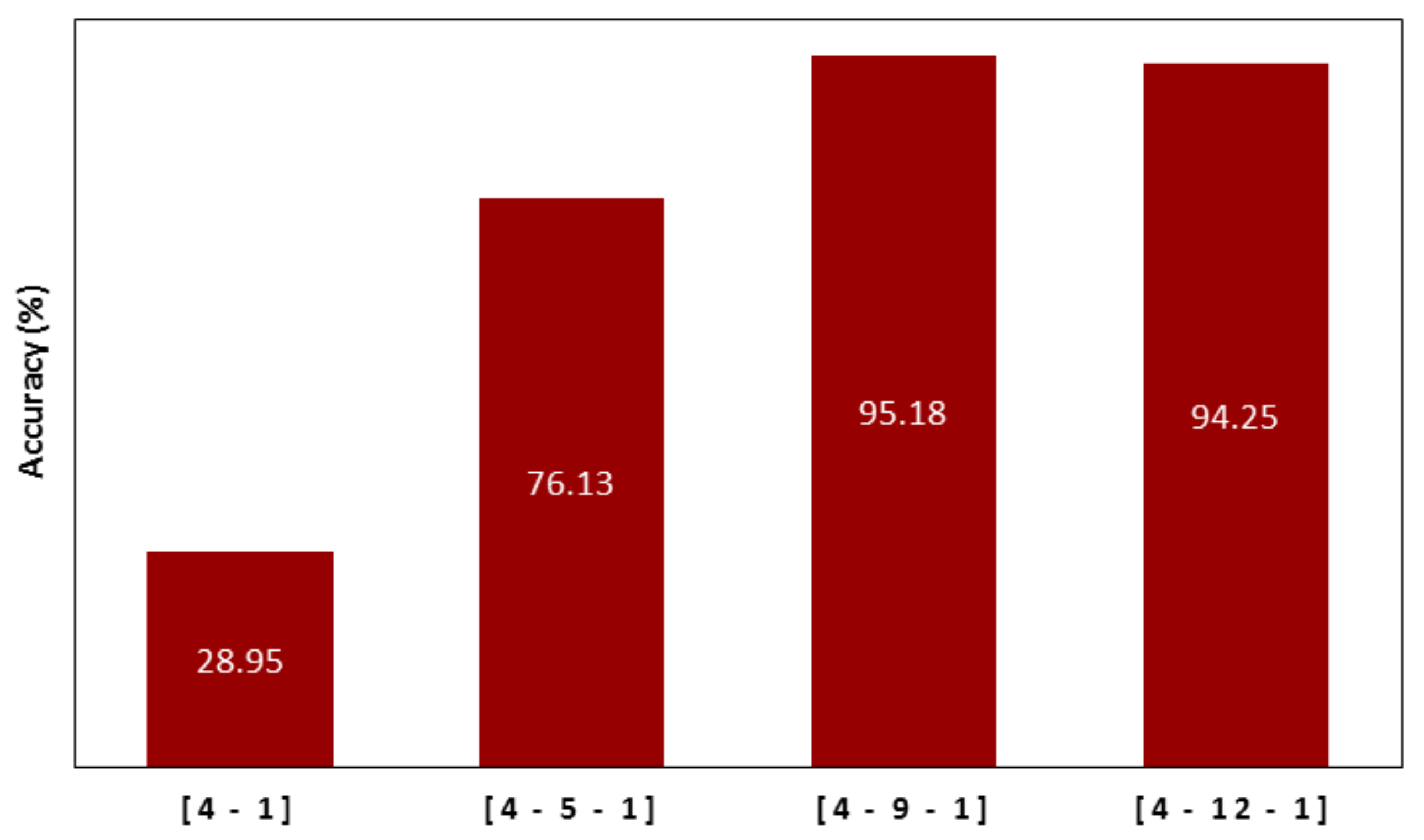
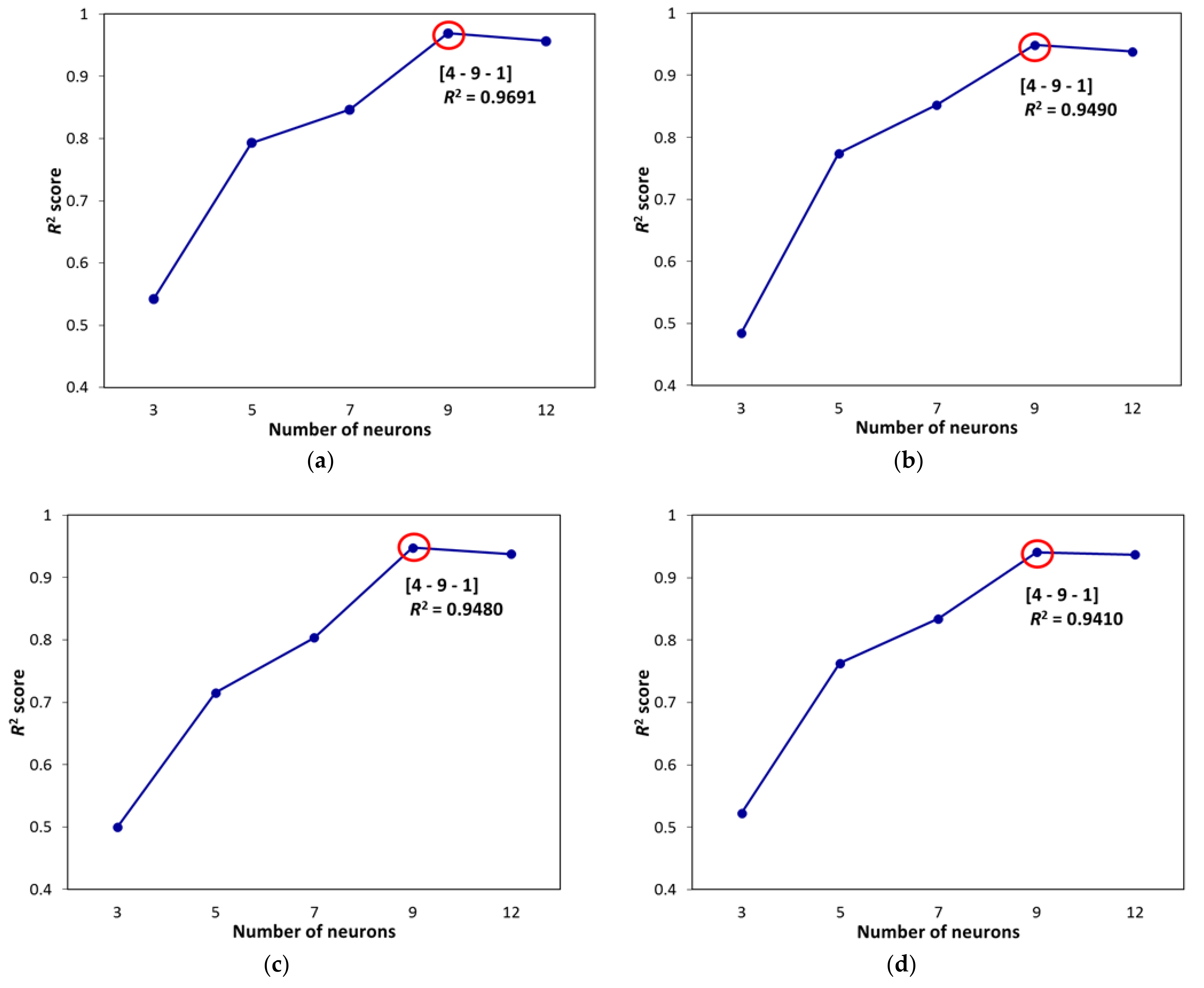
4.1. Artificial Neural Network Design
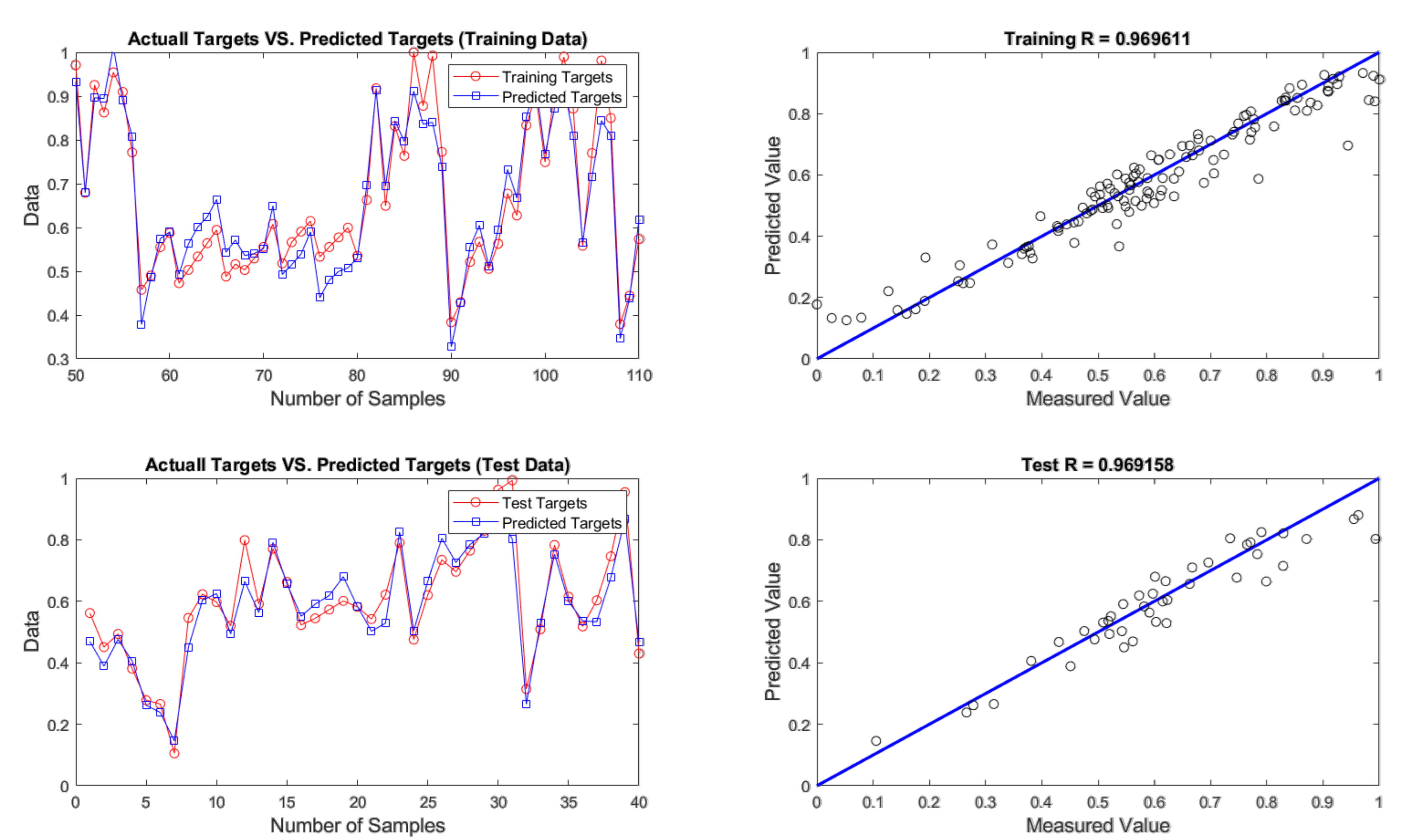
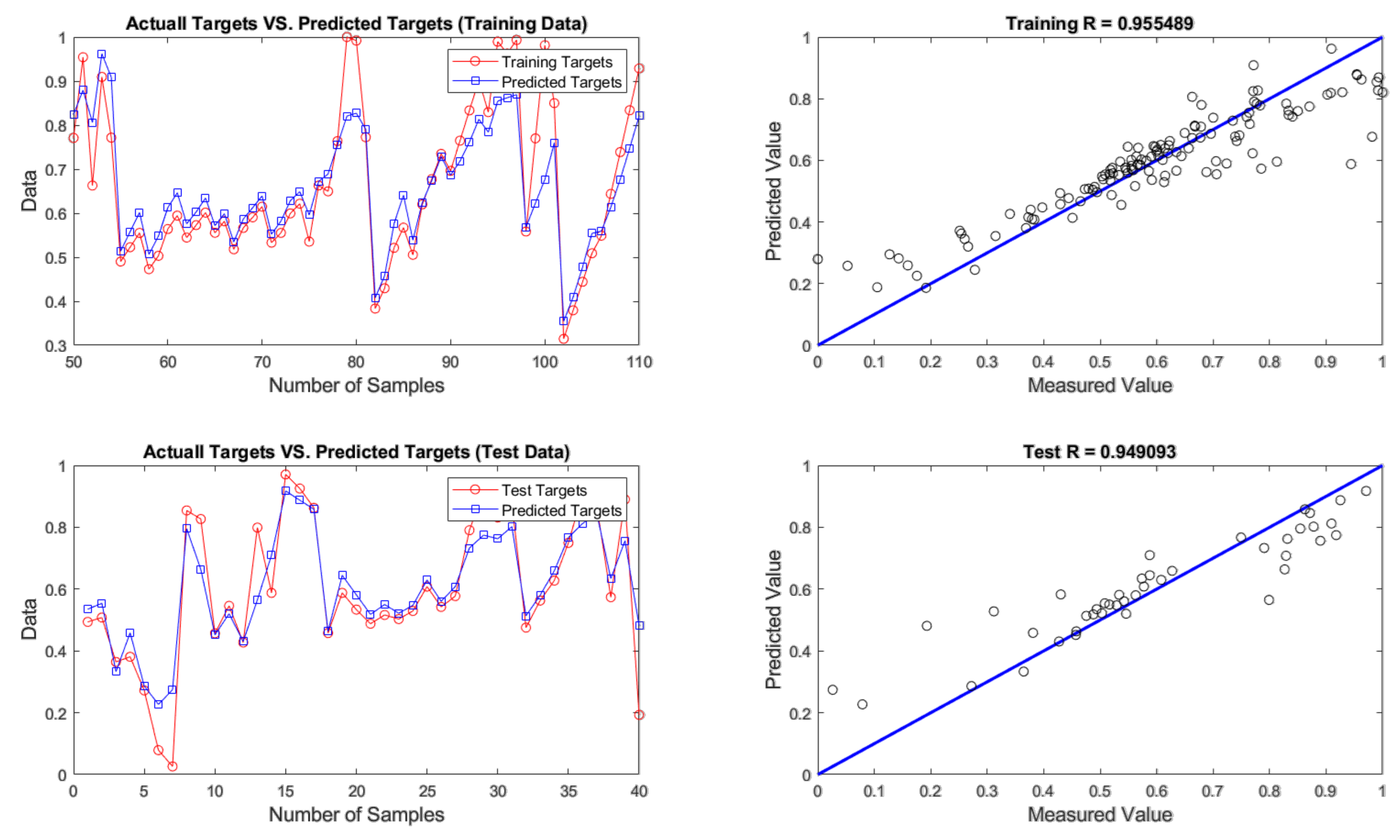
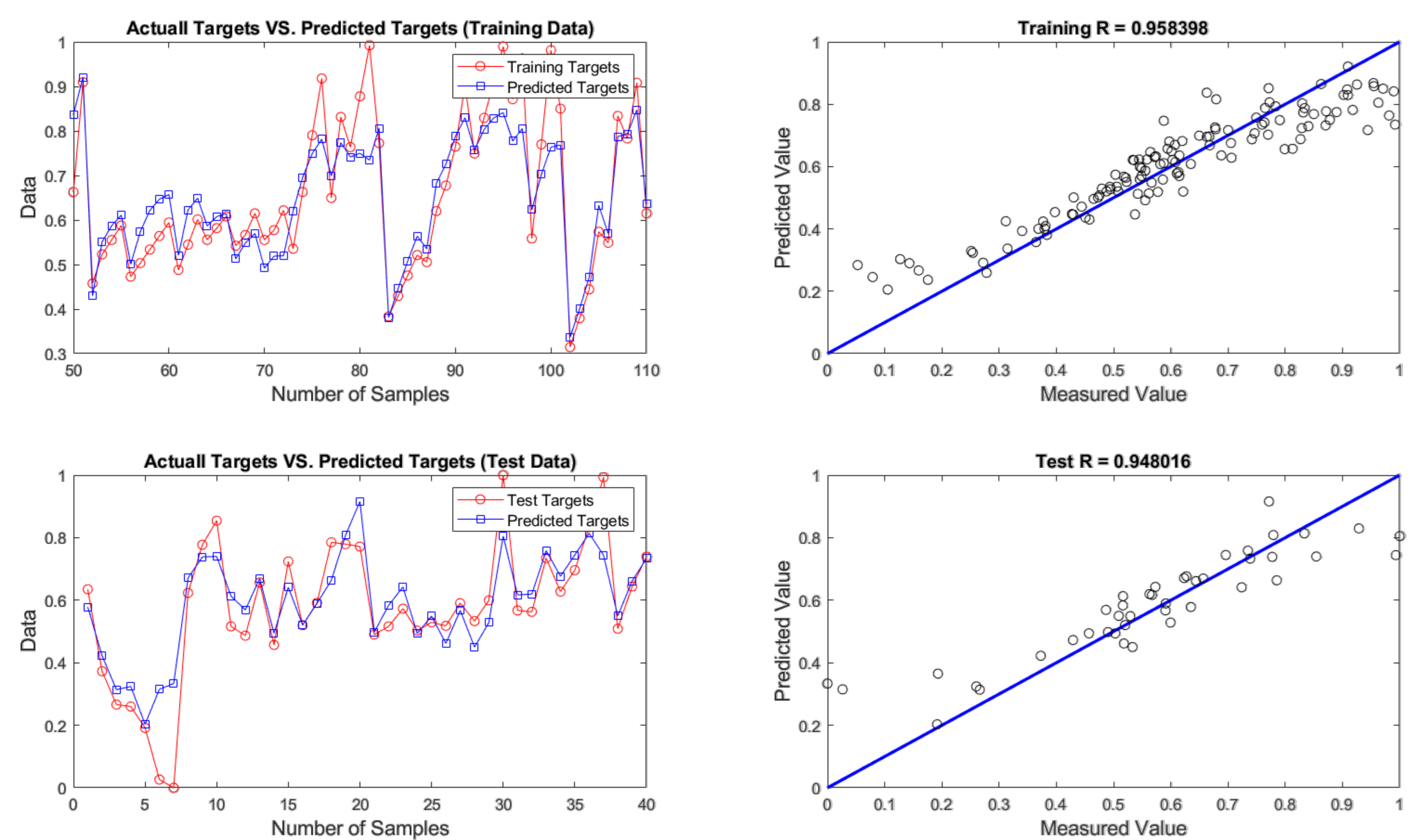
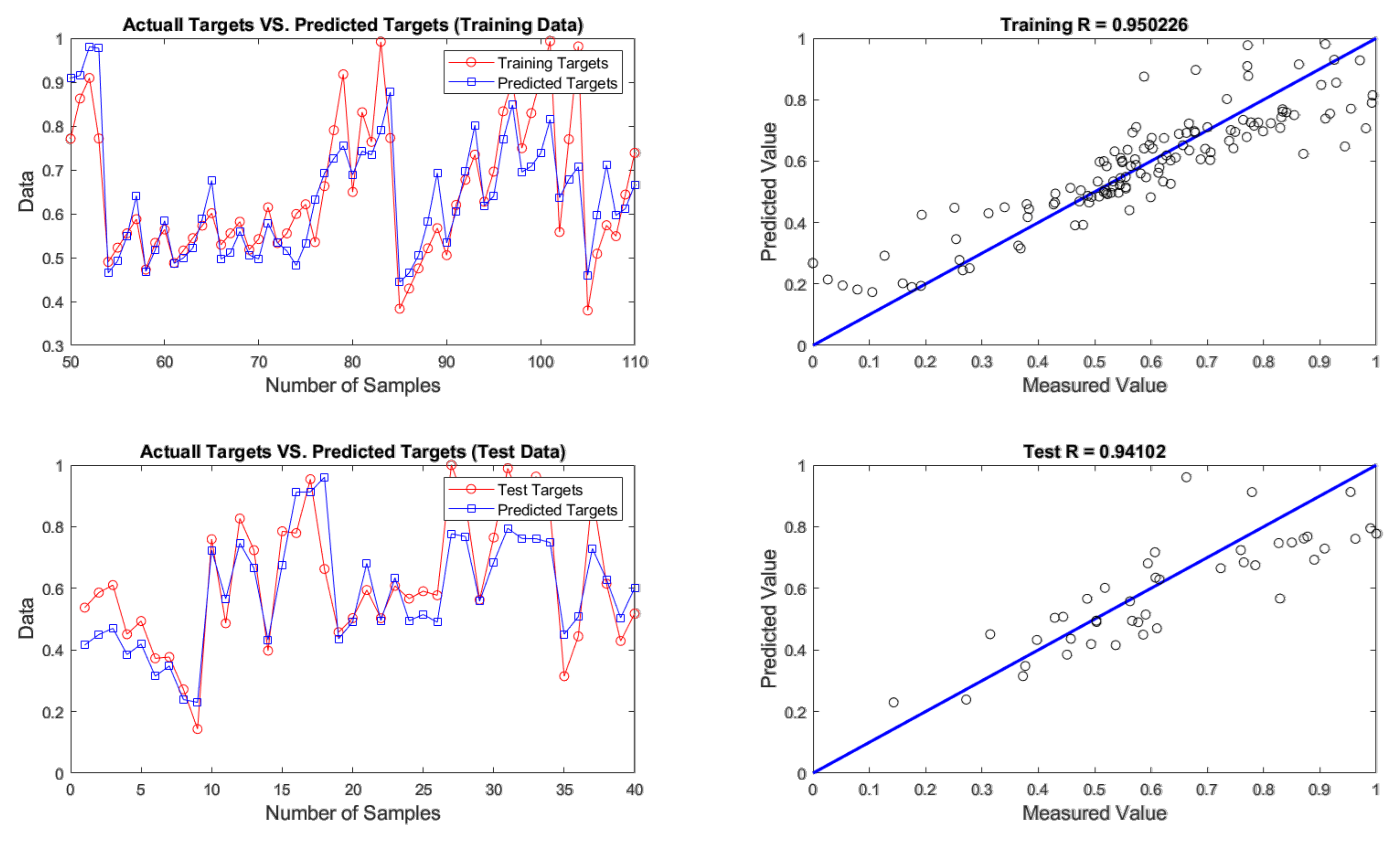
4.2. Evaluation of ANN Prediction Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitra, S.; Sarkar, A.; Se, S. Removal of chromium from industrial effluents using nanotechnology: A review. Nanotechnol. Environ. Eng. 2017, 2, 11. [Google Scholar] [CrossRef]
- Taj, R.Y.; Renganathan, S.; Partha, N.; Baskar, G.; Velan, M. Removal of chromium from synthetic effluent using Nymphaea rubra. Clean 2009, 37, 787–792. [Google Scholar]
- Fahim, N.F.; Barsoum, B.N.; Eid, A.E.; Khalil, M.S. Removal of chromium(III) from tannery wastewater using activated carbon from sugar industrial waste. J. Hazard. Mater. 2006, 136, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Hołda, A.; Kisielowska, E.; Niedoba, T. Bioaccumulation of Cr(VI) ions from aqueos solutions by Aspergilus niger. Pol. J. Environ. Stud. 2011, 20, 345–349. [Google Scholar]
- Lv, X.; Xu, J.; Jiang, G.; Tang, J.; Xu, X. Highly active nanoscale zero-valent iron (nZVI)-Fe3O4 nanocomposites for the removal of chromium(VI) from aqueous solutions. J. Colloid Interface Sci. 2012, 369, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Exerowa, D. Surface forces and properties of foam films from rhamnolipid biosurfactants. Adv. Colloid Interface 2007, 134–135, 24–34. [Google Scholar] [CrossRef]
- Vilinska, A.; Rao, K.H.; Forssberg, K.S.E. Microorganisms in flotation and flocculation of minerals-an overview. In Proceedings of the 24th International Mineral Processing Congress, Beijing, China, 24–28 September 2008; pp. 22–39. [Google Scholar]
- Fazaelipoor, M.H.; Khoshdast, H.; Ranjbar, M. Coal flotation using a biosurfactant from Pseudomonas aeruginosa as a frother. Korean J. Chem. Eng. 2010, 27, 1527–1531. [Google Scholar] [CrossRef]
- Hari, O.; Upadhyay, S.K. Rhamnolipid–metal ions (CrVI and PbII) complexes: Spectrophotometric, conductometric, and surface tension measurement studies. J. Surfact. Deterger. 2021, 24, 281–288. [Google Scholar] [CrossRef]
- Khoshdast, H.; Abbasi, H.; Sam, A.; Akbari Noghabi, K. Frothability and surface behavior of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa MA01. Biochem. Eng. J. 2012, 64, 127–134. [Google Scholar] [CrossRef]
- Renfro, T.D.; Xie, W.; Yang, G.; Chen, G. Rhamnolipid surface thermodynamic properties and transport in agricultural soil. Colloid Surf. B 2014, 115, 317–322. [Google Scholar] [CrossRef]
- İkizler, B.; Arslan, G.; Kipcak, E.; Dirik, C.; Çelenk, D.; Aktuğlu, T.; Helvacı, S.S.; Peker, S. Surface adsorption and spontaneous aggregation of rhamnolipid mixtures in aqueous solutions. Colloid Surf. A 2017, 519, 125–136. [Google Scholar] [CrossRef]
- Zhao, F.; Han, S.; Zhang, Y. Comparative studies on the structural composition, surface/interface activity and application potential of rhamnolipids produced by Pseudomonas aeruginosa using hydrophobic or hydrophilic substrates. Bioresource Technol. 2020, 295, 122269. [Google Scholar] [CrossRef] [PubMed]
- Rekiel, E.; Zdziennicka, A.; Jańczuk, B. Adsorption properties of rhamnolipid and ethanol at water/ethanol solution-air interface. J. Mol. Liq. 2020, 308, 113080. [Google Scholar] [CrossRef]
- Ahmad, Z.; Zhang, X.; Imran, M.; Zhong, H.; Andleeb, S.; Zulekha, R.; Liu, G.; Ahmad, I.; Coulon, F. Production, functional stability, and effect of rhamnolipid biosurfactant from Klebsiella sp. on phenanthrene degradation in various medium systems. Ecotox. Environ. Saf. 2021, 207, 111514. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Dubey, K.V.; Nair, A.; Singh, S.K. Bioremediation of multi-metal contaminated soil using biosurfactant—A novel approach. Indian J. Microbiol. 2008, 48, 142–146. [Google Scholar]
- Ozturk, S.; Kaya, T.; Aslim, B.; Tan, S. Removal and reduction of chromium by Pseudomonas spp. and their correlation to rhamnolipid production. J. Hazard. Mater. 2012, 231–232, 64–69. [Google Scholar] [CrossRef]
- Abbasi-Garravand, E.; Mulligan, C.N. Using micellar enhanced ultrafiltration and reduction techniques for removal of Cr(VI) and Cr(III) from water. Sep. Purif. Technol. 2014, 132, 505–512. [Google Scholar] [CrossRef]
- Chen, W.; Qu, Y.; Xu, Z.; He, F.; Chen, Z.; Huang, S.; Li, Y. Heavy metal (Cu, Cd, Pb, Cr) washing from river sediment using biosurfactant rhamnolipid. Environ. Sci. Pollut. Res. Int. 2017, 24, 16344–16350. [Google Scholar] [CrossRef]
- Shojaei, V.; Khoshdast, H. Efficient chromium removal from aqueous solutions by precipitate flotation using rhamnolipid biosurfactants. Physicochem. Probl. Miner. Process. 2018, 54, 1014–1025. [Google Scholar]
- Abyaneh, A.S.; Fazaelipoor, M.H. Evaluation of rhamnolipid (RL) as a biosurfactant for the removal of chromium from aqueous solutions by precipitate flotation. J. Environ. Manag. 2016, 165, 184–187. [Google Scholar] [CrossRef]
- Yang, R.; Wang, H.; Shi, M.; Jiang, Y.; Dong, Y.; Shi, L. Biosurfactant rhamnolipid affacts the desorption of sorbed As(III), As(V), Cr(VI), Cd(II) and Pb(II) on iron (oxyhydr)oxides and clay minerals. Int. Biodeter. Biodegr. 2020, 153, 105019. [Google Scholar] [CrossRef]
- Gorucu, F.B. Artificial neural network modeling for forecasting gas consumption. Energy Source Part A 2004, 26, 299–307. [Google Scholar] [CrossRef]
- Armaghani, D.J.; Hajihassani, M.; Marto, A.; Faradonbeh, R.S.; Mohamad, E.T. Prediction of blast-induced air overpressure: A hybrid AI-based predictive model. Environ. Monit. Assess. 2015, 187, 666. [Google Scholar] [CrossRef]
- Altıner, M.; Kuvvetli, Y. Prediction of grinding behavior of low-grade coal based on its moisture loss by neural networks. Energy Source Part A 2017, 39, 1250–1257. [Google Scholar] [CrossRef]
- Khandelwal, M.; Faradonbeh, R.S.; Monjezi, M.; Armaghani, D.J.; Majid, M.Z.B.A.; Yagiz, S. Function development for appraising brittleness of intact rocks using genetic programming and non-linear multiple regression models. Eng. Comput. 2017, 33, 13–21. [Google Scholar] [CrossRef]
- Yildiz, S. Artificial neural network (ANN) approach for modeling Zn (II) adsorption in batch process. Korean J. Chem. Eng. 2017, 34, 2423–2434. [Google Scholar] [CrossRef]
- Sobouti, A.; Hoseinian, F.S.; Rezai, B.; Jalili, S. The lead recovery prediction from lead concentrate by an artificial neural network and particle swarm optimization. Geosyst. Eng. 2019, 22, 319–327. [Google Scholar] [CrossRef]
- Hoseinian, F.S.; Rezai, B.; Kowsari, E.; Safari, M. A hybrid neural network/genetic algorithm to predict Zn (II) removal by ion flotation. Sep. Sci. Technol. 2020, 55, 1197–1206. [Google Scholar] [CrossRef]
- Vyas, S.; Das, S.; Ting, Y.-P. Predictive modeling and response analysis of spent catalyst bioleaching using artificial neural network. Biores. Technol. Rep. 2020, 9, 100389. [Google Scholar] [CrossRef]
- Khoshdast, H.; Sam, A.; Vali, H.; Akbari Noghabi, K. Effect of rhamnolipid biosurfactants on performance of coal and mineral flotation. Int. Biodeter. Biodegr. 2011, 65, 1238–1243. [Google Scholar] [CrossRef]
- Khoshdast, H.; Shojaei, V. Ash removal from a sample coal by flotation using rhamnolipid biosurfactants. J. Min. World Express 2012, 1, 39–45. [Google Scholar]
- Yuan, X.Z.; Meng, Y.T.; Zeng, G.M.; Fang, Y.Y.; Shi, J.G. Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation. Colloid Surf. A 2008, 317, 256–261. [Google Scholar] [CrossRef]
- Amnieh, H.B.; Siamaki, A.; Soltani, S. Design of blasting pattern in proportion to the peak particle velocity (PPV): Artificial neural networks approach. Saf. Sci. 2012, 50, 1913–1916. [Google Scholar] [CrossRef]
- Abdollahi, A.; Shams, M.; Abdollahi, A. Artificial neural network modeling of a deflector in a grooved channel as well as optimization of its effective parameters. Heat Mass Transf. 2017, 54, 1–10. [Google Scholar] [CrossRef]
- Hoseinian, F.S.; Irannajad, M.; Safari, M. Effective factors and kinetics study of zinc ion removal from synthetic wastewater by ion flotation. Sep. Sci. Technol. 2017, 52, 892–902. [Google Scholar] [CrossRef]
- Lai, K.C.; Lim, S.K.; Teh, P.C.; Yeap, K.H. An Artificial Neural Network approach to predicting electrostatic separation performance for food waste recovery. Pol. J. Environ. Stud. 2017, 26, 1921–1926. [Google Scholar] [CrossRef]
- Samavati, V.; Emam-Djomeh, Z.; Omid, M. Prediction of rheological properties of multi-component dispersions by using artificial neural networks. J. Disper. Sci. Technol. 2014, 35, 428–434. [Google Scholar] [CrossRef]
- Khajeh, M.; Jahanbin, E. Application of cuckoo optimization algorithm–artificial neural network method of zinc oxide nanoparticles–chitosan for extraction of uranium from water samples. Chemometr. Intell. Lab. 2014, 135, 70–75. [Google Scholar] [CrossRef]
- Hosseini-Moghari, S.M.; Morovati, R.; Moghadas, M.; Araghinejad, S. Optimum operation of reservoir using two evolutionary algorithms: Imperialist competitive algorithm (ICA) and cuckoo optimization algorithm (COA). Water Resour. Manag. 2015, 29, 3749–3769. [Google Scholar] [CrossRef]
- Yang, X.S.; Deb, S. Cuckoo search via Lévy flights. In Proceedings of the 2009 World Congress on Nature & Biologically Inspired Computing (NaBIC), Coimbatore, India, 9–11 December 2009; pp. 210–214. [Google Scholar]
- Rajabioun, R. Cuckoo optimization algorithm. Appl. Soft Comput. 2011, 11, 5508–5518. [Google Scholar] [CrossRef]
- Ding, S.; Su, C.; Yu, J. An optimizing BP neural network algorithm based on genetic algorithm. Artif. Intell. Rev. 2011, 36, 153–162. [Google Scholar] [CrossRef]
- Holland, J. Genetic algorithms. Sci. Am. 1992, 267, 66–73. [Google Scholar] [CrossRef]
- Gupta, J.N.; Sexton, R.S. Comparing backpropagation with a genetic algorithm for neural network training. Omega 1999, 27, 679–684. [Google Scholar] [CrossRef]
- Bui, D.K.; Nguyen, T.; Chou, J.S.; Nguyen-Xuan, H.; Ngo, T.D. A modified firefly algorithm-artificial neural network expert system for predicting compressive and tensile strength of high-performance concrete. Constr. Build. Mater. 2018, 180, 320–333. [Google Scholar] [CrossRef]
- Alweshah, M.; Abdullah, S. Hybridizing firefly algorithms with a probabilistic neural network for solving classification problems. Appl. Soft Comput. 2015, 35, 513–524. [Google Scholar] [CrossRef]
- Sánchez, D.; Melin, P.; Castillo, O. Optimization of modular granular neural networks using a firefly algorithm for human recognition. Eng. Appl. Artif. Intell. 2017, 64, 172–186. [Google Scholar] [CrossRef]
- Yang, X.S. Nature-Inspired Metaheuristic Algorithms; Luniver Press: New York, NY, USA, 2010. [Google Scholar]
- Simon, D. A probabilistic analysis of a simplified biogeography-based optimization algorithm. Evol. Comput. 2011, 19, 167–188. [Google Scholar] [CrossRef]
- Simon, D. Biogeography-based optimization. IEEE Trans. Evol. Comput. 2008, 12, 702–713. [Google Scholar] [CrossRef]
- Pham, B.T.; Nguyen, M.D.; Bui, K.T.T.; Prakash, I.; Chapi, K.; Bui, D.T. A novel artificial intelligence approach based on Multi-layer Perceptron Neural Network and Biogeography-based Optimization for predicting coefficient of consolidation of soil. Catena 2019, 173, 302–311. [Google Scholar] [CrossRef]
- Bishop, C. Pattern Recognition and Machine Learning, Information Science and Statistics; Springer: New York, NY, USA, 2006. [Google Scholar]
- Badawy, M.F.; Msekh, M.A.; Hamdia, K.M.; Steiner, M.K.; Lahmer, T.; Rabczuk, T. Hybrid nonlinear surrogate models for fracture behavior of polymeric nanocomposites. Probabilist. Eng. Mech. 2017, 50, 64–75. [Google Scholar] [CrossRef]
- Hassanzadeh, A. A new statistical view to modeling of particle residence time distribution in full-scale overflow ball mill operating in closed-circuit. Geosyst. Eng. 2018, 21, 199–209. [Google Scholar] [CrossRef]
- Corder, G.W.; Foreman, D.I. Nonparametric Statistics for Non-Statisticians: A Step-by-Step Approach; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Özdemir, G.; Peker, S.; Helvaci, S.S. Effect of pH on the surface and interfacial behavior of rhamnolipids R1 and R2. Colloid Surf. A 2004, 234, 135–143. [Google Scholar] [CrossRef]
- Ishigami, Y.; Gama, Y.; Nagahora, H.; Yamaguchi, M.; Nakahara, H.; Kamata, T. The pH sensitive conversion of molecular aggregates of rhamnolipid biosurfactant. Chem. Lett. 1987, 16, 763–766. [Google Scholar] [CrossRef]
- Helvaci, S.S.; Peker, S.; Özdemir, G. Effect of electrolytes on the surface behavior of rhamnolipids R1 and R2. Colloid Surf. B 2004, 35, 225–233. [Google Scholar] [CrossRef]
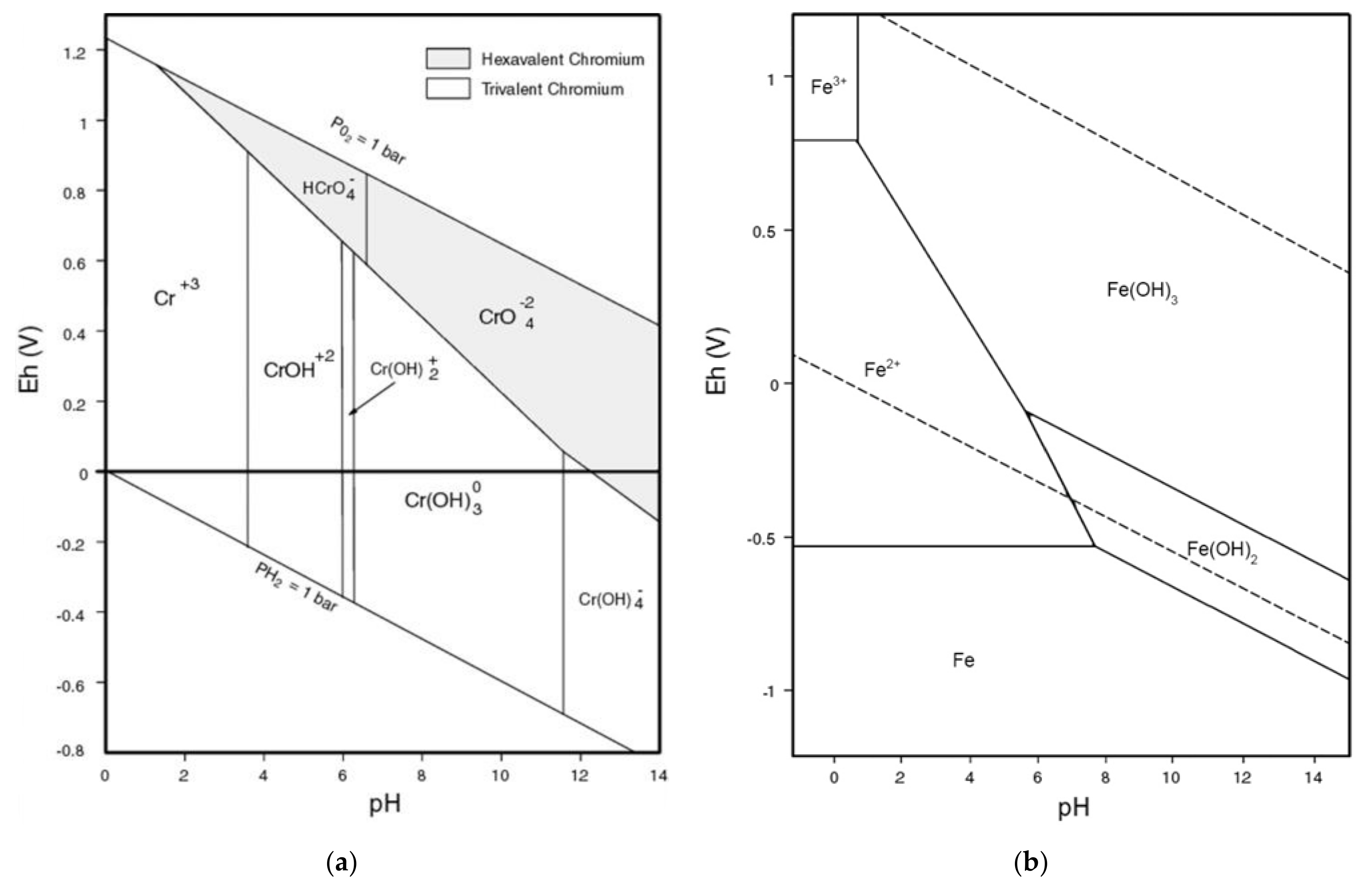
- Palmer, C.D.; Wittbrodt, P.R. Processes affecting the remediation of chromium-contaminated sites. Environ. Health Persp. 1991, 92, 25–40. [Google Scholar] [CrossRef]
- Monhemius, A.J. The iron elephant: A brief history of hydrometallurgists’ struggles with element no. 26. CIM J. 2017, 8, 197–206. [Google Scholar] [CrossRef]
- Pinfold, T.A. Precipitate flotation. In Adsorptive Bubble Separation Techniques; Academic Press: Johannesburg, South Africa, 1972; pp. 75–90. [Google Scholar]
- Hassanzadeh, A.; Firouzi, M.; Albijanic, B.; Celik, M.S. A review on determination of particle-bubble encounter using analytical, experimental and numerical methods. Miner. Eng. 2018, 122, 296–311. [Google Scholar] [CrossRef]
- Hoang, D.H.; Hassanzadeh, A.; Peuker, U.A.; Rudolph, M. Impact of flotation hydrodynamics on the optimization of fine-grained carbonaceous sedimentary apatite ore beneficiation. Powder Techol. 2019, 345, 223–233. [Google Scholar]
- Azizi, A.; Masdarian, M.; Hassanzadeh, A.; Bahri, Z.; Niedoba, T.; Surowiak, A. Parametric optimization in rougher flotation performance of a sulfidized mixed copper ore. Minerals—Basel 2020, 10, 660. [Google Scholar] [CrossRef]
- Liu, H.L.; Chiou, Y.R. Optimal decolorization efficiency of reactive red 239 by UV/TiO2 photocatalytic process coupled with response surface methodology. Chem. Eng. J. 2005, 112, 173–179. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Azizi, A.; Kouachi, S.; Karimi, M.; Celik, M.S. Estimation of flotation rate constant and particle-bubble interactions considering key hydrodynamic parameters and their interrelations. Miner. Eng. 2019, 41, 105836. [Google Scholar] [CrossRef]
- Azizi, A.; Hassanzadeh, A.; Fadaei, B. Investigating the first-order flotation kinetics models for Sarcheshmeh copper sulfide ore. Int. J. Min. Sci. Technol. 2015, 25, 849–854. [Google Scholar]
- Medina, B.Y.; Torem, M.L.; De Mesquita, L.M.S. On the kinetics of precipitate flotation of Cr III using sodium dodecylsulfate and ethanol. Miner. Eng. 2005, 18, 225–231. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Hoang, D.H.; Brockman, M. Assessment of flotation kinetics modeling using information criteria; case studies of elevated-pyritic copper sulfide and high-grade carbonaceous sedimentary apatite ores. J. Disper. Sci. Technol. 2020, 41, 1083–1094. [Google Scholar] [CrossRef]
- Karlik, B.; Olgac, A.V. Performance analysis of various activation functions in generalized MLP architectures of neural networks. Int. J. Artif. Intell. Exp. Syst. 2011, 1, 111–122. [Google Scholar]
- Debnath, A.; Deb, K.; Das, N.S.; Chattopadhyay, K.K.; Saha, B. Simple chemical route synthesis of Fe2O3 nanoparticles and its application for adsorptive removal of Congo red from aqueous media: Artificial neural network modeling. J. Disper. Sci. Technol. 2016, 37, 775–785. [Google Scholar] [CrossRef]
- Karadurmuş, E.; Akyazı, H.; Göz, E.; Yüceer, M. Prediction of characteristic properties of crude oil blending with ANN. J. Disper. Sci. Technol. 2018, 39, 1236–1243. [Google Scholar] [CrossRef]
- Yang, X.S. Firefly algorithms for multimodal optimization. In Proceedings of the 5th International Conference on Stochastic Algorithms, Foundations and Applications, Sapporo, Japan, 26–28 October 2009; Springer: Berlin/Heidelberg, Germany, 2009; pp. 169–178. [Google Scholar]
- Fateen, S.E.K.; Bonilla-Petriciolet, A. Intelligent firefly algorithm for global optimization. In Cuckoo Search and Firefly Algorithm; Springer: Cham, Switzerland, 2014; pp. 315–330. [Google Scholar]
- Mo, Y.B.; Ma, Y.Z.; Zheng, Q.Y. Optimal choice of parameters for firefly algorithm. In Proceedings of the 4th International Conference on Digital Manufacturing & Automation, Qindao, China, 29–30 June 2013; IEEE; pp. 887–892. [Google Scholar]





| Chemical Component | MgCl2 | K2SO4 | KH2PO4 | Na2HPO4 | NaNO3 | MgSO4·H2O | CaCl2·2H2O | Agar |
|---|---|---|---|---|---|---|---|---|
| Dosage (g/L) | 1.4 | 10 | 0.7 | 0.9 | 2 | 0.4 | 0.1 | 20 |
| Parameter | Minimum | Mean Value | Maximum | Standard Deviation |
|---|---|---|---|---|
| pH | 2 | 4.53 | 12 | 2.9153 |
| RL/Cr ratio | 0.01 | 0.02 | 0.1 | 0.0137 |
| Air flowrate (mL/min) | 50 | 131.76 | 250 | 71.4793 |
| Fe/Cr ratio | 0.5 | 1.40 | 3 | 0.8414 |
| Chromium removal (%) | 45.10 | 77.89 | 99.96 | 11.9887 |
| Analysis | Normality Analysis | ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|
| Measures | AD | p-value | Status | SS | MS | F Value | p-value | Status |
| Solution pH | 0.504 | 0.402 | Normal | 2315 | 463 | 3.46 | 0.005 | Significant |
| RL:Cr ratio | 0.459 | 0.437 | Normal | 4591 | 1148 | 9.61 | 0.000 | Significant |
| Aeration rate | 0.391 | 0.163 | Normal | 46 | 11 | 0.08 | 0.989 | Insignificant |
| Fe:Cr ratio | 0.412 | 0.420 | Normal | 1941 | 388 | 2.85 | 0.017 | Significant |
| Algorithm | Network Structure | Training | Test | ||||
|---|---|---|---|---|---|---|---|
| MSE | RMSE | % Error | MSE | RMSE | % Error | ||
| FFA | 4 - 9 - 1 | 0.0037 | 0.0608 | 3.0389 | 0.0038 | 0.0617 | 3.0842 |
| GA | 4 - 9 - 1 | 0.0079 | 0.0890 | 4.4511 | 0.0104 | 0.1018 | 5.0907 |
| BBO | 4 - 9 - 1 | 0.0069 | 0.0832 | 4.1602 | 0.0108 | 0.1040 | 5.1984 |
| COA | 4 - 9 - 1 | 0.0099 | 0.0995 | 4.9774 | 0.0139 | 0.1180 | 5.8980 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoshdast, H.; Gholami, A.; Hassanzadeh, A.; Niedoba, T.; Surowiak, A. Advanced Simulation of Removing Chromium from a Synthetic Wastewater by Rhamnolipidic Bioflotation Using Hybrid Neural Networks with Metaheuristic Algorithms. Materials 2021, 14, 2880. https://doi.org/10.3390/ma14112880
Khoshdast H, Gholami A, Hassanzadeh A, Niedoba T, Surowiak A. Advanced Simulation of Removing Chromium from a Synthetic Wastewater by Rhamnolipidic Bioflotation Using Hybrid Neural Networks with Metaheuristic Algorithms. Materials. 2021; 14(11):2880. https://doi.org/10.3390/ma14112880
Chicago/Turabian StyleKhoshdast, Hamid, Alireza Gholami, Ahmad Hassanzadeh, Tomasz Niedoba, and Agnieszka Surowiak. 2021. "Advanced Simulation of Removing Chromium from a Synthetic Wastewater by Rhamnolipidic Bioflotation Using Hybrid Neural Networks with Metaheuristic Algorithms" Materials 14, no. 11: 2880. https://doi.org/10.3390/ma14112880
APA StyleKhoshdast, H., Gholami, A., Hassanzadeh, A., Niedoba, T., & Surowiak, A. (2021). Advanced Simulation of Removing Chromium from a Synthetic Wastewater by Rhamnolipidic Bioflotation Using Hybrid Neural Networks with Metaheuristic Algorithms. Materials, 14(11), 2880. https://doi.org/10.3390/ma14112880










