Silver Doped Magnesium Ferrite Nanoparticles: Physico-Chemical Characterization and Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization Techniques
2.3. Antibacterial Activity
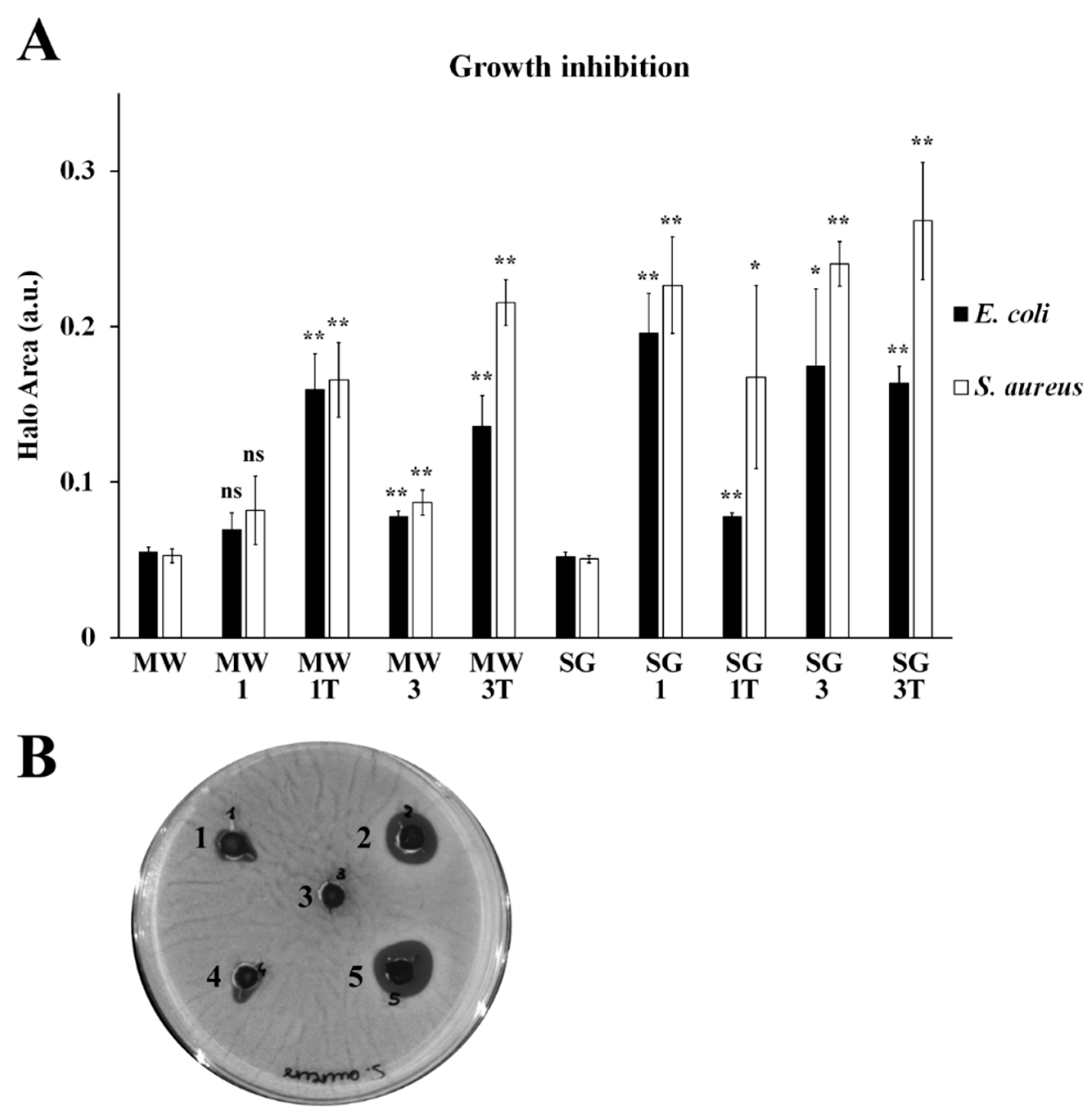
3. Results
3.1. XRPD and Rietveld Refinement
3.2. Morphological and Compositional Analysis
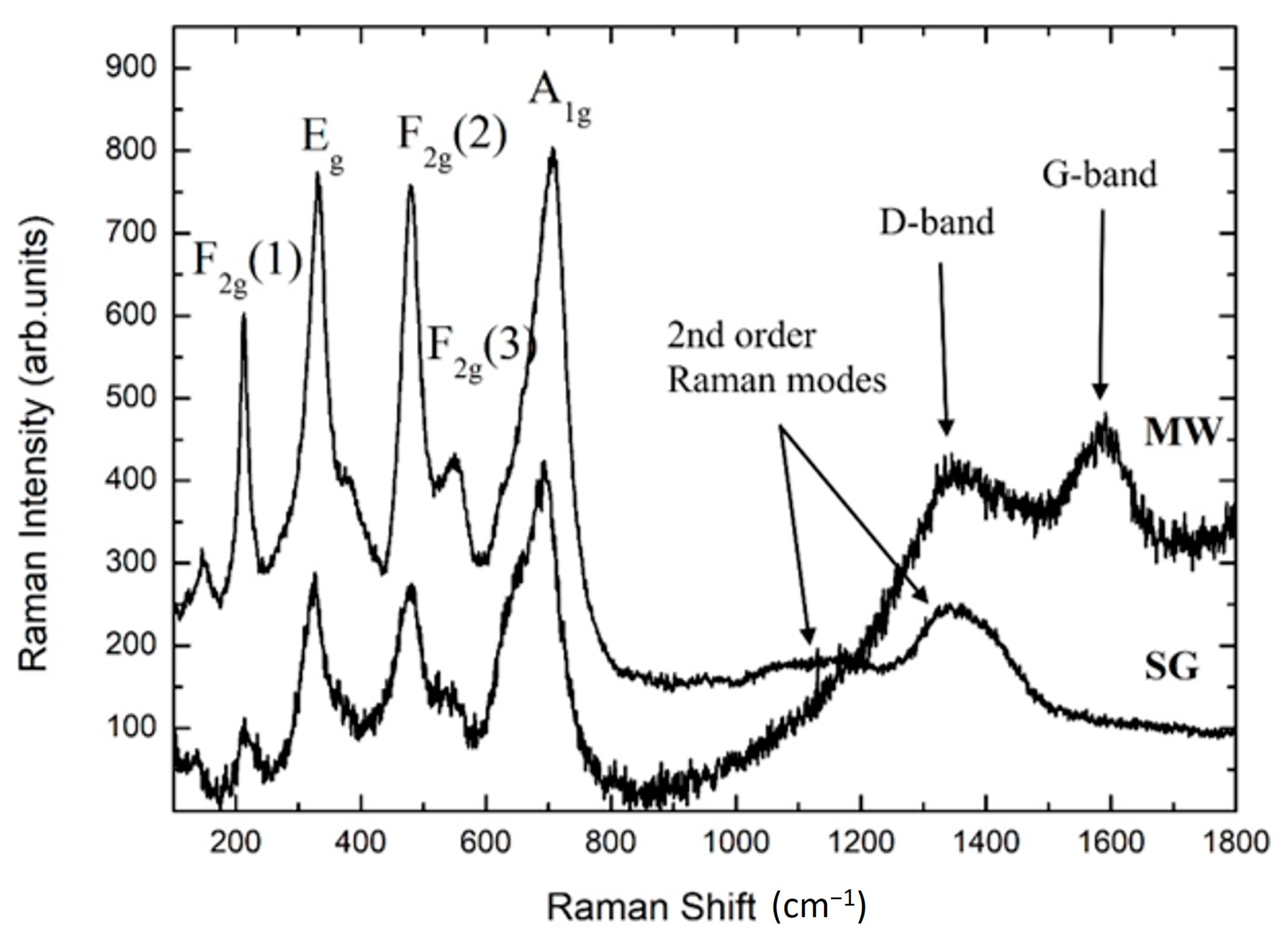
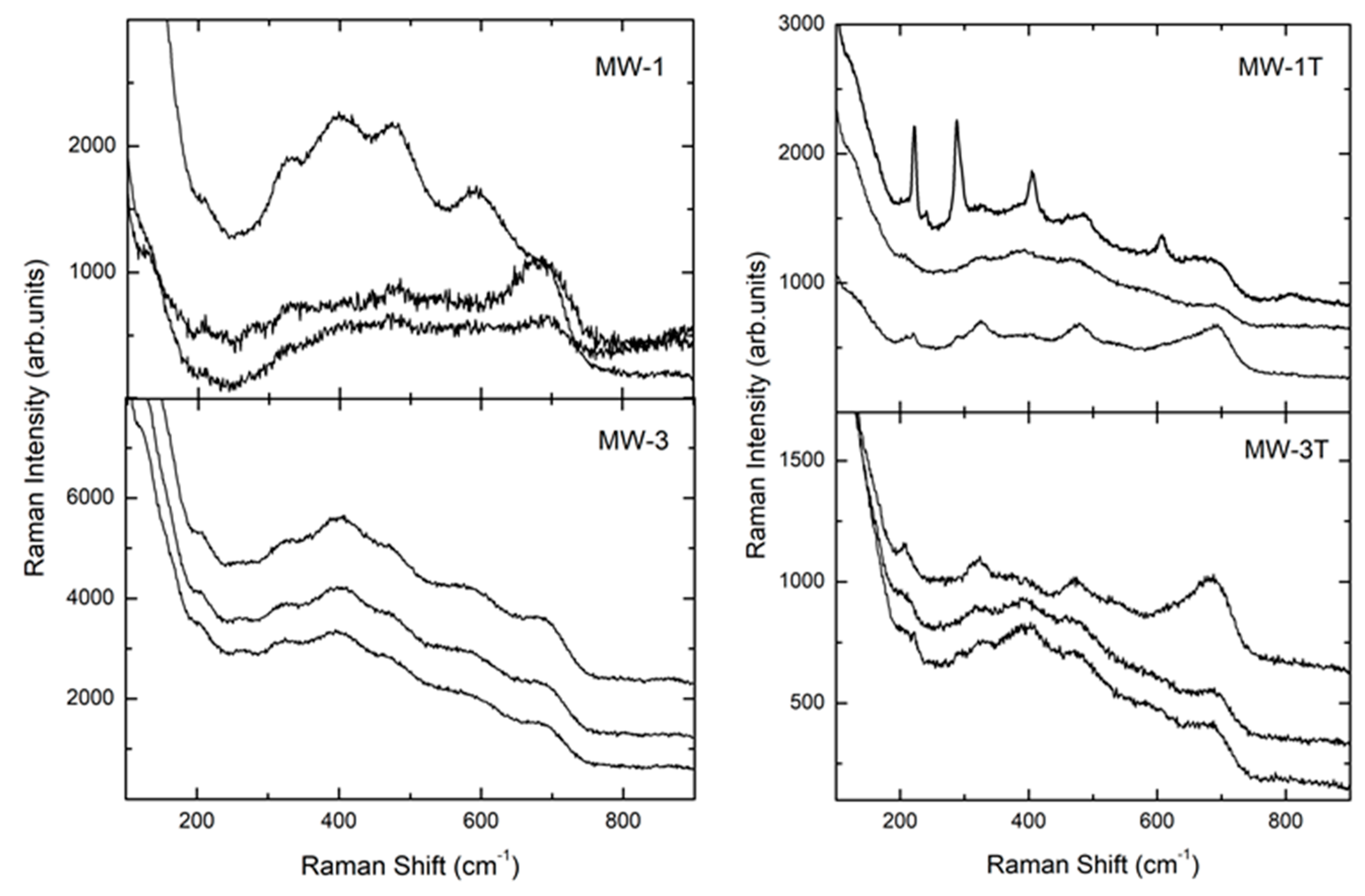
3.3. Raman Spectroscopy
3.4. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saidin, S.; Jumat, M.A.; Amin, N.A.A.M.; Al-Hammadi, A.S.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C 2021, 118, 111382. [Google Scholar] [CrossRef] [PubMed]
- Adeleke Ojo, O.; Idowu Olayide, I.; Akalabu, M.C.; Ajiboye, B.O.; Busola Ojo, A.; Oyinloye, B.E.; Ramalingam, M. Nanopar-ticles and their Biomedical Applications. Biointerface Res. Appl. Chem. 2021, 11, 8431–8445. [Google Scholar]
- Khalid, K.; Tan, X.; Zaid, H.F.M.; Tao, Y.; Chew, C.L.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Wei, L.C. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineering 2020, 11, 328–355. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.; Salam, A.; Khan, A.; Khan, S.U.; Khanzada, H.; Wasim, M.; Khan, M.Q.; Kim, I.S. Functional nanocomposites and their potential applications: A review. J. Polym. Res. 2021, 28, 1–22. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Li, G.; Su, M.; Chen, X.; Zhang, H.; Zhang, Y.; Jiao, W.; He, Y.; Yi, J.; et al. Engineering ferrite nanoparticles with enhanced magnetic response for advanced biomedical applications. Mater. Today Adv. 2020, 8, 100119. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.; Nkambule, T.T.; Mamba, B.B. Spinel ferrite nanoparticles and nanocomposites for biomedical applications and their toxicity. Mater. Sci. Eng. C 2020, 107, 110314. [Google Scholar] [CrossRef]
- Lima-Tenório, M.K.; Tenório-Neto, E.T.; Hechenleitner, A.A.W.; Fessi, H.; Pineda, E.A.G. CoFe2O4 and ZnFe2O4 Nanopar-ticles: An Overview About Structure, Properties, Synthesis and Biomedical Applications. J. Colloid Sci. Biotech. 2016, 5, 45–54. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Meng, F. Spinel-Type Materials Used for Gas Sensing: A Review. Sensors 2020, 20, 5413. [Google Scholar] [CrossRef]
- Thakur, P.; Chahar, D.; Taneja, S.; Bhalla, N.; Thakur, A. A review on MnZn ferrites: Synthesis, characterization and applications. Ceram. Int. 2020, 46, 15740–15763. [Google Scholar] [CrossRef] [PubMed]
- Rajabathar, J.R.; Periyasamy, G.; Alanazi, A.M.; Govindasamy, M.; Arunachalam, P. Review on Carbon Nanotube Varieties for Healthcare Application: Effect of Preparation Methods and Mechanism Insight. Processing 2020, 8, 1654. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 1–19. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Kuthati, Y.; Kankala, R.K.; Busa, P.; Lin, S.-X.; Deng, J.-P.; Mou, C.-Y.; Lee, C.-H. Phototherapeutic spectrum expansion through synergistic effect of mesoporous silica trio-nanohybrids against antibiotic-resistant gram-negative bacterium. J. Photochem. Photobiol. B Biol. 2017, 169, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Kuthati, Y.; Kumar Kankala, R.; Lin, S.X.; Weng, C.F.; Lee, C.H. pH-Triggered Controllable Release of Silver−Indole-3 Acetic Acid Complexes from Mesoporous Silica Nanoparticles (IBN-4) for Effectively Killing Malignant Bacteria. Mol. Pharm. 2015, 12, 2289–2304. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Losasso, C.; Patuzzi, I.; Rigo, L.; Conficoni, D.; Gallocchio, F.; Cibin, V.; Catellani, P.; Segato, S.; Ricci, A. Silver as antibacterial toward Listeria monocytogenes. Front. Microbiol. 2016, 7, 307. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Conte, M.; Pontico, M.; Pani, A.; Pani, R.; De Vincentis, G. New Frontiers in Molecular Imaging with Super-paramagnetic Iron Oxide Nanoparticles (SPIONs): Efficacy, Toxicity, and Future Applications. Nucl. Med. Mol. Imaging 2020, 54, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Pettibone, J.M.; Gorham, J.M.; Nguyen, T.M.; MacCuspie, R.I.; Gigault, J.; Hackley, V.A. Unexpected Changes in Functionality and Surface Coverage for Au Nanoparticle PEI Conjugates: Implications for Stability and Efficacy in Biological Systems. Langmuir 2015, 31, 7673–7683. [Google Scholar] [CrossRef]
- WHO, (World Health Organization). Ambient (Outdoor) Air Quality and Health. Available online: http://www.who.int (accessed on 31 January 2021).
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Lagashetty, A.; Pattar, A.; Ganiger, S.K. Synthesis, characterization and antibacterial study of Ag doped magnesium ferrite nanocomposite. Heliyon 2019, 5, e01760. [Google Scholar] [CrossRef] [PubMed]
- El-Molla, S.A.; Ali, L.I.; Amin, N.H.; Ebrahim, A.A.; Mahmoud, H.R. Effect of Ag-doping of nanosized FeMgO system on its structural, surface, spectral, and catalytic properties. Chem. Pap. 2012, 66, 722–732. [Google Scholar] [CrossRef]
- Shetty, K.; Prathibha, B.; Rangappa, D.; Anantharaju, K.; Nagaswarupa, H.; Nagabhushana, H.; Prashantha, S. Photocatalytic study for fabricated Ag doped and undoped MgFe2O4 nanoparticles. Mater. Today Proc. 2017, 4, 11764–11772. [Google Scholar] [CrossRef]
- Naaz, F.; Dubey, H.K.; Kumari, C.; Lahiri, P. Structural and magnetic properties of MgFe2O4 nanopowder synthesized via co-precipitation route. SN Appl. Sci. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Okasha, N. Influence of silver doping on the physical properties of Mg ferrites. J. Mater. Sci. 2008, 43, 4192–4197. [Google Scholar] [CrossRef]
- Bini, M.; Tondo, C.; Capsoni, D.; Mozzati, M.C.; Albini, B.; Galinetto, P. Superparamagnetic ZnFe2O4 nanoparticles: The effect of Ca and Gd doping. Mater. Chem. Phys. 2018, 204, 72–82. [Google Scholar] [CrossRef]
- Bruker AXS. TOPAS V3.0: General Profile and Structural Analysis Software for Powder Diffraction Data; User Manual Bruker AXS: Karlsruhe, Germany, 2005. [Google Scholar]
- Minogue, T.D.; Daligault, H.A.; Davenport, K.W.; Bishop-Lilly, K.A.; Broomall, S.M.; Bruce, D.C.; Chain, P.S.; Chertkov, O.; Coyne, S.R.; Freitas, T.; et al. Complete Genome Assembly of Escherichia coli ATCC 25922, a Serotype O6 Reference Strain. Genome Announc. 2014, 2, 00969-14. [Google Scholar] [CrossRef] [PubMed]
- Treangen, T.J.; Maybank, R.A.; Enke, S.; Friss, M.B.; Diviak, L.F.; Karaolis, D.K.R.; Koren, S.; Ondov, B.; Phillippy, A.M.; Bergman, N.H.; et al. Complete Genome Sequence of the Quality Control Strain Staphylococcus aureus subsp. aureus ATCC 25923. Genome Announc. 2014, 2, e01110-14. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ. U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 25 May 2021).
- Manigold Intraclass Correlation Coefficient (ICC) Calculator. Available online: http://raterreliability.com/ (accessed on 25 May 2021).
- White, W.; DeAngelis, B. Interpretation of the vibrational spectra of spinels. Spectrochim. Acta Part A Mol. Spectrosc. 1967, 23, 985–995. [Google Scholar] [CrossRef]
- D’Ippolito, V.; Andreozzi, G.B.; Bersani, D.; Lottici, P.P. Raman fingerprint of chromate, aluminate and ferrite spinels. J. Raman Spectrosc. 2015, 46, 1255–1264. [Google Scholar] [CrossRef]
- Galinetto, P.; Albini, B.; Bini, M.; Mozzati, M.C. Raman spectroscopy in Zinc Ferrites Nanoparticles. In Raman Spectroscopy; IntechOpen: London, UK, 2018. [Google Scholar]
- da Silva, S.W.; Nakagomi, F.; Silva, M.S.; Franco, A., Jr.; Garg, W.K.; Oliveira, A.C.; Morais, P.C. Raman study of cations’ distribution in ZnxMg12xFe2O4 nanoparticles. J. Nanopart. Res. 2012, 14, 798. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A Guide to and Review of the Use of Multiwavelength Raman Spectroscopy for Characterizing Defective Aromatic Carbon Solids: From Graphene to Amorphous Carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Sangaiya, ·P.; Jayaprakash, R. A Review on Iron Oxide Nanoparticles and Their Biomedical Applications. J. Supercond. Novel Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]

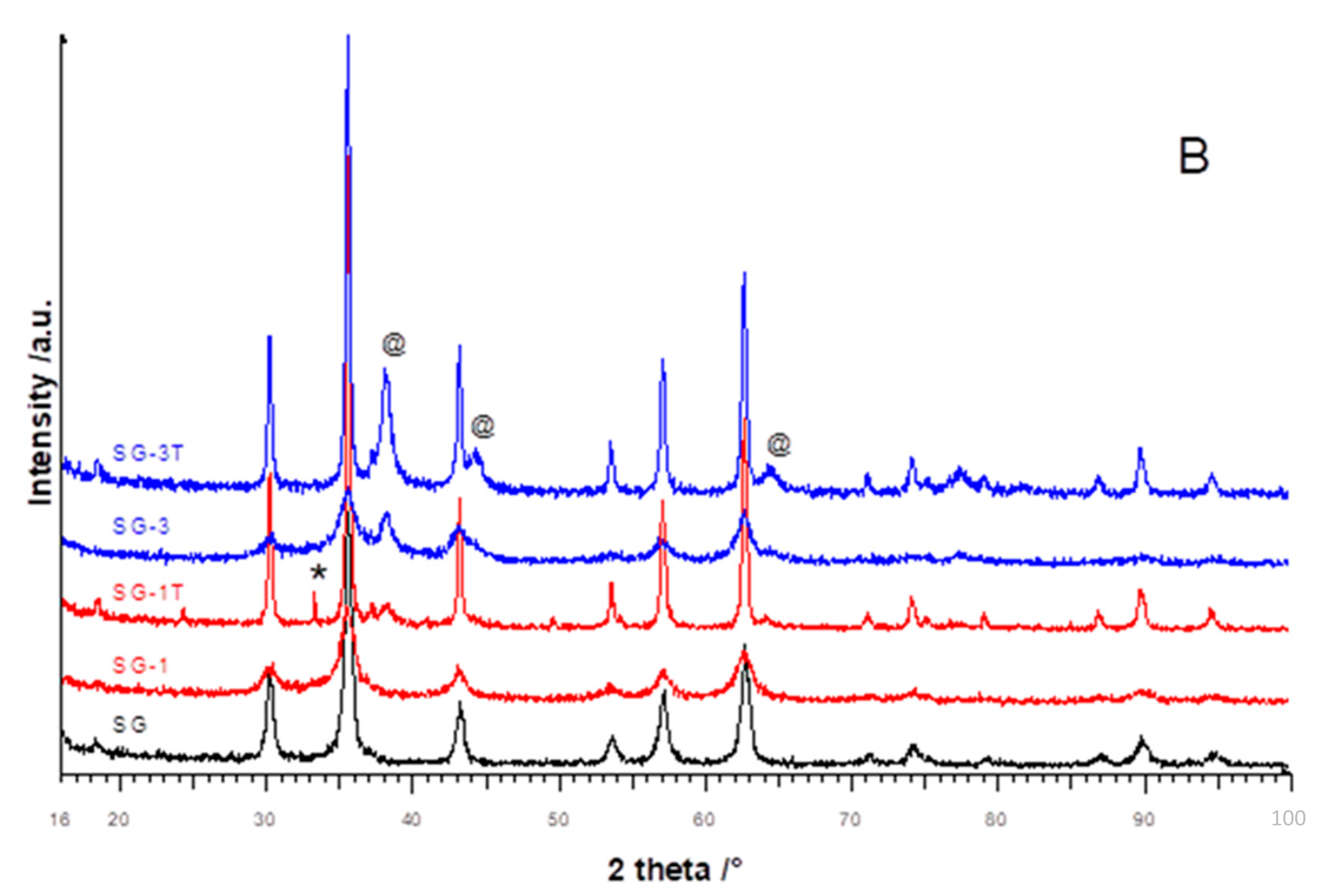
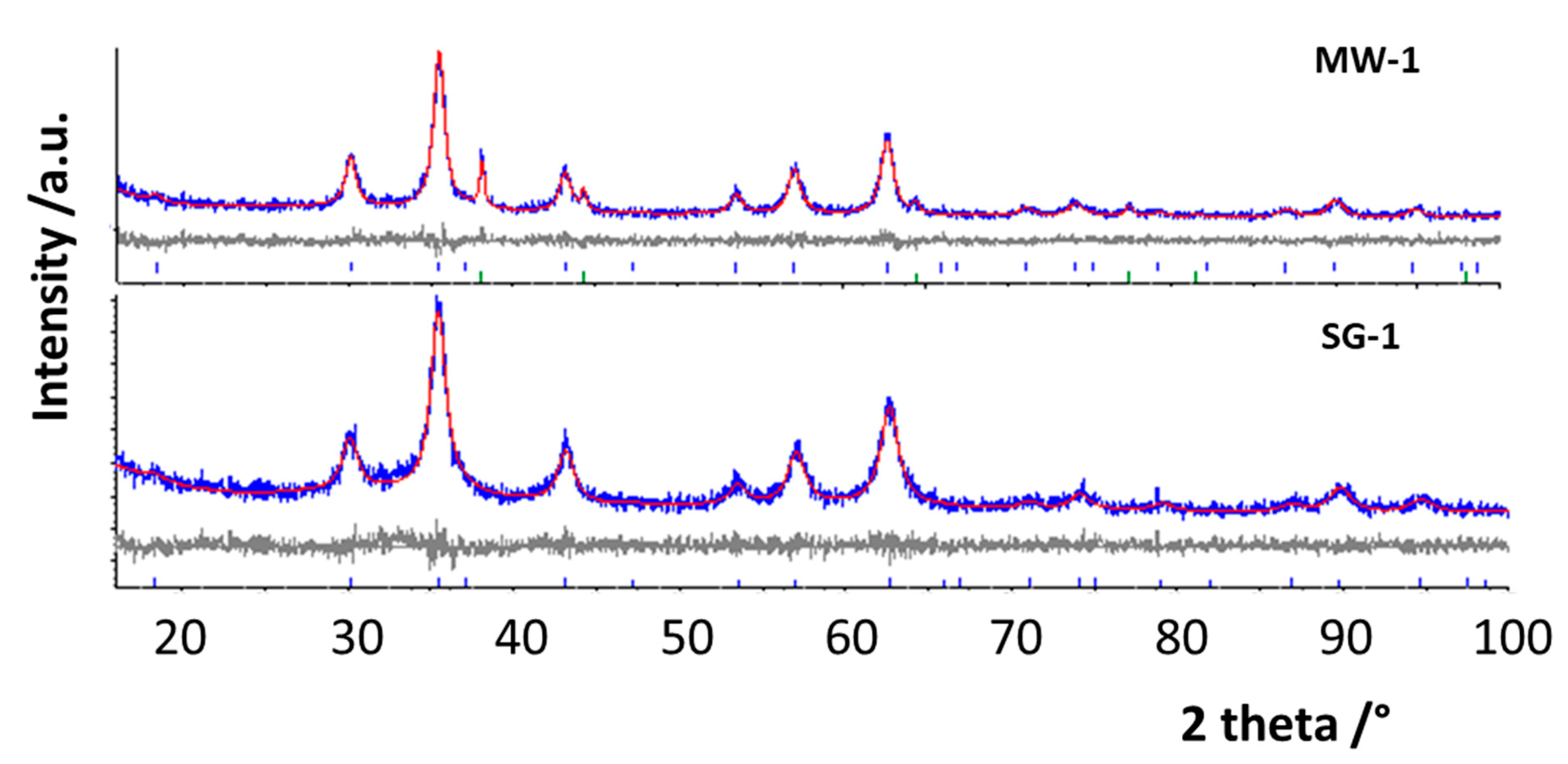


| Samples | a/Å | Cry Size Spinel (nm) | Cry Size Silver (nm) | Cation Distribution on T and O Sites | I | wt.% Fe2O3 | Rwp/ GoF |
|---|---|---|---|---|---|---|---|
| MW | 8.3734(13) | 13(1) | - | [Mg0.64Fe0.36]T [Fe1.64Mg0.36]O | 0.36 | - | 14.0/ 1.15 |
| MW-1 | 8.3765(12) | 12(1) | 22(2) | [Mg0.29Fe0.71]T [Fe1.28Mg0.71]O | 0.71 | - | 12.8/ 1.06 |
| MW-3 | 8.3777(12) | 11(1) | 24(1) | [Mg0.25Fe0.75]T [Fe1.25Mg0.75]O | 0.75 | - | 12.1/ 1.07 |
| MW-1T | 8.3868(7) | 19(1) | 35(1) | [Mg0.22Fe0.78]T [Fe1.22Mg0.78]O | 0.78 | 5 | 14.0/ 1.08 |
| MW-3T | 8.3861(7) | 19(1) | 36(2) | [Mg0.23Fe0.77]T [Fe1.23Mg0.77]O | 0.77 | 3 | 12.5/ 1.06 |
| Samples | a/Å | Cry Size (nm) | Cry Size Silver (nm) | Cation Distribution on T and O Sites | I | wt.% Fe2O3 | Rwp/ GoF |
|---|---|---|---|---|---|---|---|
| SG | 8.3792(7) | 18(1) | - | [Mg0.53Fe0.47]T [Fe1.53Mg0.47]O | 0.47 | - | 16.4/ 1.23 |
| SG-1 | 8.3849(20) | 8(1) | - | [Mg0.23Fe0.77]T [Fe1.13Mg0.77Ag0.1]O | 0.77 | - | 13.4/ 1.03 |
| SG-3 | 8.3930(25) | 8(1) | 11(1) | [Mg0.26Fe0.74]T [Fe1.26Mg0.74]O | 0.74 | - | 13.3/ 1.07 |
| SG-1T | 8.3885(3) | 48(1) | 9(1) | [Mg0.26Fe0.74]T [Fe1.26Mg0.74]O | 0.74 | 4 | 14/ 1.05 |
| SG-3T | 8.3888(4) | 44(1) | 11(1) | [Mg0.25Fe0.75]T [Fe1.25Mg0.75]O | 0.75 | - | 10.5/ 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantozzi, E.; Rama, E.; Calvio, C.; Albini, B.; Galinetto, P.; Bini, M. Silver Doped Magnesium Ferrite Nanoparticles: Physico-Chemical Characterization and Antibacterial Activity. Materials 2021, 14, 2859. https://doi.org/10.3390/ma14112859
Fantozzi E, Rama E, Calvio C, Albini B, Galinetto P, Bini M. Silver Doped Magnesium Ferrite Nanoparticles: Physico-Chemical Characterization and Antibacterial Activity. Materials. 2021; 14(11):2859. https://doi.org/10.3390/ma14112859
Chicago/Turabian StyleFantozzi, Erika, Erlinda Rama, Cinzia Calvio, Benedetta Albini, Pietro Galinetto, and Marcella Bini. 2021. "Silver Doped Magnesium Ferrite Nanoparticles: Physico-Chemical Characterization and Antibacterial Activity" Materials 14, no. 11: 2859. https://doi.org/10.3390/ma14112859
APA StyleFantozzi, E., Rama, E., Calvio, C., Albini, B., Galinetto, P., & Bini, M. (2021). Silver Doped Magnesium Ferrite Nanoparticles: Physico-Chemical Characterization and Antibacterial Activity. Materials, 14(11), 2859. https://doi.org/10.3390/ma14112859










