Repair of Critical Size Bone Defects Using Synthetic Hydroxyapatite or Xenograft with or without the Bone Marrow Mononuclear Fraction: A Histomorphometric and Immunohistochemical Study in Rat Calvaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
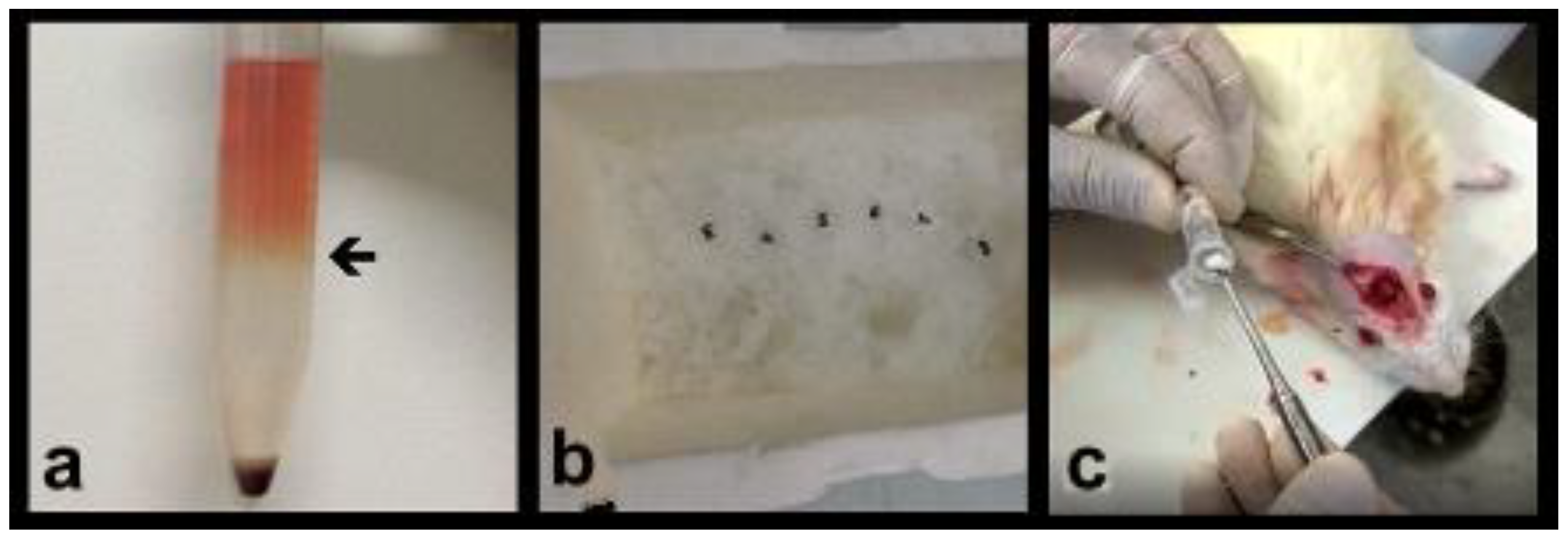
2.2. Obtaining the Bone Marrow Mononuclear Fraction (BMMF)
2.3. Bone Grafts Tested in Groups
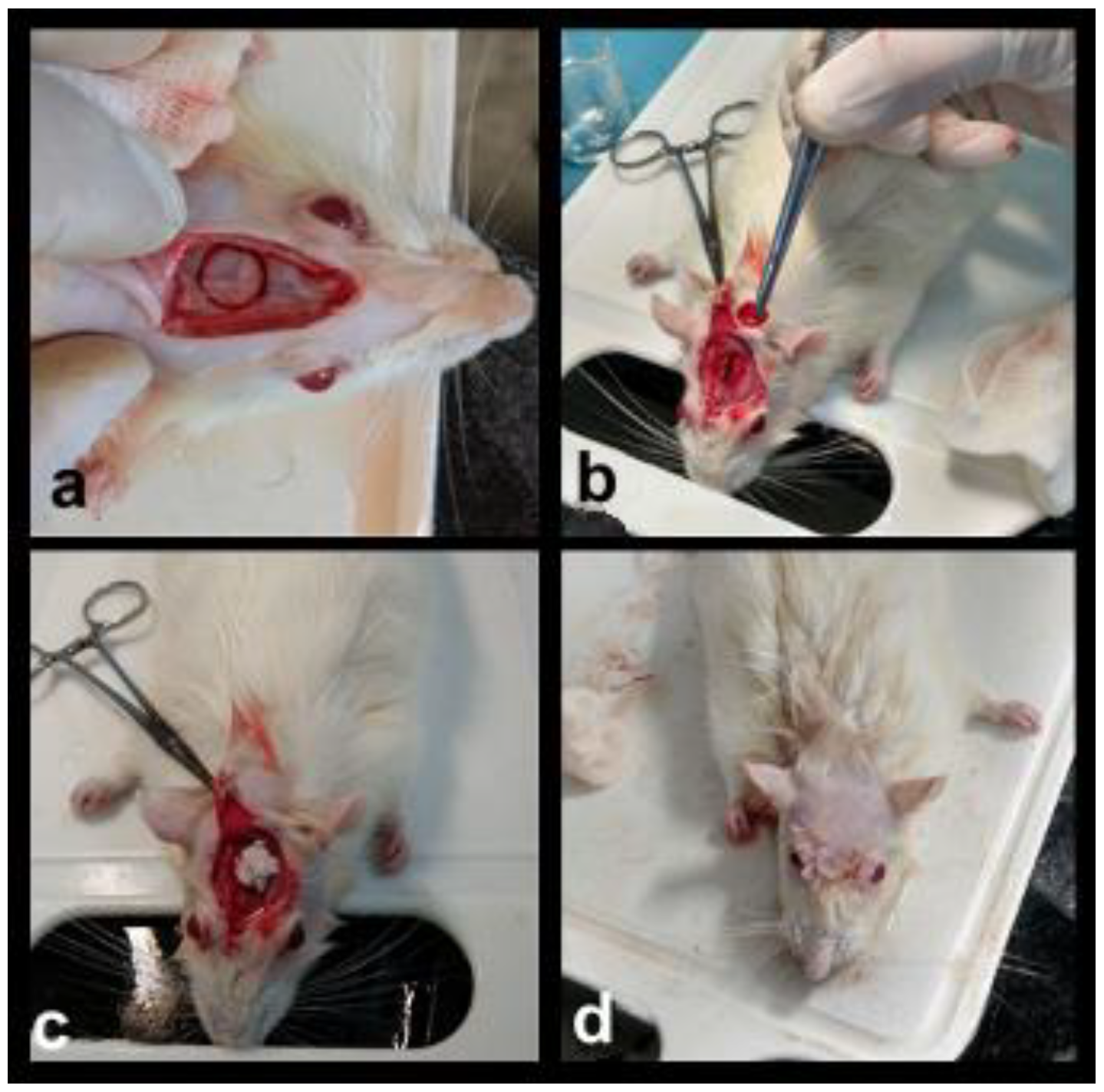
2.4. Surgical Protocol and Sample Preparation
2.5. Masson Trichromic Staining Protocol
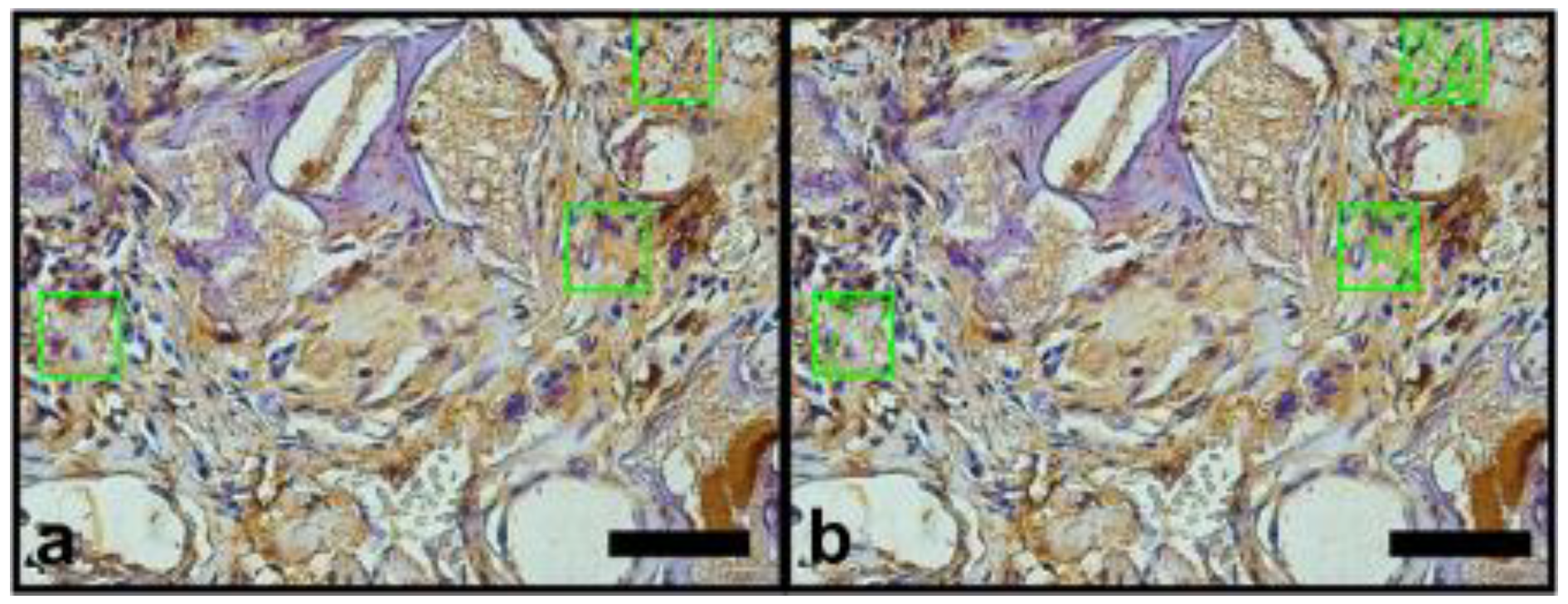
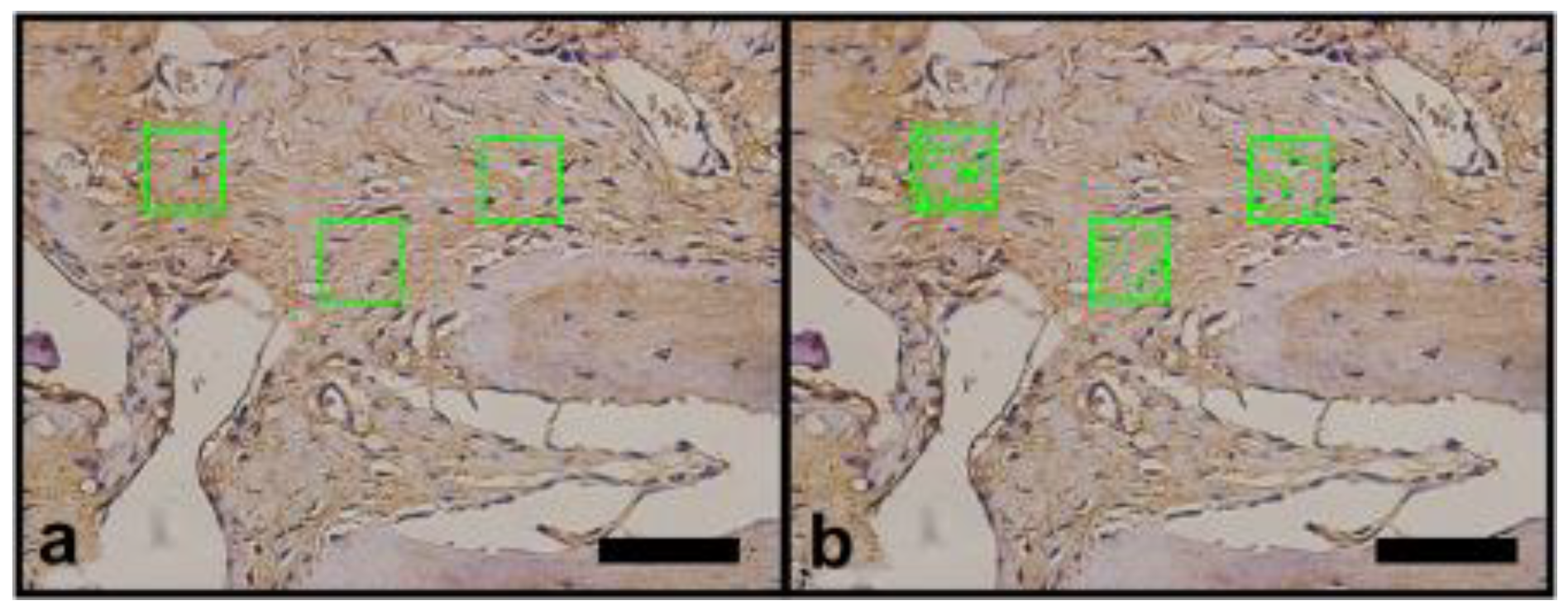
2.6. Vascular Endothelial Growth Factor (VEGF) and Osteopomtin (OPN) Immunohistochemical Protocol
2.7. Image Analysis
2.8. Statistical Analysis
3. Results
3.1. General Observations
3.2. Histomorphometric Results
3.2.1. Histomorphometric Masson’s Trichrome Results
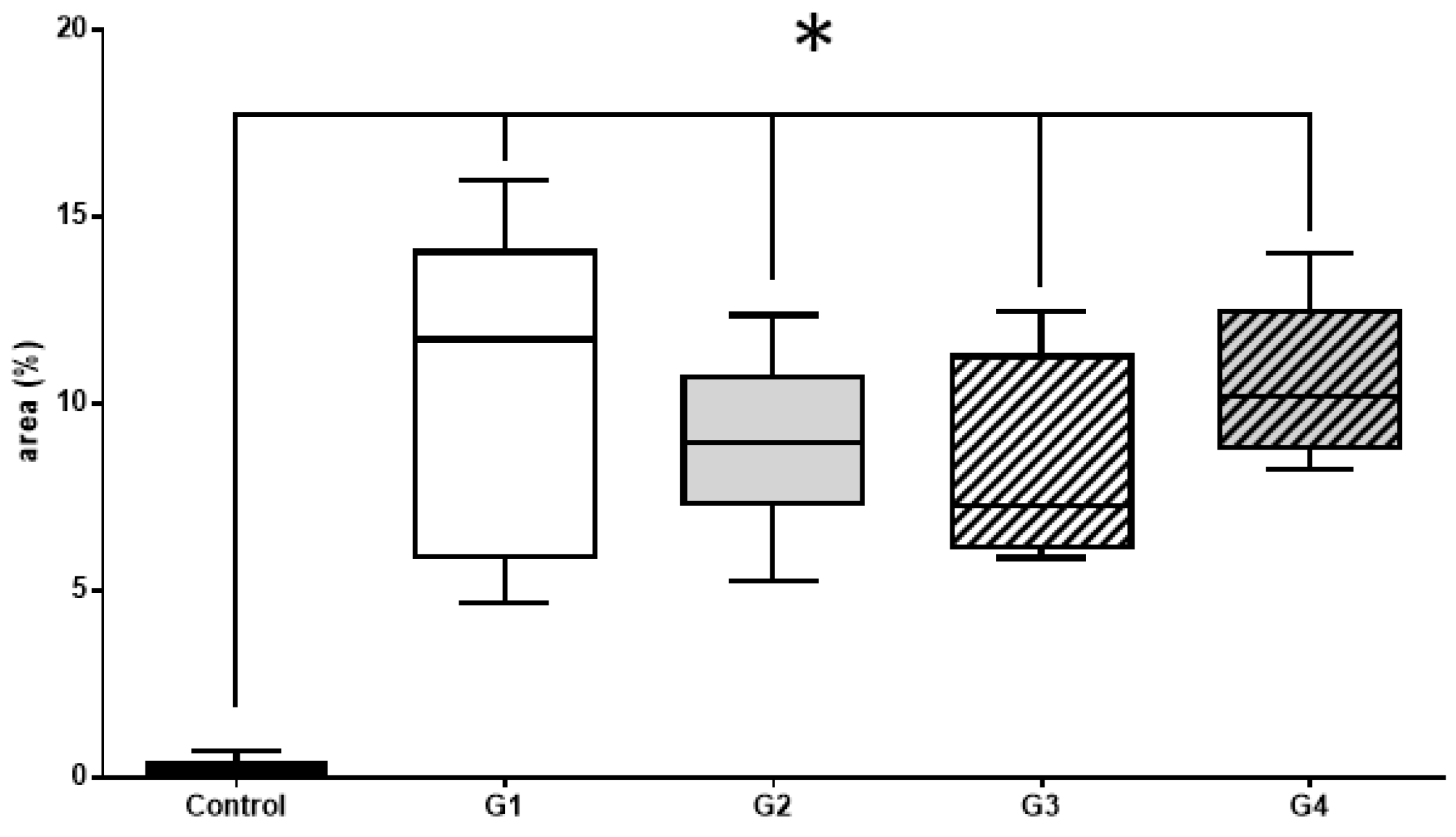
3.2.2. Histomorphometric VEGF Results
3.2.3. Histomorphometric OPN Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Willenbacher, M.; Al-Nawas, B.; Berres, M.; Kämmerer, P.W.; Schiegnitz, E. The Effects of Alveolar Ridge Preservation: A Meta-Analysis. Clin. Implant. Dent. Relat. Res. 2016, 18, 1248–1268. [Google Scholar] [CrossRef]
- Sapata, V.M.; Llanos, A.H.; Neto, J.B.C.; Jung, R.E.; Thoma, D.S.; Christoph, H.F.H.; Pannuti, C.M.; Romito, G.A. Deproteinized bovine bone mineral is non-inferior to deproteinized bovine bone mineral with 10% collagen in maintaining the soft tissue contour post-extraction: A randomized trial. Clin. Oral Implant. Res. 2019, 31, 294–301. [Google Scholar] [CrossRef]
- Araújo, M.G.; da Silva, J.C.C.; de Mendonça, A.F.; Lindhe, J. Ridge alterations following grafting of fresh extraction sockets in man: A randomized clinical trial. Clin. Oral Implants Res. 2015, 26, 407–412. [Google Scholar] [CrossRef]
- Araújo, M.G.; Silva, C.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontology 2000 2015, 68, 122–134. [Google Scholar] [CrossRef]
- Lee, J.-E.; Heo, S.-J.; Koak, J.-Y.; Kim, S.-K.; Han, C.-H. Bone regeneration with rabbit bone marrow-derived mesenchymal stem cells and bone graft materials. Int. J. Oral Maxillofac. Implant. 2012, 27, 1389–1399. [Google Scholar]
- Vignoletti, F.; Matesanz, P.; Rodrigo, D.; Figuero, E.; Martin, C.; Sanz, M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral Implant. Res. 2011, 23, 22–38. [Google Scholar] [CrossRef]
- Pelegrine, A.A.; Aloise, A.C.; Zimmermann, A.; Ferreira, L.M.; Oliveira, R.D.M.E. Repair of critical-size bone defects using bone marrow stromal cells: A histomorphometric study in rabbit calvaria. Part I: Use of fresh bone marrow or bone marrow mononuclear fraction. Clin. Oral Implant. Res. 2013, 25, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Conz, M.B.; Granjeiro, J.M.; de Almeida Soares, G. Physiochemical Characterization of six Commercial Hydroxyapatites for Medical-Dental Applications as Bone Graft. J. Appl. Oral Sci. 2005, 13, 136–140. [Google Scholar] [CrossRef]
- de Faria, C.A.; Chiantia, F.; Teixeira, M.; Aloise, A.; Pelegrine, A. Comparative Study between Mesenchymal Stem Cells Derived from Bone Marrow and from Adipose Tissue, Associated with Xenograft, in Appositional Reconstructions: Histomorphometric Study in Rabbit Calvaria. Int J. Oral Maxillofac. Implant. 2016, 31, e155–e161. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.D.M.E.; Pelegrine, A.A.; Aloise, A.C.; Ferreira, L.M. Xenograft impregnated with bone marrow mononuclear fraction for appositional bone regeneration in rabbit calvaria: A clinical and histomorphometric study. Int. J. Oral Maxillofac. Implant. 2014, 29, 962–968. [Google Scholar] [CrossRef][Green Version]
- Jafarian, M.; Eslaminejad, M.B.; Khojasteh, A.; Abbas, F.M.; Dehghan, M.M.; Hassanizadeh, R.; Houshmand, B. Marrow-derived mesenchymal stem cells-directed bone regeneration in the dog mandible: A comparison between biphasic calcium phosphate and natural bone mineral. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 105, e14–e24. [Google Scholar] [CrossRef]
- Ito, K.; Yamada, Y.; Nakamura, S.; Ueda, M. Osteogenic potential of effective bone engineering using dental pulp stem cells, bone marrow stem cells, and periosteal cells for osseointegration of dental implants. Int. J. Oral Maxillofac. Implant. 2011, 26, 947–954. [Google Scholar]
- Katagiri, W.; Kawai, T.; Osugi, M.; Sugimura-Wakayama, Y.; Sakaguchi, K.; Kojima, T.; Kobayashi, T. Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 8. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Tamai, N.; Naka, N.; Yoshikawa, H.; Myoui, A. Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model. J. Artif. Organs 2013, 16, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.I.; Li, G.; Guo, J.I.A.; Yang, L.E.I.; Liu, Y.; Sun, Q.; Li, R.; Yu, W. Hybrid composites of mesenchymal stem cell sheets, hydroxyapatite, and platelet—Rich fibrin granules for bone regeneration in a rabbit calvarial critical—Size defect model. Exp. Med. 2017, 13, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, Z.; Xing, X.; Li, D.-Q.; Li, Z.-B. Multiple Inoculations of Bone Marrow Stromal Cells into Beta-Tricalcium Phosphate/Chitosan Scaffolds Enhances the Formation and Reconstruction of New Bone. Int. J. Oral Maxillofac. Implant. 2016, 31, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Salvador, E.; Ruaro, M.E.; Melato, M.; Tromba, G.; Angerame, D.; Bevilacqua, L. Bone regeneration with adipose derived stem cells in a rabbit model. J. Biomed. Res. 2017, 33, 38–45. [Google Scholar] [CrossRef]
- Zimmermann, A.; Pelegrine, A.A.; Peruzzo, D.; Martinez, E.F.; Oliveira, R.D.M.E.; Aloise, A.C.; Ferreira, L.M. Adipose mesenchymal stem cells associated with xenograft in a guided bone regeneration model: A histomorphometric study in rabbit calvaria. Int. J. Oral Maxillofac. Implant. 2015, 30, 1415–1422. [Google Scholar] [CrossRef]
- Pereira, L.D.V. A importância do uso das células tronco para a saúde pública. Cienc. Saude Coletiva 2008, 13, 7–14. [Google Scholar] [CrossRef]
- Pelegrine, A.; Aloise, A.; Sorgi, E. Células Tronco em Implantodontia, 1st ed.; Editora Napoleão LTDA: São Paulo, Brazil, 2013; pp. 126–152. [Google Scholar]
- Pelegrine, A.A.; Da Costa, C.E.S.; Sendyk, W.R.; Gromatzky, A. The comparative analysis of homologous fresh frozen bone and autogenous bone graft, associated or not with autogenous bone marrow, in rabbit calvaria: A clinical and histomorphometric study. Cell Tissue Bank. 2010, 12, 171–184. [Google Scholar] [CrossRef]
- De Oliveira e Silva, M.; Pelegrine, A.A.; Da Silva, A.A.P.; Manhães, L.R., Jr.; De Mello e Oliveira, R.; Gaiba França, S.; Aloise, A.C.; Ferreira, L.M. Xenograft enriched with autologous bone marrow in inlay reconstructions: A tomographic and histomorphometric study in rabbit calvaria. Int. J. Biomater. 2012, 2012. [Google Scholar] [CrossRef]
- Pelegrine, A.A.; Da Costa, C.E.S.; Correa, M.E.P.; Marques, J.F.C. Clinical and histomorphometric evaluation of extraction sockets treated with an autologous bone marrow graft. Clin. Oral Implant. Res. 2010, 21, 535–542. [Google Scholar] [CrossRef]
- Takeda, Y.; Katsutoshi, K.; Matsuzaka, K.; Inoue, T. The Effect of Concentrated Growth Factor on Rat Bone Marrow Cells In Vitro and on Calvarial Bone Healing In Vivo. Int. J. Oral Maxillofac. Implant. 2015, 30, 1187–1196. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Hara, K.; Ikeno, M.; Okamoto, Y.; Hibi, H.; Ueda, M. Rat Bone Marrow Stromal Cell–Conditioned Medium Promotes Early Osseointegration of Titanium Implants. Int. J. Oral Maxillofac. Implant. 2013, 28, 1360–1369. [Google Scholar] [CrossRef]
- Veis, A.; Kougias, K.; Tsirlis, A.; Parisis, N.; Papadopoulou, C.; Romanos, G.E. Evaluation of the osteogenic potential in experimental defects, with and without bone marrow, in the rabbit tibia: A pilot study. Int. J. Oral Maxillofac. Implant. 2010, 24, 1054–1060. [Google Scholar]
- Houshmand, B.; Behnia, H.; Khoshzaban, A.; Morad, G.; Behrouzi, G.; Dashti, S.G.; Khojasteh, A. Osteoblastic differentiation of human stem cells derived from bone marrow and periodontal ligament under the effect of enamel matrix derivative and transforming growth factor-beta. Int. J. Oral Maxillofac. Implant. 2013, 28, e440–e450. [Google Scholar] [CrossRef]
- Dalapicula, S.S.; Vidigal, G.M., Jr.; Conz, M.E.C. Características físico-químicas dos biomateriais utilizados em enxertias ósseas. Implant. News. 2006, 3, 487–491. [Google Scholar]
- Conz, M.B.; Campos, C.N.; Serrão, S.D.; Soares, G.A.; Vidigal, G.M. Caracterização físico-química de 12 biomateriaisutilizados como enxertos ósseos na Implantodontia. Implant. News 2010, 7, 541–546. [Google Scholar]
- Luo, Z.-B.; Zhang, Q.-B.; Zhang, Z.-Q.; Chen, D.; Yan, W.-X.; Li, K.-F.; Chen, Y. Performance of coralline hydroxyapatite in sinus floor augmentation: A retrospective study. Clin. Oral Investig. 2013, 17, 2003–2010. [Google Scholar] [CrossRef]
- Sadeghi, M.; Bakhshandeh, B.; Dehghan, M.M.; Mehrnia, M.R.; Khojasteh, A. Functional synergy of anti-mir221 and nanohydroxyapatite scaffold in bone tissue engineering of rat skull. J. Mater. Sci. Mater. Electron. 2016, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, M.; Gian, D.D.S.; Favero, A.; Scarano, A.; Orsini, D.D.S.G.; Piattelli, D.D.S.A. Bone Reactions to Anorganic Bovine Bone (Bio-Oss) Used in Sinus Augmentation Procedures: A Histologic Long-Term Report of 20 Cases in Humans. Int. J. Oral Maxillofac. Implant. 1999, 14, 835–840. [Google Scholar]
- Chaves, M.D.; Nunes, L.S.D.S.; de Oliveira, R.V.; Holgado, L.A.; Filho, H.N.; Matsumoto, M.A.; Ribeiro, D.A. Bovine hydroxyapatite (Bio-Oss®) induces osteocalcin, RANK-L and osteoprotegerin expression in sinus lift of rabbits. J. Cranio Maxillofac. Surg. 2012, 40, e315–e320. [Google Scholar] [CrossRef]
- Salehi, M.; Somayeh, M.N.; Ebrahimi-Barough, S.; Nourani, M.; Vaez, A.; Farzamfar, S.; Ai, J. Regeneration of sciatic nerve crush injury by a hydroxyapatite nanoparticle-containing collagen type I hydrogel. J. Physiol. Sci. 2018, 68, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Pil Lim, H.-J.Y. Purpose: The Influence of Cortical Perforation on Guided Bone Regeneration Using Synthetic Bone Substitutes: A Study of Rabbit Cranial Defects. Int. J. Oral Maxillofac. Implant. 2014, 29, 464–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, R.-W.; Kim, J.-H.; Moon, S.-Y. Effect of hydroxyapatite on critical-sized defect. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.B.; Vidigal, G.M., Jr.; Noleri, A.B.; Conz, M.B. Análise histomorfométrica de dois diferentes biomateriais instalados em alvéolos de coelhos Histomorphometric evaluation of two different biomaterials applied to the rabbit extraction socket model. Implant. News 2012, 9, 75–80. [Google Scholar]
- Stacchi, C.; Lombardi, T.; Oreglia, F.; Maltoni, A.A.; Traini, T. Histologic and Histomorphometric Comparison between Sintered Nanohydroxyapatite and Anorganic Bovine Xenograft in Maxillary Sinus Grafting: A Split-Mouth Randomized Controlled Clinical Trial. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Kruse, A.; Ghayor, C.; Jung, R.E.; Weber, F.E. Bone augmentation using a synthetic hydroxyapatite/silica oxide-based and a xenogenic hydroxyapatite-based bone substitute materials with and without recombinant human bone morphogenetic protein-2. Clin. Oral Implant. Res. 2014, 26, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Takauti, C.A.Y.; Futema, F.; Junior, R.B.D.B.; Abrahão, A.C.; Costa, C.; Queiroz, C.S. Assessment of Bone Healing in Rabbit Calvaria Grafted with Three Different Biomaterials. Braz. Dent. J. 2014, 25, 379–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duttenhoefer, F.; Hieber, S.F.; Stricker, A.; Schmelzeisen, R.; Gutwald, R.; Sauerbier, S. Follow-up of implant survival comparing ficoll and bone marrow aspirate concentrate methods for hard tissue regeneration with mesenchymal stem cells in humans. Biores. Open Access 2014, 3, 75–76. [Google Scholar] [CrossRef]
- Sakai, S.; Mishima, H.; Ishii, T.; Akaogi, H.; Uemura, T.; Ochiai, N. Technical note Concentration of bone marrow aspirate for osteo- genic repair using simple centrifugal methods. Acta Orthop. 2008, 79, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Mishima, H.; Akaogi, H.; Sakai, S.; Li, M.; Ochiai, N. Concentrated autologous bone marrow aspirate transplantation treatment for corticosteroid-induced osteonecrosis of the femoral head in systemic lupus erythematosus. Int. Orthop. 2011, 35, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, S.; Rickert, D.; Gutwald, R.; Nagursky, H.; Oshima, T.; Xavier, S.P.; Christmann, J.; Kurz, P.; Menne, D.; Vissink, A.; et al. Bone Marrow Concentrate and Bovine Bone Mineral for Sinus Floor Augmentation: A Controlled, Randomized, Single-Blinded Clinical and Histological Trial—Per-Protocol Analysis. Tissue Eng. Part A 2011, 17, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.K.; Alford, A.I.; Goldstein, S.A.; Stegemann, J.P. Comparison of Uncultured Marrow Mononuclear Cells and Culture-Expanded Mesenchymal Stem Cells in 3D Collagen-Chitosan Microbeads for Orthopedic Tissue Engineering. Tissue Eng. Part A 2014, 20, 210–224. [Google Scholar] [CrossRef]
- Krzymanski, G.; Kalczak, M.; Wiktor-Jedrzejczak, W. The use of bone-marrow-derived fibroblastoid cells and fresh bone marrow in the treatment of bone defects: An experimental study. Int. J. Oral Maxillofac. Surg. 1997, 26, 55–60. [Google Scholar] [CrossRef]
- Clarke, S.; Hoskins, N.; Jordan, G.; Marsh, D. Healing of an ulnar defect using a proprietary TCP bone graft substitute, JAX™, in association with autologous osteogenic cells and growth factors. Bone 2007, 40, 939–947. [Google Scholar] [CrossRef]
- Lucarelli, E.; Donati, D.; Cenacchi, A.; Fornasari, P.M. Bone reconstruction of large defects using bone marrow derived autologous stem cells. Transfus. Apher. Sci. 2004, 30, 169–174. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Oginni, F.; Holkom, M.A.M.; Mohamed, A.A.S.; Al-Sharani, H. Tissue-engineered bone using mesenchymal stem cells versus conventional bone grafts in the regeneration of maxillary alveolar bone: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2020, 35, 79–90. [Google Scholar] [CrossRef]

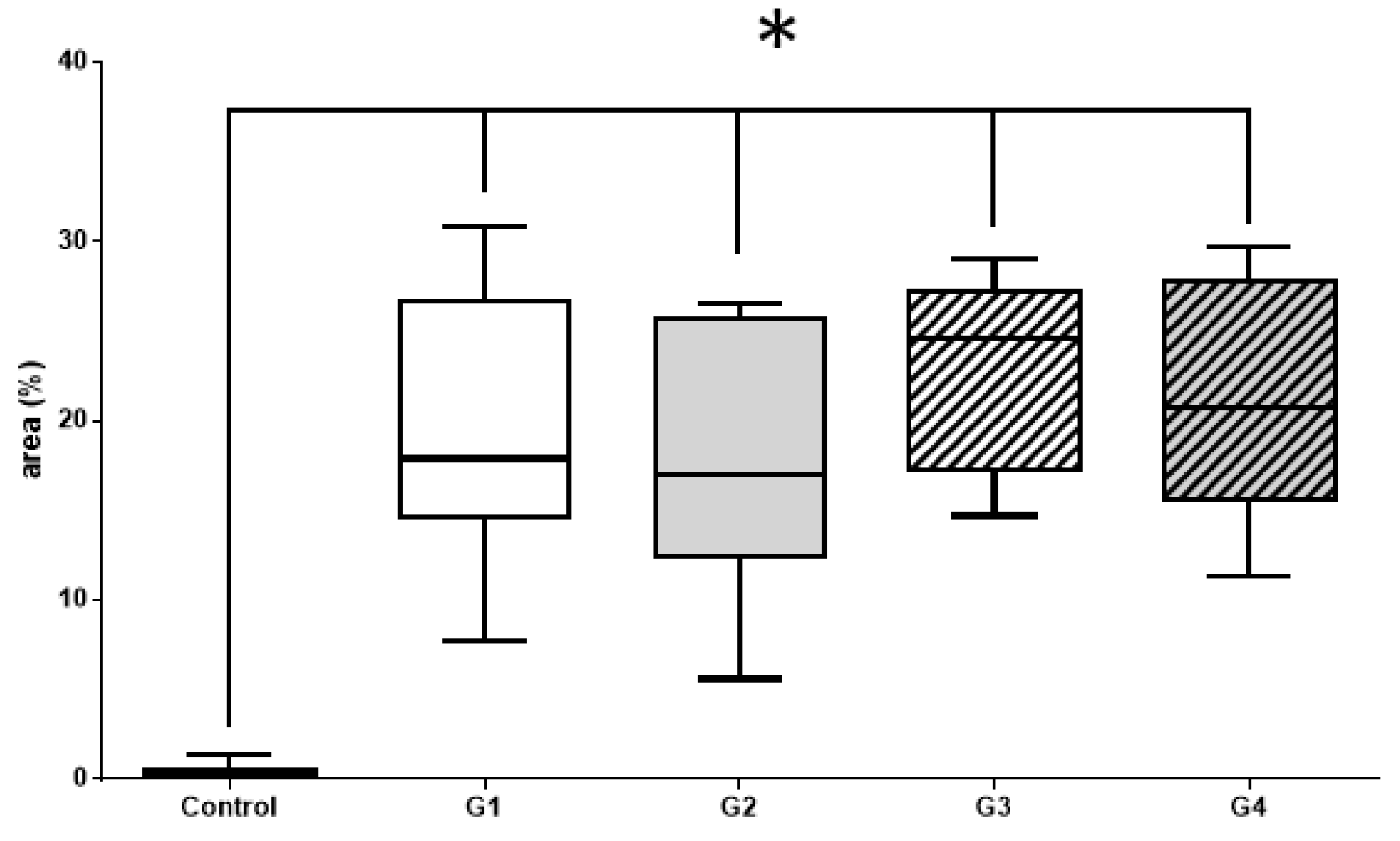

| Masson’s Trichrome Histomorphometry—Mann–Whitney Test | p Value |
|---|---|
| Control vs. G1 | <0.001 |
| Control vs. G2 | <0.001 |
| Control vs. G3 | <0.001 |
| Control vs. G4 | <0.001 |
| G1 vs. G2 | 0.6294 |
| G1 vs. G3 | 0.3231 |
| G2 vs. G4 | 0.4945 |
| G3 vs. G4 | 0.7023 |
| VEGF—Mann–Whitney Test | p Value |
|---|---|
| Control vs. G1 | <0.001 |
| Control vs. G2 | <0.001 |
| Control vs. G3 | <0.001 |
| Control vs. G4 | <0.001 |
| G1 vs. G2 | 0.3754 |
| G1 vs. G3 | 0.1930 |
| G2 vs. G4 | 0.4331 |
| G3 vs. G4 | 0.8541 |
| Osteopontin—Mann–Whitney Test | p Value |
|---|---|
| Control vs. G1 | <0.001 |
| Control vs. G2 | <0.001 |
| Control vs. G3 | <0.001 |
| Control vs. G4 | <0.001 |
| G1 vs. G2 | 0.4945 |
| G1 vs. G3 | 0.4331 |
| G2 vs. G4 | 0.2317 |
| G3 vs. G4 | 0.1593 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, J.L.d.S.; de Carvalho, J.J.; Pereira, M.J.d.S.; Brum, I.d.S.; Nascimento, A.L.R.; dos Santos, P.G.P.; Frigo, L.; Fischer, R.G. Repair of Critical Size Bone Defects Using Synthetic Hydroxyapatite or Xenograft with or without the Bone Marrow Mononuclear Fraction: A Histomorphometric and Immunohistochemical Study in Rat Calvaria. Materials 2021, 14, 2854. https://doi.org/10.3390/ma14112854
Pires JLdS, de Carvalho JJ, Pereira MJdS, Brum IdS, Nascimento ALR, dos Santos PGP, Frigo L, Fischer RG. Repair of Critical Size Bone Defects Using Synthetic Hydroxyapatite or Xenograft with or without the Bone Marrow Mononuclear Fraction: A Histomorphometric and Immunohistochemical Study in Rat Calvaria. Materials. 2021; 14(11):2854. https://doi.org/10.3390/ma14112854
Chicago/Turabian StylePires, Jorge Luís da Silva, Jorge José de Carvalho, Mario José dos Santos Pereira, Igor da Silva Brum, Ana Lucia Rosa Nascimento, Paulo Gonçalo Pinto dos Santos, Lucio Frigo, and Ricardo Guimaraes Fischer. 2021. "Repair of Critical Size Bone Defects Using Synthetic Hydroxyapatite or Xenograft with or without the Bone Marrow Mononuclear Fraction: A Histomorphometric and Immunohistochemical Study in Rat Calvaria" Materials 14, no. 11: 2854. https://doi.org/10.3390/ma14112854
APA StylePires, J. L. d. S., de Carvalho, J. J., Pereira, M. J. d. S., Brum, I. d. S., Nascimento, A. L. R., dos Santos, P. G. P., Frigo, L., & Fischer, R. G. (2021). Repair of Critical Size Bone Defects Using Synthetic Hydroxyapatite or Xenograft with or without the Bone Marrow Mononuclear Fraction: A Histomorphometric and Immunohistochemical Study in Rat Calvaria. Materials, 14(11), 2854. https://doi.org/10.3390/ma14112854





