Impact of Sandblasting on Morphology, Structure and Conductivity of Zirconia Dental Ceramics Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Surface Investigation Methods
2.3. Structural Investigation Methods
2.4. Electrical Properties
3. Results and Discussion
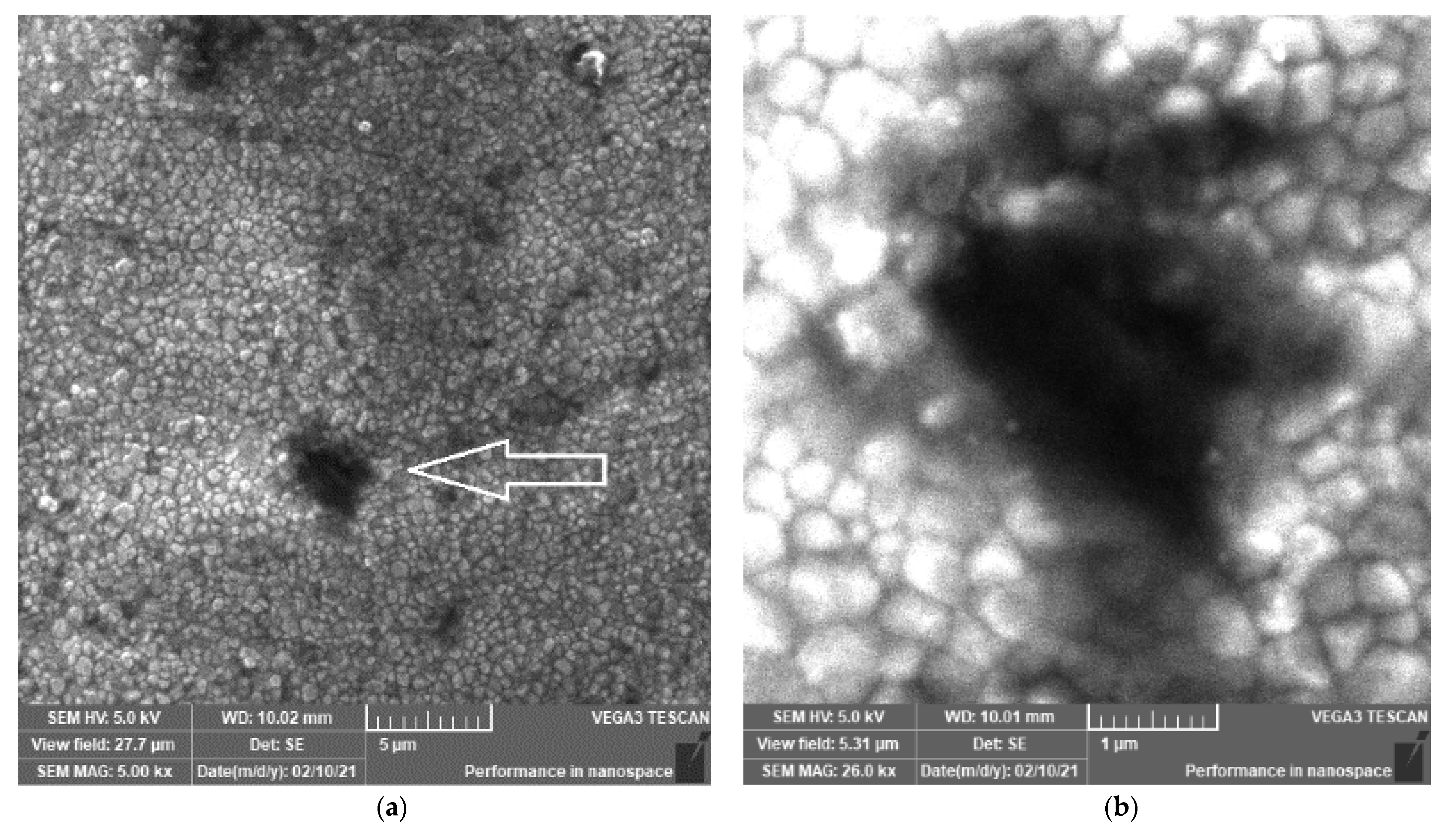
3.1. SEM Investigations
3.2. EDX Investigations
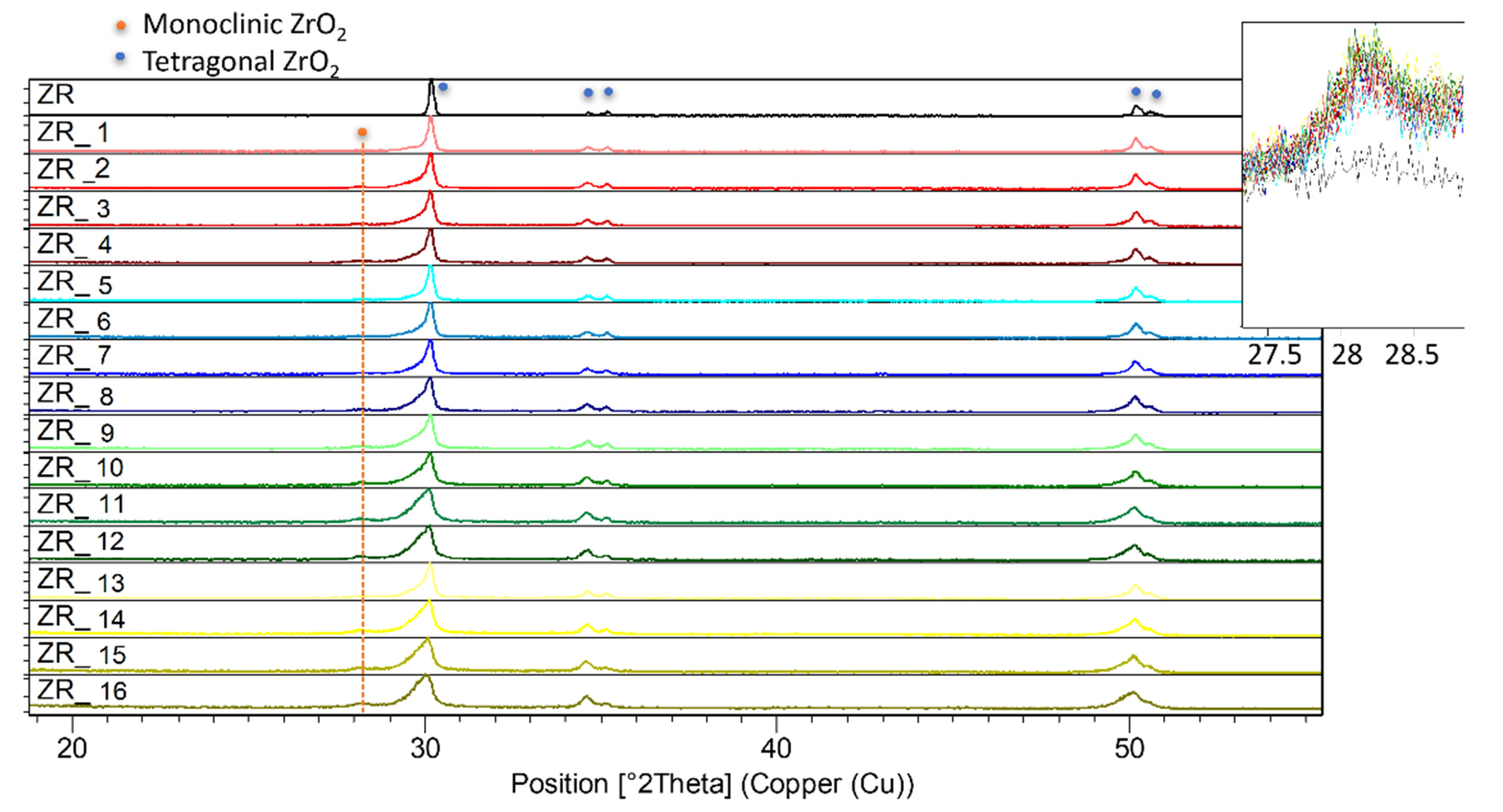
3.3. Structural Investigations
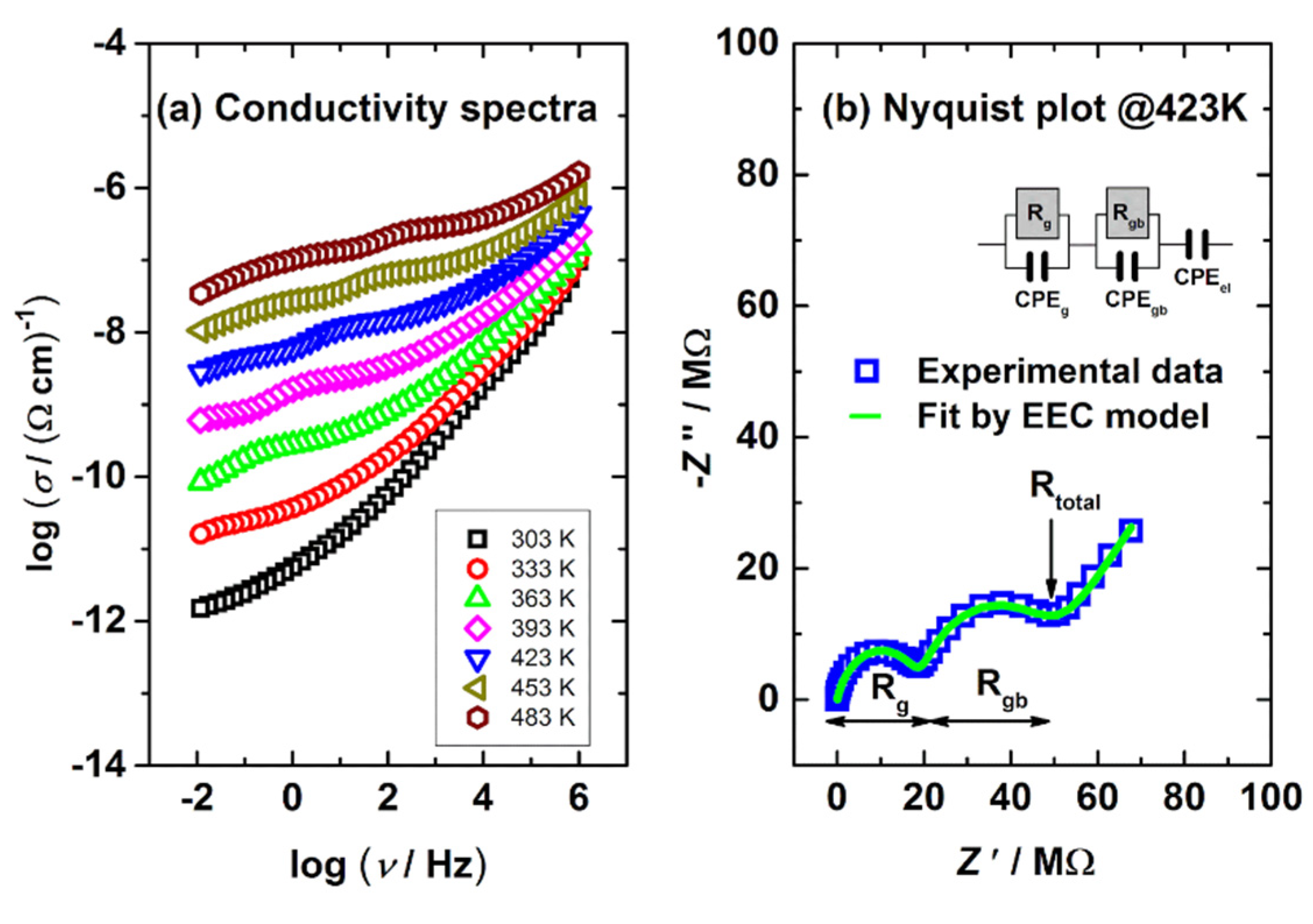
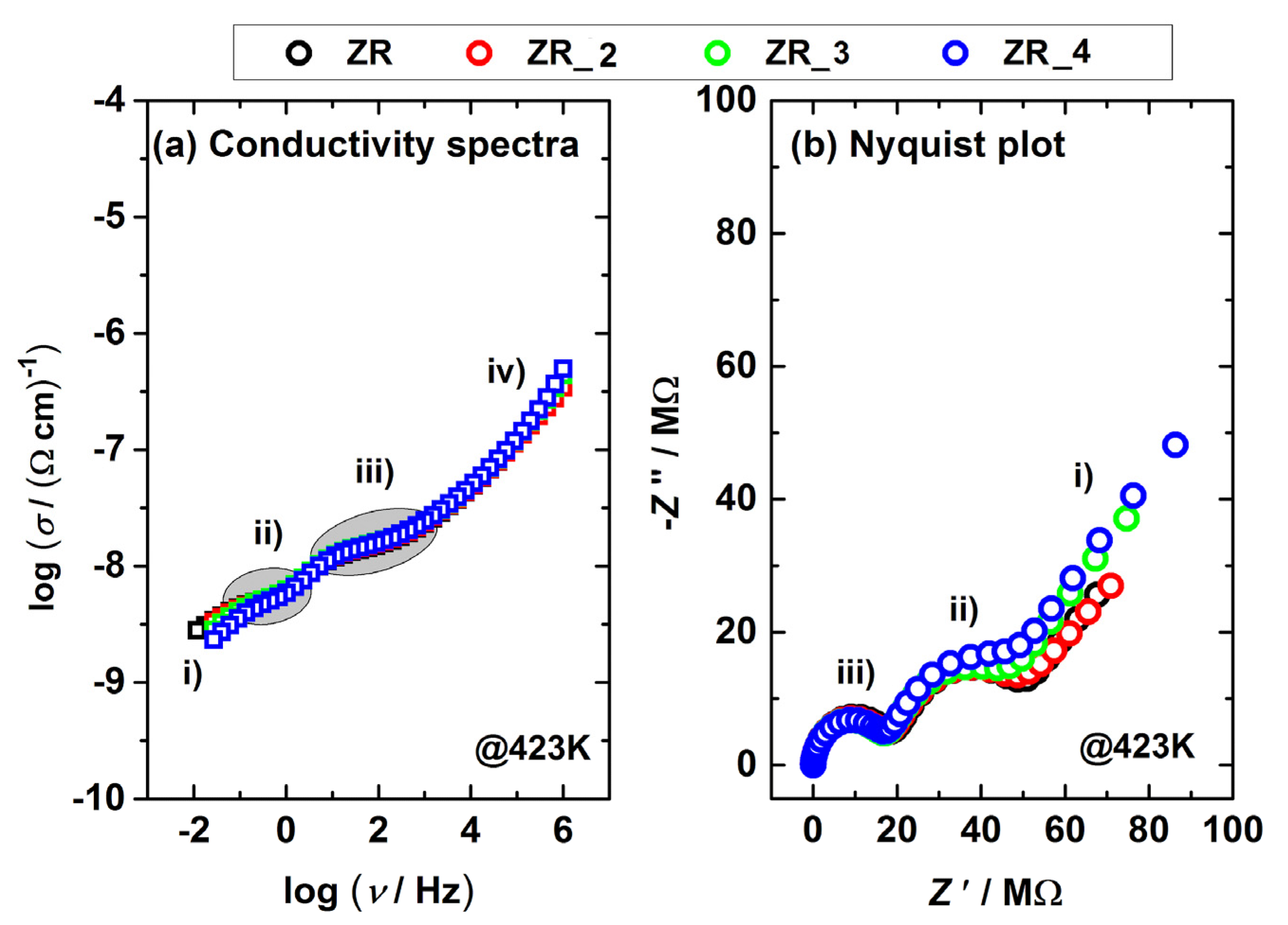
3.4. Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crespi, R.; Capparè, P.; Gherlone, E. Sinus Floor Elevation by Osteotome: Hand Mallet versus Electric Mallet. A Prospective Clinical Study. Int. J. Oral Maxillofac. Implant. 2012, 27, 1144–1150. [Google Scholar]
- Zizzari, V.L.; Zara, S.; Tetè, G.; Vinci, R.; Gherlone, E.; Cataldi, A. Biologic and Clinical Aspects of Integration of Different Bone Substitutes in Oral Surgery: A Literature Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Ciancaglini, R.; Gherlone, E.F.; Redaelli, S.; Radaelli, G. The Distribution of Occlusal Contacts in the Intercuspal Position and Temporomandibular Disorder. J. Oral Rehabil. 2002, 29, 1082–1090. [Google Scholar] [CrossRef]
- Mörmann, W.H. State of the Art of CAD/CAM Restorations: 20 Years of CEREC; Quintessence: Batavia, IL, USA, 2006. [Google Scholar]
- van Noort, R. The Future of Dental Devices Is Digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Albelasy, E.; Hamama, H.H.; Tsoi, J.K.H.; Mahmoud, S.H. Influence of Material Type, Thickness and Storage on Fracture Resistance of CAD/CAM Occlusal Veneers. J. Mech. Behav. Biomed. Mater. 2021, 119, 104485. [Google Scholar] [CrossRef]
- Ilie, N. Frequency-Related Viscoelastic Properties in High Translucent CAD-CAM Resin-Based Composites. J. Mech. Behav. Biomed. Mater. 2021, 118, 104427. [Google Scholar] [CrossRef]
- Machry, R.V.; Borges, A.L.S.; Pereira, G.K.R.; Kleverlaan, C.J.; Venturini, A.B.; Valandro, L.F. Influence of the Foundation Substrate on the Fatigue Behavior of Bonded Glass, Zirconia Polycrystals, and Polymer Infiltrated Ceramic Simplified CAD-CAM Restorations. J. Mech. Behav. Biomed. Mater. 2021, 117, 104391. [Google Scholar] [CrossRef]
- Conrad, H.J.; Seong, W.-J.; Pesun, I.J. Current Ceramic Materials and Systems with Clinical Recommendations: A Systematic Review. J. Prosthet. Dent. 2007, 98, 389–404. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J.R. State of the Art of Zirconia for Dental Applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef]
- Manicone, P.F.; Rossi Iommetti, P.; Raffaelli, L. An Overview of Zirconia Ceramics: Basic Properties and Clinical Applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a Ceramic Biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Špehar, D.; Jakovac, M. New Knowledge about Zirconium-Ceramic as a Structural Material in Fixed Prosthodontics. Acta Stomatol. Croat. 2015, 49, 137–144. [Google Scholar] [CrossRef]
- Craciunescu, E.; Sinescu, C.; Negrutiu, M.L.; Pop, D.M.; Lauer, H.-C.; Rominu, M.; Hutiu, G.; Bunoiu, M.; Duma, V.-F.; Antoniac, I. Shear Bond Strength Tests of Zirconia Veneering Ceramics after Chipping Repair. J. Adhes. Sci. Technol. 2016, 30, 666–676. [Google Scholar] [CrossRef]
- Strasser, T.; Preis, V.; Behr, M.; Rosentritt, M. Roughness, Surface Energy, and Superficial Damages of CAD/CAM Materials after Surface Treatment. Clin. Oral Investig. 2018, 22, 2787–2797. [Google Scholar] [CrossRef]
- Ahamer, C.; Opitz, A.K.; Rupp, G.M.; Fleig, J. Revisiting the Temperature Dependent Ionic Conductivity of Yttria Stabilized Zirconia (YSZ). J. Electrochem. Soc. 2017, 164, F790–F803. [Google Scholar] [CrossRef]
- Badwal, S. Zirconia-Based Solid Electrolytes: Microstructure, Stability and Ionic Conductivity. Solid State Ionics 1992, 52, 23–32. [Google Scholar] [CrossRef]
- Chen, X.J.; Khor, K.A.; Chan, S.H.; Yu, L.G. Influence of Microstructure on the Ionic Conductivity of Yttria-Stabilized Zirconia Electrolyte. Mater. Sci. Eng. A Struct. Mater. 2002, 335, 246–252. [Google Scholar] [CrossRef]
- Kelly, R.J.; Denry, I. Stabilized Zirconia as a Structural Ceramic: An Overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kosmac, T.; Oblak, C.; Jevnikar, P.; Funduk, N.; Marion, L. The Effect of Surface Grinding and Sandblasting on Flexural Strength and Reliability of Y-TZP Zirconia Ceramic. Dent. Mater. 1999, 15, 426–433. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Okada, M.; Taketa, H.; Torii, Y.; Irie, M.; Matsumoto, T. Optimal Sandblasting Conditions for Conventional-Type Yttria-Stabilized Tetragonal Zirconia Polycrystals. Dent. Mater. 2019, 35, 169–175. [Google Scholar] [CrossRef]
- Inokoshi, M.; Zhang, F.; De Munck, J.; Minakuchi, S.; Naert, I.; Vleugels, J.; Van Meerbeek, B.; Vanmeensel, K. Influence of Sintering Conditions on Low-Temperature Degradation of Dental Zirconia. Dent. Mater. 2014, 30, 669–678. [Google Scholar] [CrossRef]
- Eriksson, C.; Masaki, N.; Yao, I.; Hayasaka, T.; Setou, M. MALDI Imaging Mass Spectrometry—A Mini Review of Methods and Recent Developments. Mass Spectrom. 2013, 2, S0022. [Google Scholar] [CrossRef] [PubMed]
- Badwal, S.P.S. Electrical Conductivity of Single Crystal and Polycrystalline Yttria-Stabilized Zirconia. J. Mater. Sci. 1984, 19, 1767–1776. [Google Scholar] [CrossRef]
- Jakovac, M.; Klaser, T.; Radatović, B.; Skoko, Ž.; Pavić, L.; Žic, M. Surface Characterization and Conductivity of Two Types of Lithium-Based Glass Ceramics after Accelerating Ageing. Materials 2020, 13, 5632. [Google Scholar] [CrossRef]
- Kim, H.-K.; Ahn, B. Effect of AlO Sandblasting Particle Size on the Surface Topography and Residual Compressive Stresses of Three Different Dental Zirconia Grades. Materials 2021, 14, 610. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-M.; Min, B.K.; Kim, Y.K.; Kwon, T.-Y. Influence of Sandblasting Particle Size and Pressure on Resin Bonding Durability to Zirconia: A Residual Stress Study. Materials 2020, 13, 5629. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-A.; Ahn, J.-S.; Park, Y.-J.; Jun, S.-H.; Lee, I.-B.; Cho, B.-H.; Son, H.-H.; Seo, D.-G. The Effect of Sandblasting and Different Primers on Shear Bond Strength between Yttria-Tetragonal Zirconia Polycrystal Ceramic and a Self-Adhesive Resin Cement. Oper. Dent. 2015, 40, 63–71. [Google Scholar] [CrossRef]
- Kern, M.; Wegner, S.M. Bonding to Zirconia Ceramic: Adhesion Methods and Their Durability. Dent. Mater. 1998, 14, 64–71. [Google Scholar] [CrossRef]
- Guazzato, M.; Quach, L.; Albakry, M.; Swain, M.V. Influence of Surface and Heat Treatments on the Flexural Strength of Y-TZP Dental Ceramic. J. Dent. 2005, 33, 9–18. [Google Scholar] [CrossRef]
- Rigolin, F.J.; Negreiros, W.M.; Giannini, M.; Rizzatti Barbosa, C.M. Effects of Sandblasting and Hydrofluoric Acid Etching on Surface Topography, Flexural Strength, Modulus and Bond Strength of Composite Cement to Ceramics. J. Adhes. Dent. 2021, 23, 113–119. [Google Scholar] [CrossRef]
- Inokoshi, M.; Shimizubata, M.; Nozaki, K.; Takagaki, T.; Yoshihara, K.; Minakuchi, S.; Vleugels, J.; Van Meerbeek, B.; Zhang, F. Impact of Sandblasting on the Flexural Strength of Highly Translucent Zirconia. J. Mech. Behav. Biomed. Mater. 2021, 115, 104268. [Google Scholar] [CrossRef]
- Inokoshi, M.; Poitevin, A.; De Munck, J.; Minakuchi, S.; Van Meerbeek, B. Bonding Effectiveness to Different Chemically Pre-Treated Dental Zirconia. Clin. Oral Investig. 2014, 18, 1803–1812. [Google Scholar] [CrossRef]
- Ban, S.; Okuda, Y.; Noda, M.; Tsuruki, J.; Kawai, T.; Kono, H. Contamination of Dental Zirconia before Final Firing: Effects on Mechanical Properties. Dent. Mater. J. 2013, 32, 1011–1019. [Google Scholar] [CrossRef]
- Ghoveizi, R.; Parsirad, R.; Tavakolizadeh, S.; Beyabanaki, E. Effect of Different Nd:YAG Laser Power Outputs on Bond Strength of Resin Cement to Zirconia in Comparison to Sandblasting. J. Lasers Med. Sci. 2021, 12, e6. [Google Scholar] [CrossRef]
- Chintapalli, R.K.; Mestra Rodriguez, A.; Garcia Marro, F.; Anglada, M. Effect of Sandblasting and Residual Stress on Strength of Zirconia for Restorative Dentistry Applications. J. Mech. Behav. Biomed. Mater. 2014, 29, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Derand, T.; Molin, M.; Kleven, E.; Haag, P.; Karlsson, S. Bond Strength of Luting Materials to Ceramic Crowns after Different Surface Treatments. Eur. J. Prosthodont. Restor. Dent. 2008, 16, 35–38. [Google Scholar] [PubMed]
- Tzanakakis, E.-G.C.; Tzoutzas, I.G.; Koidis, P.T. Is There a Potential for Durable Adhesion to Zirconia Restorations? A Systematic Review. J. Prosthet. Dent. 2016, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Dündar, M.; Ozcan, M.; Gökçe, B.; Cömlekoğlu, E.; Leite, F.; Valandro, L.F. Comparison of Two Bond Strength Testing Methodologies for Bilayered All-Ceramics. Dent. Mater. 2007, 23, 630–636. [Google Scholar] [CrossRef]
- Altan, B.; Cinar, S.; Tuncelli, B. Evaluation of Shear Bond Strength of Zirconia-Based Monolithic CAD-CAM Materials to Resin Cement after Different Surface Treatments. Niger. J. Clin. Pract. 2019, 22, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Paranhos, M.P.G.; Burnett, L.H., Jr.; Magne, P. Effect Of Nd:YAG Laser and CO2 Laser Treatment on the Resin Bond Strength to Zirconia Ceramic. Quintessence Int. 2011, 42, 79–89. [Google Scholar] [PubMed]
- Shahmiri, R.; Standard, O.C.; Hart, J.N.; Sorrell, C.C. Optical Properties of Zirconia Ceramics for Esthetic Dental Restorations: A Systematic Review. J. Prosthet. Dent. 2018, 119, 36–46. [Google Scholar] [CrossRef]
- Liu, D.; Matinlinna, J.P.; Tsoi, J.K.-H.; Pow, E.H.N.; Miyazaki, T.; Shibata, Y.; Kan, C.-W. A New Modified Laser Pretreatment for Porcelain Zirconia Bonding. Dent. Mater. 2013, 29, 559–565. [Google Scholar] [CrossRef]
- Blatz, M.B.; Sadan, A.; Martin, J.; Lang, B. In Vitro Evaluation of Shear Bond Strengths of Resin to Densely-Sintered High-Purity Zirconium-Oxide Ceramic after Long-Term Storage and Thermal Cycling. J. Prosthet. Dent. 2004, 91, 356–362. [Google Scholar] [CrossRef]
- Kern, M.; Barloi, A.; Yang, B. Surface Conditioning Influences Zirconia Ceramic Bonding. J. Dent. Res. 2009, 88, 817–822. [Google Scholar] [CrossRef]
- Magne, P.; Paranhos, M.P.G.; Burnett, L.H., Jr. New Zirconia Primer Improves Bond Strength of Resin-Based Cements. Dent. Mater. 2010, 26, 345–352. [Google Scholar] [CrossRef]
- Nishigawa, G.; Maruo, Y.; Irie, M.; Maeda, N.; Yoshihara, K.; Nagaoka, N.; Matsumoto, T.; Minagi, S. Various Effects of Sandblasting of Dental Restorative Materials. PLoS ONE 2016, 11, e0147077. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Heuer, A.H. Mechanisms of toughening partially stabilized zirconia (PSZ). J. Am. Ceram. Soc. 1977, 8. [Google Scholar] [CrossRef]
- Garvie, R.C.; Nicholson, P.S. Structure and Thermomechanical Properties of Partially Stabilized Zirconia in the CaO-ZrO2 System. J. Am. Ceram. Soc. 1972, 55, 152–157. [Google Scholar] [CrossRef]
- Belli, R.; Wendler, M.; de Ligny, D.; Cicconi, M.R.; Petschelt, A.; Peterlik, H.; Lohbauer, U. Chairside CAD/CAM Materials. Part 1: Measurement of Elastic Constants and Microstructural Characterization. Dent. Mater. 2017, 33, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Hannink, R.H.J.; Swain, M.V. Transformation Toughening of Ceramics; CRC-Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Eichler, J.; Eisele, U.; Rödel, J. Mechanical Properties of Monoclinic Zirconia. J. Am. Ceram. Soc. 2004, 87, 1401–1403. [Google Scholar] [CrossRef]
- Guillon, O.; Dash, A.; Lenser, C.; Uhlenbruck, S.; Mauer, G. Tuning the Microstructure and Thickness of Ceramic Layers with Advanced Coating Technologies Using Zirconia as an Example. Adv. Eng. Mater. 2020, 22, 2000529. [Google Scholar] [CrossRef]
- Kilo, M.; Argirusis, C.; Borchardt, G.; Jackson, R.A. Oxygen Diffusion in Yttria Stabilised Zirconia—Experimental Results and Molecular Dynamics Calculations. Phys. Chem. Chem. Phys. 2003, 5, 2219–2224. [Google Scholar] [CrossRef]
- Ross Macdonald, J.; Kenan, W.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; Wiley-Interscience: Hoboken, NJ, USA, 1987. [Google Scholar]
- Pavić, L.; Skoko, Ž.; Gajović, A.; Su, D.; Moguš-Milanković, A. Electrical Transport in Iron Phosphate Glass-Ceramics. J. Non-Cryst. Solids 2018, 502, 44–53. [Google Scholar] [CrossRef]
- Androš Dubraja, L.; Žilić, D.; Olujić, K.; Pavić, L.; Molčanov, K.; Pajić, D. Targeted Synthesis of a CrIII–O–VV Core Oxo-Bridged Complex: Spectroscopic, Magnetic and Electrical Properties. New J. Chem. 2021, 14. [Google Scholar] [CrossRef]
- Ishii, R.; Tsujimoto, A.; Takamizawa, T.; Tsubota, K.; Suzuki, T.; Shimamura, Y.; Miyazaki, M. Influence of Surface Treatment of Contaminated Zirconia on Surface Free Energy and Resin Cement Bonding. Dent. Mater. J. 2015, 34, 91–97. [Google Scholar] [CrossRef]
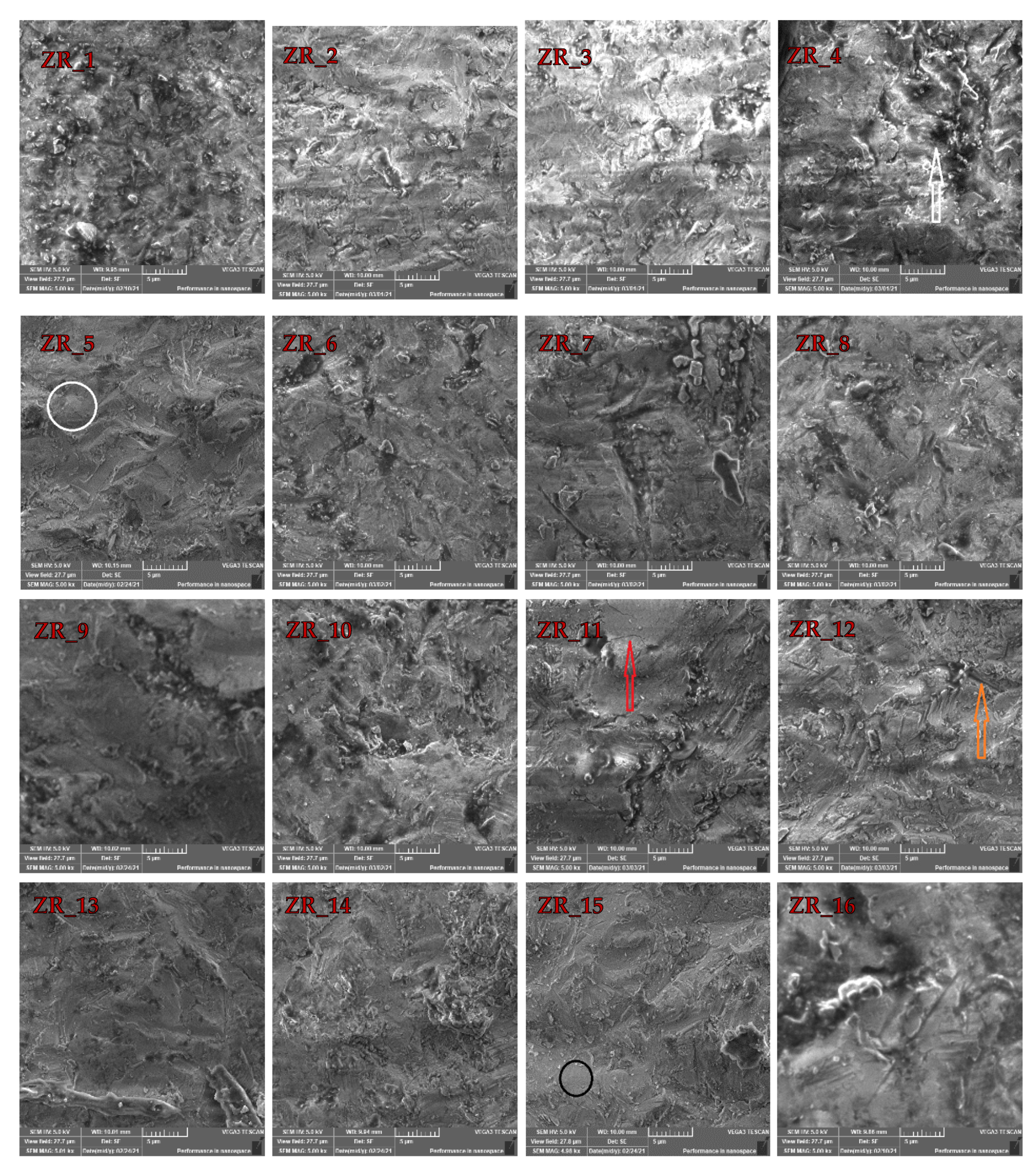
| Size (Al2O3)/µm | Pressure/MPa | |||
|---|---|---|---|---|
| 0.1 | 0.2 | 0.4 | 0.6 | |
| 25 | ZR_1 | ZR_2 | ZR_3 | ZR_4 |
| 50 | ZR_5 | ZR_6 | ZR_7 | ZR_8 |
| 110 | ZR_9 | ZR_10 | ZR_11 | ZR_12 |
| 125 | ZR_13 | ZR_14 | ZR_15 | ZR_16 |
| Samples | ||||||||
|---|---|---|---|---|---|---|---|---|
| ZR_1 | ZR_2 | ZR_3 | ZR_4 | ZR_5 | ZR_6 | ZR_7 | ZR_8 | |
| Zr/% | 15.36 | 15.36 | 15.36 | 15.36 | 21.184 | 21.184 | 21.184 | 21.184 |
| Al/% | 10.87 | 10.87 | 10.87 | 10.87 | 9.024 | 9.024 | 9.024 | 9.024 |
| Samples | ||||||||
| ZR_9 | ZR_10 | ZR_11 | ZR_12 | ZR_13 | ZR_14 | ZR_15 | ZR_16 | |
| Zr/% | 17.34 | 17.34 | 17.34 | 17.34 | 17.26 | 17.26 | 17.26 | 17.26 |
| Al/% | 10.31 | 10.31 | 10.31 | 10.31 | 9.83 | 9.83 | 9.83 | 9.83 |
| EEC Parameters | Samples | |||
|---|---|---|---|---|
| ZR | ZR_2 | ZR_3 | ZR_4 | |
| Rg/Ω | 1.88 × 107 | 1.72 × 107 | 1.68 × 107 | 1.63 × 107 |
| Rgb/Ω | 2.56 × 107 | 2.42 × 107 | 2.00 × 107 | 1.69 × 107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovac, M.; Klaser, T.; Radatović, B.; Bafti, A.; Skoko, Ž.; Pavić, L.; Žic, M. Impact of Sandblasting on Morphology, Structure and Conductivity of Zirconia Dental Ceramics Material. Materials 2021, 14, 2834. https://doi.org/10.3390/ma14112834
Jakovac M, Klaser T, Radatović B, Bafti A, Skoko Ž, Pavić L, Žic M. Impact of Sandblasting on Morphology, Structure and Conductivity of Zirconia Dental Ceramics Material. Materials. 2021; 14(11):2834. https://doi.org/10.3390/ma14112834
Chicago/Turabian StyleJakovac, Marko, Teodoro Klaser, Borna Radatović, Arijeta Bafti, Željko Skoko, Luka Pavić, and Mark Žic. 2021. "Impact of Sandblasting on Morphology, Structure and Conductivity of Zirconia Dental Ceramics Material" Materials 14, no. 11: 2834. https://doi.org/10.3390/ma14112834
APA StyleJakovac, M., Klaser, T., Radatović, B., Bafti, A., Skoko, Ž., Pavić, L., & Žic, M. (2021). Impact of Sandblasting on Morphology, Structure and Conductivity of Zirconia Dental Ceramics Material. Materials, 14(11), 2834. https://doi.org/10.3390/ma14112834








