A Self-Bleaching Electrochromic Mirror Based on Metal Organic Frameworks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Preparation of the MOF/Ag films
2.2.2. Preparation of the WO3 thin Films with SnO2 Interface
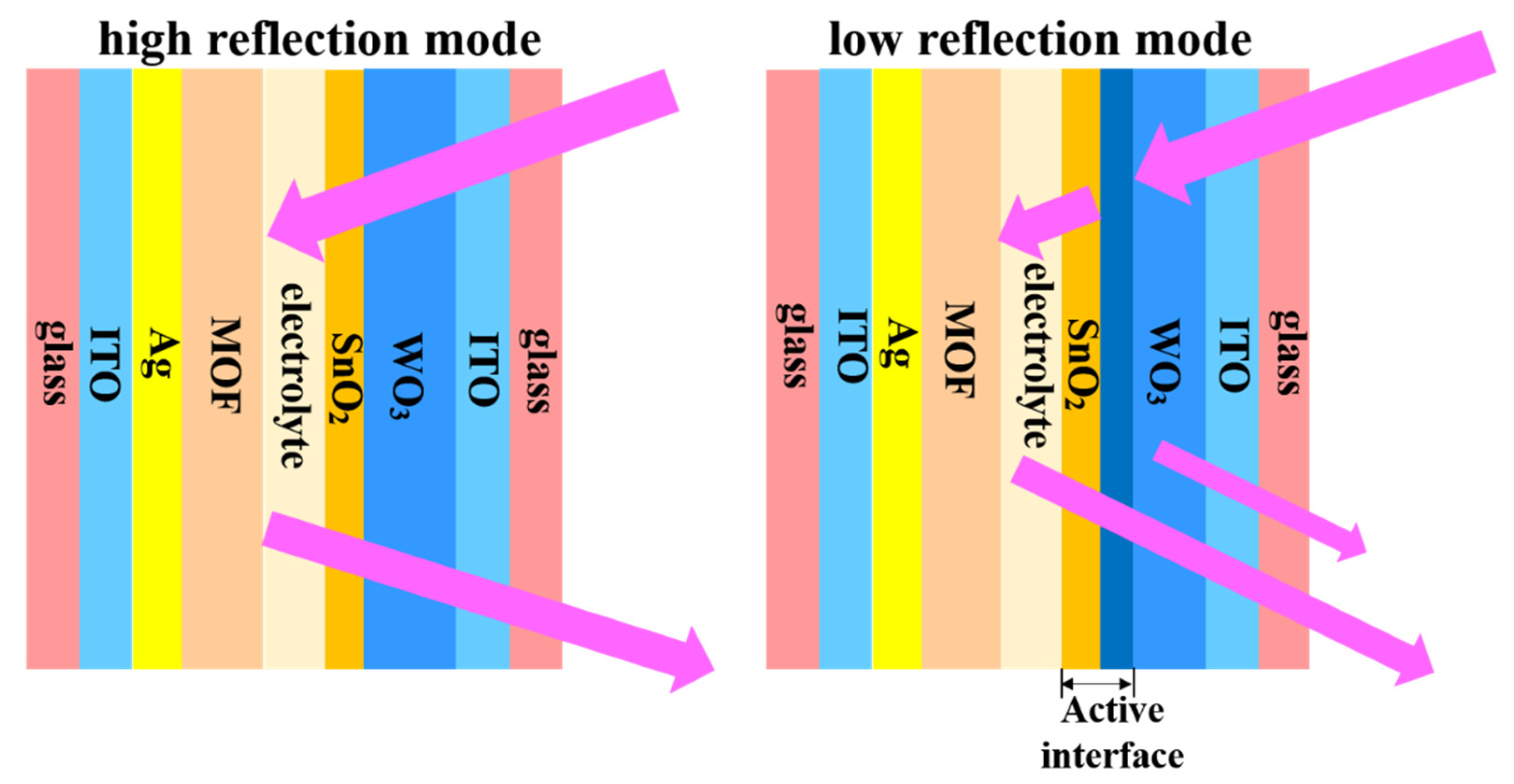
2.2.3. Assembly of the Mirrors
2.3. Measurements
3. Results and Discussion
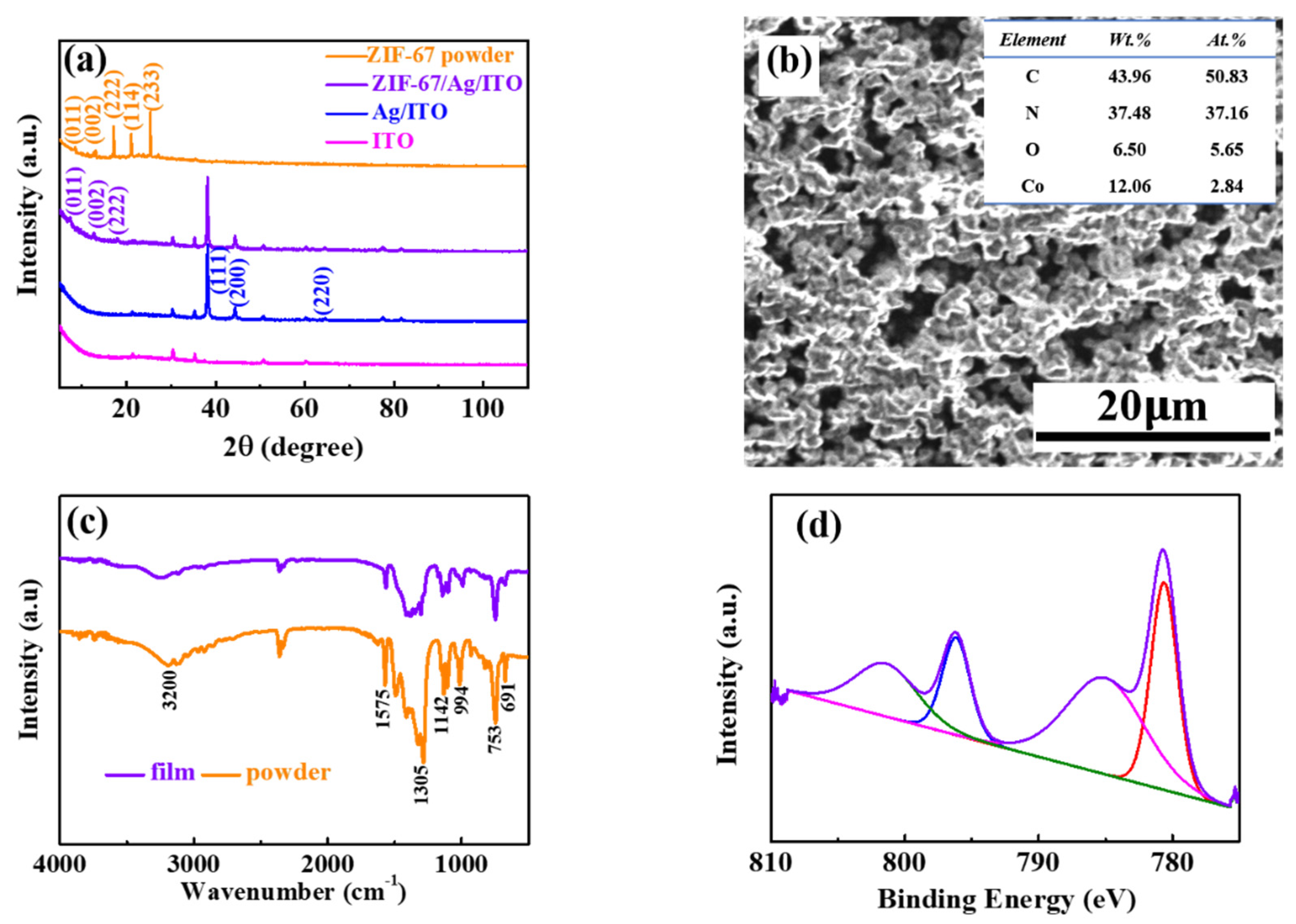
3.1. Characterization of Films
3.2. Electrochemical Properties of the ZIF-67/Ag Films
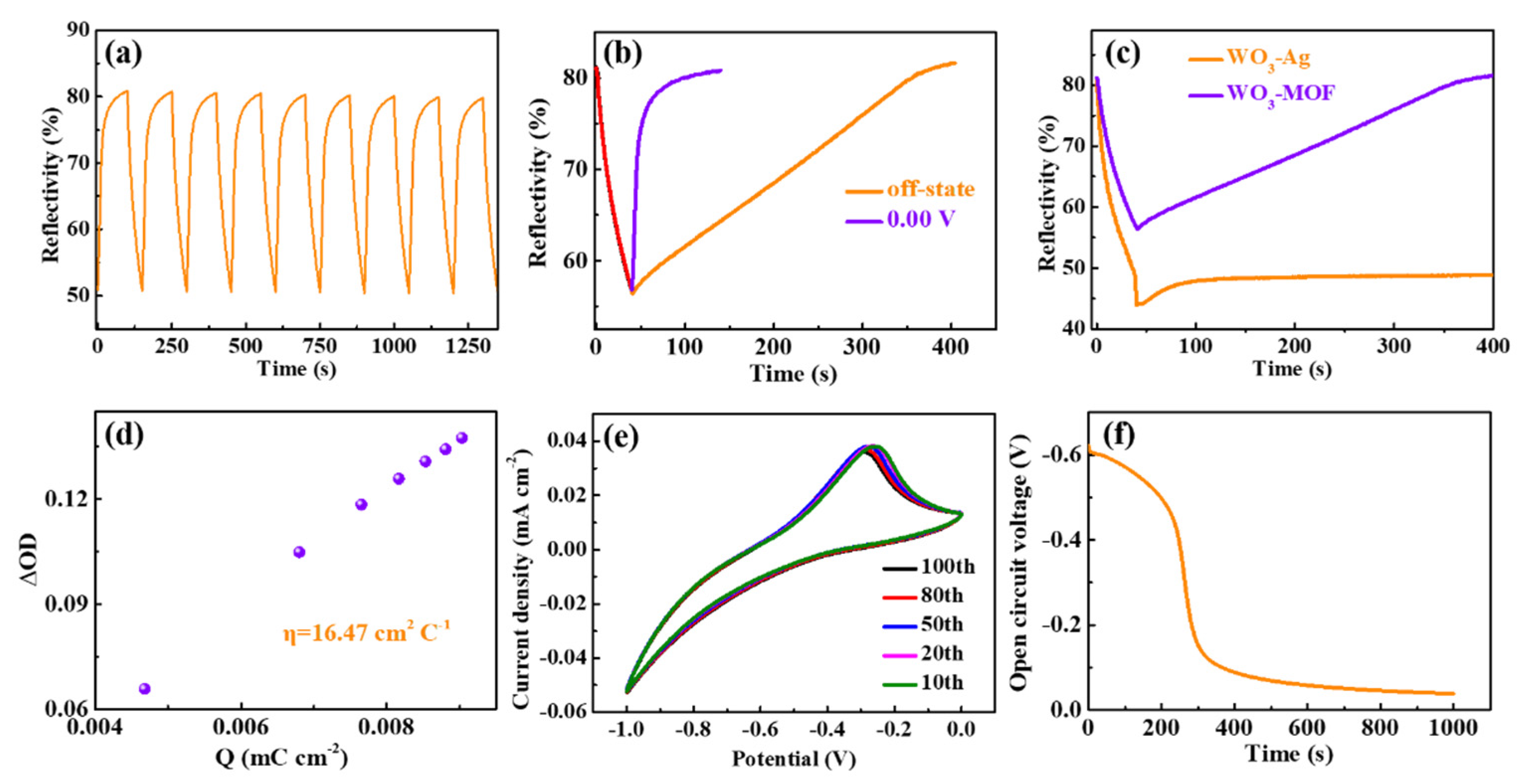
3.3. Electrochromic Performance of the WO3//ZIF-67 Mirrors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic systems and the prospects for devices. Adv. Mater. 2001, 13, 783. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Granqvist, C.G. Electrochromics for smart windows: Thin films of tungsten oxide and nickel oxide, and devices based on these. J. Mater. Chem. 2006, 17, 127–156. [Google Scholar] [CrossRef]
- Cheng, W.; Moreno-Gonzalez, M.; Hu, K.; Krzyszkowski, C.; Dvorak, D.J.; Weekes, D.M.; Tam, B.; Berlinguette, C.P. Solution-Deposited Solid-State Electrochromic Windows. iScience 2018, 10, 80–86. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Wang, X.; Zhang, W.; Zheng, W.; Zhang, Y.-M.; Zhang, S.X.-A. A multicolour bistable electronic shelf label based on intramolecular proton-coupled electron transfer. Nat. Mater. 2019, 18, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.C.; Kim, C.-H.; Lodge, T.P.; Frisbie, C.D. Multicolored, Low-Power, Flexible Electrochromic Devices Based on Ion Gels. ACS Appl. Mater. Interfaces 2016, 8, 6252–6260. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Lee, P.S. Next-Generation Multifunctional Electrochromic Devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Xue, Q.; Zhi, C. Multifunctional Energy Storage and Conversion Devices. Adv. Mater. 2016, 28, 8344–8364. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, J.; Ke, L.; Liu, X.; Demir, H.V.; Yang, M.F.; Sun, X.W. Electrochromic properties of nanostructured tungsten trioxide (hydrate) films and their applications in a complementary electrochromic device. Electrochim. Acta 2012, 63, 153–160. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, J.; Wang, J.-L.; Xu, J.; Li, H.-H.; Yu, S.-H. Ultrathin W18O49 Nanowire Assemblies for Electrochromic Devices. Nano Lett. 2013, 13, 3589–3593. [Google Scholar] [CrossRef]
- Invernale, M.A.; Seshadri, V.; Mamangun, D.M.D.; Ding, Y.; Filloramo, J.; Sotzing, G. Polythieno[3,4-b]thiophene as an Optically Transparent Ion-Storage Layer. Chem. Mater. 2009, 21, 3332–3336. [Google Scholar] [CrossRef]
- Choi, D.; Lee, M.; Kim, H.; Chu, W.-S.; Chun, D.-M.; Ahn, S.-H.; Lee, C.S. Investigation of dry-deposited ion storage layers using various oxide particles to enhance electrochromic performance. Sol. Energy Mater. Sol. Cells 2018, 174, 599–606. [Google Scholar] [CrossRef]
- Kim, C.-Y.; Cho, S.-G.; Lim, T.-Y. Cycle test and degradation analysis of WO3/PC+LiClO4/CeO2 center dot TiO2 electrochromic device. Sol. Energy Mater. Sol. Cells 2009, 93, 2056–2061. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Chen, Y.; Xu, Y.; Pan, H.; Sun, W.; Liu, S.; Yan, M.; Jiang, Y. Gradient substitution: An intrinsic strategy towards high performance sodium storage in Prussian blue-based cathodes. J. Mater. Chem. A 2018, 6, 8947–8954. [Google Scholar] [CrossRef]
- Wade, C.R.; Li, M.; Dincă, M. Facile Deposition of Multicolored Electrochromic Metal-Organic Framework Thin Films. Angew. Chem. Int. Ed. 2013, 52, 13377–13381. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, M.; Sun, W.; Wang, Y. Bimetal-Organic Framework: One-Step Homogenous Formation and its Derived Mesoporous Ternary Metal Oxide Nanorod for High-Capacity, High-Rate, and Long-Cycle-Life Lithium Storage. Adv. Funct. Mater. 2016, 26, 1098–1103. [Google Scholar] [CrossRef]
- Liu, X.B.; Li, W.X.; Zhao, X.D.; Liu, Y.C.; Nan, C.W.; Fan, L.Z. Two Birds with One Stone: Metal-Organic Framework Derived Micro-/Nanostructured Ni2P/Ni Hybrids Embedded in Porous Carbon for Electrocatalysis and Energy Storage. Adv. Funct. Mater. 2019, 29, 9. [Google Scholar] [CrossRef]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-Organic Frameworks: A Rapidly Growing Class of Versatile Nanoporous Materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Pramanik, A.; Manju, U.; Mahanty, S. Reversible Lithium Storage in Manganese 1,3,5-Benzenetricarboxylate Metal–Organic Framework with High Capacity and Rate Performance. ACS Appl. Mater. Interfaces 2015, 7, 16357–16363. [Google Scholar] [CrossRef]
- Anonymous. Materials: Supercapacitor made from MOF. Nature 2016, 538, 143. [Google Scholar] [CrossRef]
- An, T.; Wang, Y.; Tang, J.; Zhang, L.; Zheng, G. A flexible ligand-based wavy layered metal–organic framework for lithium-ion storage. J. Colloid Interface Sci. 2015, 445, 320–325. [Google Scholar] [CrossRef]
- Jia, Z.; Tan, Y.; Cui, Z.; Zhang, L.; Guo, X. Construction of NiCo2O4@graphene nanorods by tuning the compositional chemistry of metal–organic frameworks with enhanced lithium storage properties. J. Mater. Chem. A 2018, 6, 19604–19610. [Google Scholar] [CrossRef]
- Li, Z.; Huang, X.; Sun, C.; Chen, X.; Hu, J.; Stein, A.; Tang, B. Thin-film electrode based on zeolitic imidazolate frameworks (ZIF-8 and ZIF-67) with ultra-stable performance as a lithium-ion battery anode. J. Mater. Sci. 2017, 52, 3979–3991. [Google Scholar] [CrossRef]
- Xu, C.; Ma, C.; Kong, X.; Taya, M. Vacuum filling process for electrolyte in enhancing electrochromic polymer window assembly. Polym. Adv. Technol. 2009, 20, 178–182. [Google Scholar] [CrossRef]
- Isfahani, V.B.; Memarian, N.; Dizaji, H.R.; Arab, A.; Silva, M. The physical and electrochromic properties of Prussian Blue thin films electrodeposited on ITO electrodes. Electrochim. Acta 2019, 304, 282–291. [Google Scholar] [CrossRef]
- Azizian-Kalandaragh, Y.; Nouhi, S.; Amiri, M. Effect of post-annealing treatment on the wetting, optical and structural properties of Ag/Indium tin oxide thin films prepared by electron beam evaporation technique. Mater. Express 2015, 5, 137–145. [Google Scholar] [CrossRef]
- Gross, A.F.; Sherman, E.; Vajo, J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalton Trans. 2012, 41, 5458–5460. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Shi, Y.; Zhang, M.; Fan, S.; Yin, Z.; Qin, M.; Lian, T.; Li, X. Rational design of cobalt and nitrogen co-doped carbon hollow frameworks for efficient photocatalytic degradation of gaseous toluene. J. Colloid Interface Sci. 2018, 528, 45–52. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Yan, W.; Liu, X.; Yang, X.; Miao, S.; Wang, W.; Wang, A.; Zhang, T. Single-atom dispersed Co–N–C catalyst: Structure identification and performance for hydrogenative coupling of nitroarenes. Chem. Sci. 2016, 7, 5758–5764. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, H.; Jiang, X.; Gui, R. Assembly of Black Phosphorus Nanosheets and MOF to Form Functional Hybrid Thin-Film for Precise Protein Capture, Dual-Signal and Intrinsic Self-Calibration Sensing of Specific Cancer-Derived Exosomes. Anal. Chem. 2020, 92, 2866–2875. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Y.; Han, C.; Chen, J.; He, Z.; Zhu, J.; Dai, L.; Meng, W.; Wang, L. Application of porous biomass carbon materials in vanadium redox flow battery. J. Colloid Interface Sci. 2020, 566, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Lo, W.C.; Genc, A.; Lebeau, J.; Augustyn, V. Transition from Battery to Pseudocapacitor Behavior via Structural Water in Tungsten Oxide. Chem. Mater. 2017, 29, 3928–3937. [Google Scholar] [CrossRef]
- Li, K.; Shao, Y.; Liu, S.; Zhang, Q.; Wang, H.; Li, Y.; Kaner, R.B. Aluminum-Ion-Intercalation Supercapacitors with Ultrahigh Areal Capacitance and Highly Enhanced Cycling Stability: Power Supply for Flexible Electrochromic Devices. Small 2017, 13, 13. [Google Scholar] [CrossRef]
- Solomon, G.; Mazzaro, R.; You, S.; Natile, M.M.; Morandi, V.; Concina, I.; Vomiero, A. Ag2S/MoS2 Nanocomposites Anchored on Reduced Graphene Oxide: Fast Interfacial Charge Transfer for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 22380–22389. [Google Scholar] [CrossRef]
- Nobili, F.; Tossici, R.; Marassi, R.; Croce, F.; Scrosati, B. An AC Impedance Spectroscopic Study of LixCoO2 at Different Temperatures. J. Phys. Chem. B 2002, 106, 3909–3915. [Google Scholar] [CrossRef]
- Kim, M.; Oh, I. Superior electric double layer capacitors using micro- and mesoporous silicon carbide sphere. J. Mater. Chem. A 2015, 3, 3944–3951. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Ai, L.; Jiang, J. Mechanistic insight into the interaction and adsorption of Cr(VI) with zeolitic imidazolate framework-67 microcrystals from aqueous solution. Chem. Eng. J. 2015, 274, 238–246. [Google Scholar] [CrossRef]
- Yan, C.; Kang, W.; Wang, J.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Stretchable and Wearable Electrochromic Devices. ACS Nano 2014, 8, 316–322. [Google Scholar] [CrossRef]
- Guzel, M.; Karatas, E.; Ak, M. Synthesis and Fluorescence Properties of Carbazole Based Asymmetric Functionalized Star Shaped Polymer. J. Electrochem. Soc. 2016, 164, H49–H55. [Google Scholar] [CrossRef]
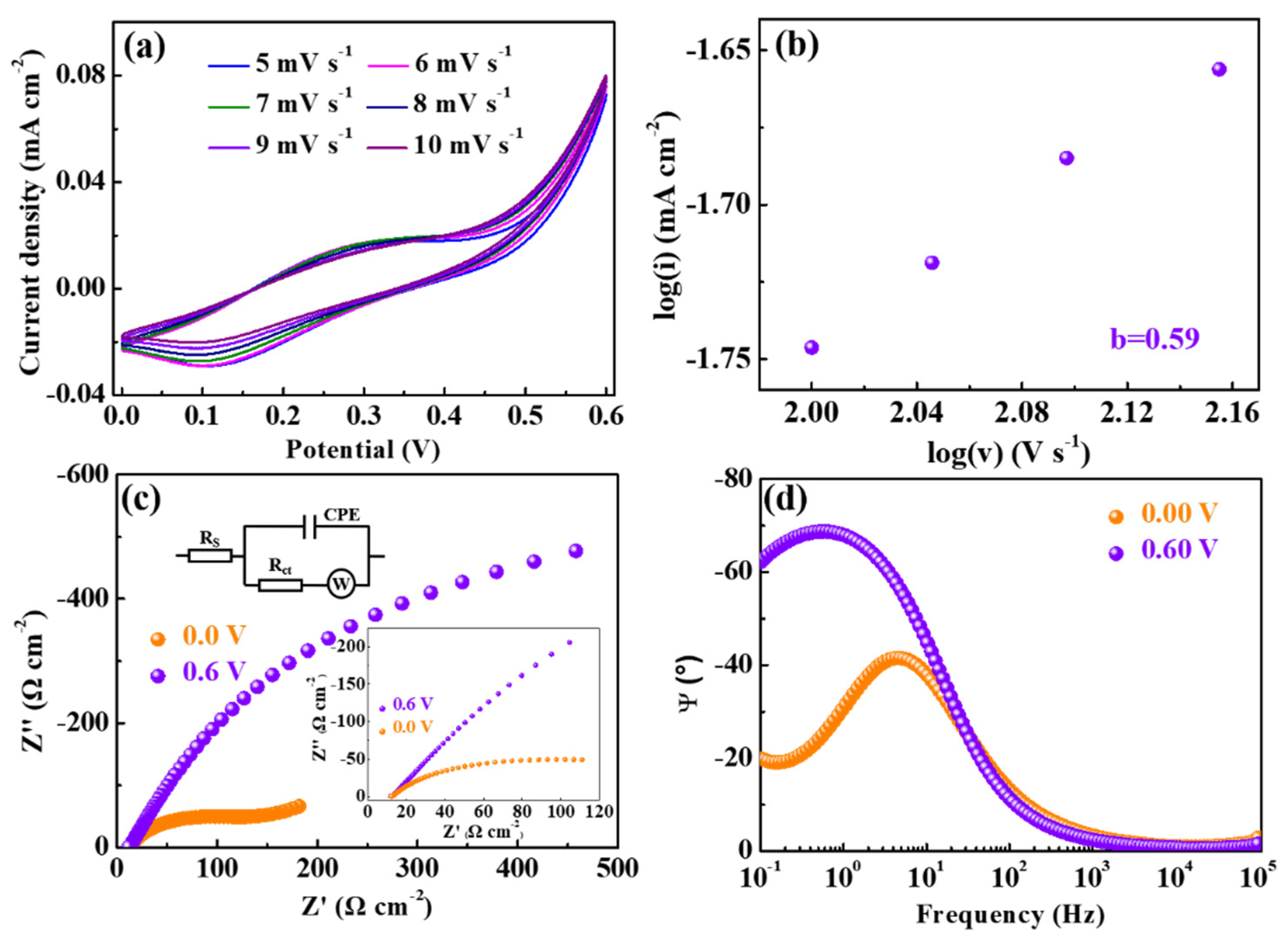
| Applied Potentials (V) | Electrical Parameters | |
|---|---|---|
| Rs (Ω/cm2) | Rct (Ω/cm2) | |
| +0.0 | 15.07 | 82.81 |
| +0.6 | 14.47 | 6.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Tao, K.; Jiang, R.; Zhang, H.; Liang, L.; Gao, J.; Cao, H. A Self-Bleaching Electrochromic Mirror Based on Metal Organic Frameworks. Materials 2021, 14, 2771. https://doi.org/10.3390/ma14112771
Wang K, Tao K, Jiang R, Zhang H, Liang L, Gao J, Cao H. A Self-Bleaching Electrochromic Mirror Based on Metal Organic Frameworks. Materials. 2021; 14(11):2771. https://doi.org/10.3390/ma14112771
Chicago/Turabian StyleWang, Kun, Kai Tao, Ran Jiang, Hongliang Zhang, Lingyan Liang, Junhua Gao, and Hongtao Cao. 2021. "A Self-Bleaching Electrochromic Mirror Based on Metal Organic Frameworks" Materials 14, no. 11: 2771. https://doi.org/10.3390/ma14112771
APA StyleWang, K., Tao, K., Jiang, R., Zhang, H., Liang, L., Gao, J., & Cao, H. (2021). A Self-Bleaching Electrochromic Mirror Based on Metal Organic Frameworks. Materials, 14(11), 2771. https://doi.org/10.3390/ma14112771






