Anodic Electrodeposition of Chitosan–AgNP Composites Using In Situ Coordination with Copper Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
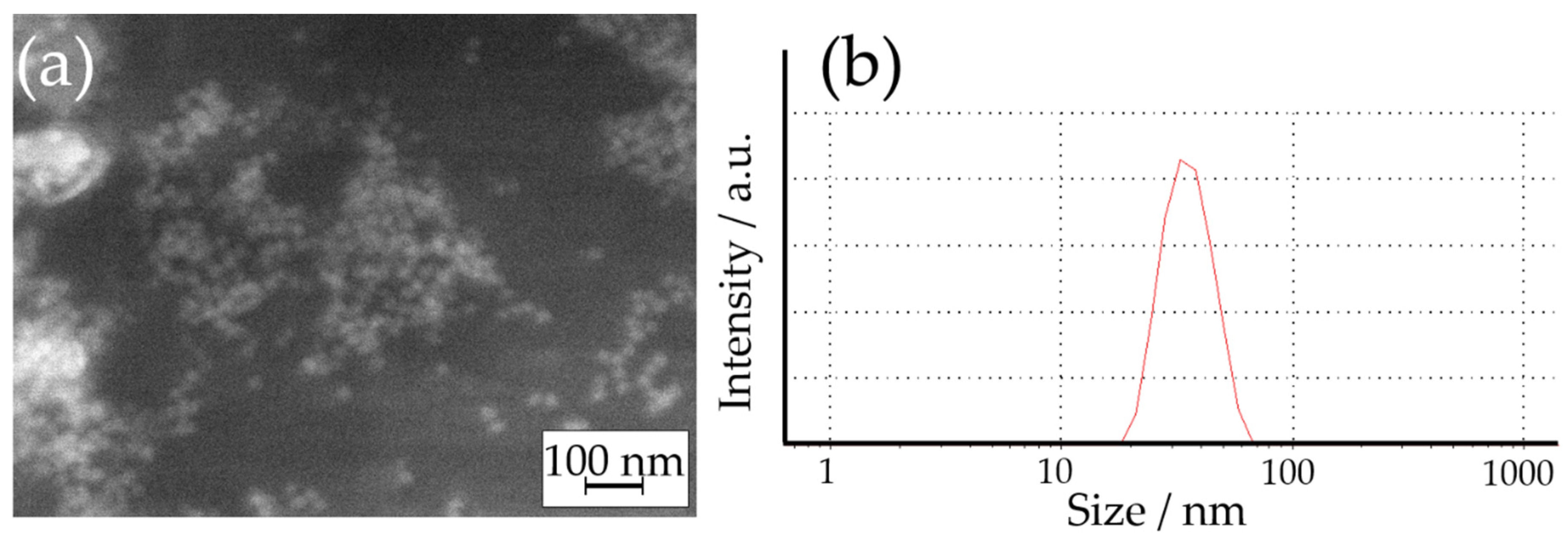
2.2. Synthesis of AgNPs
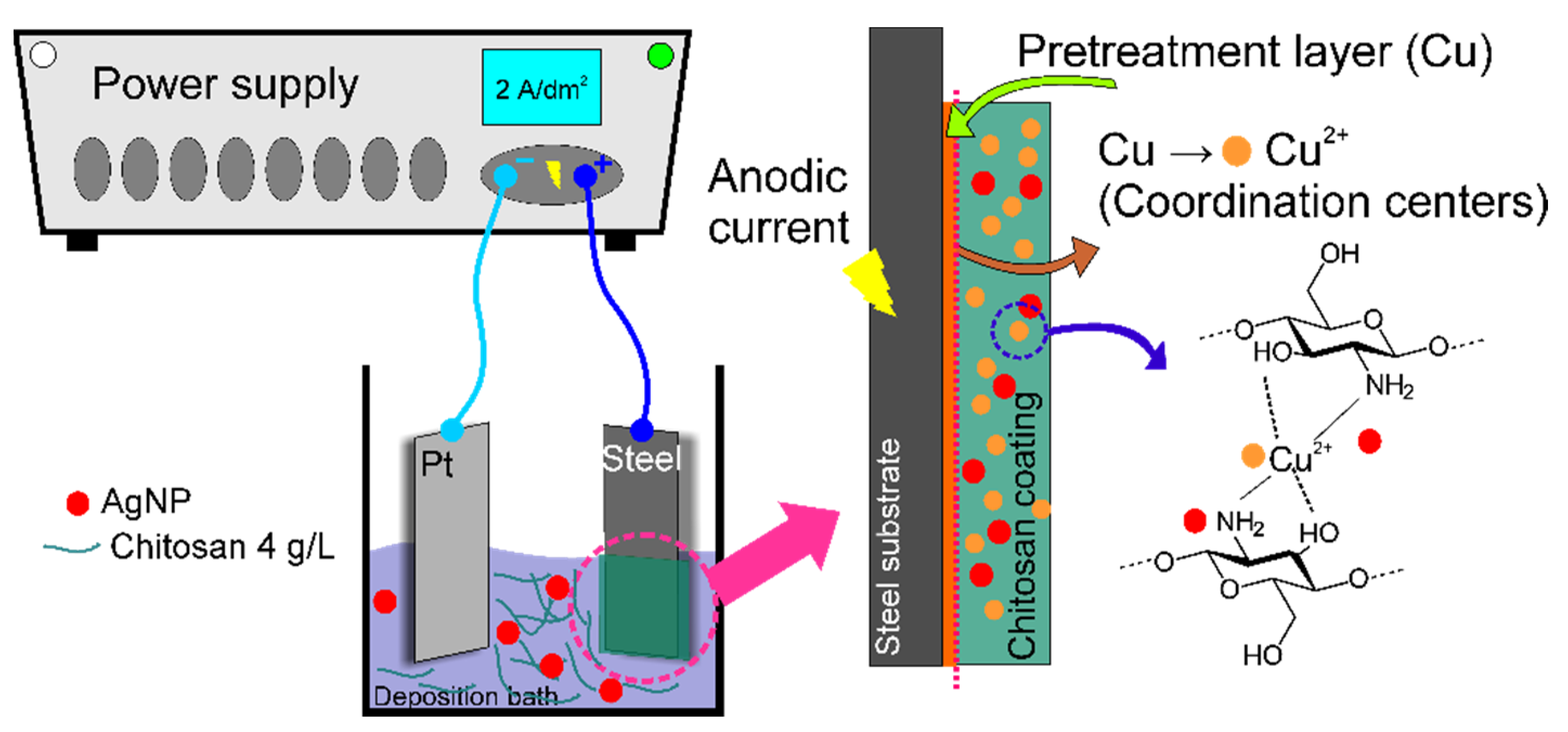
2.3. Electrodeposition of CS and CS-Based Coatings
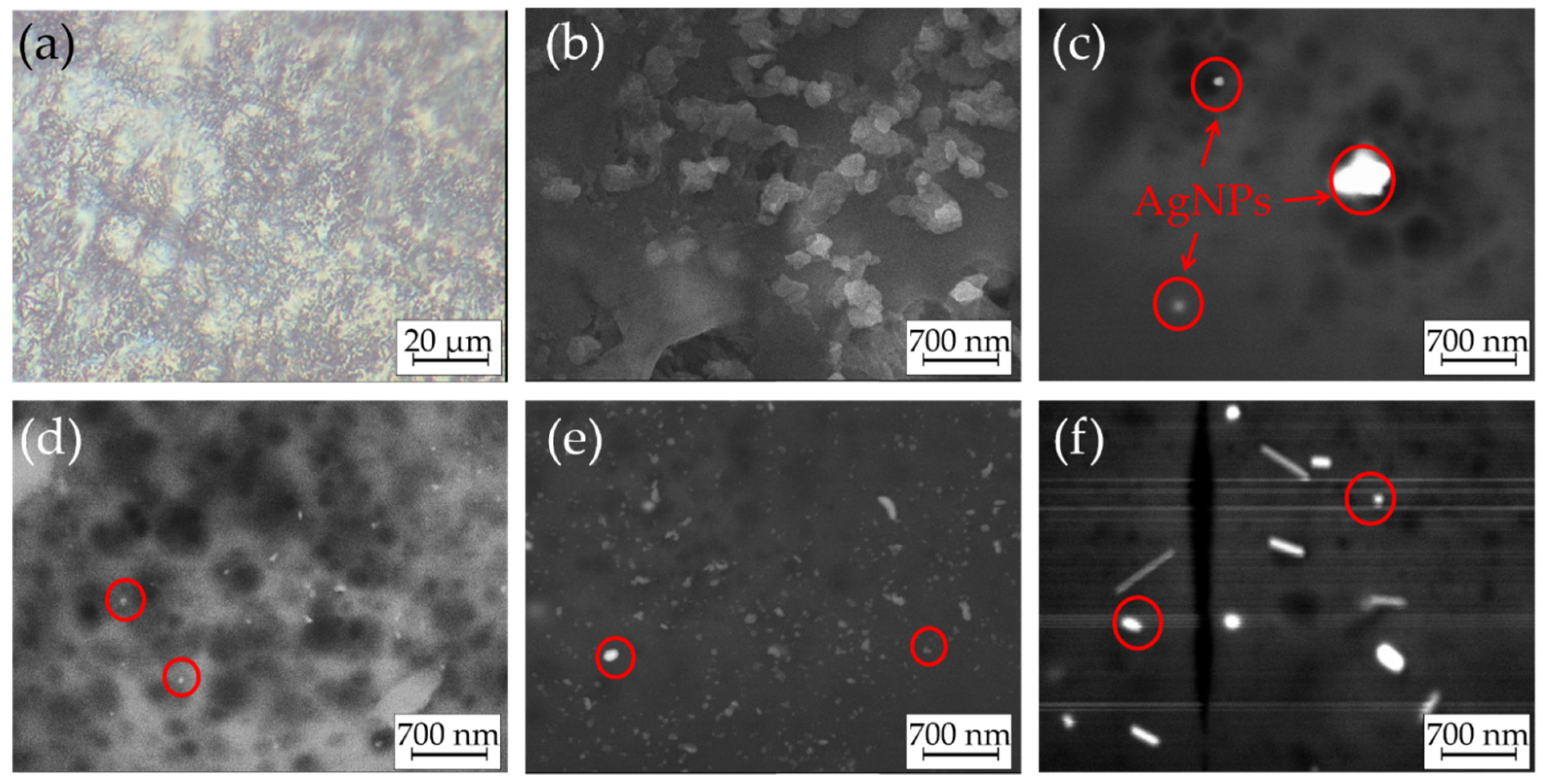
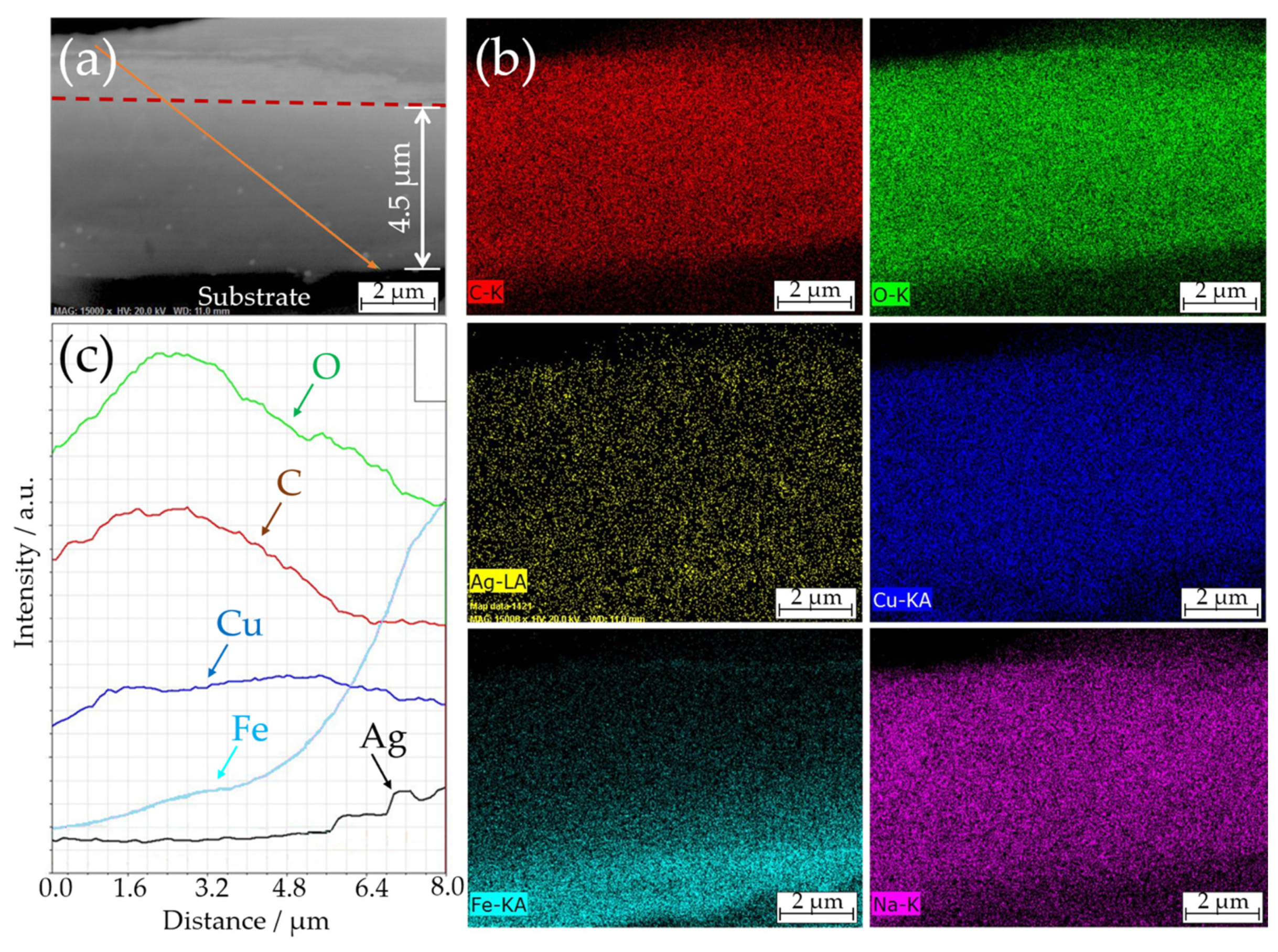
2.4. Coatings Characterization
2.5. Antibacterial Properties
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanpichai, S.; Witayakran, S.; Wootthikanokkhan, J.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Mechanical and antibacterial properties of the chitosan coated cellulose paper for packaging applications: Effects of molecular weight types and concentrations of chitosan. Int. J. Biol. Macromol. 2020, 155, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Saleh, A.S.; Elsharma, E.M.; Metwally, E.; Siyam, T. Chitosan-g-maleic acid for effective removal of copper and nickel ions from their solutions. Int. J. Biol. Macromol. 2019, 121, 1287–1294. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Y.; Zhang, Y.; Yu, C.; Cao, S. Physicochemical, mechanical and thermal properties of chitosan films with and without sorbitol. Int. J. Biol. Macromol. 2014, 70, 340–346. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Vieira, R.P.; Jamróz, E.; Anjos, C.A.R. Furcellaran: An innovative biopolymer in the production of films and coatings. Carbohydr. Polym. 2021, 252, 117221. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, J.; Ge, S.; Jiang, C.; Guo, T.; Peng, T.; Huang, T.; Xie, L. Biocompatible, hydrophobic and resilience graphene/chitosan composite aerogel for efficient oil−water separation. Surf. Coat. Technol. 2020, 385, 125361. [Google Scholar] [CrossRef]
- Li, W.W.; Wang, H.Y.; Zhang, Y.Q. A novel chitosan hydrogel membrane by an improved electrophoretic deposition and its characteristics in vitro and in vivo. Mater. Sci. Eng. C 2017, 74, 287–297. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Functional Chitosan; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.J.R.; Butt, J. Chitosan films are NOT antimicrobial. Biotechnol. Lett. 2011, 33, 417–421. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Rozmysłowska-Wojciechowska, A.; Karwowska, E.; Gloc, M.; Woźniak, J.; Petrus, M.; Przybyszewski, B.; Wojciechowski, T.; Jastrzębska, A.M. Controlling the Porosity and Biocidal Properties of the Chitosan-Hyaluronate Matrix Hydrogel Nanocomposites by the Addition of 2D Ti3C2Tx MXene. Materials 2020, 13, 4587. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Baruzzi, F.; de Candia, S.; Giangregorio, M.M.; Giannossa, L.C.; Dicarlo, M.; Mattioli-Belmonte, M.; Sabbatini, L.; De Giglio, E. Silver-loaded chitosan coating as an integrated approach to face titanium implant-associated infections: Analytical characterization and biological activity. Anal. Bioanal. Chem. 2017, 409, 7211–7221. [Google Scholar] [CrossRef] [PubMed]
- Titov, V.; Nikitin, D.; Naumova, I.; Losev, N.; Lipatova, I.; Kosterin, D.; Pleskunov, P.; Perekrestov, R.; Sirotkin, N.; Khlyustova, A.; et al. Dual-Mode Solution Plasma Processing for the Production of Chitosan/Ag Composites with the Antibacterial Effect. Materials 2020, 13, 4821. [Google Scholar] [CrossRef] [PubMed]
- Ciraldo, F.; Schnepf, K.; Goldmann, W.; Boccaccini, A. Development and Characterization of Bioactive Glass Containing Composite Coatings with Ion Releasing Function for Antibiotic-Free Antibacterial Surgical Sutures. Materials 2019, 12, 423. [Google Scholar] [CrossRef]
- Arjunan, N.; Singaravelu, C.M.; Kulanthaivel, J.; Kandasamy, J. A potential photocatalytic, antimicrobial and anticancer activity of chitosan-copper nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1774–1782. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Sanpui, P.; Murugadoss, A.; Prasad, P.; Ghosh, S.; Chattopadhyay, A. The antibacterial properties of a novel chitosan–Ag-nanoparticle composite. Int. J. Food Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Jung, J.; Kim, D.; Seo, J. Microwave assisted antibacterial chitosan–silver nanocomposite films. Int. J. Biol. Macromol. 2016, 84, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Virgili, A.H.; Laranja, D.C.; Malheiros, P.S.; Pereira, M.B.; Costa, T.M.H.; de Menezes, E.W. Nanocomposite film with antimicrobial activity based on gold nanoparticles, chitosan and aminopropylsilane. Surf. Coat. Technol. 2021, 127086. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Ilyas, K.; Dlouhý, I.; Siska, F.; Boccaccini, A.R. Electrophoretic Deposition of Copper(II)–Chitosan Complexes for Antibacterial Coatings. Int. J. Mol. Sci. 2020, 21, 2637. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Bentley, W.E.; Payne, G.F. Biofabrication to build the biology-device interface. Biofabrication 2010, 2, 022002. [Google Scholar] [CrossRef] [PubMed]
- Saleem, O.; Wahaj, M.; Akhtar, M.A.; Ur Rehman, M.A. Fabrication and Characterization of Ag–Sr-Substituted Hydroxyapatite/Chitosan Coatings Deposited via Electrophoretic Deposition: A Design of Experiment Study. ACS Omega 2020, 5, 22984–22992. [Google Scholar] [CrossRef] [PubMed]
- Nawrotek, K.; Grams, J. Understanding Electrodeposition of Chitosan–Hydroxyapatite Structures for Regeneration of Tubular-Shaped Tissues and Organs. Materials 2021, 14, 1288. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Wang, X.; Guo, X.; Zhang, Z.; Chen, Y.; Wang, Y. Electrodeposition of chitosan based on coordination with metal ions: In situ-generated by electrochemical oxidation. J. Mater. Chem. B 2016, 4, 3331–3338. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic deposition of hydroxyapatite–chitosan nanocomposite coatings in different alcohols. Surf. Coat. Technol. 2013, 216, 106–114. [Google Scholar] [CrossRef]
- Blanda, G.; Brucato, V.; Carfì, F.; Conoscenti, G.; La Carrubba, V.; Piazza, S.; Sunseri, C.; Inguanta, R. Chitosan-Coating Deposition via Galvanic Coupling. ACS Biomater. Sci. Eng. 2019, 5, 1715–1724. [Google Scholar] [CrossRef]
- Gamage, A.; Shahidi, F. Use of chitosan for the removal of metal ion contaminants and proteins from water. Food Chem. 2007, 104, 989–996. [Google Scholar] [CrossRef]
- Gritsch, L.; Maqbool, M.; Mouriño, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: Copper and strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Sun, C.; Li, K.; Guan, F.; Liu, X.; Duan, J.; Hou, B. Synthesis and characterization of chitosan–zinc composite electrodeposits with enhanced antibacterial properties. RSC Adv. 2016, 6, 46081–46088. [Google Scholar] [CrossRef]
- Santini, E.; Jarek, E.; Ravera, F.; Liggieri, L.; Warszynski, P.; Krzan, M. Surface properties and foamability of saponin and saponin-chitosan systems. Colloids Surf. B Biointerfaces 2019, 181, 198–206. [Google Scholar] [CrossRef]
- Kasach, A.A.; Kharitonov, D.S.; Makarova, I.V.; Wrzesińska, A.; Zharskii, I.M.; Kurilo, I.I. Effect of thiourea on electrocrystallization of Cu–Sn alloys from sulphate electrolytes. Surf. Coat. Technol. 2020, 399, 126137. [Google Scholar] [CrossRef]
- Mofidfar, M.; Kim, E.S.; Larkin, E.L.; Long, L.; Jennings, W.D.; Ahadian, S.; Ghannoum, M.A.; Wnek, G.E. Antimicrobial Activity of Silver Containing Crosslinked Poly(Acrylic Acid) Fibers. Micromachines 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Kruk, T.; Gołda-Cępa, M.; Szczepanowicz, K.; Szyk-Warszyńska, L.; Brzychczy-Włoch, M.; Kotarba, A.; Warszyński, P. Nanocomposite multifunctional polyelectrolyte thin films with copper nanoparticles as the antimicrobial coatings. Colloids Surf. B Biointerfaces 2019, 181, 112–118. [Google Scholar] [CrossRef]
- Oliveira, J.A.M.; de Santana, R.A.C.; de OliveiraWanderley Neto, A. Electrophoretic deposition and characterization of chitosan-molybdenum composite coatings. Carbohydr. Polym. 2021, 255. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.A.M.; de Santana, R.A.C.; de OliveiraWanderley Neto, A. Characterization of the chitosan-tungsten composite coating obtained by electrophoretic deposition. Prog. Org. Coat. 2020, 143, 105631. [Google Scholar] [CrossRef]
- Gebhardt, F.; Seuss, S.; Turhan, M.C.; Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Characterization of electrophoretic chitosan coatings on stainless steel. Mater. Lett. 2012, 66, 302–304. [Google Scholar] [CrossRef]
- Cordero-Arias, L.; Cabanas-Polo, S.; Gao, H.; Gilabert, J.; Sanchez, E.; Roether, J.A.; Schubert, D.W.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of nanostructured-TiO2/chitosan composite coatings on stainless steel. RSC Adv. 2013, 3, 11247. [Google Scholar] [CrossRef]
- Sun, F.; Pang, X.; Zhitomirsky, I. Electrophoretic Deposition of Composite Hydroxyapatite–Chitosan–Heparin Coatings. J. Mater. Process. Technol. 2009, 209, 1597–1606. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A. Contribution to the study of the complexation of copper by chitosan and oligomers. Polymer 2002, 43, 1267–1276. [Google Scholar] [CrossRef]
- Vold, I.M.N.; Vårum, K.M.; Guibal, E.; Smidsrød, O. Binding of ions to chitosan—selectivity studies. Carbohydr. Polym. 2003, 54, 471–477. [Google Scholar] [CrossRef]
- Bhattarai, N.; Khanal, S.; Pudasaini, P.R.; Pahl, S.; Romero-Urbina, D. Citrate Stabilized Silver Nanoparticles. Int. J. Nanotechnol. Mol. Comput. 2011, 3, 15–28. [Google Scholar] [CrossRef]
- Ryan, C.; Alcock, E.; Buttimer, F.; Schmidt, M.; Clarke, D.; Pemble, M.; Bardosova, M. Synthesis and Characterisation of Cross-Linked Chitosan Composites Functionalised with Silver and Gold Nanoparticles for Antimicrobial Applications. Sci. Technol. Adv. Mater. 2017, 18, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and Antibacterial Activity of Silver Nanoparticles against Gram-Positive and Gram-Negative Bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Miller, C.J.; Waite, T.D. Fenton-like zero-valent silver nanoparticle-mediated hydroxyl radical production. J. Catal. 2014, 317, 198–205. [Google Scholar] [CrossRef]

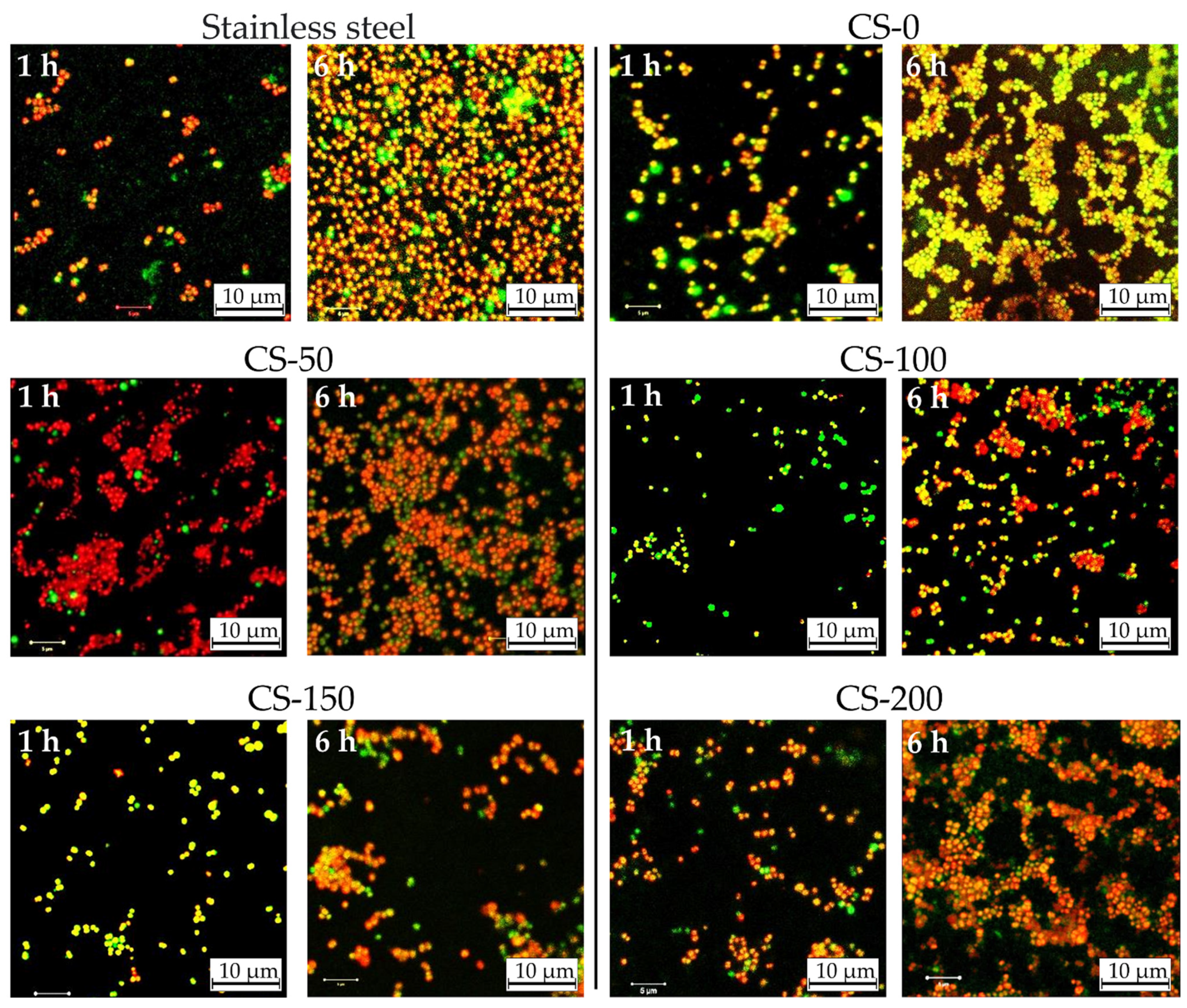
| Sample | Average Dead-to-Live Cells Ratio | Surface Coverage by Biofilm, % | ||
|---|---|---|---|---|
| 1 h | 6 h | 1 h | 6 h | |
| AISI 304 Steel | 7.4 | 25.0 | 7 | 65 |
| CS-0 | 5.8 | 15.3 | 14 | 72 |
| CS-50 | 6.3 | 14.7 | 20 | 67 |
| CS-100 | 9.3 | 6.0 | 12 | 35 |
| CS-150 | 41.0 | 3.5 | 11 | 37 |
| CS-200 | 10.4 | 198.0 | 20 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharitonov, D.S.; Kasach, A.A.; Gibala, A.; Zimowska, M.; Kurilo, I.I.; Wrzesińska, A.; Szyk-Warszyńska, L.; Warszyński, P. Anodic Electrodeposition of Chitosan–AgNP Composites Using In Situ Coordination with Copper Ions. Materials 2021, 14, 2754. https://doi.org/10.3390/ma14112754
Kharitonov DS, Kasach AA, Gibala A, Zimowska M, Kurilo II, Wrzesińska A, Szyk-Warszyńska L, Warszyński P. Anodic Electrodeposition of Chitosan–AgNP Composites Using In Situ Coordination with Copper Ions. Materials. 2021; 14(11):2754. https://doi.org/10.3390/ma14112754
Chicago/Turabian StyleKharitonov, Dmitry S., Aliaksandr A. Kasach, Agnieszka Gibala, Małgorzata Zimowska, Irina I. Kurilo, Angelika Wrzesińska, Lilianna Szyk-Warszyńska, and Piotr Warszyński. 2021. "Anodic Electrodeposition of Chitosan–AgNP Composites Using In Situ Coordination with Copper Ions" Materials 14, no. 11: 2754. https://doi.org/10.3390/ma14112754
APA StyleKharitonov, D. S., Kasach, A. A., Gibala, A., Zimowska, M., Kurilo, I. I., Wrzesińska, A., Szyk-Warszyńska, L., & Warszyński, P. (2021). Anodic Electrodeposition of Chitosan–AgNP Composites Using In Situ Coordination with Copper Ions. Materials, 14(11), 2754. https://doi.org/10.3390/ma14112754









