Nanostructured Surface Finishing and Coatings: Functional Properties and Applications
Abstract
:1. Introduction
2. Advance in Halochromic Smart Textiles
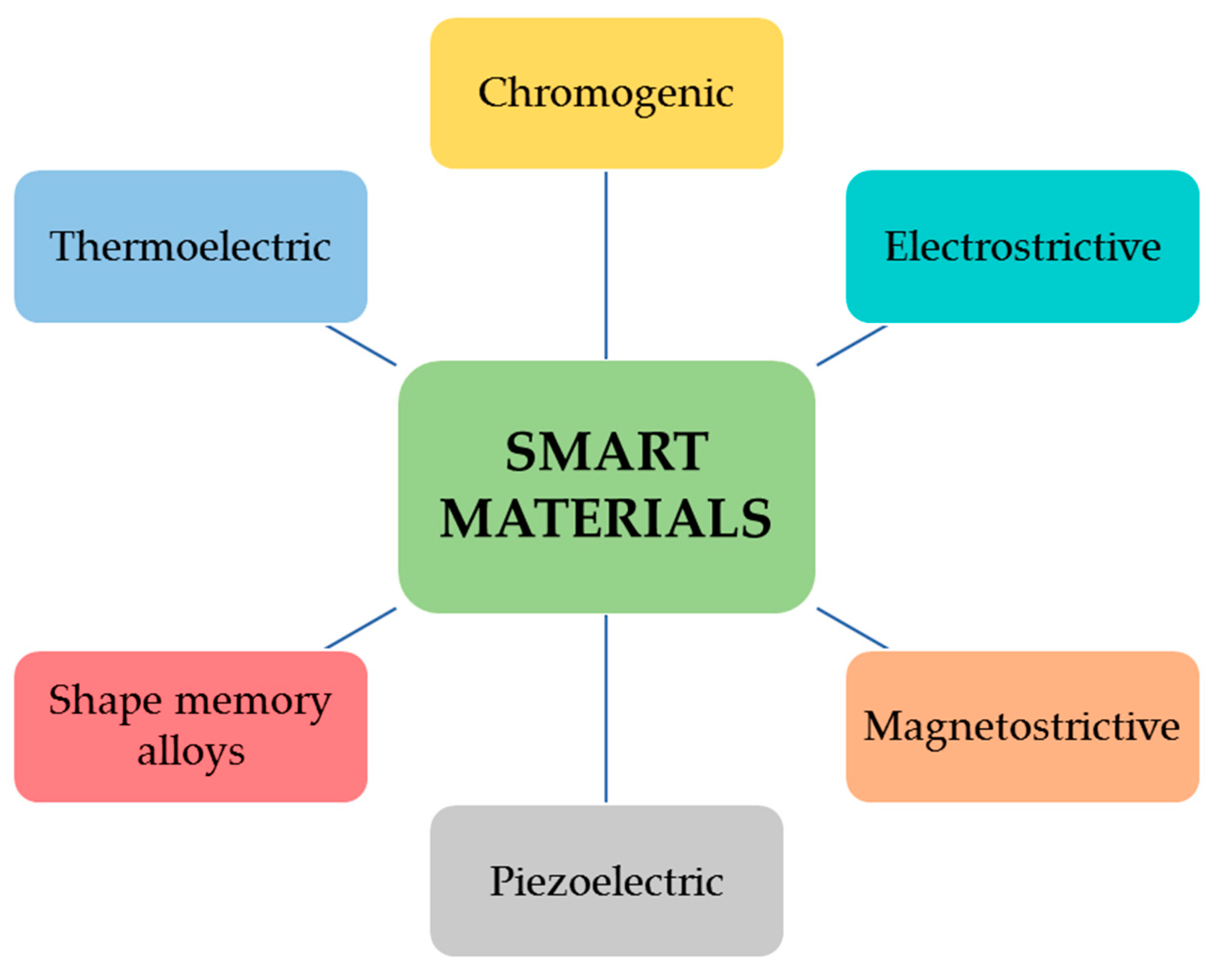
2.1. Smart Materials: An Introduction
2.2. Textile-Based Smart Materials
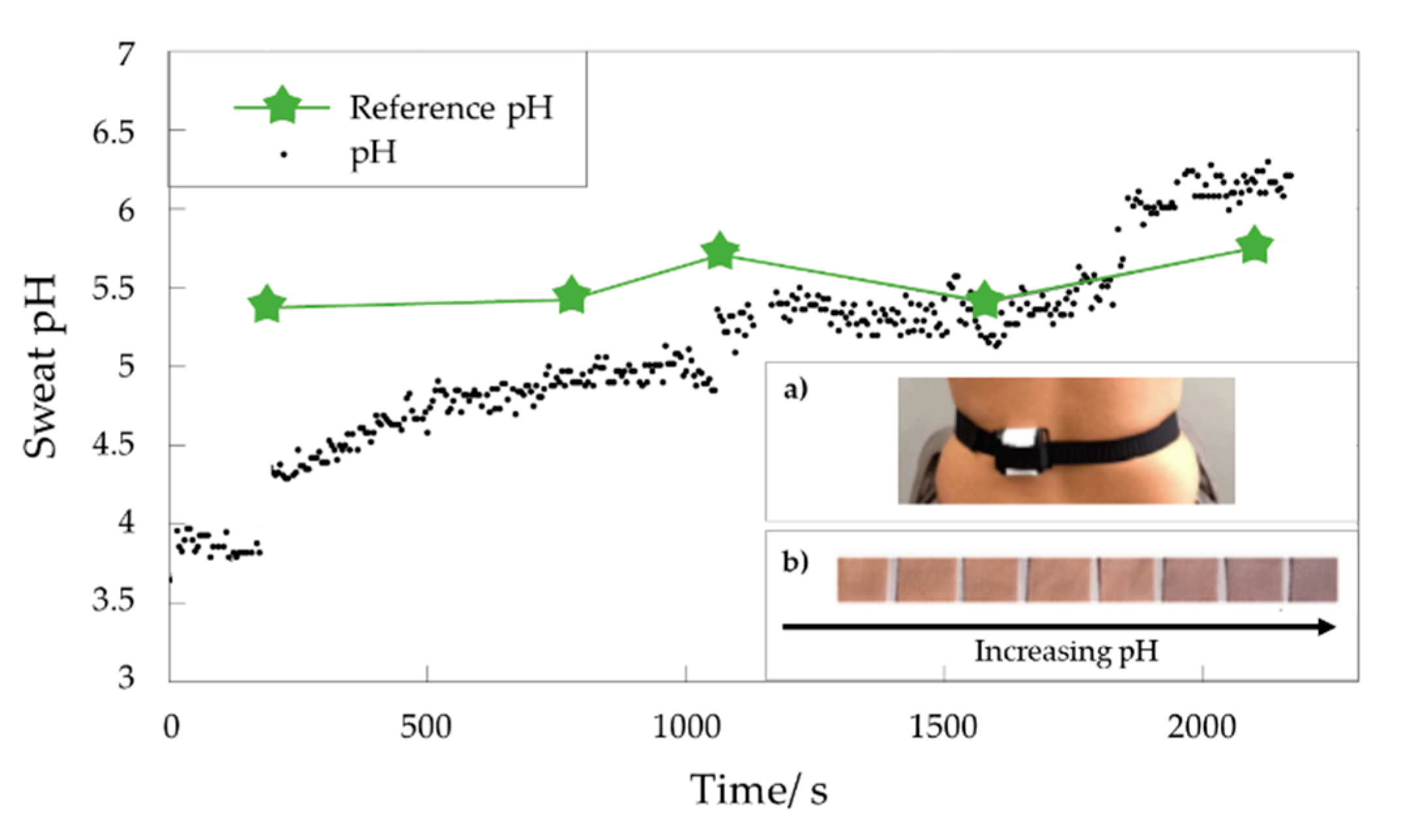
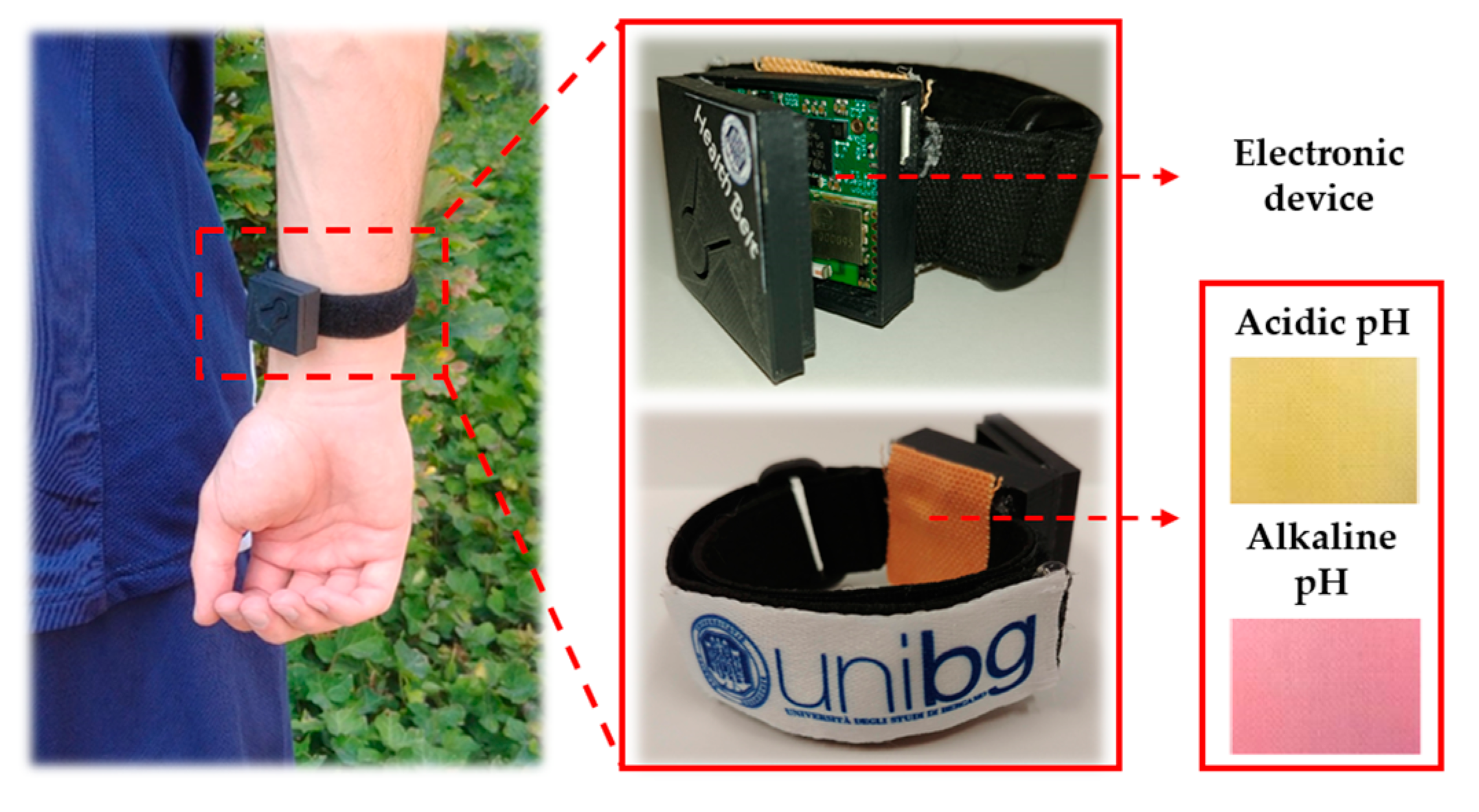
2.3. Textile-Based Optical pH Sensors
2.3.1. Development Methods of Halochromic Coatings for Optical Textile-Based pH Sensors
Electrospinning
Grafting Polymerization
Sol–Gel Technique
3. Drug-Delivery/Release Nanosystems
3.1. Functional Coating for Drug-Delivery Nanosystems: An Introduction
- constant release into the blood of quantities of therapeutic compounds, avoiding drug waste;
- repeatable and scheduled long-term release rates;
- reduction of side effects;
- personalized therapy;
- drug stabilization [59]
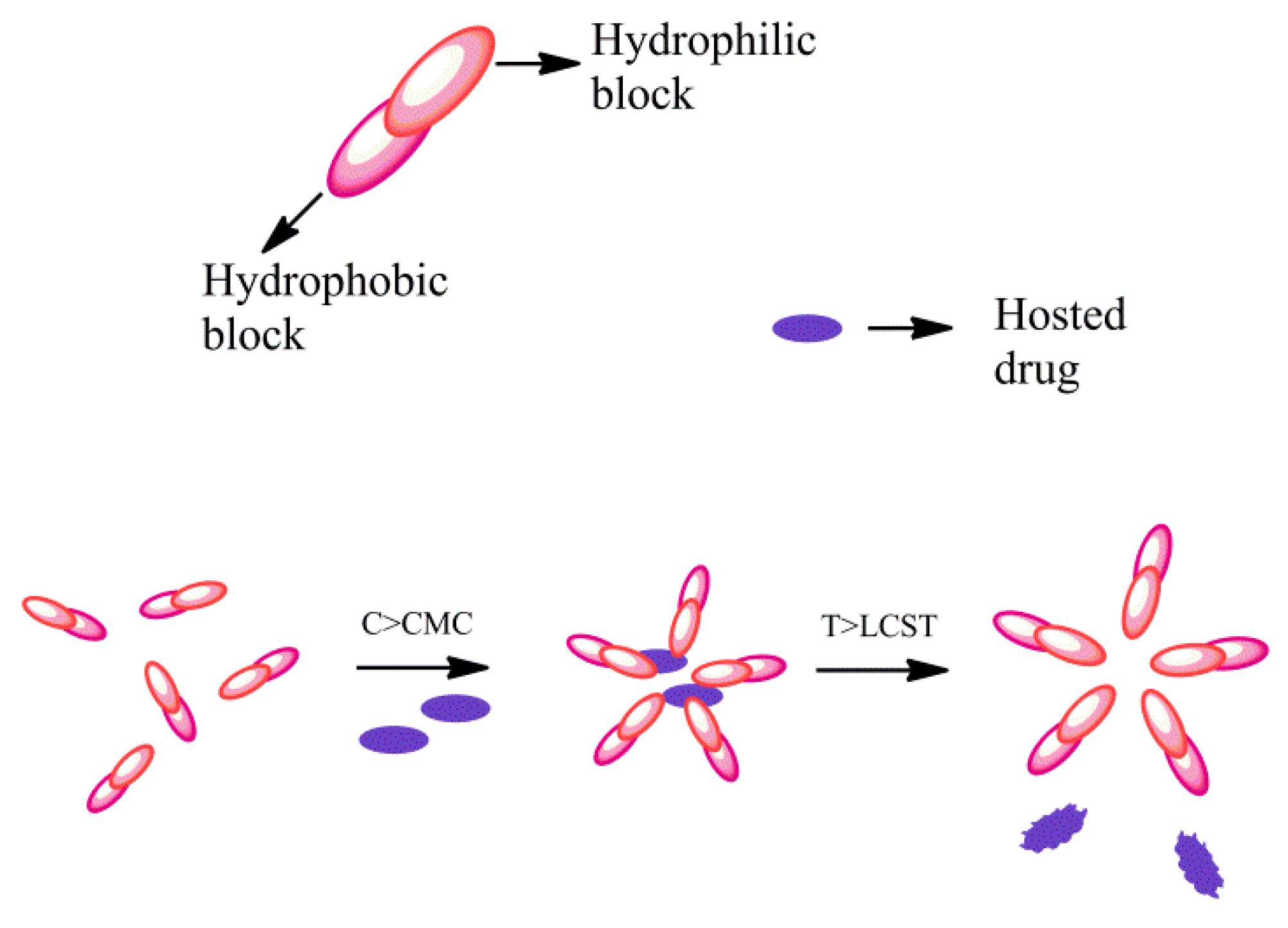
3.1.1. Self-Assembled Polymers for Nanocarriers
3.1.2. Functional or Coated Nanofillers
3.2. Smart Polymers for Drug Nanocarriers
3.2.1. Temperature-Sensitive Smart Polymers
3.2.2. Phase-Sensitive Smart Polymers
3.2.3. Light-Sensitive Smart Polymers
3.2.4. Biomolecule-Sensitive Smart Polymers
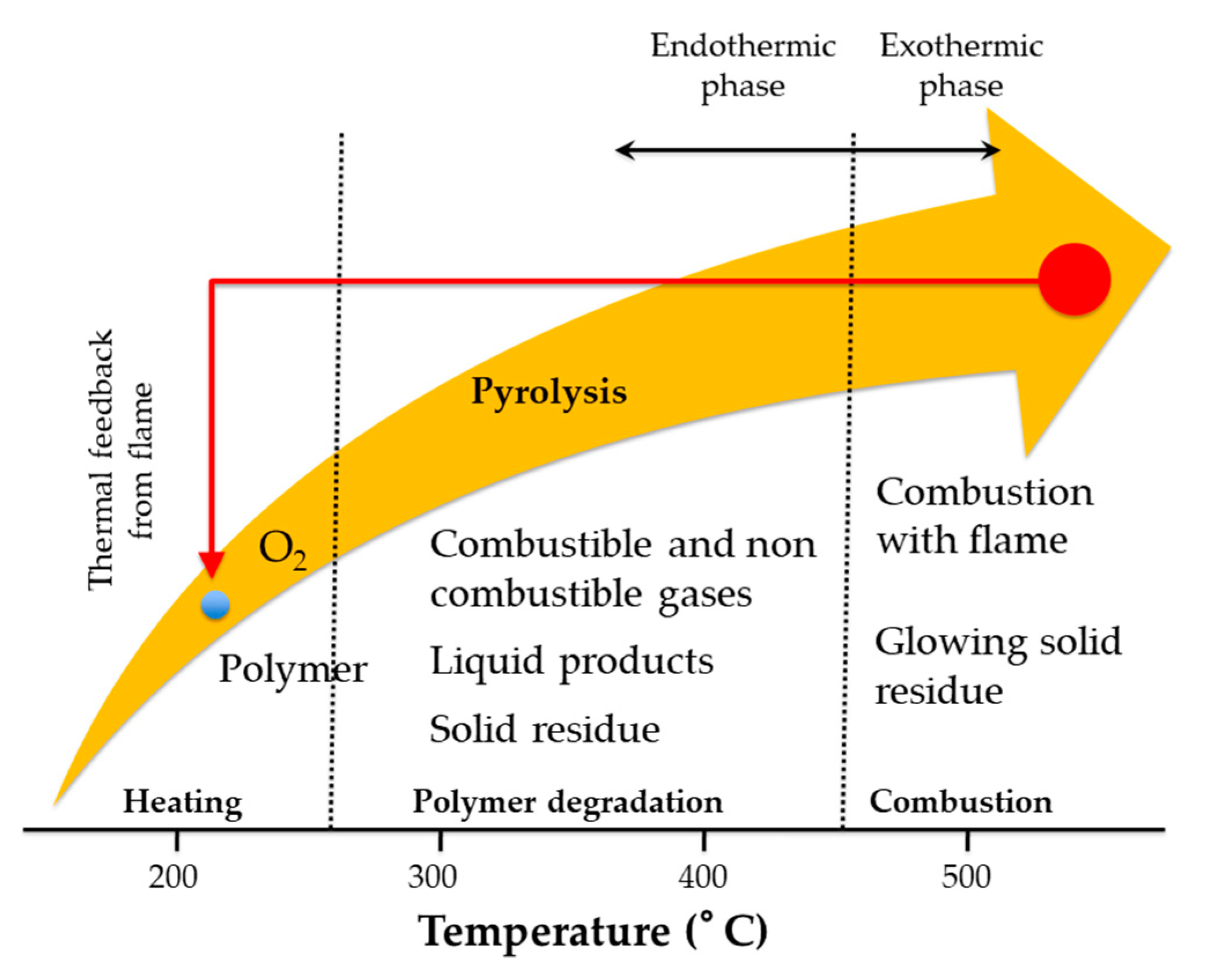
4. Flame-Retardant Coatings
- use of additives that release radicals [111];
- promote endothermic reactions;
- generate inert gases that dilute the concentration of the atmospheric oxygen;
- form a protecting impermeable coating.
- Inorganic flame retardants;
- Halogenated flame retardants;
- Phosphorus-based flame retardants, along with nitrogenous compounds due to their synergistic effect.
5. Industrial Coatings
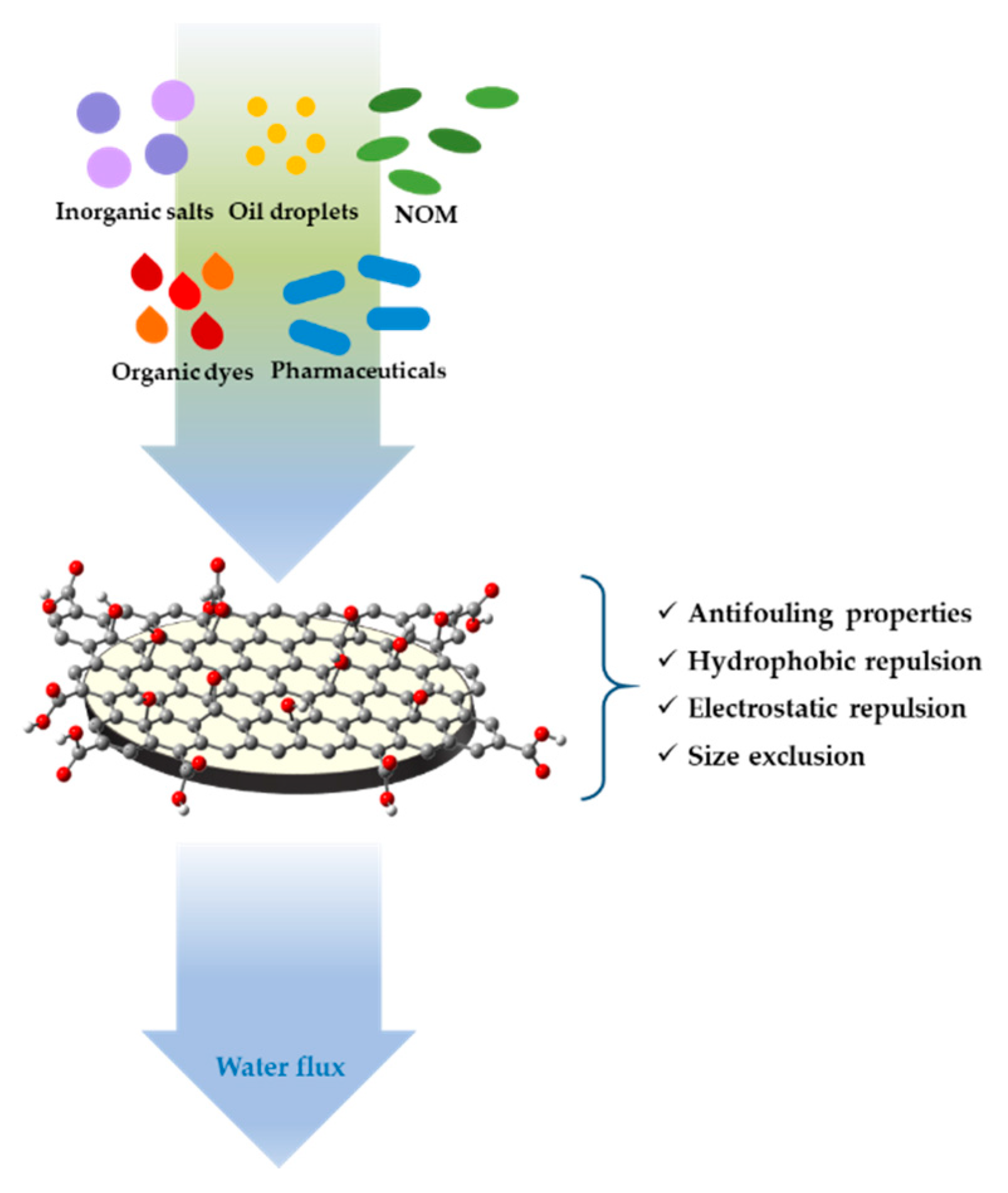
6. Nano/Ultrafiltration Membrane Coatings
7. Antifouling (AF) Coatings
- Adsorption of organic molecules such as proteins, polysaccharides and glycoproteins that form a conditioning film.
- Primary colonization with a settlement of microorganisms such as bacteria and diatoms, by creating a biofilm matrix.
- Secondary colonization consisting mainly of a biofilm of multicellular species (e.g., visible algae and invertebrates) generally called microfouling.
- Tertiary colonization including macrofoulants which consists of shelled invertebrates like barnacles, mollusks and sponges.
7.1. Tributyltin-Based Antifouling Solutions
7.2. Eco-Friendly Silica-Based AF Formulations
7.3. Nanostructured Biocide-Based AF Formulations
7.4. Hydrorepellent Coatings
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, J.; Schoenung, J.M. Nanostructured coatings. Mater. Sci. Eng. A 2002, 336, 274–319. [Google Scholar] [CrossRef]
- Schuh, C.A.; Nieh, T.G. Hardness and Abrasion Resistance of Nanocrystalline Nickel Alloys near the Hall-Petch Breakdown Regime. MRS Proc. 2002, 740, 18. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Patel, S.H.; Young, M.-Y.; Zunino, J.L.; Xanthos, M. Smart polymeric coatings—recent advances. Adv. Polym. Technol. 2007, 26, 1–13. [Google Scholar] [CrossRef]
- Mekhzoum, M.E.M.; el kacem Qaiss, A.; Bouhfid, R. 1-Introduction: Different types of smart materials and their practical applications. In Woodhead Publishing Series in Composites Science and Engineering; Bouhfid, R., el kacem Qaiss, A., Jawaid, M.B.T.-P.N.-B.S.M., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 1–19. [Google Scholar]
- Diamond, D.; Coyle, S.; Scarmagnani, S.; Hayes, J. Wireless Sensor Networks and Chemo-/Biosensing. Chem. Rev. 2008, 108, 652–679. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Kantiani, L.; Pérez, S.; Barceló, D. Sensors and biosensors in support of EU Directives. TrAC Trends Anal. Chem. 2009, 28, 170–185. [Google Scholar] [CrossRef]
- Baghdachi, J. Smart Coatings. ACS Symp. Ser. 2009, 3–24. [Google Scholar] [CrossRef]
- Ebara, M.; Kotsuchibashi, Y.; Narain, R.; Idota, N.; Kim, Y.-J.; Hoffman, J.M.; Uto, K.; Aoyagi, T. Smart Biomaterials; Springer: Tokyo, Japan, 2014. [Google Scholar]
- Bratek-Skicki, A. Towards a new class of stimuli-responsive polymer-based materials—Recent advances and challenges. Appl. Surf. Sci. Adv. 2021, 4, 100068. [Google Scholar] [CrossRef]
- Shyr, T.-W.; Shie, J.-W.; Jiang, C.-H.; Li, J.-J. A Textile-Based Wearable Sensing Device Designed for Monitoring the Flexion Angle of Elbow and Knee Movements. Sensors 2014, 14, 4050–4059. [Google Scholar] [CrossRef] [Green Version]
- Rosace, G.; Trovato, V.; Colleoni, C.; Caldara, M.; Re, V.; Brucale, M.; Piperopoulos, E.; Mastronardo, E.; Milone, C.; De Luca, G.; et al. Structural and morphological characterizations of MWCNTs hybrid coating onto cotton fabric as potential humidity and temperature wearable sensor. Sens. Actuators B Chem. 2017, 252, 428–439. [Google Scholar] [CrossRef]
- Trovato, V.; Teblum, E.; Kostikov, Y.; Pedrana, A.; Re, V.; Nessim, G.D.; Rosace, G. Sol-gel approach to incorporate millimeter-long carbon nanotubes into fabrics for the development of electrical-conductive textiles. Mater. Chem. Phys. 2020, 240, 122218. [Google Scholar] [CrossRef]
- Qian, Z.; Ginger, D.S. Reversibly Reconfigurable Colloidal Plasmonic Nanomaterials. J. Am. Chem. Soc. 2017, 139, 5266–5276. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Kim, A.; Huang, W.; Braun, P.V.; Li, X.; Chen, Q. Reconfigurable nanoscale soft materials. Curr. Opin. Solid State Mater. Sci. 2019, 23, 41–49. [Google Scholar] [CrossRef]
- Raeis-Hosseini, N.; Rho, J. Metasurfaces Based on Phase-Change Material as a Reconfigurable Platform for Multifunctional Devices. Materials 2017, 10, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, P.; Sarangan, A.M.; Agha, I. A Review of Germanium-Antimony-Telluride Phase Change Materials for Non-Volatile Memories and Optical Modulators. Appl. Sci. 2019, 9, 530. [Google Scholar] [CrossRef] [Green Version]
- Van Langenhove, L.; Hertleer, C. Smart clothing: A new life. Int. J. Cloth. Sci. Technol. 2004, 16, 63–72. [Google Scholar] [CrossRef]
- Persson, N.-K.; Martinez, J.G.; Zhong, Y.; Maziz, A.; Jager, E.W.H. Actuating Textiles: Next Generation of Smart Textiles. Adv. Mater. Technol. 2018, 3, 1700397. [Google Scholar] [CrossRef]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [Green Version]
- Harifi, T.; Montazer, M. Application of nanotechnology in sports clothing and flooring for enhanced sport activities, performance, efficiency and comfort: A review. J. Ind. Text. 2017, 46, 1147–1169. [Google Scholar] [CrossRef]
- Bamfield, P. Chromic Phenomena, Technological Applications of Color Chemistry; Royal Society of Chemistry: Cambridge, UK, 2001. [Google Scholar]
- Wencel, D.; Abel, T.; McDonagh, C. Optical Chemical pH Sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef]
- Patterson, M.J.; Galloway, S.D.R.; Nimmo, M.A. Variations in regional sweat composition in normal human males. Exp. Physiol. 2000, 85, 869–875. [Google Scholar] [CrossRef]
- Morgan, R.M.; Patterson, M.J.; Nimmo, M.A. Acute effects of dehydration on sweat composition in men during prolonged exercise in the heat. Acta Physiol. Scand. 2004, 182, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.L. Adhesion Measurement of Thin Films. Electrocompon. Sci. Technol. 1976, 3, 21–42. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K.; Brancatelli, G.; Rosace, G.; Van Damme, E.; De Vos, W. Novel cellulose and polyamide halochromic textile sensors based on the encapsulation of Methyl Red into a sol–gel matrix. Sens. Actuators B Chem. 2012, 162, 27–34. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Wang, N.; Burugapalli, K.; Song, W.; Halls, J.; Moussy, F.; Ray, A.; Zheng, Y. Electrospun fibro-porous polyurethane coatings for implantable glucose biosensors. Biomaterials 2013, 34, 888–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Schueren, L.; De Clerck, K. Halochromic Textile Materials as Innovative pH-Sensors. Adv. Sci. Technol. 2012, 80, 47–52. [Google Scholar] [CrossRef]
- Van der Schueren, L.; Hemelsoet, K.; Van Speybroeck, V.; De Clerck, K. The influence of a polyamide matrix on the halochromic behaviour of the pH-sensitive azo dye Nitrazine Yellow. Dye. Pigment. 2012, 94, 443–451. [Google Scholar] [CrossRef]
- Hong, K.H.; Liu, N.; Sun, G. UV-induced graft polymerization of acrylamide on cellulose by using immobilized benzophenone as a photo-initiator. Eur. Polym. J. 2009, 45, 2443–2449. [Google Scholar] [CrossRef]
- Monica, P.; Abdul, B.; Ada, F.; Roberta, B. Wettability and comfort of cellulosic materials modified by photo grafting of non-fluorinated oligomers. Cellulose 2016, 23, 1447–1458. [Google Scholar] [CrossRef]
- Kianfar, P.; Abate, M.T.; Trovato, V.; Rosace, G.; Ferri, A.; Bongiovanni, R.; Vitale, A. Surface Functionalization of Cotton Fabrics by Photo-Grafting for pH Sensing Applications. Front. Mater. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Trovato, V.; Vitale, A.; Bongiovanni, R.; Ferri, A.; Rosace, G.; Plutino, M.R. Development of a Nitrazine Yellow-glycidyl methacrylate coating onto cotton fabric through thermal-induced radical polymerization reactions: A simple approach towards wearable pH sensors applications. Cellulose 2021, 28, 1–22. [Google Scholar] [CrossRef]
- Rosace, G.; Colleoni, C.; Trovato, V.; Iacono, G.; Malucelli, G. Vinylphosphonic acid/methacrylamide system as a durable intumescent flame retardant for cotton fabric. Cellulose 2017, 24, 3095–3108. [Google Scholar] [CrossRef]
- Trovato, V.; Colleoni, C.; Castellano, A.; Plutino, M.R. The key role of 3-glycidoxypropyltrimethoxysilane sol–gel precursor in the development of wearable sensors for health monitoring. J. Sol Gel Sci. Technol. 2018, 87, 27–40. [Google Scholar] [CrossRef]
- Mahltig, B.; Haufe, H.; Böttcher, H. Functionalisation of textiles by inorganic sol–gel coatings. J. Mater. Chem. 2005, 15, 4385–4398. [Google Scholar] [CrossRef]
- Štular, D.; Simončič, B.; Tomšič, B. Stimuli-responsive Hydrogels for Textile Functionalisation: A Review. Tekstilec 2017, 60, 76–96. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Tarimala, S.; Dai, L.L. Cotton fabric surface modification for improved UV radiation protection using sol–gel process. J. Appl. Polym. Sci. 2007, 104, 111–117. [Google Scholar] [CrossRef]
- Mahltig, B.; Textor, T. Combination of silica sol and dyes on textiles. J. Sol Gel Sci. Technol. 2006, 39, 111–118. [Google Scholar] [CrossRef]
- Poli, R.; Colleoni, C.; Calvimontes, A.; Polášková, H.; Dutschk, V.; Rosace, G. Innovative sol–gel route in neutral hydroalcoholic condition to obtain antibacterial cotton finishing by zinc precursor. J. Sol Gel Sci. Technol. 2014, 74, 151–160. [Google Scholar] [CrossRef]
- Colleoni, C.; Guido, E.; Migani, V.; Rosace, G. Hydrophobic behaviour of non-fluorinated sol–gel based cotton and polyester fabric coatings. J. Ind. Text. 2015, 44, 815–834. [Google Scholar] [CrossRef]
- Colleoni, C.; Massafra, M.; Rosace, G. Photocatalytic properties and optical characterization of cotton fabric coated via sol–gel with non-crystalline TiO2 modified with poly(ethylene glycol). Surf. Coat. Technol. 2012, 207, 79–88. [Google Scholar] [CrossRef]
- Vasiljević, J.; Hadžić, S.; Jerman, I.; Černe, L.; Tomšič, B.; Medved, J.; Godec, M.; Orel, B.; Simončič, B. Study of flame-retardant finishing of cellulose fibres: Organic–inorganic hybrid versus conventional organophosphonate. Polym. Degrad. Stab. 2013, 98, 2602–2608. [Google Scholar] [CrossRef]
- Guido, E.; Alongi, J.; Colleoni, C.; Di Blasio, A.; Carosio, F.; Verelst, M.; Malucelli, G.; Rosace, G. Thermal stability and flame retardancy of polyester fabrics sol–gel treated in the presence of boehmite nanoparticles. Polym. Degrad. Stab. 2013, 98, 1609–1616. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Colleoni, C.; Guido, E.; Botteri, L.; Rosace, G. Thermal behaviour and flame retardancy of monoethanolamine-doped sol-gel coatings of cotton fabric. Prog. Org. Coat. 2017, 103, 174–181. [Google Scholar] [CrossRef]
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation. Appl. Sci. 2020, 10, 2287. [Google Scholar] [CrossRef] [Green Version]
- Plutino, M.; Colleoni, C.; Donelli, I.; Freddi, G.; Guido, E.; Maschi, O.; Mezzi, A.; Rosace, G. Sol-gel 3-glycidoxypropyltriethoxysilane finishing on different fabrics: The role of precursor concentration and catalyst on the textile performances and cytotoxic activity. J. Colloid Interface Sci. 2017, 506, 504–517. [Google Scholar] [CrossRef]
- Guido, E.; Colleoni, C.; De Clerck, K.; Plutino, M.R.; Rosace, G. Influence of catalyst in the synthesis of a cellulose-based sensor: Kinetic study of 3-glycidoxypropyltrimethoxysilane epoxy ring opening by Lewis acid. Sens. Actuators B Chem. 2014, 203, 213–222. [Google Scholar] [CrossRef]
- Rosace, G.; Guido, E.; Colleoni, C.; Brucale, M.; Piperopoulos, E.; Milone, C.; Plutino, M.R. Halochromic resorufin-GPTMS hybrid sol-gel: Chemical-physical properties and use as pH sensor fabric coating. Sens. Actuators B Chem. 2017, 241, 85–95. [Google Scholar] [CrossRef]
- Plutino, M.R.; Guido, E.; Colleoni, C.; Rosace, G. Effect of GPTMS functionalization on the improvement of the pH-sensitive methyl red photostability. Sens. Actuators B Chem. 2017, 238, 281–291. [Google Scholar] [CrossRef]
- Caldara, M.; Colleoni, C.; Guido, E.; Re, V.; Rosace, G. Development of a textile-optoelectronic pH meter based on hybrid xerogel doped with Methyl Red. Sens. Actuators B Chem. 2012, 171–172, 1013–1021. [Google Scholar] [CrossRef]
- Caldara, M.; Colleoni, C.; Guido, E.; Rosace, G.; Re, V.; Vitali, A. A wearable sensor platform to monitor sweat pH and skin temperature. In Proceedings of the 2013 IEEE International Conference on Body Sensor Networks, Cambridge, MA, USA, 6–9 May 2013; pp. 1–6. [Google Scholar] [CrossRef]
- Caldara, M.; Colleoni, C.; Guido, E.; Re, V.; Rosace, G. Optical monitoring of sweat pH by a tex-tile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sens. Actuators B Chem. 2016, 222, 213–220. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, W.; Zheng, M.; Meng, F.; Zhong, Z. pH-sensitive degradable chimaeric polymersomes for the intracellular release of doxorubicin hydrochloride. Biomaterials 2012, 33, 7291–7299. [Google Scholar] [CrossRef]
- Wagenaar, B.; Müller, B. Piroxicam release from spray-dried biodegradable microspheres. Biomaterials 1994, 15, 49–54. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Godfrin, P.D.; Myerson, A.S.; Trout, B.L.; Doyle, P.S. Core-Shell Composite Hydrogels for Controlled Nanocrystal Formation and Release of Hydrophobic Active Pharmaceutical Ingredients. Adv. Healthcare Mater. 2016, 5, 1960–1968. [Google Scholar] [CrossRef]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.R. Dendrimer-based nanoparticles for cancer therapy. Hematology 2009, 1, 708–719. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J. Drug Deliv. 2014, 2014, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Dinesh, B.; Bianco, A.; Ménard-Moyon, C. Designing multimodal carbon nanotubes by covalent multi-functionalization. Nanoscale 2016, 8, 18596–18611. [Google Scholar] [CrossRef]
- Murugesan, S.; Mousa, S.A.; O’Connor, L.J.; Lincoln, D.W.; Linhardt, R.J. Carbon inhibits vascular endothelial growth factor- and fibroblast growth factor-promoted angiogenesis. FEBS Lett. 2007, 581, 1157–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Yang, W.; Man, N.; Zheng, F.; Shen, Y.; Sun, K.; Li, Y.; Wen, L.-P. Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy 2009, 5, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prylutska, S.V.; Skivka, L.M.; Didenko, G.V.; Prylutskyy, Y.I.; Evstigneev, M.P.; Potebnya, G.P.; Panchuk, R.R.; Stoika, R.S.; Ritter, U.; Scharff, P. Complex of C60 Fullerene with Doxorubicin as a Promising Agent in Antitumor Therapy. Nanoscale Res. Lett. 2015, 10, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, B.; Ma, Y.; Yu, S.; Ji, C. Smart Multifunctional Magnetic Nanoparticle-Based Drug Delivery System for Cancer Thermo-Chemotherapy and Intracellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 24502–24508. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.; Rotello, V.M. Gold nanoparticles in delivery applications☆. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Saturnino, C.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Caruso, A.; Muià, N.; Longo, P.; Rosace, G.; Galletta, M.; Ielo, I.; et al. N-Thiocarbazole-based gold nanoparticles: Synthesis, characterization and anti-proliferative activity evaluation. IOP Conf. Series Mater. Sci. Eng. 2018, 459, 012023. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan Reduced Gold Nanoparticles as Novel Carriers for Transmucosal Delivery of Insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef] [Green Version]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 206–212. [Google Scholar] [CrossRef]
- Baban, D.F.; Seymour, L.W. Control of tumour vascular permeability. Adv. Drug Deliv. Rev. 1998, 34, 109–119. [Google Scholar] [CrossRef]
- Bergen, J.M.; Von Recum, H.A.; Goodman, T.T.; Massey, A.P.; Pun, S.H. Gold Nanoparticles as a Versatile Platform for Optimizing Physicochemical Parameters for Targeted Drug Delivery. Macromol. Biosci. 2006, 6, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.; Bossche, J.V.D.; Sherman, D.M.; Thompson, D.H.; Andres, R.P. Synthesis and Grafting of Thioctic Acid−PEG−Folate Conjugates onto Au Nanoparticles for Selective Targeting of Folate Receptor-Positive Tumor Cells. Bioconjugate Chem. 2006, 17, 603–609. [Google Scholar] [CrossRef]
- De La Fuente, J.M.; Berry, C.C. Tat Peptide as an Efficient Molecule to Translocate Gold Nanoparticles into the Cell Nucleus. Bioconjugate Chem. 2005, 16, 1176–1180. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive Intelligent Drug Delivery Systems. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-H.; Jeon, O.; Kwon, I.C.; Park, K. Engineered polymers for advanced drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef]
- Rahamathullah, S. Design and Evaluation of Controlled Release Layered Matrix Tablets of Parace-Tamol and Verapamil HCL. 2009; pp. 1–58. Available online: http://eprints.usm.my/10158/%5Cnhttp://eprints.usm.my/10158/1/DESIGN_AND_EVALUATION_OF_CONTROLLED_RELEASE_LAYERED_MATRIX_TABLETS_OF_PARACETAMOL_AND_VERAPAMIL_HCl.pdf (accessed on 21 May 2021).
- Jeong, B.; Gutowska, A. Lessons from nature: Stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002, 20, 305–311. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Stayton, P.S. Bioconjugates of smart polymers and proteins: Synthesis and applications. Macromol. Symp. 2004, 207, 139–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Kim, S.W.; Lee, D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release 2008, 127, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Hrubý, M.; Filippov, S.; Štěpánek, P. Smart polymers in drug delivery systems on crossroads: Which way deserves following? Eur. Polym. J. 2015, 65, 82–97. [Google Scholar] [CrossRef]
- Adhikari, U.; Goliaei, A.; Tsereteli, L.; Berkowitz, M.L. Properties of Poloxamer Molecules and Poloxamer Micelles Dissolved in Water and Next to Lipid Bilayers: Results from Computer Simulations. J. Phys. Chem. B 2016, 120, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.A.; Shukla, S.R. Adsorptive removal of cobalt ions on raw and alkali-treated lemon peels. Int. J. Environ. Sci. Technol. 2015, 13, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Singh, J. Controlled delivery of testosterone from smart polymer solution based systems: In vitro evaluation. Int. J. Pharm. 2005, 295, 183–190. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef]
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef]
- Sumaru, K.; Ohi, K.; Takagi, T.; Kanamori, T.; Shinbo, T. Photoresponsive Properties of Poly(N-isopropylacrylamide) Hydrogel Partly Modified with Spirobenzopyran. Langmuir 2006, 22, 4353–4356. [Google Scholar] [CrossRef]
- Jiang, J.; Tong, X.; Morris, D.; Zhao, Y. Toward Photocontrolled Release Using Light-Dissociable Block Copolymer Micelles. Macromolecules 2006, 39, 4633–4640. [Google Scholar] [CrossRef]
- McCoy, C.P.; Rooney, C.; Edwards, C.R.; Jones, A.D.S.; Gorman, S.P. Light-Triggered Molecule-Scale Drug Dosing Devices. J. Am. Chem. Soc. 2007, 129, 9572–9573. [Google Scholar] [CrossRef]
- Klohs, J.; Wunder, A.; Licha, K. Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Res. Cardiol. 2008, 103, 144–151. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Shao, R.; Wei, X.; Gupta, S.; Li, C. Near-Infrared Light Triggers Release of Paclitaxel from Biodegradable Microspheres: Photothermal Effect and Enhanced Antitumor Activity. Small 2010, 6, 1022–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mano, J.F. Stimuli-Responsive Polymeric Systems for Biomedical Applications. Adv. Eng. Mater. 2008, 10, 515–527. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Thermal and fire stability of cotton fabrics coated with hybrid phosphorus-doped silica films. J. Therm. Anal. Calorim. 2011, 110, 1207–1216. [Google Scholar] [CrossRef]
- Visakh, P.M.; Arao, Y. Flame Retardants; Springer International Publishing Switzerland: Basel, Switzerland, 2015. [Google Scholar] [CrossRef]
- Vijay Kumar, V.; Balaganesan, G.; Lee JK, Y.; Neisiany, R.E.; Surendran, S.; Ramakrishna, S. A Review of Recent Advances in Nanoengineered Polymer Composites. Polymers 2019, 11, 644. [Google Scholar] [CrossRef] [Green Version]
- Massaroni, C.; Saccomandi, P.; Schena, E. Medical Smart Textiles Based on Fiber Optic Technology: An Overview. J. Funct. Biomater. 2015, 6, 204–221. [Google Scholar] [CrossRef]
- Luo, J.; Gao, S.; Luo, H.; Wang, L.; Huang, X.; Guo, Z.; Lai, X.; Lin, L.; Li, R.K.; Gao, J. Superhydrophobic and breathable smart MXene-based textile for multifunctional wearable sensing electronics. Chem. Eng. J. 2021, 406, 126898. [Google Scholar] [CrossRef]
- Ferrer-Vilanova, A.; Alonso, Y.; Dietvorst, J.; Pérez-Montero, M.; Rodríguez-Rodríguez, R.; Ivanova, K.; Tzanov, T.; Vigués, N.; Mas, J.; Guirado, G.; et al. Sonochemical coating of Prussian Blue for the production of smart bacterial-sensing hospital textiles. Ultrason. Sonochemistry 2021, 70, 105317. [Google Scholar] [CrossRef]
- Norouzi, M.; Zare, Y.; Kiany, P. Nanoparticles as Effective Flame Retardants for Natural and Synthetic Textile Polymers: Application, Mechanism, and Optimization. Polym. Rev. 2015, 55, 531–560. [Google Scholar] [CrossRef]
- Liang, S.; Neisius, M.; Mispreuve, H.; Naescher, R.; Gaan, S. Flame retardancy and thermal decomposition of flexible polyurethane foams: Structural influence of organophosphorus compounds. Polym. Degrad. Stab. 2012, 97, 2428–2440. [Google Scholar] [CrossRef]
- Lessan, F.; Montazer, M.; Moghadam, M. A novel durable flame-retardant cotton fabric using sodium hypophosphite, nano TiO2 and maleic acid. Thermochim. Acta 2011, 520, 48–54. [Google Scholar] [CrossRef]
- Malucelli, G. Sol-Gel and Layer-by-Layer Coatings for Flame-Retardant Cotton Fabrics: Recent Advances. Coatings 2020, 10, 333. [Google Scholar] [CrossRef] [Green Version]
- Westbrook, C.K. Chemical kinetics of hydrocarbon ignition in practical combustion systems. Proc. Combust. Inst. 2000, 28, 1563–1577. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Li, Z.; Li, X.; Zhang, Z. Flame retardant coatings prepared using layer by layer assembly: A review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, S.; Cai, J.; Zhou, B.; Xin, Y. Risk forecasting for spontaneous combustion of coals at different ranks due to free radicals and functional groups reaction. Process. Saf. Environ. Prot. 2018, 118, 195–202. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, S.; Jiang, S.; He, X.; Shao, H.; Wang, K.; Fan, D.; Li, W. Experimental study on prevention and control of coal spontaneous combustion with heat control inhibitor. J. Loss Prev. Process. Ind. 2018, 56, 272–277. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, C.; Yang, Y.; Zhang, X. Experimental study on the inhibitory effect of ethylenediaminetetraacetic acid (EDTA) on coal spontaneous combustion. Fuel Process. Technol. 2018, 178, 312–321. [Google Scholar] [CrossRef]
- Zhu, C.; Li, S.; Li, J.; Clement, M.; Rudd, C.; Yi, X.; Liu, X. Fire performance of sandwich composites with intumescent mat protection: Evolving thermal insulation, post-fire performance and rail industry testing. Fire Saf. J. 2020, 116, 103205. [Google Scholar] [CrossRef]
- Lucherini, A.; Hidalgo, J.P.; Torero, J.L.; Maluk, C. Influence of heating conditions and initial thickness on the effectiveness of thin intumescent coatings. Fire Saf. J. 2021, 120, 103078. [Google Scholar] [CrossRef]
- Howard, P.H.; Muir, D.C.G. Identifying New Persistent and Bioaccumulative Organics Among Chemicals in Commerce. Environ. Sci. Technol. 2010, 44, 2277–2285. [Google Scholar] [CrossRef]
- Daniel, Y.; Howell, B. Phosphorus flame retardants from isosorbide bis-acrylate. Polym. Degrad. Stab. 2018, 156, 14–21. [Google Scholar] [CrossRef]
- Chen, R.; Hu, K.; Tang, H.; Wang, J.; Zhu, F.; Zhou, H. A novel flame retardant derived from DOPO and piperazine and its application in epoxy resin: Flame retardance, thermal stability and pyrolysis behavior. Polym. Degrad. Stab. 2019, 166, 334–343. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brehme, S.; Schartel, B.; Goebbels, J.; Fischer, O.; Pospiech, D.; Bykov, Y.; Döring, M. Phosphorus polyester versus aluminium phosphinate in poly(butylene terephthalate) (PBT): Flame retardancy performance and mechanisms. Polym. Degrad. Stab. 2011, 96, 875–884. [Google Scholar] [CrossRef]
- Pawlowski, K.H.; Schartel, B. Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol A bis(diphenyl phosphate) in polycarbon- ate/acrylonitrile–butadiene–styrene blends. Polym. Int. 2007, 56, 1404–1414. [Google Scholar] [CrossRef]
- Schartel, D.P.B.; Balabanovich, A.I.; Braun, U.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.W.; Altstädt, V.; et al. Pyrolysis of Epoxy Resins and Fire Behavior of Epoxy Resin Composites Flame-Retarded with 9,10-Dihydro-9- oxa-10-phosphaphenanthrene-10-oxide Additives. J. Appl. Polym. Sci. 2007, 104, 2260–2269. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Wu, X.; Xu, B. The influence of nano ZnO coated by phosphazene/triazine bi-group molecular on the flame retardant property and mechanical property of intumescent flame retardant poly (lactic acid) composites. Thermochim. Acta 2019, 679. [Google Scholar] [CrossRef]
- Yang, G.; Wu, W.-H.; Wang, Y.-H.; Jiao, Y.-H.; Lu, L.-Y.; Qu, H.-Q.; Qin, X.-Y. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of Epoxy Resin. J. Hazard. Mater. 2019, 366, 78–87. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X. Synthesis, characterization, thermal properties and flame retardancy of a novel nonflammable phosphazene-based epoxy resin. Polym. Degrad. Stab. 2009, 94, 617–624. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Wu, D. Novel Spirocyclic Phosphazene-Based Epoxy Resin for Halogen-Free Fire Resistance: Synthesis, Curing Behaviors, and Flammability Characteristics. ACS Appl. Mater. Interfaces 2012, 4, 4047–4061. [Google Scholar] [CrossRef]
- Qian, L.; Ye, L.; Qiu, Y.; Qu, S. Thermal degradation behavior of the compound containing phosphaphenanthrene and phosphazene groups and its flame retardant mechanism on epoxy resin. Polymer 2011, 52, 5486–5493. [Google Scholar] [CrossRef]
- Horacek, H.; Grabner, W. Nitrogen Based Flame Retardants for Nitrogen Containing Polymers. In Makromolekulare Chemie. Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, Switzerland, 1993; Volume 74, pp. 271–276. [Google Scholar]
- Xie, K.; Gao, A.; Zhang, Y. Flame retardant finishing of cotton fabric based on synergistic compounds containing boron and nitrogen. Carbohydr. Polym. 2013, 98, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Castellano, A.; Colleoni, C.; Iacono, G.; Mezzi, A.; Plutino, M.R.; Malucelli, G.; Rosace, G. Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen- and formaldehyde-free flame retardant finishing for cotton fabric. Polym. Degrad. Stab. 2019, 162, 148–159. [Google Scholar] [CrossRef]
- Tian, P.; Lu, Y.; Wang, D.; Zhang, G.; Zhang, F. Synthesis of a new N–P durable flame retardant for cotton fabrics. Polym. Degrad. Stab. 2019, 165, 220–228. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.; Naik, Y.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Lewin, M. Synergism and catalysis in flame retardancy of polymers. Polym. Adv. Technol. 2001, 12, 215–222. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Smart, G.; Nazaré, S.; Kandola, B.; Price, D. Quantification of Zinc Hydroxystannate** and Stannate** Synergies in Halogen-containing Flame-retardant Polymeric Formulations. J. Fire Sci. 2009, 28, 217–248. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Phosphorus- and nitrogen-doped silica coatings for enhancing the flame retardancy of cotton: Synergisms or additive effects? Polym. Degrad. Stab. 2013, 98, 579–589. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; He, Y.; Xu, M.; Shi, Z.; Hu, H.; Yang, Z.; Li, B. An effective mono-component intumescent flame retardant for the enhancement of water resistance and fire safety of thermoplastic polyurethane composites. Polym. Degrad. Stab. 2019, 167, 146–156. [Google Scholar] [CrossRef]
- Jia, D.; Guo, X.; He, J.; Yang, R. An anti-melt dripping, high char yield and flame-retardant polyether rigid polyurethane foam. Polym. Degrad. Stab. 2019, 167, 189–200. [Google Scholar] [CrossRef]
- Chiu, S.-H.; Wang, W.-K. Dynamic flame retardancy of polypropylene filled with ammonium polyphosphate, pentaerythritol and melamine additives. Polymer 1998, 39, 1951–1955. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.-A.; Mülhaupt, R.; Schartel, B. Flame-Retardancy Properties of Intumescent Ammonium Poly (Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polymer 2014, 6, 2875–2895. [Google Scholar] [CrossRef] [Green Version]
- Alongi, J.; Carosio, F.; Malucelli, G. Current emerging techniques to impart flame retardancy to fabrics: An overview. Polym. Degrad. Stab. 2014, 106, 138–149. [Google Scholar] [CrossRef]
- Bourbigot, S.; Devaux, E.; Flambard, X. Flammability of polyamide-6/clay hybrid nanocomposite textiles. Polym. Degrad. Stab. 2002, 75, 397–402. [Google Scholar] [CrossRef]
- Durin-France, A.; Ferry, L.; Cuesta, J.M.L.; Crespy, A. Magnesium hydroxide/zinc borate/talc compositions as flame-retardants in EVA copolymer. Polym. Int. 2000, 49, 1101–1105. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Use of Turkish Huntite/Hydromagnesite Mineral in Plastic Materials as a Flame Retardant. Polym. Compos. 2008, 16, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, J.; Song, S. Preparation of a novel type of flame retardant diatomite and its application in silicone rubber composites. Adv. Powder Technol. 2019, 30, 1567–1575. [Google Scholar] [CrossRef]
- Hayashida, K.; Tsuge, S.; Ohtani, H. Flame retardant mechanism of polydimethylsiloxane material containing platinum compound studied by analytical pyrolysis techniques and alkaline hydrolysis gas chromatography. Polymer 2003, 44, 5611–5616. [Google Scholar] [CrossRef]
- Nodera, A.; Kanai, T. Flame retardancy of polycarbonate–polydimethylsiloxane block copolymer/silica nanocomposites. J. Appl. Polym. Sci. 2006, 101, 3862–3868. [Google Scholar] [CrossRef]
- Mosurkal, R.; Kirby, R.; Muller, W.S.; Soares, J.W.; Kumar, J. Simple green synthesis of polyborosiloxanes as environmentally-safe, non-halogenated flame retardant polymers. Green Chem. 2011, 13, 659–665. [Google Scholar] [CrossRef]
- Carosio, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Layer by layer nanoarchitectures for the surface protection of polycarbonate. Eur. Polym. J. 2013, 49, 397–404. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. α-Zirconium phosphate-based nanoarchitectures on polyester fabrics through layer-by-layer assembly. J. Mater. Chem. 2011, 21, 10370–10376. [Google Scholar] [CrossRef]
- American Society for Testing Materials. Standard Test Method for Flame Resistance of Textiles (Vertical Test). ASTM D6413; ASTM International: West Conshohocken, PA, USA.
- Alongi, J.; Ciobanu, M.; Malucelli, G. Sol–gel treatments for enhancing flame retardancy and thermal stability of cotton fabrics: Optimisation of the process and evaluation of the durability. Cellulose 2011, 18, 167–177. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Sol–gel treatments on cotton fabrics for improving thermal and flame stability: Effect of the structure of the alkoxysilane precursor. Carbohydr. Polym. 2012, 87, 627–635. [Google Scholar] [CrossRef]
- Brancatelli, G.; Colleoni, C.; Massafra, M.; Rosace, G. Effect of hybrid phosphorus-doped silica thin films produced by sol-gel method on the thermal behavior of cotton fabrics. Polym. Degrad. Stab. 2011, 96, 483–490. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Tang, R.-C.; Guan, J.-P.; Zhou, S.-Q. An eco-friendly and effective flame retardant coating for cotton fabric based on phytic acid doped silica sol approach. Prog. Org. Coat. 2020, 141, 105539. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, H.; He, Y.; Liu, Y.; Dong, C.; Zhu, P. Flame retardancy and thermal behavior of cotton fabrics based on a novel phosphorus-containing siloxane. Appl. Surf. Sci. 2019, 479, 765–775. [Google Scholar] [CrossRef]
- Cunningham, M.F.; Campbell, J.D.; Fu, Z.; Bohling, J.; Leroux, J.G.; Mabee, W.; Robert, T. Future green chemistry and sustainability needs in polymeric coatings. Green Chem. 2019, 21, 4919–4926. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The Sol–Gel Route to Advanced Silica-Based Materials and Recent Applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef]
- Ielo, I.; Galletta, M.; Rando, G.; Sfameni, S.; Cardiano, P.; Sabatino, G.; Drommi, D.; Rosace, G.; Plutino, M.R. Design, synthesis and characterization of hybrid coatings suitable for geopolymeric-based supports for the restoration of cultural heritage. IOP Conf. Series: Mater. Sci. Eng. 2020, 777. [Google Scholar] [CrossRef]
- Alcantara-Garcia, A.; Garcia-Casas, A.; Jimenez-Morales, A. The effect of the organosilane content on the barrier features of sol-gel anticorrosive coatings applied on carbon steel. Prog. Org. Coat. 2020, 139, 105418. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Figueira, R.M.B.B.M.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, M.; Borhani, G.H.; Bakhshi, S.R.; Ghasemi, A. Preparation and evaluation of corrosion behavior of GPTMS–TEOS hybrid coatings containing Zr and Ce on aluminum alloy 6061-T6. J. Sol Gel Sci. Technol. 2015, 76, 552–561. [Google Scholar] [CrossRef]
- Taheri, M.; Saremi, M.; Mahdavian, M.; Naderi, R. Development of an ecofriendly silane sol-gel coating with zinc acetylacetonate corrosion inhibitor for active protection of mild steel in sodium chloride solution. J. Sol Gel Sci. Technol. 2016, 81, 154–166. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [Green Version]
- Zadeh, M.A.; Van Der Zwaag, S.; Garcia, S. Routes to extrinsic and intrinsic self-healing corrosion protective sol-gel coatings: A review. Self Heal. Mater. 2013, 1, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Alaneme, K.; Bodunrin, M. Self-healing using metallic material systems—A review. Appl. Mater. Today 2017, 6, 9–15. [Google Scholar] [CrossRef]
- Adsul, S.H.; Siva, T.; Sathiyanarayanan, S.; Sonawane, S.H.; Subasri, R. Self-healing ability of nanoclay-based hybrid sol-gel coatings on magnesium alloy AZ91D. Surf. Coat. Technol. 2017, 309, 609–620. [Google Scholar] [CrossRef]
- Oldani, V.; Sergi, G.; Pirola, C.; Sacchi, B.; Bianchi, C.L. Sol-gel hybrid coatings containing silica and a perfluoropolyether derivative with high resistance and anti-fouling properties in liquid media. J. Fluor. Chem. 2016, 188, 43–49. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ansary, J.; Ghoreishi, R. Sol-gel process applications: A mini-review. Proc. Nat. Res. Soc. 2018, 2, 02008. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Durán, A.; Castro, Y.; Aparicio, M.; Conde, A.; De Damborenea, J.J. Protection and surface modification of metals with sol–gel coatings. Int. Mater. Rev. 2007, 52, 175–192. [Google Scholar] [CrossRef] [Green Version]
- Tedim, J.; Poznyak, S.K.; Kuznetsova, A.; Raps, D.; Hack, T.; Zheludkevich, M.L.; Ferreira, M.G.S. Enhancement of Active Corrosion Protection via Combination of Inhibitor-Loaded Nanocontainers. ACS Appl. Mater. Interfaces 2010, 2, 1528–1535. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Tedim, J.; Ferreira, M. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Tan, A.L.K.; Soutar, A.M. Hybrid sol-gel coatings for corrosion protection of copper. Thin Solid Film. 2008, 516, 5706–5709. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. PTMS/OH-MWCNT sol-gel nanocomposite for corrosion protection of magnesium alloy. Surf. Coat. Technol. 2018, 335, 228–240. [Google Scholar] [CrossRef]
- Rivero, P.J.; Maeztu, J.D.; Berlanga, C.; Miguel, A.; Palacio, J.F.; Rodriguez, R. Hydrophobic and Corrosion Behavior of Sol-Gel Hybrid Coatings Based on the Combination of TiO2 NPs and Fluorinated Chains for Aluminum Alloys Protection. Metals 2018, 8, 1076. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, J.; Liu, R. Organic–inorganic hybrid sol–gel coatings for corrosion protection of aluminum alloys. Surf. Innov. 2016, 4, 51–69. [Google Scholar] [CrossRef]
- Hernández, M.; Barba, A.; Genesca, J.; Covelo, A.; Bucio, E.; Torres, V.; Marín-Romero, D.; Galván-Martínez, R.; Orozco-Cruz, R. Characterization of Hybrid Sol-Gel Coatings Doped with Hydrotalcite-likeCompounds on Steel and Stainless Steel Alloys. ECS Trans. 2013, 47, 195–206. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Hybrid sol–gel coatings for corrosion protection of galvanized steel in simulated concrete pore solution. J. Coat. Technol. Res. 2016, 13, 355–373. [Google Scholar] [CrossRef]
- Balan, P.; Ng, A.; Siang, C.B.; Raman, R.S.; Chan, E.S.; Seng, C.E. Effect of Nanoparticle Addition in Hybrid Sol-Gel Silane Coating on Corrosion Resistance of Low Carbon Steel. Adv. Mater. Res. 2013, 686, 244–249. [Google Scholar] [CrossRef]
- Wojcik, A.B.; Klein, L.C. Transparent Organic/Inorganic Hybrid Gels: A Classification Scheme. Appl. Organomet. Chem. 1997, 11, 129–135. [Google Scholar] [CrossRef]
- Perrin, F.; Ziarelli, F.; Dupuis, A. Relation between the corrosion resistance and the chemical structure of hybrid sol-gel coatings with interlinked inorganic-organic network. Prog. Org. Coat. 2020, 141, 105532. [Google Scholar] [CrossRef]
- Hamidon, T.S.; Hussin, M.H. Susceptibility of hybrid sol-gel (TEOS-APTES) doped with caffeine as potent corrosion protective coatings for mild steel in 3.5 wt.% NaCl. Prog. Org. Coat. 2020, 140, 105478. [Google Scholar] [CrossRef]
- Aziz, A.H.A.; Jamil, T.S.; Shalaby, M.S.; Shaban, A.M.; Souaya, E.R.; Ghany, N.A.A. Application of (polyaniline/zeolite X) composite as anticorrosion coating for energy recovery devices in RO desalination water plants. Int. J. Ind. Chem. 2019, 10, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Izadi, M.; Shahrabi, T.; Mohammadi, I.; Ramezanzadeh, B. Synthesis of impregnated Na+-montmorillonite as an eco-friendly inhibitive carrier and its subsequent protective effect on silane coated mild steel. Prog. Org. Coat. 2019, 135, 135–147. [Google Scholar] [CrossRef]
- Mora, L.V.; Taylor, A.; Paul, S.; Dawson, R.; Wang, C.; Taleb, W.; Owen, J.; Neville, A.; Barker, R. Impact of silica nanoparticles on the morphology and mechanical properties of sol-gel derived coatings. Surf. Coat. Technol. 2018, 342, 48–56. [Google Scholar] [CrossRef]
- Agustín-Sáenz, C.; Martín-Ugarte, E.; Jorcin, J.B.; Imbuluzqueta, G.; Coloma, P.S.; Izagirre-Etxeberria, U. Effect of organic precursor in hybrid sol–gel coatings for corrosion protection and the application on hot dip galvanised steel. J. Sol Gel Sci. Technol. 2018, 89, 264–283. [Google Scholar] [CrossRef]
- Tiringer, U.; Milošev, I.; Durán, A.; Castro, Y. Hybrid sol–gel coatings based on GPTMS/TEOS containing colloidal SiO2 and cerium nitrate for increasing corrosion protection of aluminium alloy 7075-T6. J. Sol Gel Sci. Technol. 2018, 85, 546–557. [Google Scholar] [CrossRef]
- Aparicio, M.; Jitianu, A.; Rodriguez, G.; Degnah, A.; Al-Marzoki, K.; Mosa, J.; Klein, L. Corrosion Protection of AISI 304 Stainless Steel with Melting Gel Coatings. Electrochim. Acta 2016, 202, 325–332. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Enhancement of the hydrophobic and anti-corrosion properties of a composite zeolite coating on Al6061 substrate by modification of silane matrix. Corros. Eng. Sci. Technol. 2016, 52, 1–12. [Google Scholar] [CrossRef]
- Maeztu, J.D.; Rivero, P.J.; Berlanga, C.; Bastidas, D.M.; Palacio, J.F.; Rodriguez, R. Effect of graphene oxide and fluorinated polymeric chains incorporated in a multilayered sol-gel nanocoating for the design of corrosion resistant and hydrophobic surfaces. Appl. Surf. Sci. 2017, 419, 138–149. [Google Scholar] [CrossRef]
- Dalmoro, V.; Dos Santos, J.H.; Baibich, I.M.; Butler, I.S.; Armelin, E.; Aleman, C.; Azambuja, D.S. Improving the corrosion performance of hybrid sol–gel matrix by modification with phosphonic acid. Prog. Org. Coat. 2015, 80, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Kirtay, S. Preparation of hybrid silica sol–gel coatings on mild steel surfaces and evaluation of their corrosion resistance. Prog. Org. Coat. 2014, 77, 1861–1866. [Google Scholar] [CrossRef]
- Figueira, R.B. Hybrid Sol–gel Coatings for Corrosion Mitigation: A Critical Review. Polymer 2020, 12, 689. [Google Scholar] [CrossRef] [Green Version]
- Düzgün, O.A.; Gül, R.; Aydin, A.C. Effect of steel fibers on the mechanical properties of natural lightweight aggregate concrete. Mater. Lett. 2005, 59, 3357–3363. [Google Scholar] [CrossRef]
- Xu, L.; Min, M.; Yang, S.; Jiang, A.; Yang, Z.; Chu, S. Improvement of high-temperature resistance on carbon fiber felt/portland cement composite friction material by Al2O3 sol–gel coating. J. Sol Gel Sci. Technol. 2019, 91, 471–484. [Google Scholar] [CrossRef]
- Constantino, J.C.; Garcia, D.C.; Palhares, H.G.; Houmard, M.; Figueiredo, R.B. Development of functional TiO2 coatings deposited on cementitious materials. Constr. Build. Mater. 2020, 250, 118732. [Google Scholar] [CrossRef]
- Lin, J.; Tang, C.Y.; Ye, W.; Sun, S.-P.; Hamdan, S.H.; Volodin, A.; Van Haesendonck, C.; Sotto, A.; Luis, P.; Van der Bruggen, B. Unraveling flux behavior of superhydrophilic loose nanofiltration membranes during textile wastewater treatment. J. Membr. Sci. 2015, 493, 690–702. [Google Scholar] [CrossRef]
- Cho, J.; Amya, G.; Pellegrinob, J. Membrane filtration of natural organic matter: Initial comparison of rejection and flux decline characteristics with ultrafiltration and nanofiltration membranes. Water Res. 1999, 33, 2517–2526. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Mohammad, A.; Teow, Y.; Ang, W.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Chu, K.H.; Huang, Y.; Yu, M.; Heo, J.; Flora, J.R.; Jang, A.; Jang, M.; Jung, C.; Park, C.M.; Kim, D.-H.; et al. Evaluation of graphene oxide-coated ultrafiltration membranes for humic acid removal at different pH and conductivity conditions. Sep. Purif. Technol. 2017, 181, 139–147. [Google Scholar] [CrossRef]
- Mi, B. Graphene Oxide Membranes for Ionic and Molecular Sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Chu, K.H.; Fathizadeh, M.; Yu, M.; Flora, J.R.V.; Jang, A.; Jang, M.; Park, C.M.; Yoo, S.S.; Her, N.; Yoon, Y. Evaluation of Removal Mechanisms in a Graphene Oxide-Coated Ceramic Ultrafiltration Membrane for Retention of Natural Organic Matter, Pharmaceuticals, and Inorganic Salts. ACS Appl. Mater. Interfaces 2017, 9, 40369–40377. [Google Scholar] [CrossRef]
- Song, J.J.; Huang, Y.; Nam, S.-W.; Yu, M.; Heo, J.; Her, N.; Flora, J.R.; Yoon, Y. Ultrathin graphene oxide membranes for the removal of humic acid. Sep. Purif. Technol. 2015, 144, 162–167. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, L.; Qiao, Y.; Tang, C.; Jung, C.; Yoon, Y.; Li, S.; Yu, M. Ultrafiltration Membranes with Structure-Optimized Graphene-Oxide Coatings for Antifouling Oil/Water Separation. Adv. Mater. Interfaces 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Mao, Y.; Xu, W.L.; Ploehn, H.J.; Yu, M. Tuning the underwater oleophobicity of graphene oxide coatings via UV irradiation. Chem. Commun. 2014, 50, 9849–9851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, C.H.; Baek, Y.; Lee, C.; Kim, S.O.; Kim, S.; Lee, S.; Kim, S.-H.; Bae, S.S.; Park, J.; Yoon, J. Carbon nanotube-based membranes: Fabrication and application to desalination. J. Ind. Eng. Chem. 2012, 18, 1551–1559. [Google Scholar] [CrossRef]
- Sianipar, M.; Kim, S.H.; Min, C.; Tijing, L.D.; Shon, H.K. Potential and performance of a polydopamine-coated multiwalled carbon nanotube/polysulfone nanocomposite membrane for ultrafiltration application. J. Ind. Eng. Chem. 2016, 34, 364–373. [Google Scholar] [CrossRef]
- Dudchenko, A.V.; Rolf, J.; Russell, K.; Duan, W.; Jassby, D. Organic fouling inhibition on electrically conducting carbon nanotube–polyvinyl alcohol composite ultrafiltration membranes. J. Membr. Sci. 2014, 468, 1–10. [Google Scholar] [CrossRef]
- Duan, W.; Ronen, A.; Walker, S.; Jassby, D. Polyaniline-Coated Carbon Nanotube Ultrafiltration Membranes: Enhanced Anodic Stability for In Situ Cleaning and Electro-Oxidation Processes. ACS Appl. Mater. Interfaces 2016, 8, 22574–22584. [Google Scholar] [CrossRef] [PubMed]
- Bosc, F.; Ayral, A.; Guizard, C. Mesoporous anatase coatings for coupling membrane separation and photocatalyzed reactions. J. Membr. Sci. 2005, 265, 13–19. [Google Scholar] [CrossRef]
- Byun, S.; Davies, S.; Alpatova, A.; Corneal, L.; Baumann, M.; Tarabara, V.; Masten, S. Mn oxide coated catalytic membranes for a hybrid ozonation–membrane filtration: Comparison of Ti, Fe and Mn oxide coated membranes for water quality. Water Res. 2011, 45, 163–170. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A simple and eco-friendly route for fabricating iron-based coating on metal mesh for efficient oil/water separation. Sep. Purif. Technol. 2019, 226, 31–38. [Google Scholar] [CrossRef]
- Nghiem, L.; Mornane, P.; Potter, I.; Perera, J.; Cattrall, R.; Kolev, S. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Garcia-Rodríguez, A.; Matamoros, V.; Kolev, S.; Fontàs, C. Development of a polymer inclusion membrane (PIM) for the preconcentration of antibiotics in environmental water samples. J. Membr. Sci. 2015, 492, 32–39. [Google Scholar] [CrossRef]
- Vázquez, M.; Romero, V.; Fontas, C.; Antico, E.; Benavente, J. Polymer inclusion membranes (PIMs) with the ionic liquid (IL) Aliquat 336 as extractant: Effect of base polymer and IL concentration on their physical–chemical and elastic characteristics. J. Membr. Sci. 2014, 455, 312–319. [Google Scholar] [CrossRef]
- Laudelout, H.; Van Bladel, R.; Bolt, G.H.; Page, A.L. Thermodynamics of heterovalent cation exchange reactions in a montmorillonite clay. Trans. Faraday Soc. 1968, 64, 1477–1488. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.-Y.; Lau, S.C.K.; Dahms, H.-U.; Dobretsov, S.V.; Harder, T. Marine Biofilms as Mediators of Colonization by Marine Macroorganisms: Implications for Antifouling and Aquaculture. Mar. Biotechnol. 2007, 9, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Trovato, V.; Rosace, G.; Colleoni, C.; Sfameni, S.; Migani, V.; Plutino, M.R. Sol-gel based coatings for the protection of cultural heritage textiles. IOP Conf. Series: Mater. Sci. Eng. 2020, 777. [Google Scholar] [CrossRef]
- Detty, M.R.; Ciriminna, R.; Bright, F.V.; Pagliaro, M. Environmentally Benign Sol–Gel Antifouling and Foul-Releasing Coatings. Acc. Chem. Res. 2013, 47, 678–687. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO Nanoparticles toEscherichia coli: Mechanism and the Influence of Medium Components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Sathe, P.; Laxman, K.; Myint, M.T.Z.; Dobretsov, S.; Richter, J.; Dutta, J. Bioinspired nanocoatings for biofouling prevention by photocatalytic redox reactions. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Gittens, J.E.; Smith, T.J.; Suleiman, R.; Akid, R. Current and emerging environmentally-friendly systems for fouling control in the marine environment. Biotechnol. Adv. 2013, 31, 1738–1753. [Google Scholar] [CrossRef] [PubMed]
- Nurioglu, A.G.; Esteves, A.C.C.; De With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef] [Green Version]
- Rosace, G.; Cardiano, P.; Urzì, C.; De Leo, F.; Galletta, M.; Ielo, I. Plutino Potential roles of fluorine-containing sol-gel coatings against adhesion to control microbial biofilm. IOP Conf. Series: Mater. Sci. Eng. 2018, 459, 012021. [Google Scholar] [CrossRef]
- Carve, M.; Scardino, A.; Shimeta, J. Effects of surface texture and interrelated properties on marine biofouling: A systematic review. Biofouling 2019, 35, 597–617. [Google Scholar] [CrossRef]


| Smart Polymers System | Advantages | Disadvantages | References |
|---|---|---|---|
| Temperature-sensitive | Temperature is an easily controllable parameter. Most of these systems are biodegradable and non-toxic. | The sensitivity of polymeric systems in response to changes in temperature varies according to different factors, such as molecular weight and solubility. | [81,86,87] |
| Phase-sensitive | The drug release rate can be easily modulated through functional modifications or through the use of different solvents or solvent mixtures. | To have a greater effectiveness of the release it is often necessary to associate to these systems some light sensitive components. Most of these systems are not approved by FDA. | [89,90,91] |
| Light-sensitive | Targeted drug delivery is independent of the conditions of the biological environment. The therapeutic target can be modulated by modulating the wavelength of the radiation used. | Most of these systems are not approved by FDA due to their possible toxicity. | [79,92,93] |
| Biomolecule-sensitive | These systems have a high specificity. | Biomolecules are substances that are difficult to immobilize in drug delivery systems, which leads to poor control of the release of host species | [80,98] |
| Sol–Gel Coating | Additive Agent | Substrate | Average Thickness | Ref. |
|---|---|---|---|---|
| GPTMS/TEOS | none | Carbon Steel | 47.6–92.8 μm | [159] |
| GPTMS/TEOS/TETA | TETA | Mild steel | 8–10 μm | [182] |
| TEOS/APTES | Caffeine | Mild steel | 5 μm | [183] |
| Zeolite | X-type zeolite/polyaniline | Carbon Steel | Not reported | [184] |
| TEOS/TEMS | MMT + Basil extract | Mild steel | 40 μm | [185] |
| GPTMS/TEOS | Silica NPs | Mild steel | 20–40 μm | [186] |
| GPTMS/TEOS | AP / BPA + TPOZ (Zr4+) | Hot dip galvanised steel (HDG) | 0.8–2.4 μm | [187] |
| GPTMS/TEOS | SiO2 Ce(NO3)3 | Aluminum Alloy AA7075 | 4.2–8.6 μm | [188] |
| DMDES/MTES | Not reported | Stainless steel AISI 304 | 580–760 μm | [189] |
| GPTMS/MTEOS | TiO2 NPs | Aluminum alloy AA6061–T6 | 2 μm | [176] |
| Zeolite / DMDMS/PTMS | None | Aluminum alloy AA6061 | 15.0 μm | [190] |
| GPTMS/MTEOS | Graphene Oxide | Aluminum alloy AA6061–T6 | 200–300 nm | [191] |
| GPTMS/TEOS + Nanotubes Halloysite | Ce3+/Zr4+ | Magnesium alloys AZ91D | 3–3.5 μm | [167] |
| TEOS/VTMS | EDTPO | Aluminum alloy AA2024–T3 | 145–200 nm | [192] |
| GLYMO/AMEO | Not reported | Mild steel | 1.8–2 μm | [193] |
| GPTMS/TEOS | Silica/alumina NPs | Carbon steel | Not reported | [180] |
| TEOS/ MTES | ZrO2 TiO2 | Stainless steel AISI 304 | 115–545 μm | [188] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M.R. Nanostructured Surface Finishing and Coatings: Functional Properties and Applications. Materials 2021, 14, 2733. https://doi.org/10.3390/ma14112733
Ielo I, Giacobello F, Sfameni S, Rando G, Galletta M, Trovato V, Rosace G, Plutino MR. Nanostructured Surface Finishing and Coatings: Functional Properties and Applications. Materials. 2021; 14(11):2733. https://doi.org/10.3390/ma14112733
Chicago/Turabian StyleIelo, Ileana, Fausta Giacobello, Silvia Sfameni, Giulia Rando, Maurilio Galletta, Valentina Trovato, Giuseppe Rosace, and Maria Rosaria Plutino. 2021. "Nanostructured Surface Finishing and Coatings: Functional Properties and Applications" Materials 14, no. 11: 2733. https://doi.org/10.3390/ma14112733
APA StyleIelo, I., Giacobello, F., Sfameni, S., Rando, G., Galletta, M., Trovato, V., Rosace, G., & Plutino, M. R. (2021). Nanostructured Surface Finishing and Coatings: Functional Properties and Applications. Materials, 14(11), 2733. https://doi.org/10.3390/ma14112733











