Ternary Nanocomposites Based on Oxidized Carbon Nanohorns as Sensing Layers for Room Temperature Resistive Humidity Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
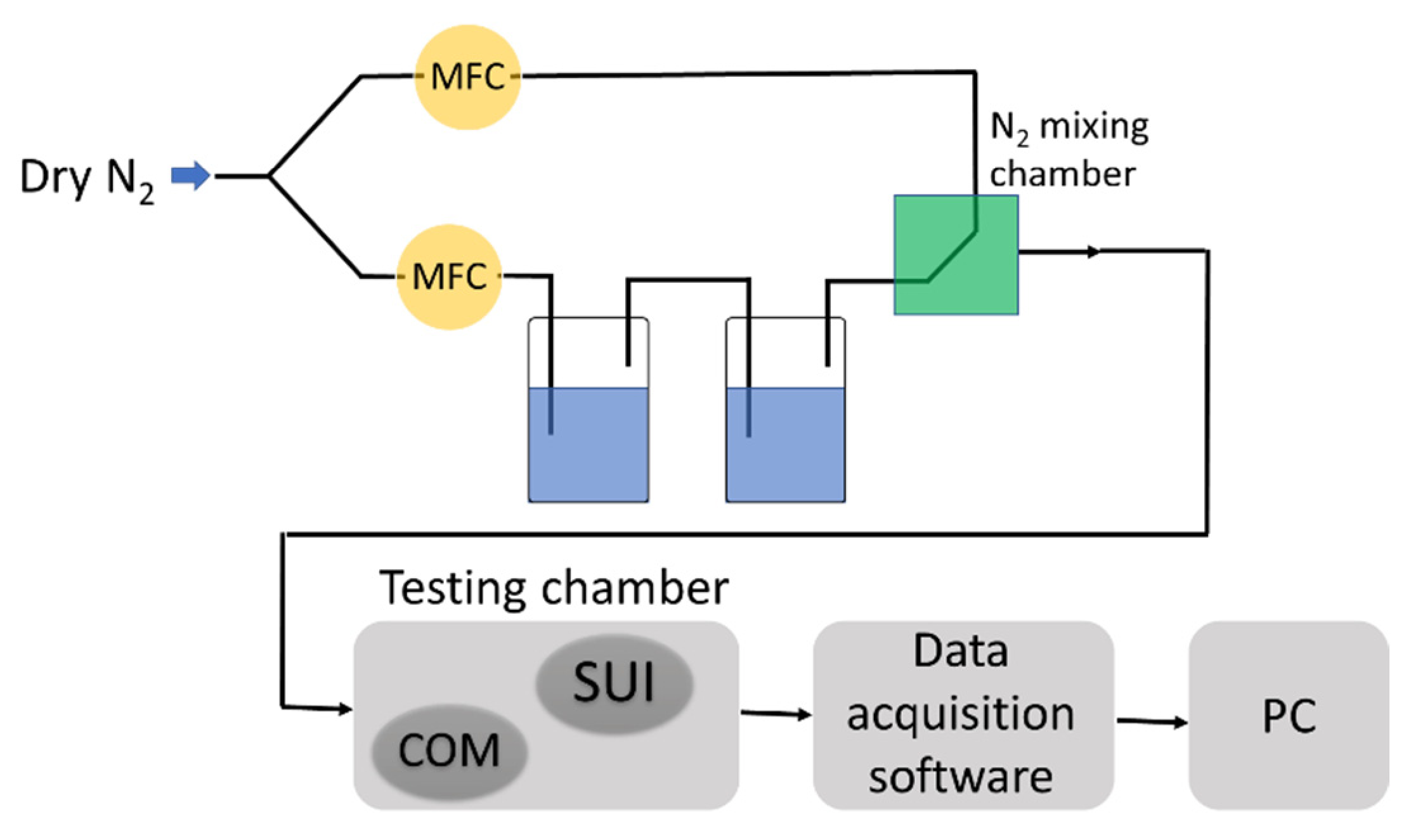
2.2. Methods
2.3. The Synthesis of the Ternary Nanocarbonic Materials-Based Nanocomposites Sensing Films
- −
- heating for 20 h at 80 ℃ under low pressure (2 mbar);
- −
- heating for 90 h at 110 ℃ under low pressure (2 mbar).
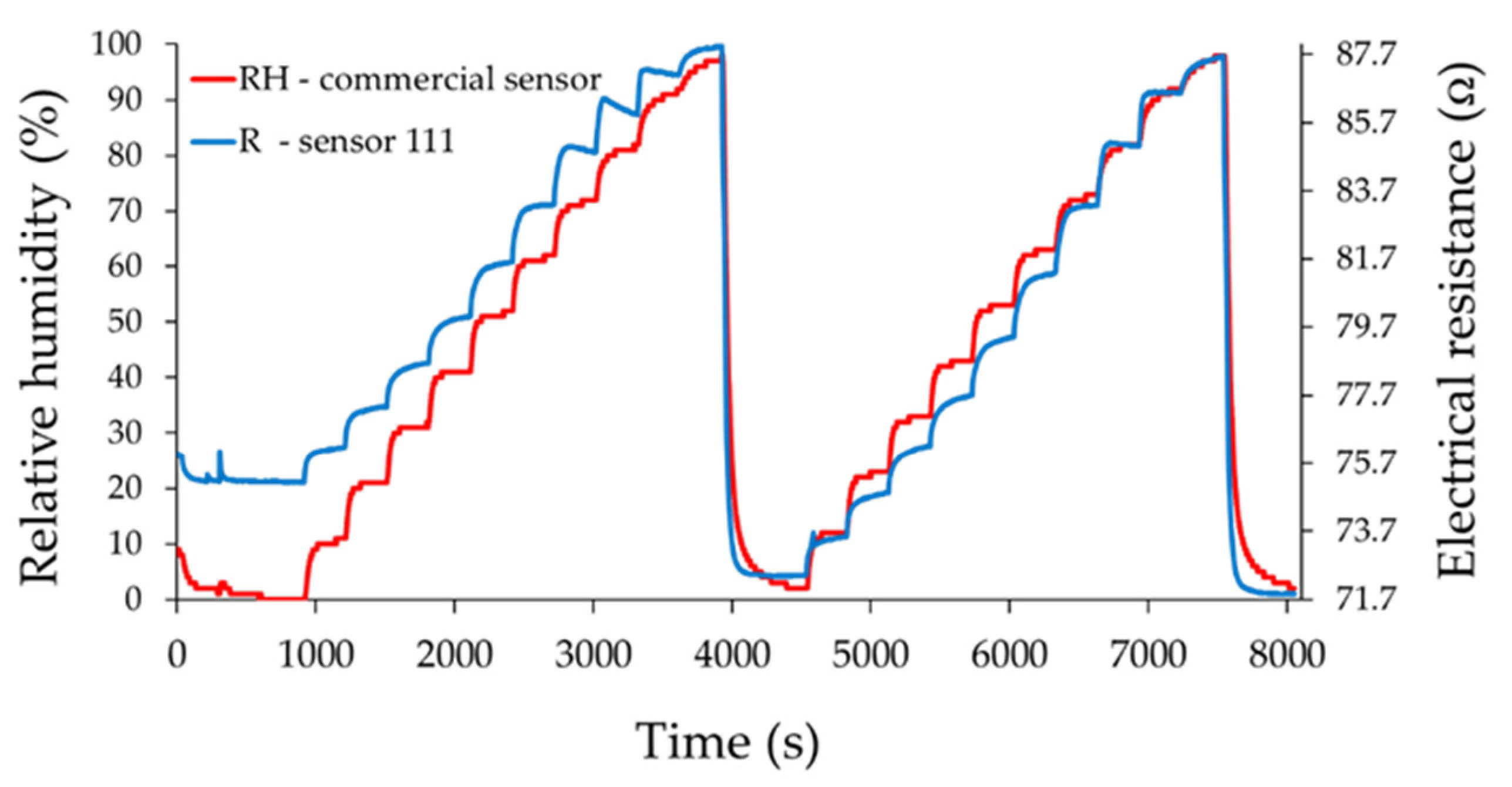
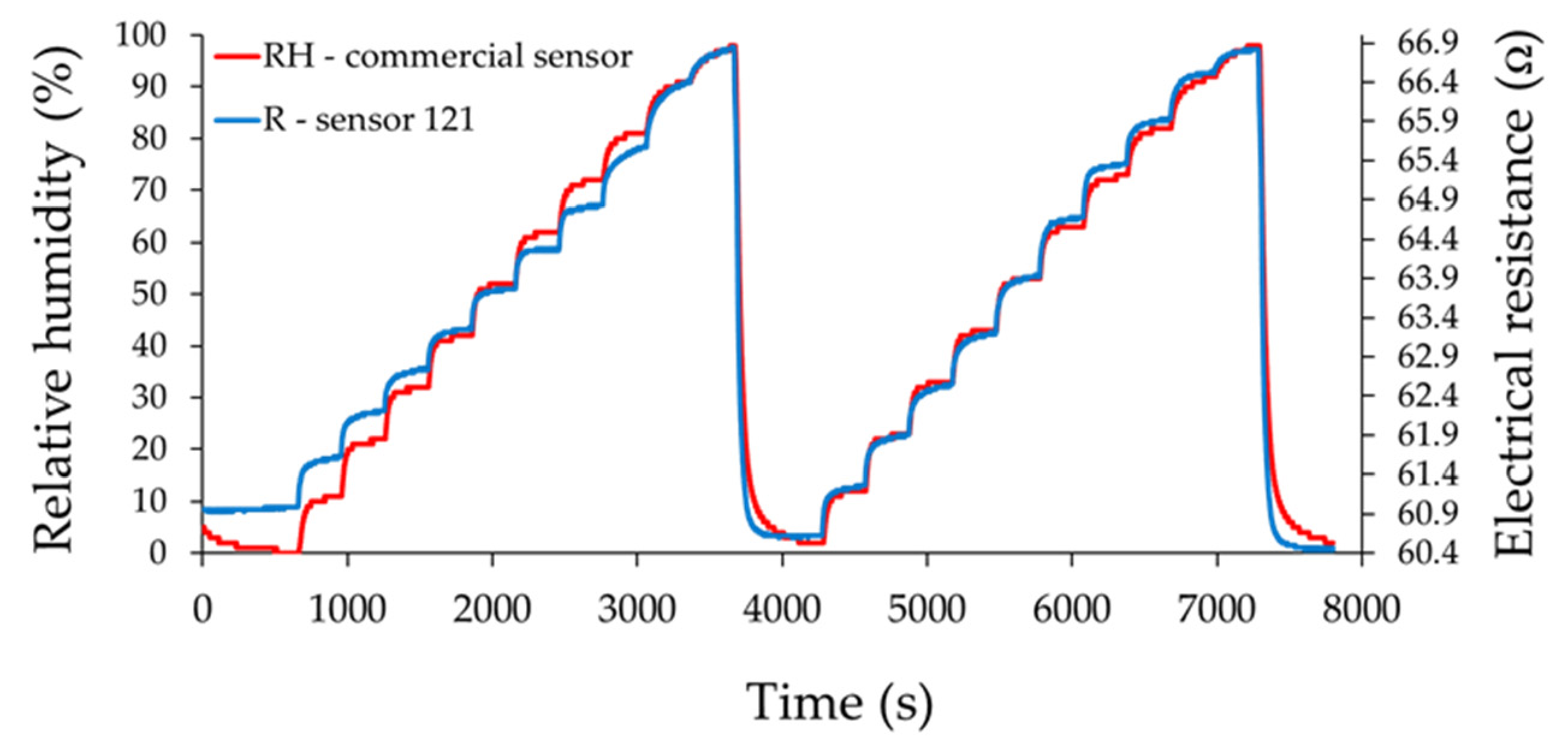
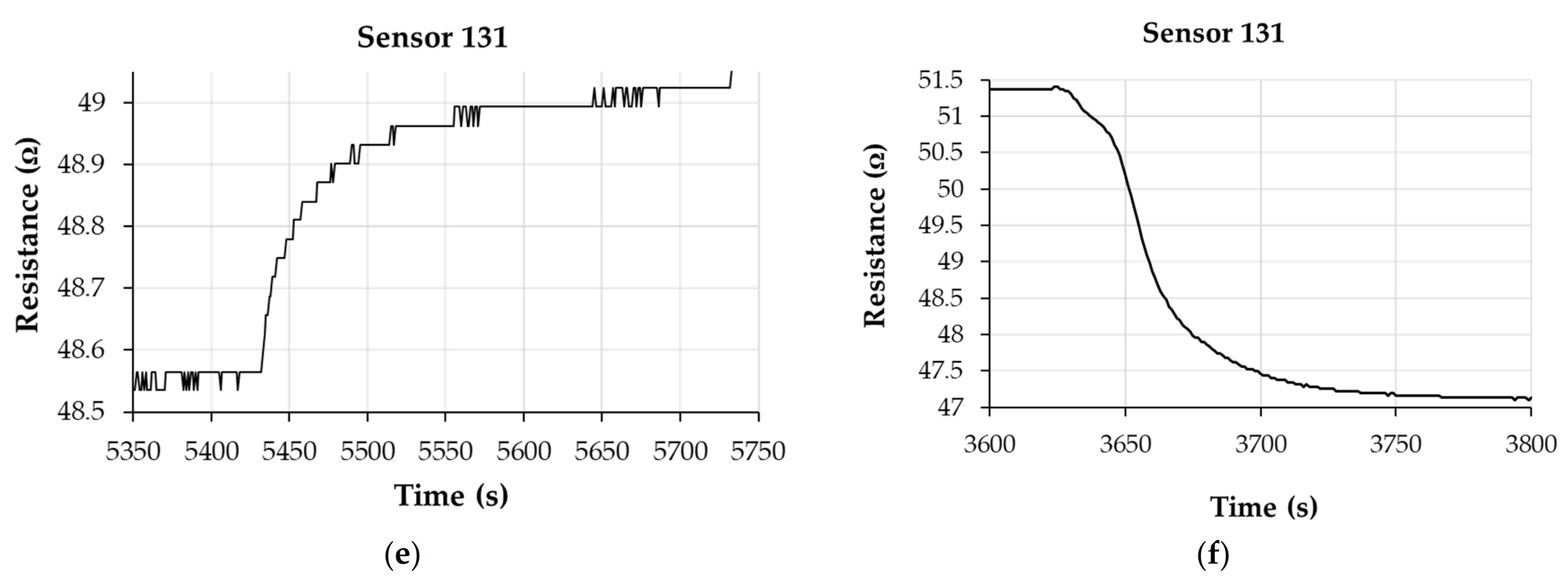
3. Results and Discussion
- Sensor 111—resisitive sensor which employed sensing layer based on GO/CNHox/PVP at 1/1/1 ratio (w/w/w);
- Sensor 121—resisitive sensor which employed sensing layer based on GO/CNHox/PVP at 1/2/1 ratio (w/w/w);
- Sensor 131—resisitive sensor which employed sensing layer based on GO/CNHox/PVP at 1/3/1 ratio (w/w/w);
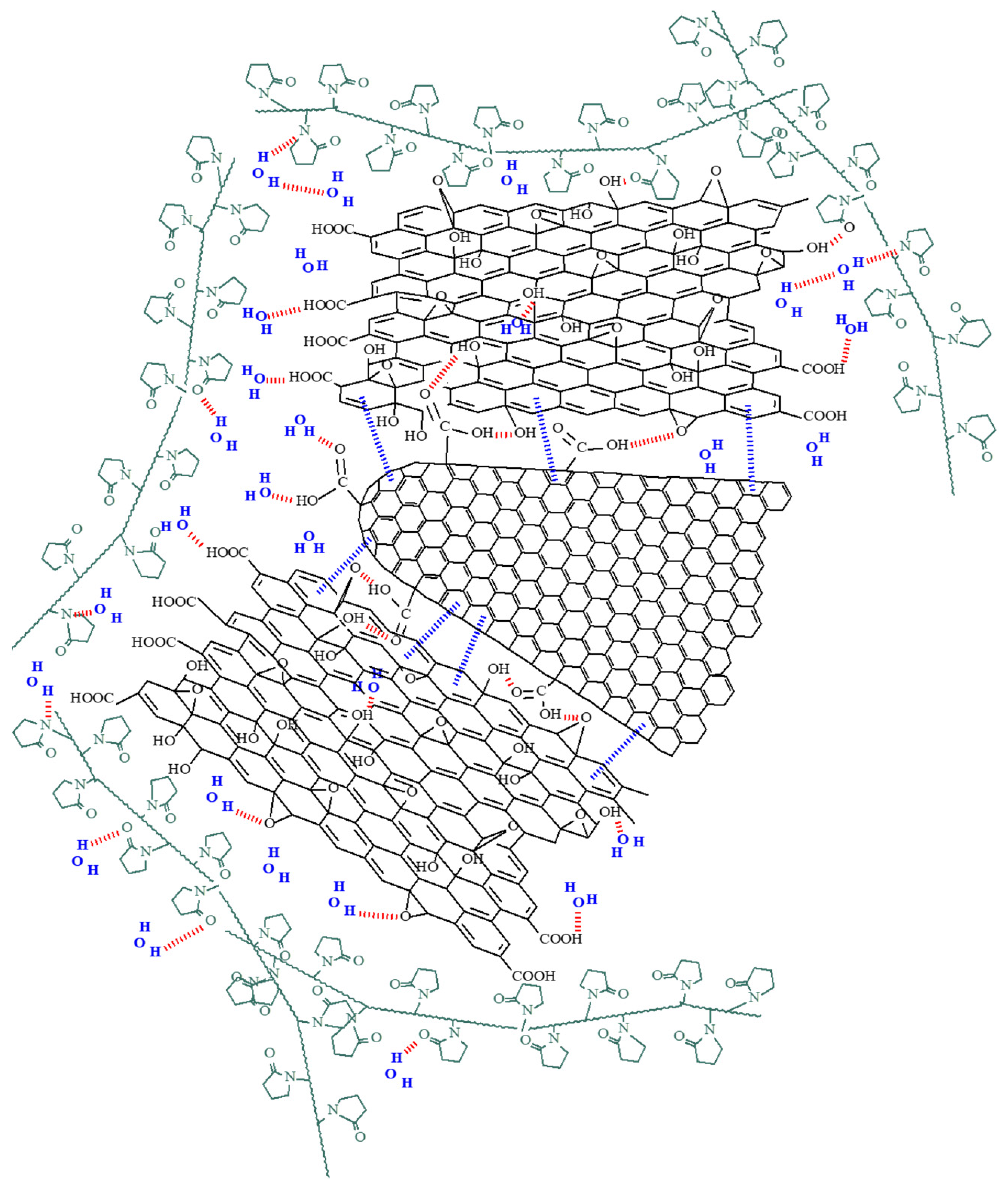
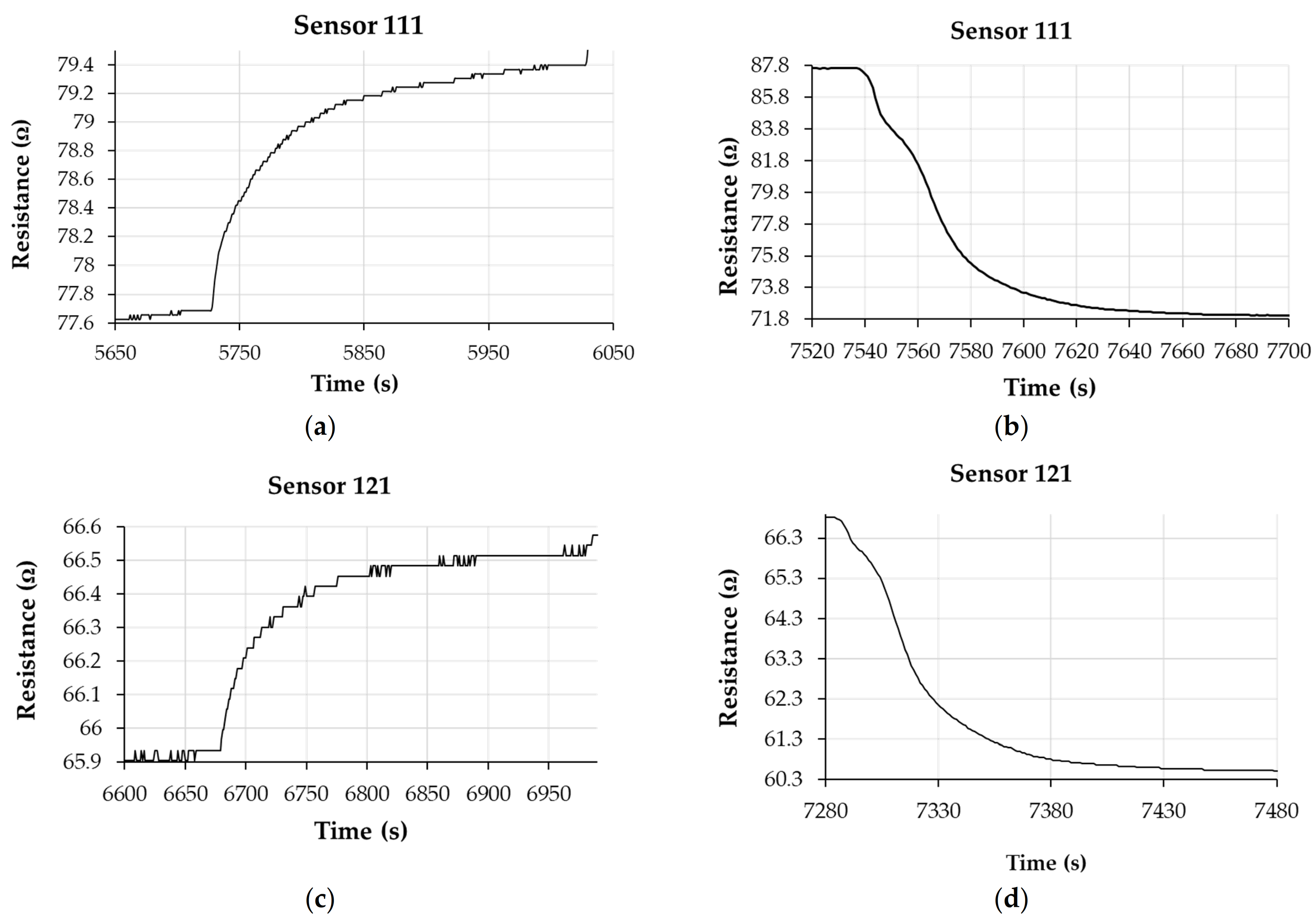
Analysis of Sensing Mechanism
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.Y.; Lee, G.B. Humidity sensors: A review. Sens. Lett. 2005, 3, 1–15. [Google Scholar] [CrossRef]
- Sikarwar, S.; Yadav, B.C. Opto-electronic humidity sensor: A review. Sens. Actuators A Phys. 2015, 233, 54–70. [Google Scholar] [CrossRef]
- De Luca, A.; Santra, S.; Ghosh, R.; Ali, S.Z.; Gardner, J.W.; Guha, P.K.; Udrea, F. Temperature-modulated graphene oxide resistive humidity sensor for indoor air quality monitoring. Nanoscale 2016, 8, 4565–4572. [Google Scholar] [CrossRef] [PubMed]
- Schubert, P.J.; Nevin, J.H. A polyimide-based capacitive humidity sensor. IEEE Trans. Electron Devices 1985, 32, 1220–1223. [Google Scholar] [CrossRef]
- Wu, T.T.; Chen, Y.Y.; Chou, T.H. A high sensitivity nanomaterial based S.A.W. humidity sensor. J. Phys. D Appl. Phys. 2008, 41, 085101. [Google Scholar] [CrossRef]
- Okcan, B.; Akin, T. A thermal conductivity based humidity sensor in a standard CMOS process. In Proceedings of the 17th IEEE International Conference on Micro Electro Mechanical Systems. Maastricht MEMS 2004 Technical Digest, Maastricht, The Netherlands, 25–29 January 2004; IEEE: Maastricht, The Netherlands, 2004; pp. 552–555. [Google Scholar]
- Xu, L.; Fanguy, J.C.; Soni, K.; Tao, S. Optical fiber humidity sensor based on evanescent-wave scattering. Opt. Lett. 2004, 29, 1191–1193. [Google Scholar] [CrossRef]
- Roveti, D.K. Choosing a humidity sensor: A review of three technologies this discussion of the operating principles of capacitive, resisitive, and thermal conductivity humidity sensors also addresses their advantages, disadvantages, and applications. Sens. J. Appl. Sens. Technol. 2001, 18, 54–58. [Google Scholar]
- Toloshniak, T.; Guhel, Y.; Besq, A.; Boudart, B. First results of humidity sensors based on CeO2 thick film deposited by a new deposition technique from a suspension of nanoparticles. Microelectron. Eng. 2019, 207, 7–14. [Google Scholar] [CrossRef]
- Kiasari, N.M.; Soltanian, S.; Gholamkhass, B.; Servati, P. Room temperature ultra-sensitive resistive humidity sensor based on single zinc oxide nanowire. Sens. Actuators A Phys. 2012, 182, 101–105. [Google Scholar] [CrossRef]
- Kuang, Q.; Lao, C.; Wang, Z.L.; Xie, Z.; Zheng, L. High-sensitivity humidity sensor based on a single SnO2 nanowire. J. Am. Chem. Soc. 2007, 129, 6070–6071. [Google Scholar] [CrossRef]
- Lee, C.W.; Kim, Y.; Joo, S.W.; Gong, M.S. Resistive humidity sensor using polyelectrolytes based on new-type mutually cross-linkable copolymers. Sens. Actuators B Chem. 2003, 88, 21–29. [Google Scholar] [CrossRef]
- Son, S. Polymeric Humidity Sensor Using Phosphonium Salt-Containing Polymers. Sens. Actuators B Chem. 2002, 86, 168–173. [Google Scholar] [CrossRef]
- Kuş, M.; Okur, S. Electrical characterization of PEDOT: P.S.S. beyond humidity saturation. Sens. Actuators B Chem. 2009, 143, 177–181. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Polyaniline nanofiber humidity sensor prepared by electrospinning. Sens. Actuators B Chem. 2012, 161, 967–972. [Google Scholar] [CrossRef]
- Wang, R.; Wang, D.; Zhang, Y.; Zheng, X. Humidity Sensing Properties of Bi0.5(Na0.85K0.15)0.5Ti0.97Zr0.03O3 Microspheres: Effect of A and B Sites Co-Substitution. Sens. Actuators B Chem. 2014, 190, 305–310. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, C.; Liu, H.; Xiong, Y.; Zhang, Z. A Rapid-Response Humidity Sensor Based on BaNbO3 Nanocrystals. Sens. Actuators B Chem. 2009, 136, 128–132. [Google Scholar] [CrossRef]
- Lee, J.; Cho, D.; Jeong, Y. A resistive-type sensor based on flexible multi-walled carbon nanotubes and polyacrylic acid composite films. Solid State Electron. 2013, 87, 80–84. [Google Scholar] [CrossRef]
- Dai, H.; Feng, N.; Li, J.; Zhang, J.; Li, W. Chemiresistive humidity sensor based on chitosan/zinc oxide/single-walled carbon nanotube composite film. Sens. Actuators B Chem. 2019, 283, 786–792. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Ma, W.; Ling, W. Quartz crystal microbalance humidity sensors based on nanodiamond sensing films. IEEE Trans. Nanotechnol. 2014, 13, 386–393. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Ding, X.; Yu, X.; Zhao, X.; Chen, X. Facile fabrication of flower-like MoS2/nanodiamond nanocomposite toward high-performance humidity detection. Sens. Actuators B Chem. 2020, 317, 128168. [Google Scholar] [CrossRef]
- Radeva, E.; Georgiev, V.; Spassov, L.; Koprinarov, N.; Kanev, S. Humidity adsorptive properties of thin fullerene layers studied by means of quartz micro-balance. Sens. Actuators B Chem. 1997, 42, 11–13. [Google Scholar] [CrossRef]
- Tang, K.; Chen, X.; Ding, X.; Zhao, X.; Yu, X.; Yu, X.; Chen, X. Humidity Sensitivity Enhancement Effects of Metal Nanoparticles Loaded Fullerene. Sens. Actuators B Chem. 2020, 329, 129086. [Google Scholar] [CrossRef]
- Smith, A.D.; Elgammal, K.; Niklaus, F.; Delin, A.; Fischer, A.C.; Vaziri, S.; Lemme, M. Resistive graphene humidity sensors with rapid and direct electrical readout. Nanoscale 2015, 7, 19099–19109. [Google Scholar] [CrossRef]
- Popov, V.I.; Nikolaev, D.V.; Timofeev, V.B.; Smagulova, S.A.; Antonova, I.V. Graphene-based humidity sensors: The origin of alternating resistance change. Nanotechnology 2017, 28, 355501. [Google Scholar] [CrossRef]
- Monereo, O.; Claramunt, S.; de Marigorta, M.M.; Boix, M.; Leghrib, R.; Prades, J.D.; Cornet, A.; Merino, P.; Merino, C.; Cirera, A. Flexible sensor based on carbon nanofibers with multifunctional sensing features. Talanta 2013, 107, 239–247. [Google Scholar] [CrossRef]
- Chu, J.; Peng, X.; Feng, P.; Sheng, Y.; Zhang, J. Study of humidity sensors based on nanostructured carbon films produced by physical vapor deposition. Sens. Actuators B Chem. 2013, 178, 508–513. [Google Scholar] [CrossRef]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Marinescu, M.R. New Sensitive Layer for Relative Humidity Sensor and Its Manufacturing Method. RO 134519 A2, 30 October 2020. [Google Scholar]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Avramescu, V.R.; Marinescu, M.R. Humidity Sensor. RO 134520 A2, 30 October 2020. [Google Scholar]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Marinescu, M.R. New Chemiresistive Sensor for Humidity Detection. RO 134521 A2, 30 October 2020. [Google Scholar]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Marinescu, M.R. Sensitive Layer for Humidity Sensor with Surface Acoustic Waves. RO 134499 A2, 30 October 2020. [Google Scholar]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, K. Nano-aggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Lodermeyer, F.; Costa, R.D.; Guldi, D.M. Single-Walled Carbon Nanohorn-Based Dye-Sensitized Solar Cells. ECS J. Solid-State Sci. Technol. 2017, 6, M3140. [Google Scholar] [CrossRef]
- Kagkoura, A.; Tagmatarchis, N. Carbon nanohorn-based electrocatalysts for energy conversion. Nanomaterials 2020, 10, 1407. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, properties, functionalization and applications of carbon nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Unni, S.M.; Ramadas, S.; Illathvalappil, R.; Bhange, S.N.; Kurungot, S. Surface-modified single wall carbon nanohorn as an effective electrocatalyst for platinum-free fuel cel cathodes. J. Mater. Chem. A 2015, 3, 4361–4367. [Google Scholar] [CrossRef]
- Xu, J.; Yudasaka, M.; Kouraba, S.; Sekido, M.; Yamamoto, Y.; Iijima, S. Single wall carbon nanohorn as a drug carrier for controlled release. Chem. Phys. Lett. 2008, 461, 189–192. [Google Scholar] [CrossRef]
- Sani, E.; Mercatelli, L.; Barison, S.; Pagura, C.; Agresti, F.; Colla, L.; Sansoni, P. Potential of carbon nanohorn-based suspensions for solar thermal collectors. Sol. Energy Mater. Sol. Cells 2011, 95, 2994–3000. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Jin, S.; Zhang, S.; Wang, H.; Hiralal, P.; Amaratunga, G.A.; Zhou, H. Carbon nanohorns/nanotubes: An effective binary conductive additive in the cathode of high energy-density zinc-ion rechargeable batteries. Carbon 2020, 167, 431–438. [Google Scholar] [CrossRef]
- Bekyarova, E.; Murata, K.; Yudasaka, M.; Kasuya, D.; Iijima, S.; Tanaka, H.; Kahoh, H.; Kaneko, K. Single-wall nanostructured carbon for methane storage. J. Phys. Chem. B 2003, 107, 4681–4684. [Google Scholar] [CrossRef]
- Sano, N.; Kinugasa, M.; Otsuki, F.; Suehiro, J. Gas sensor using single-wall carbon nanohorns. Adv. Powder Technol. 2007, 18, 455–466. [Google Scholar] [CrossRef]
- Suehiro, J.; Sano, N.; Zhou, G.; Imakiire, H.; Imasaka, K.; Hara, M. Application of dielectrophoresis to fabrication of carbon nanohorn gas sensor. J. Electrost. 2006, 64, 408–415. [Google Scholar] [CrossRef]
- Cobianu, C.; Serban, B.C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Pachiu, C.; Bita, B.; Bumbac, M.; Nicolescu, C.M.; Cobianu, C. Organic–Inorganic Ternary Nanohybrids of Single-Walled Carbon Nanohorns for Room Temperature Chemiresistive Ethanol Detection. Nanomaterials 2020, 10, 2552. [Google Scholar] [CrossRef]
- Cobianu, C.; Serban, B.C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Bumbac, M.; Nicolescu, C.M.; Cobianu, C. Room Temperature Chemiresistive Ethanol Detection by Ternary Nanocomposites of Oxidized Single Wall Carbon Nanohorn (ox-SWCNH). In Proceedings of the 2020 International Semiconductor Conference (C.A.S.), Sinaia, Romania, 7–9 October 2020; pp. 13–16. [Google Scholar]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Bumbac, M.; Nicolescu, C.M. Oxidized Carbon Nanohorns as Novel Sensing Layer for Resistive Humidity Sensor. Acta Chim. Slov. 2020, 67, 1–7. [Google Scholar] [CrossRef]
- Serban, B.C.; Bumbac, M.; Buiu, O.; Cobianu, C.; Brezeanu, M.; Nicolescu, C. Carbon nanohorns and their nanocomposites: Synthesis, properties and applications. A concise review. Ann. Acad. Rom. Sci. Ser. Math. Appl. 2018, 11, 5–18. [Google Scholar]
- Serban, B.C.; Buiu, O.; Dumbravescu, N.; Cobianu, C.; Avramescu, V.; Brezeanu, M.; Pachiu, C.; Nicolescu, C.M. Oxidized Carbon Nanohorn-Hydrophilic Polymer Nanocomposite as the Resistive Sensing Layer for Relative Humidity. Anal. Lett. 2020, 54, 527–540. [Google Scholar] [CrossRef]
- Serban, B.C.; Cobianu, C.; Dumbravescu, N.; Buiu, O.; Avramescu, V.; Bumbac, M.; Nicolescu, C.M.; Cobianu, C.; Brezeanu, M. Electrical Percolation Threshold in Oxidized Single Wall Carbon Nanohorn-Polyvinylpyrrolidone Nanocomposite: A Possible Application for High Sensitivity Resistive Humidity Sensor. In Proceedings of the International Semiconductor Conference (CAS), Sinaia, Romania, 7–9 October 2020; pp. 239–242. [Google Scholar]
- Șerban, B.C.; Cobianu, C.; Buiu, O.; Dumbrăvescu, N.; Avramescu, V.; Brezeanu, M.; Marinescu, M.R. Ternary oxidized carbon nanohorn-based nanohybrid as sensing layer for resistive humidity sensor 3RD. In Proceedings of the International Conference on Emerging Technologies in Materials Engineering, Bucharest, Romania, 6–8 November 2019; p. 83. [Google Scholar]
- Șerban, B.C.; Cobianu, C.; Buiu, O.; Dumbrăvescu, N.; Avramescu, V.; Brezeanu, M.; Marinescu, M.R. Ternary hydrophilic carbon nanohorn/ZnO/PVP nanohybrid structure for room temperature resistive humidity sensing, 3RD. In Proceedings of the International Conference on Emerging Technologies in Materials Engineering, Bucharest, Romania, 6–8 November 2019; p. 84. [Google Scholar]
- Yu, H.W.; Kim, H.K.; Kim, T.; Bae, K.M.; Seo, S.M.; Kim, J.M.; Kang, T.J.; Kim, Y.H. Self-powered humidity sensor based on graphene oxide composite film intercalated by poly (sodium 4-styrenesulfonate). ACS Appl. Mater. Interfaces 2014, 6, 8320–8326. [Google Scholar] [CrossRef]
- Borini, S.; White, R.; Wei, D.; Astley, M.; Haque, S.; Spigone, E.; Harris, N.; Kivioja, J.; Ryhanen, T. Ultrafast graphene oxide humidity sensors. ACS Nano 2013, 7, 11166–11173. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, D.; Li, P.; Zhou, X.; Zong, X.; Dong, G. Facile fabrication of high-performance Q.C.M. humidity sensor based on layer-by-layer self-assembled polyaniline/graphene oxide nanocomposite film. Sens. Actuators B Chem. 2018, 255, 1869–1877. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Guo, H.; Wu, Z.; Li, X. Humidity sensing behaviors of graphene oxide-silicon bi-layer flexible structure. Sens. Actuators B Chem. 2012, 161, 1053–1058. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Șerban, B.C.; Buiu, O.; Cobianu, C.; Avramescu, V.; Dumbrăvescu, N.; Brezeanu, M.; Nicolescu, C.M.; Marinescu, R. Ternary Carbon-Based Nanocomposite as Sensing Layer for Resistive Humidity Sensor. Multidiscip. Digit. Publ. Inst. Proc. 2019, 29, 114. [Google Scholar] [CrossRef]
- Song, P.; Xu, Z.; Wu, Y.; Cheng, Q.; Guo, Q.; Wang, H. Super-tough artificial nacre based on graphene oxide via synergistic interface interactions of π-π stacking and hydrogen bonding. Carbon 2017, 111, 807–812. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Chen, X.; Ding, X.; Zhao, X. High-sensitive humidity sensor based on graphene oxide with evenly dispersed multiwalled carbon nanotubes. Mater. Chem. Phys. 2018, 207, 135–140. [Google Scholar] [CrossRef]
- Ajito, K.; Sukamto, J.P.H.; Nagahara, L.A.; Hashimoto, K.; Fujishima, A. Strain imaging analysis of Si using Raman microscopy. J. Vac. Sci. Technol. A 1995, 13, 1234–1238. [Google Scholar] [CrossRef]
- Jabbarnia, A.; Asmatulu, R. Synthesis and Characterization of PVdF/PVP-Based Electrospun Membranes as Separators for Supercapacitor Applications. J. Mater. Sci. Technol. Res. 2015, 2, 43–51. [Google Scholar]
- Fatima, Q.; Haidry, A.A.; Yao, Z.; He, Y.; Li, Z.; Sun, L.; Xie, L. The critical role of hydroxyl groups in water vapor sensing of graphene oxide. Nanoscale Adv. 2019, 1, 1319–1330. [Google Scholar] [CrossRef]
- Santra, S.; Hu, G.; Howe RC, T.; De Luca, A.; Ali, S.Z.; Udrea, F.; Gardner, J.W.; Ray, S.K.; Guha, P.K.; Hasan, T. CMOS integration of inkjet-printed graphene for humidity sensing. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Cao, C.L.; Hu, C.G.; Fang, L.; Wang, S.X.; Tian, Y.S.; Pan, C.Y. Humidity Sensor Based on Multi-Walled Carbon Nanotube Thin Films. J. Nanomater. 2011, 2011, 5. [Google Scholar] [CrossRef]
- Li, B.; Weng, X.; Sun, X.; Zhang, Y.; Lv, X.; Gu, G. Facile synthesis of Fe3O4/reduced graphene oxide/polyvinyl pyrrolidone ternary composites and their enhanced microwave absorbing properties. J. Saudi Chem. Soc. 2018, 22, 979–984. [Google Scholar] [CrossRef]
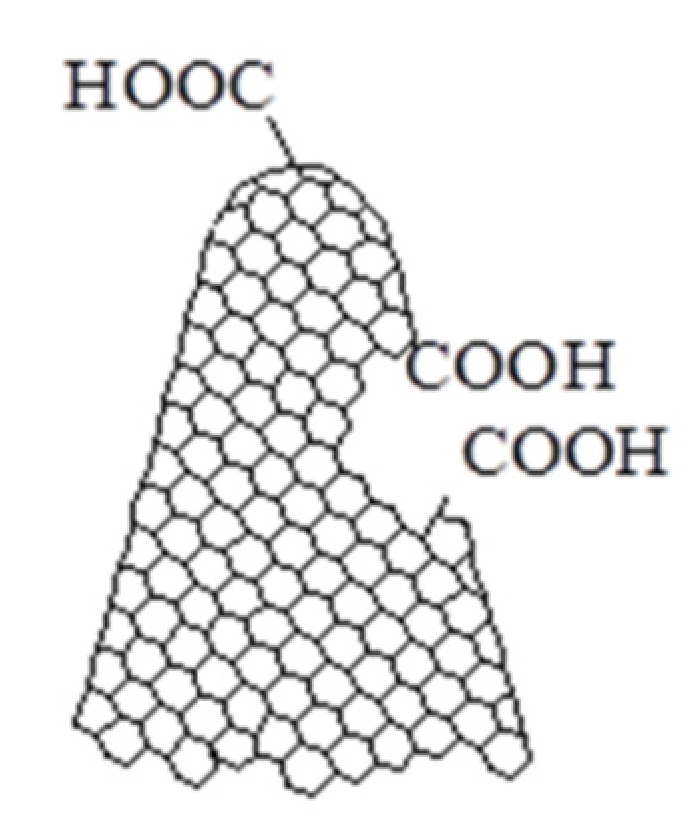
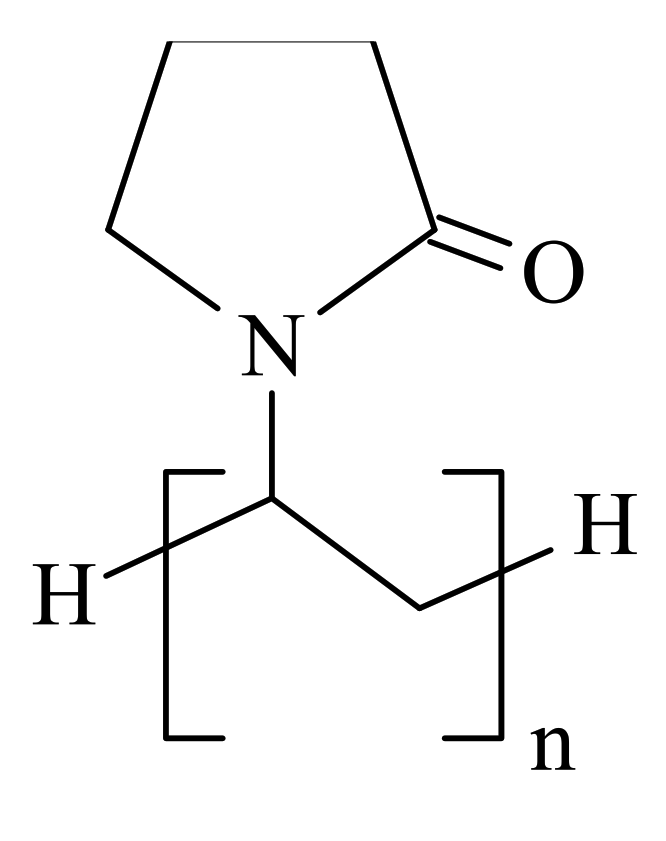
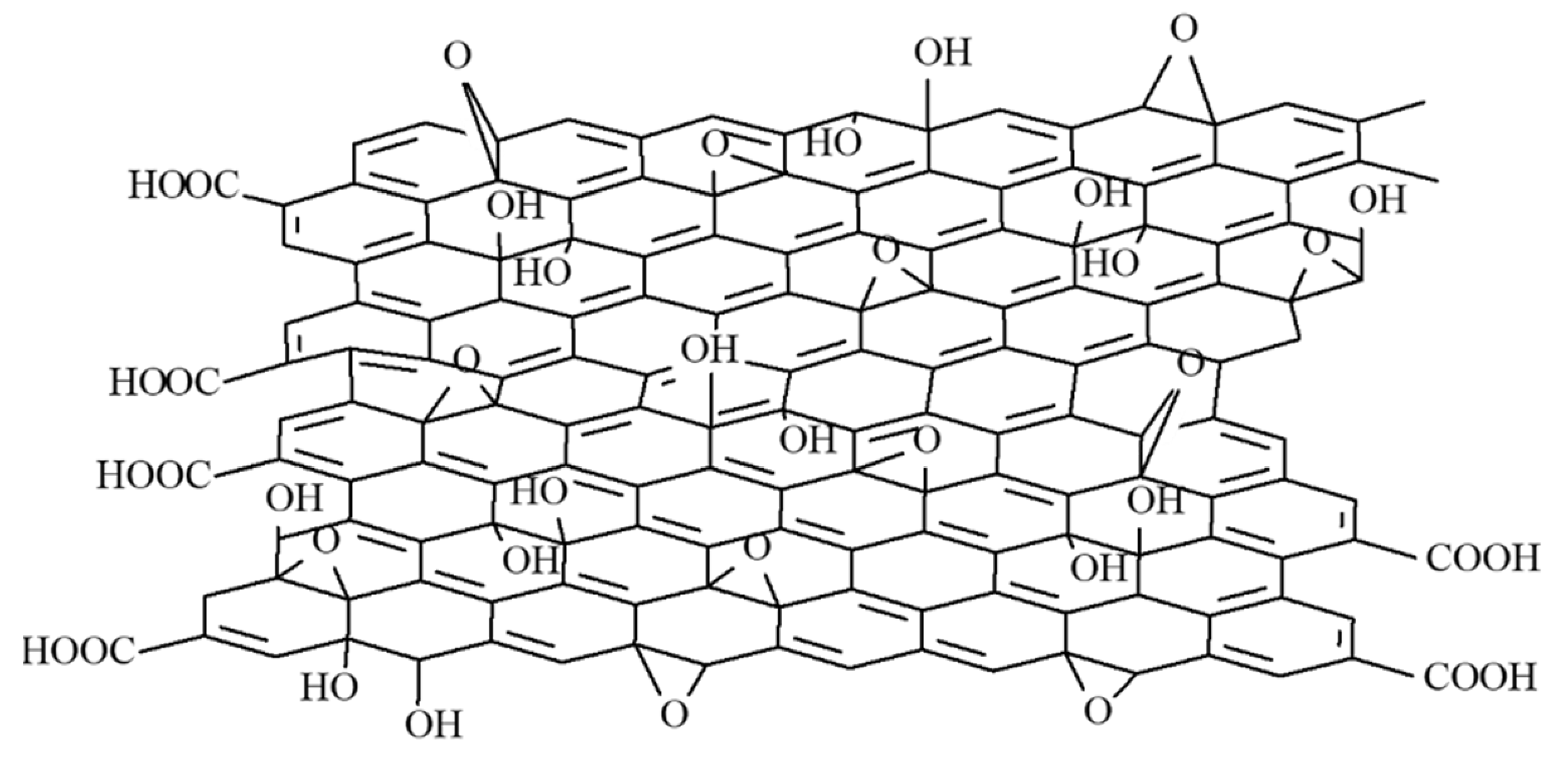
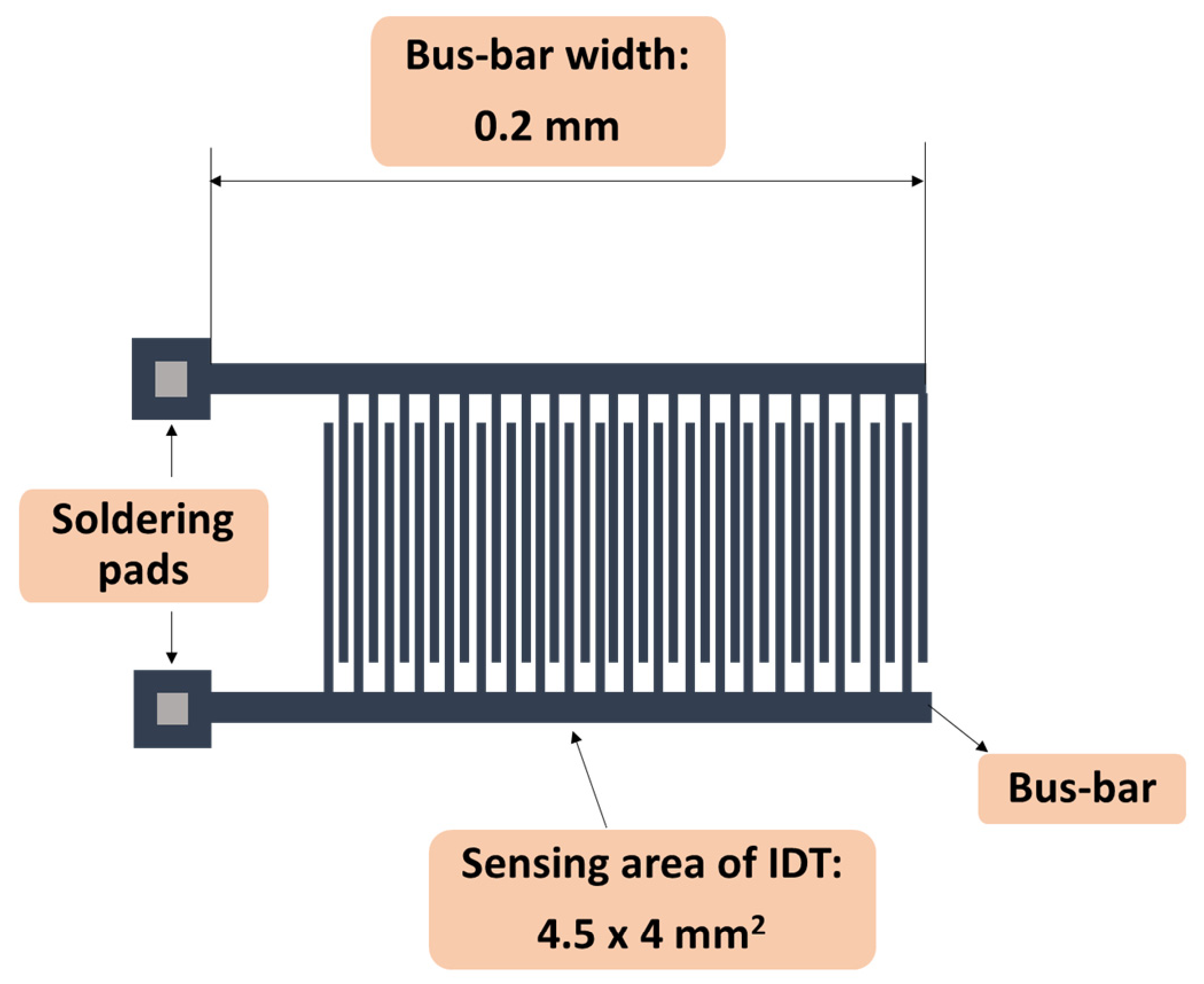
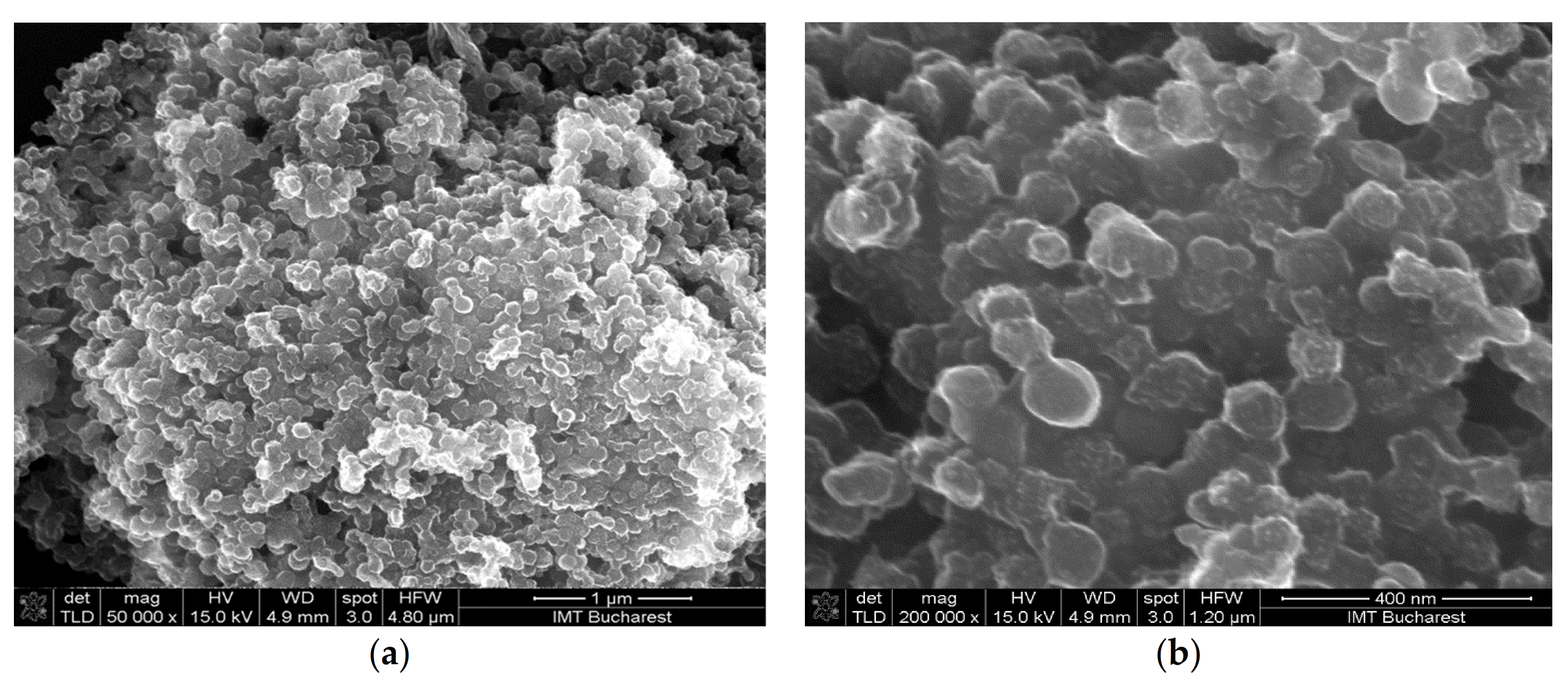
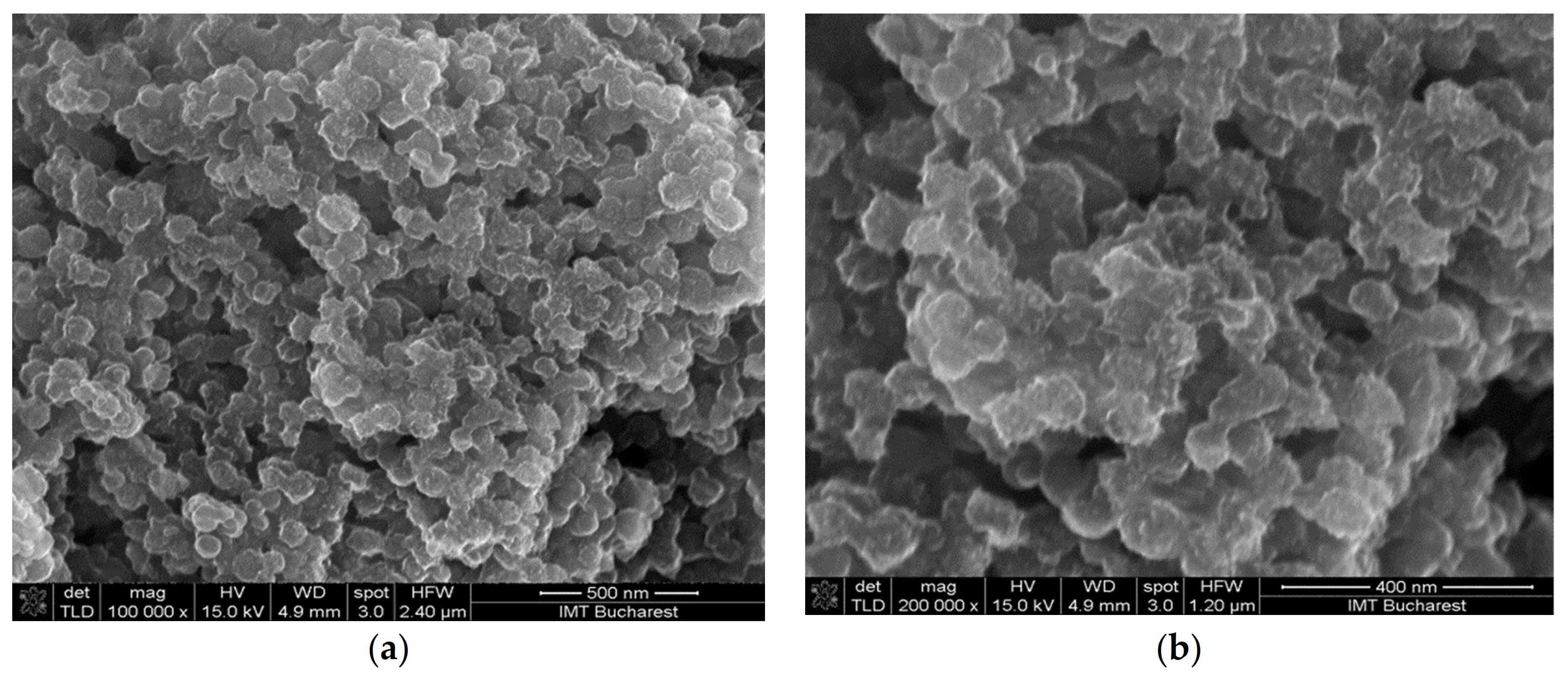
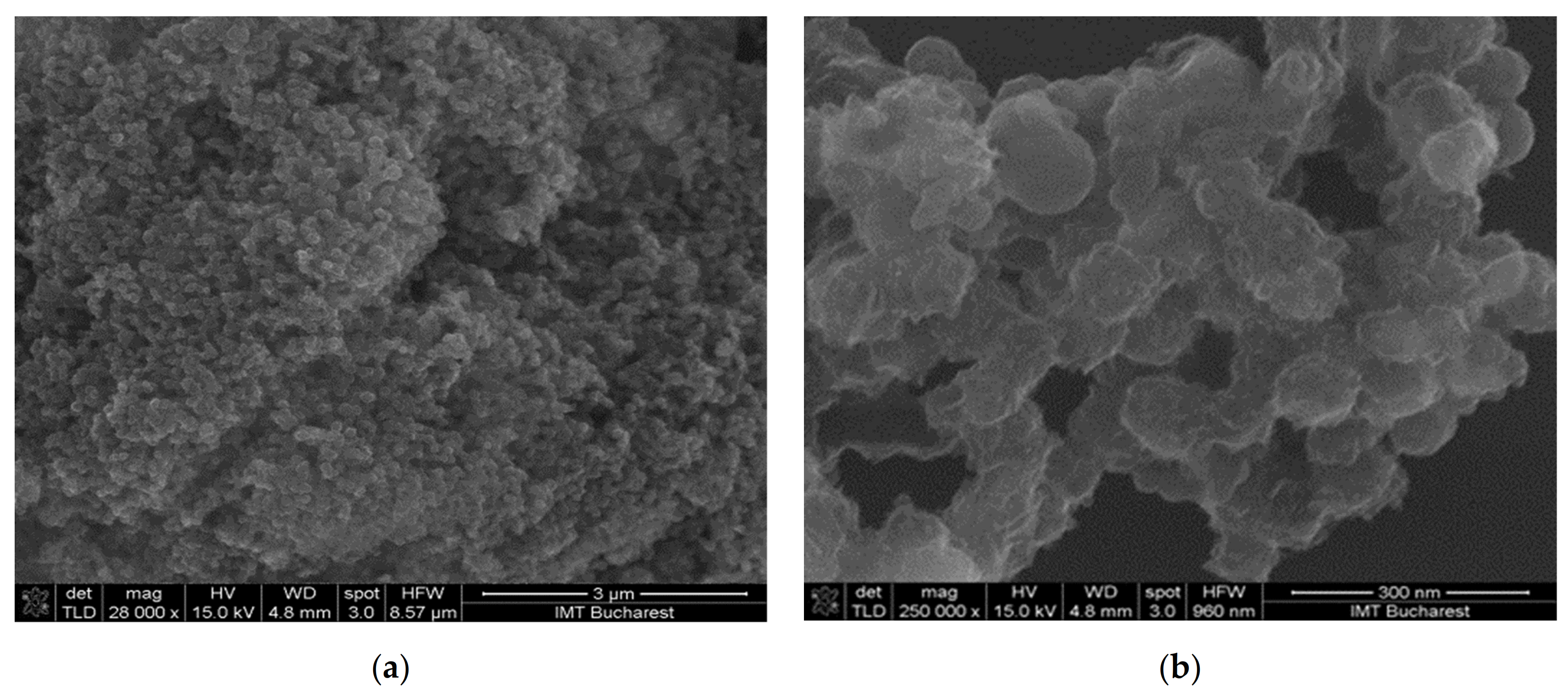





| Sensing Layer | Sensitivity |
|---|---|
| CNHox [45] | 0.013–0.021 |
| PVP + CNHox 1/1 [47] | 0.020–0.058 |
| PVP + CNHox 2/1 [47] | 0.017–0.025 |
| GO-CNHox–PVP 1/1/1 | 0.150–0.200 |
| GO-CNHox–PVP 1/2/1 | 0.063–0.070 |
| GO-CNHox–PVP 1/3/1 | 0.043–0.051 |
| Recovery Time (s) | ||||||
|---|---|---|---|---|---|---|
| Commercial Sensor | Sensor 111 | Commercial Sensor | Sensor 121 | Commercial Sensor | Sensor 131 | |
| Recovery time | 121 | 62 | 111 | 73 | 114 | 73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, B.-C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Pachiu, C.; Craciun, G.; et al. Ternary Nanocomposites Based on Oxidized Carbon Nanohorns as Sensing Layers for Room Temperature Resistive Humidity Sensing. Materials 2021, 14, 2705. https://doi.org/10.3390/ma14112705
Serban B-C, Cobianu C, Buiu O, Bumbac M, Dumbravescu N, Avramescu V, Nicolescu CM, Brezeanu M, Pachiu C, Craciun G, et al. Ternary Nanocomposites Based on Oxidized Carbon Nanohorns as Sensing Layers for Room Temperature Resistive Humidity Sensing. Materials. 2021; 14(11):2705. https://doi.org/10.3390/ma14112705
Chicago/Turabian StyleSerban, Bogdan-Catalin, Cornel Cobianu, Octavian Buiu, Marius Bumbac, Niculae Dumbravescu, Viorel Avramescu, Cristina Mihaela Nicolescu, Mihai Brezeanu, Cristina Pachiu, Gabriel Craciun, and et al. 2021. "Ternary Nanocomposites Based on Oxidized Carbon Nanohorns as Sensing Layers for Room Temperature Resistive Humidity Sensing" Materials 14, no. 11: 2705. https://doi.org/10.3390/ma14112705
APA StyleSerban, B.-C., Cobianu, C., Buiu, O., Bumbac, M., Dumbravescu, N., Avramescu, V., Nicolescu, C. M., Brezeanu, M., Pachiu, C., Craciun, G., & Radulescu, C. (2021). Ternary Nanocomposites Based on Oxidized Carbon Nanohorns as Sensing Layers for Room Temperature Resistive Humidity Sensing. Materials, 14(11), 2705. https://doi.org/10.3390/ma14112705











