Using TOF-SIMS Spectrometry to Study the Kinetics of the Interfacial Retro Diels–Alder Reaction
Abstract
1. Introduction
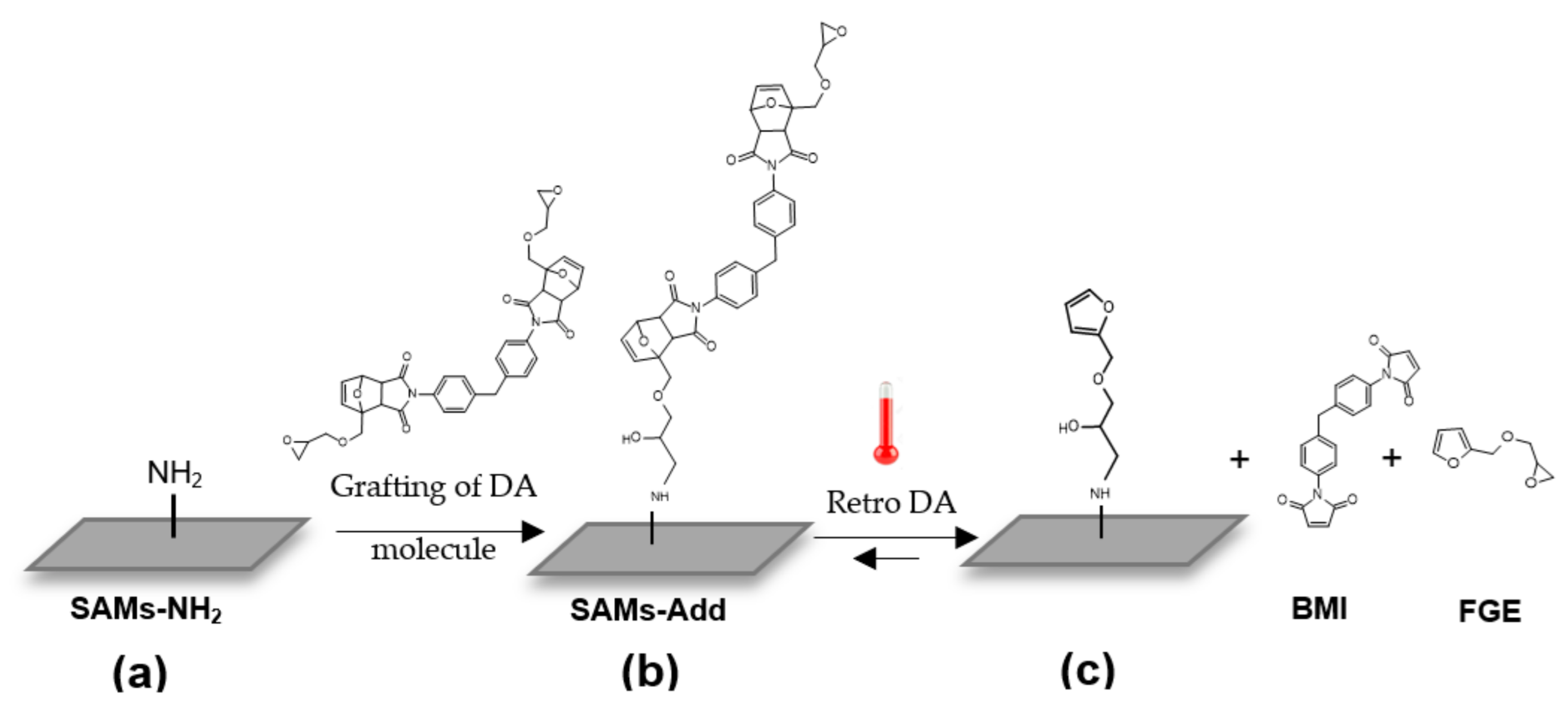
2. Materials and Methods
3. Results and Discussions
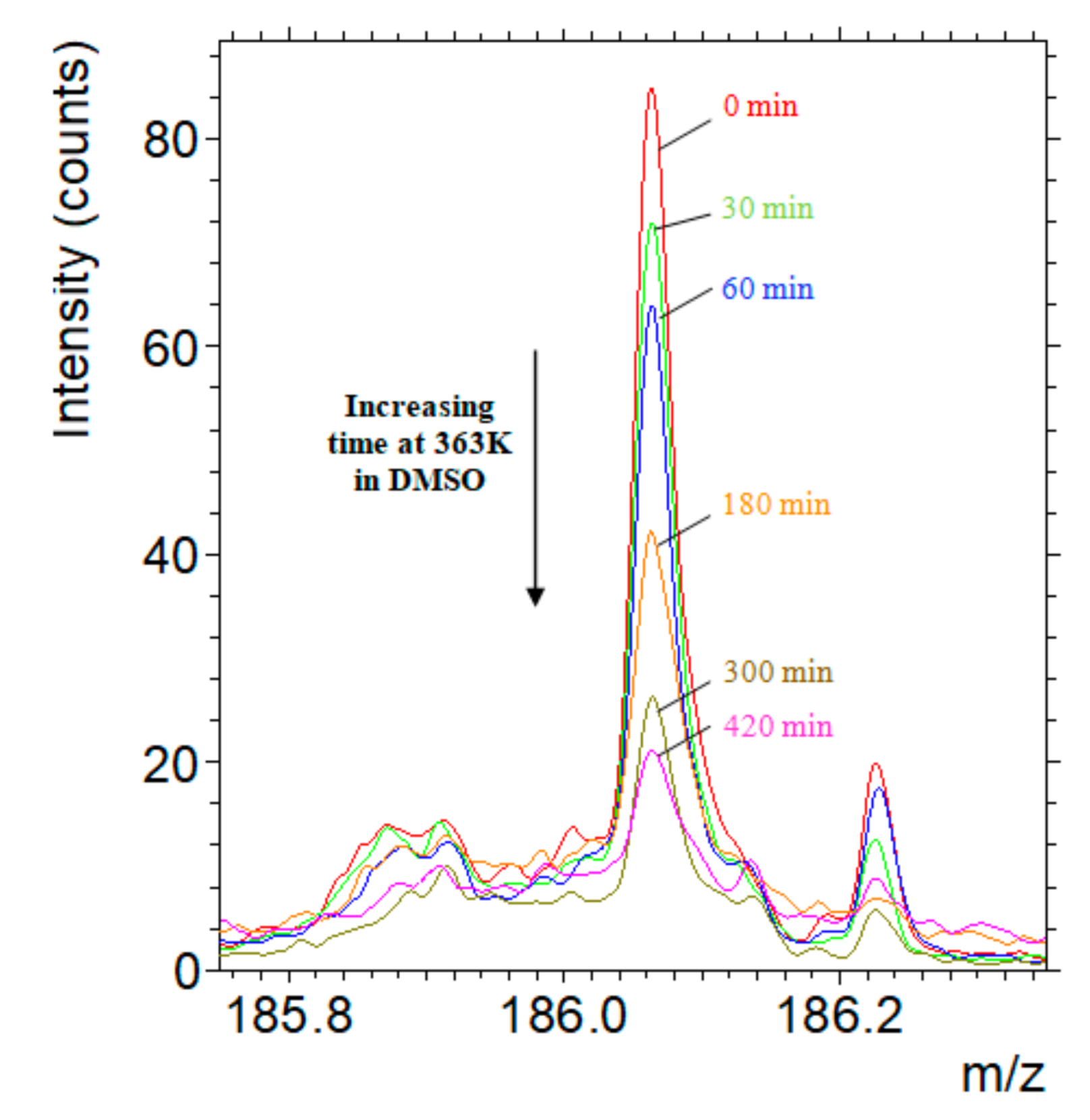
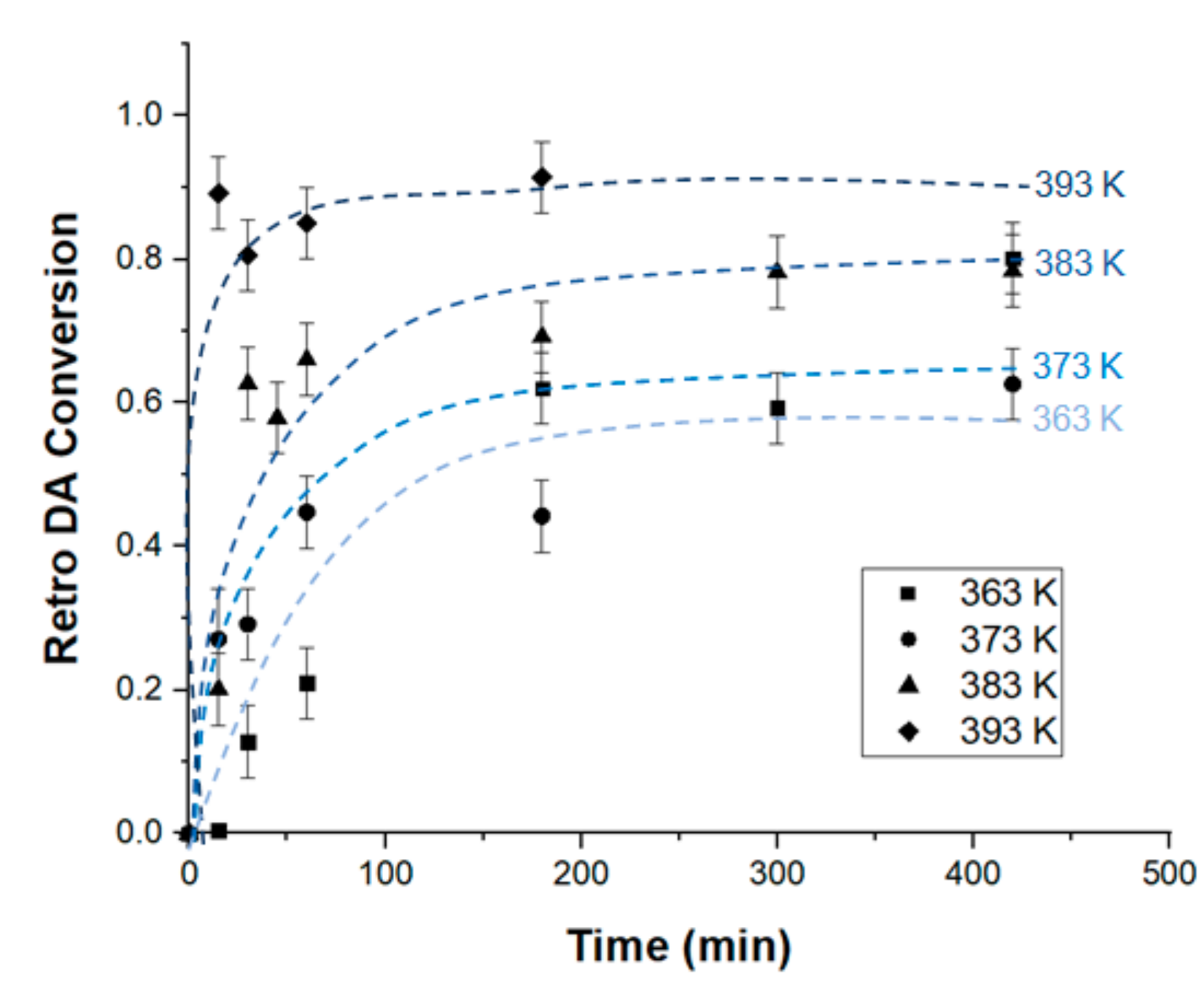
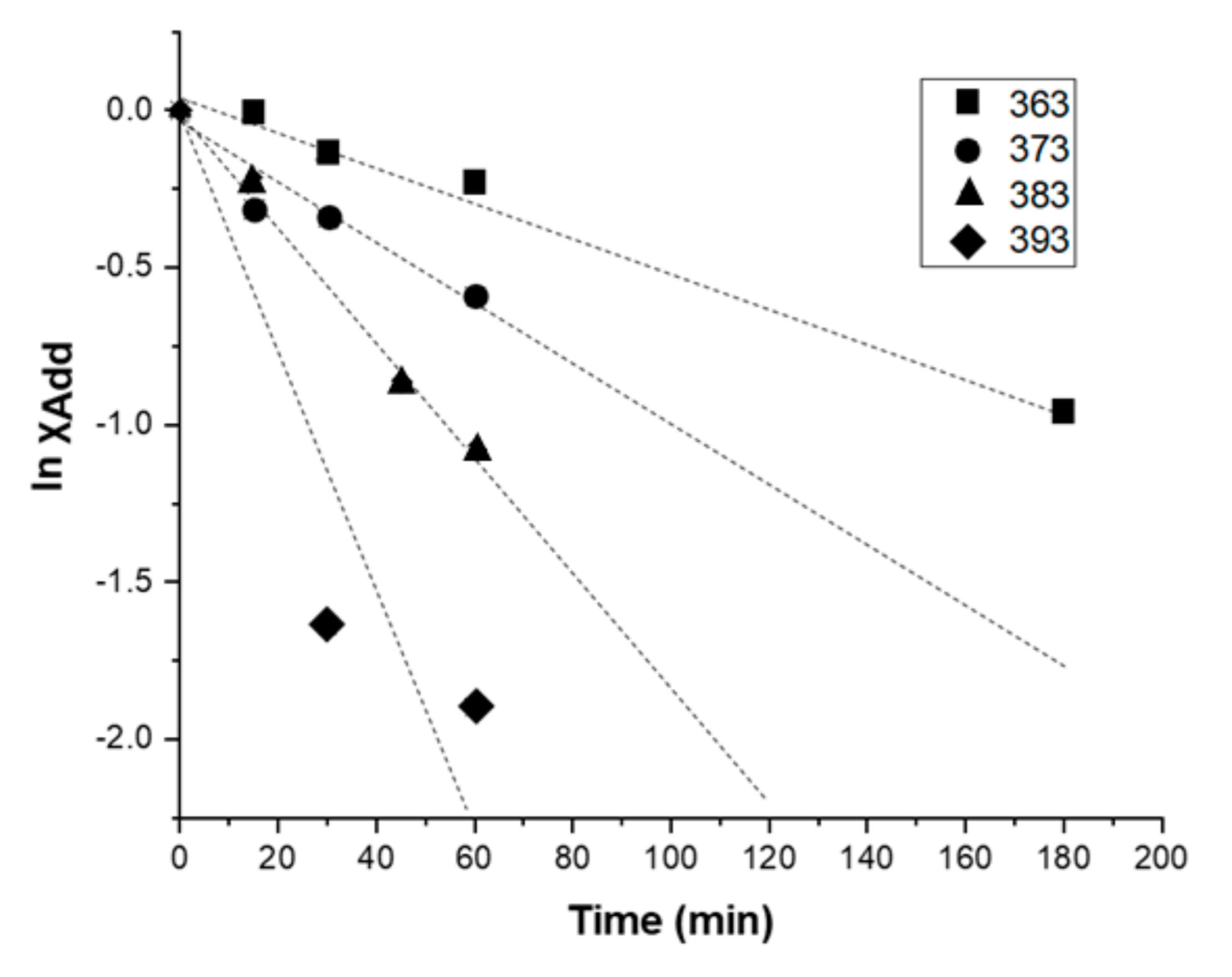
3.1. Investigation of Interfacial Retro DA Reaction Using TOF-SIMS
3.2. Investigation of Retro DA Reaction Using 1H NMR Spectroscopy
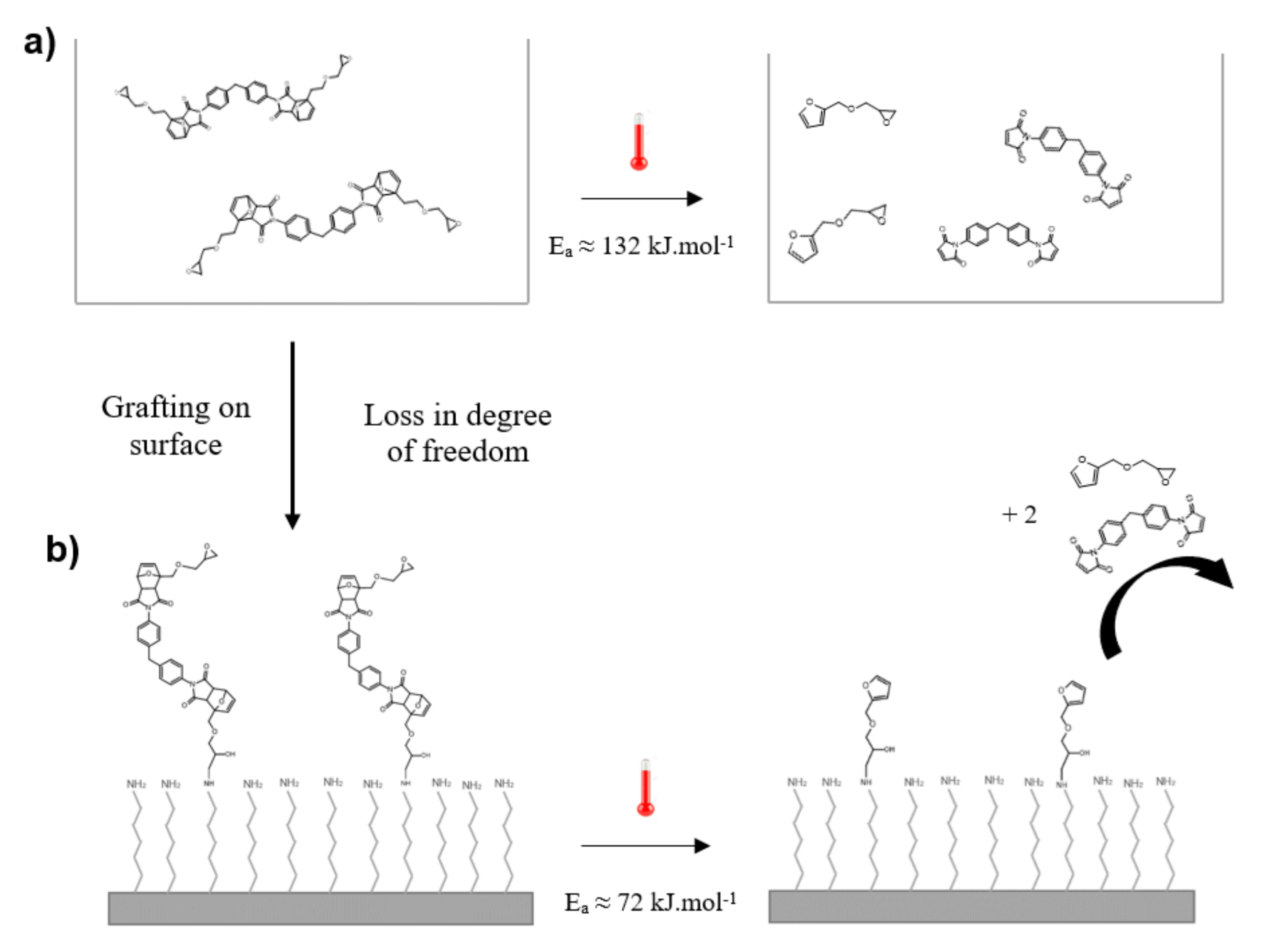
3.3. Discussion of Activation Energy Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno-Couranjou, M.; Manakhov, A.; Boscher, N.D.; Pireaux, J.-J.; Choquet, P. A Novel Dry Chemical Path Way for Diene and Dienophile Surface Functionalization toward Thermally Responsive Metal–Polymer Adhesion. ACS Appl. Mater. Interfaces 2013, 5, 8446–8456. [Google Scholar] [CrossRef] [PubMed]
- Siffer, F.; Roucoules, V.; Vallat, M.; Defoin, A. Synthesis of New Functionalized Cyclopentadienes To Reach Reversible Bonding between Two Substrates. Synthesis 2008, 4, 515–518. [Google Scholar] [CrossRef]
- Nimmo, C.M.; Shoichet, M.S. Regenerative Biomaterials that “Click”: Simple, Aqueous-Based Protocols for Hydrogel Synthesis, Surface Immobilization, and 3D Patterning. Bioconjug. Chem. 2011, 22, 2199–2209. [Google Scholar] [CrossRef]
- Preuss Corinna, M.; Goldmann Anja, S.; Trouillet, V.; Walther, A.; Barner-Kowollik, C. Biomimetic Dopamine-Diels–Alder Switches. Macromol. Rapid Commun. 2013, 34, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Nandivada, H.; Jiang, X.; Lahann, J. Click Chemistry: Versatility and Control in the Hands of Materials Scientists. Adv. Mater. 2007, 19, 2197–2208. [Google Scholar] [CrossRef]
- Vauthier, M.; Jierry, L.; Oliveira, J.C.; Hassouna, L.; Roucoules, V.; Gall, F.B. Interfacial Thermoreversible Chemistry on Functional Coatings: A Focus on the Diels–Alder Reaction. Adv. Funct. Mater. 2019, 29, 1806765. [Google Scholar] [CrossRef]
- Vauthier, M.; Jierry, L.; Mendez, M.L.M.; Durst, Y.-M.; Kelber, J.B.; Roucoules, V.; Gall, F.B.-L. Interfacial Diels–Alder Reaction between Furan-Functionalized Polymer Coatings and Maleimide-Terminated Poly(ethylene glycol). J. Phys. Chem. C 2019, 123, 4125–4132. [Google Scholar] [CrossRef]
- Chechik, V.; Crooks, R.M.; Stirling, C.J.M. Reactions and Reactivity in Self-Assembled Monolayers. Adv. Mater. 2000, 12, 1161–1171. [Google Scholar] [CrossRef]
- Vauthier, M.; Jierry, L.; Boulmedais, F.; Oliveira, J.C.; Clancy, K.F.A.; Simet, C.; Roucoules, V.; Gall, F.B.-L. Control of Interfacial Diels–Alder Reactivity by Tuning the Plasma Polymer Properties. Langmuir 2018, 34, 11960–11970. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Marzolin, C.; Terfort, A.; Whitesides, G.M. Formation and Reaction of Interchain Carboxylic Anhydride Groups on Self-Assembled Monolayers on Gold. Langmuir 1997, 13, 6704–6712. [Google Scholar] [CrossRef]
- Chan, E.W.L.; Yousaf, M.N.; Mrksich, M. Understanding the Role of Adsorption in the Reaction of Cyclopentadiene with an Immobilized Dienophile. J. Phys. Chem. A 2000, 104, 9315–9320. [Google Scholar] [CrossRef]
- Kwon, Y.; Mrksich, M. Dependence of the Rate of an Interfacial Diels−Alder Reaction on the Steric Environment of the Immobilized Dienophile: An Example of Enthalpy−Entropy Compensation. J. Am. Chem. Soc. 2002, 124, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Collman, J.P.; Devaraj, N.K.; Eberspacher, T.P.A.; Chidsey, C.E.D. Mixed Azide-Terminated Monolayers: A Platform for Modifying Electrode Surfaces. Langmuir 2006, 22, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Benninghoven, A. Chemical Analysis of Inorganic and Organic Surfaces and Thin Films by Static Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS). Angew. Chem. Int. Ed. 1994, 33, 1023–1043. [Google Scholar] [CrossRef]
- Eynde, X.V.; Bertrand, P. ToF-SIMS Quantification of Polystyrene Spectra Based on Principal Component Analysis (PCA). Surf. Interface Anal. 1997, 25, 878–888. [Google Scholar] [CrossRef]
- Zanderigo, F.; Ferrari, S.; Queirolo, G.; Pello, C.; Borgini, M. Quantitative TOF-SIMS Analysis of Metal Contamination on Silicon Wafers. Mater. Sci. Eng. B 2000, 73, 173–177. [Google Scholar] [CrossRef]
- Li, L.; Chan, C.-M.; Liu, S.; An, L.; Ng, K.-M.; Weng, L.-T.; Ho, K.-C. Surface Studies of Polymers with a Well-Defined Segmental Length by ToF-SIMS and XPS. Relationship between the Surface Chemical Composition and Segmental Length. Macromolecules 2000, 33, 8002–8005. [Google Scholar]
- Kim, Y.-P.; Hong, M.-Y.; Kim, J.; Oh, E.; Shon, H.K.; Moon, D.W.; Kim, H.-S.; Lee, T.G. Quantitative Analysis of Surface-Immobilized Protein by TOF-SIMS: Effects of Protein Orientation and Trehalose Additive. Anal. Chem. 2007, 79, 1377–1385. [Google Scholar] [CrossRef]
- Kim, J.; Shon, H.K.; Jung, D.; Moon, D.W.; Han, S.Y.; Lee, T.G. Quantitative Chemical Derivatization Technique in Time-of-Flight Secondary Ion Mass Spectrometry for Surface Amine Groups on Plasma-Polymerized Ethylenediamine Film. Anal. Chem. 2005, 77, 4137–4141. [Google Scholar] [CrossRef]
- Botreau, M.; Guignard, C.; Hoffmann, L.; Migeon, H.-N. ToF-SIMS as an Alternative Tool for the Qualitative and Quantitative Analysis of Polar Herbicides. Appl. Surf. Sci. 2004, 231–232, 533–537. [Google Scholar] [CrossRef]
- Balachander, N.; Sukenik, C.N. Monolayer transformation by nucleophilic substitution: Applications to the creation of new monolayer assemblies. Langmuir 1990, 6, 1621–1627. [Google Scholar] [CrossRef]
- Böhmler, J.; Ponche, A.; Anselme, K.; Ploux, L. Self-Assembled Molecular Platforms for Bacteria/Material Biointerface Studies: Importance to Control Functional Group Accessibility. ACS Appl. Mater. Interfaces 2013, 5, 10478–10488. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Huang, S.; Wang, Y.; Zhang, Z.; Du, B.; Zhang, X.; Fan, Z. Sonochemical Transformation of Epoxy–Amine Thermoset into Soluble and Reusable Polymers. Macromolecules 2015, 48, 316–322. [Google Scholar] [CrossRef]
- Breitbach, M.; Bathen, D. Influence of ultrasound on adsorption processes. Ultrason. Sonochem. 2001, 8, 277–283. [Google Scholar] [CrossRef]
- Gandini, A.; Coelho, D.; Silvestre, A.J. Reversible click chemistry at the service of macromolecular materials. Part 1: Kinetics of the Diels–Alder reaction applied to furan–maleimide model compounds and linear polymerizations. Eur. Polym. J. 2008, 44, 4029–4036. [Google Scholar] [CrossRef]
- Lyu, B.; Cha, W.; Mao, T.; Wu, Y.; Qian, H.-J.; Zhou, Y.; Chen, X.; Zhang, S.; Liu, L.; Yang, G.; et al. Surface Confined Retro Diels–Alder Reaction Driven by the Swelling of Weak Polyelectrolytes. ACS Appl. Mater. Interfaces 2015, 7, 6254–6259. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, V.; Borne, M.; Laborbe, E.; Auvergne, R.; Gandini, A.; Boutevin, B. Study of the Diels–Alder and retro-Diels–Alder reaction between furan derivatives and maleimide for the creation of new materials. RSC Adv. 2015, 5, 37742–37754. [Google Scholar] [CrossRef]
- Widstrom, A.L.; Lear, B.J. Structural and solvent control over activation parameters for a pair of retro Diels-Alder reactions. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pool, B.R.; White, J.M. Structural Manifestations of the Retro Diels–Alder Reaction. Org. Lett. 2000, 2, 3505–3507. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Priebe, A.; Xie, T.; Bürki, G.; Pethö, L.; Michler, J. The matrix effect in TOF-SIMS analysis of two-element inorganic thin films. J. Anal. At. Spectrom. 2020, 35, 1156–1166. [Google Scholar] [CrossRef]
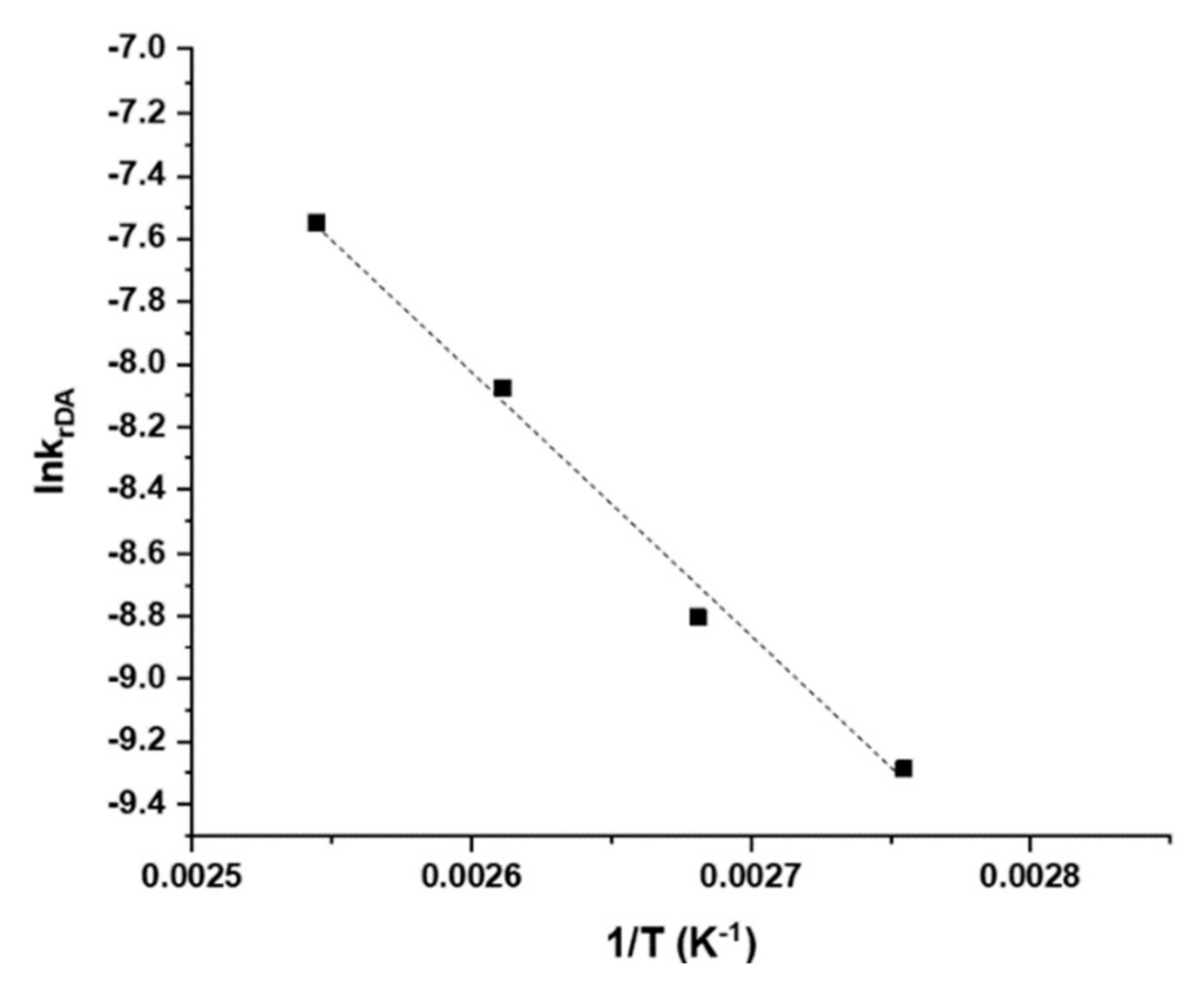

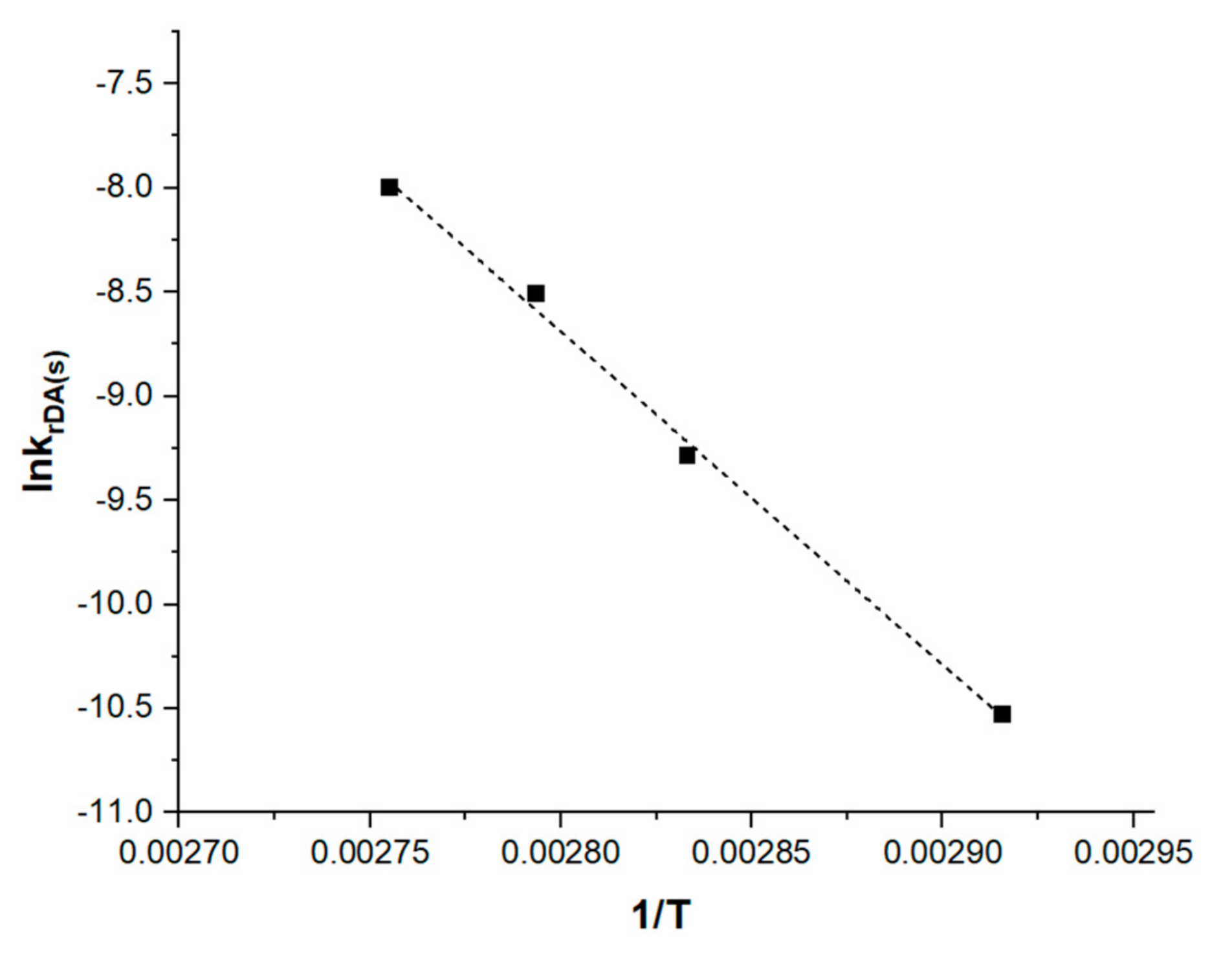
| Table (K) | krDA × 105 (s−1) |
|---|---|
| 363 | 9 ± 1 |
| 373 | 15 ± 3 |
| 383 | 31 ± 2 |
| 393 | 52 ± 10 |
| T (K) | krDA × 105 (s−1) |
|---|---|
| 343 | 2.7 ± 0.1 |
| 353 | 9.3 ± 0.5 |
| 358 | 20 ± 1 |
| 363 | 34 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassouna, L.; Enganati, S.K.; Bally-Le Gall, F.; Mertz, G.; Bour, J.; Ruch, D.; Roucoules, V. Using TOF-SIMS Spectrometry to Study the Kinetics of the Interfacial Retro Diels–Alder Reaction. Materials 2021, 14, 2674. https://doi.org/10.3390/ma14102674
Hassouna L, Enganati SK, Bally-Le Gall F, Mertz G, Bour J, Ruch D, Roucoules V. Using TOF-SIMS Spectrometry to Study the Kinetics of the Interfacial Retro Diels–Alder Reaction. Materials. 2021; 14(10):2674. https://doi.org/10.3390/ma14102674
Chicago/Turabian StyleHassouna, Lilia, Sachin Kumar Enganati, Florence Bally-Le Gall, Grégory Mertz, Jérôme Bour, David Ruch, and Vincent Roucoules. 2021. "Using TOF-SIMS Spectrometry to Study the Kinetics of the Interfacial Retro Diels–Alder Reaction" Materials 14, no. 10: 2674. https://doi.org/10.3390/ma14102674
APA StyleHassouna, L., Enganati, S. K., Bally-Le Gall, F., Mertz, G., Bour, J., Ruch, D., & Roucoules, V. (2021). Using TOF-SIMS Spectrometry to Study the Kinetics of the Interfacial Retro Diels–Alder Reaction. Materials, 14(10), 2674. https://doi.org/10.3390/ma14102674







