Application of Statistical Methods in Predicting the Properties of Glass-Ceramic Materials Obtained from Inorganic Solid Waste
Abstract
:1. Introduction
2. Experimental Procedures
2.1. The Plan for Mixtures
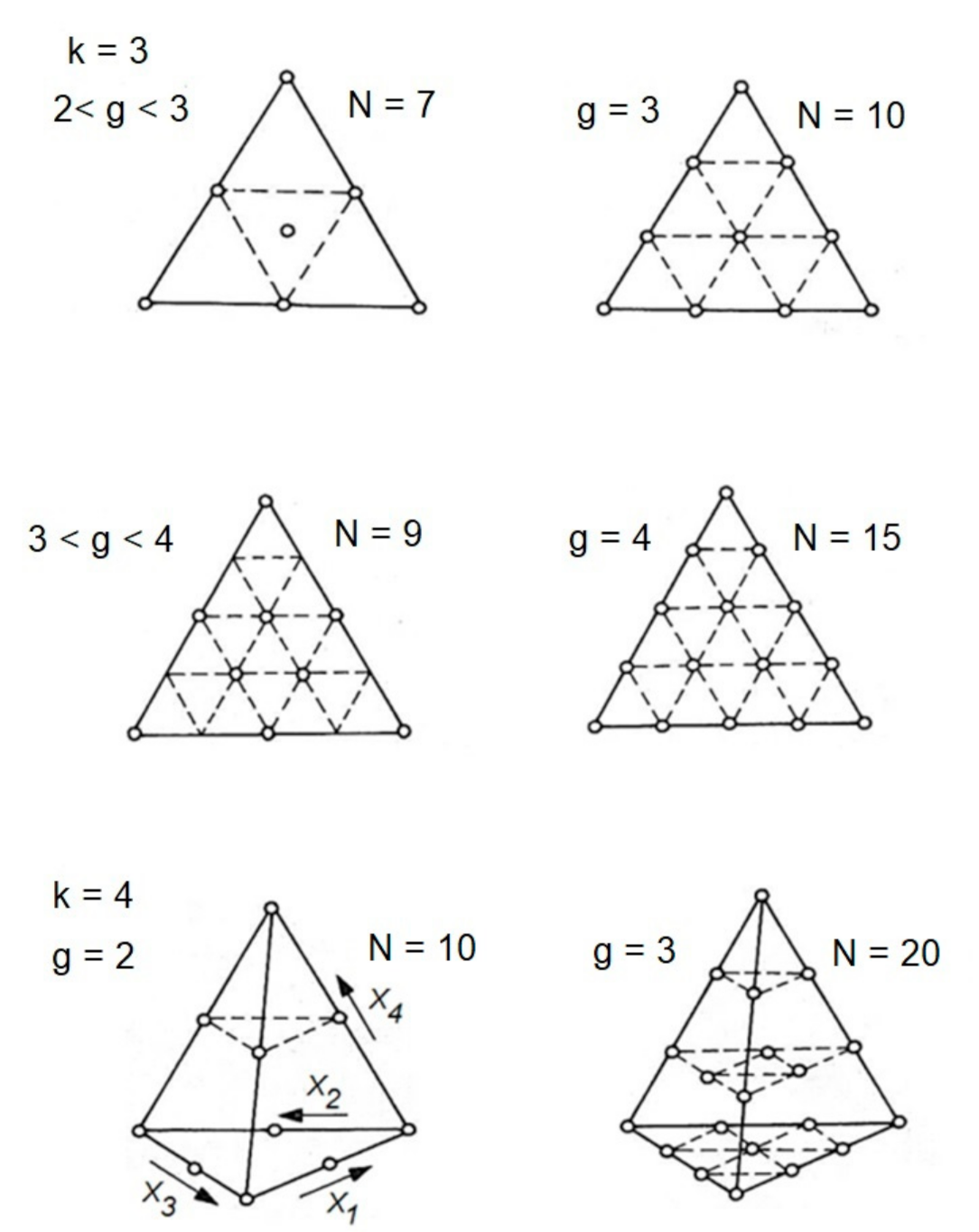
2.2. The Canonical Form of Polynomials for Mixtures
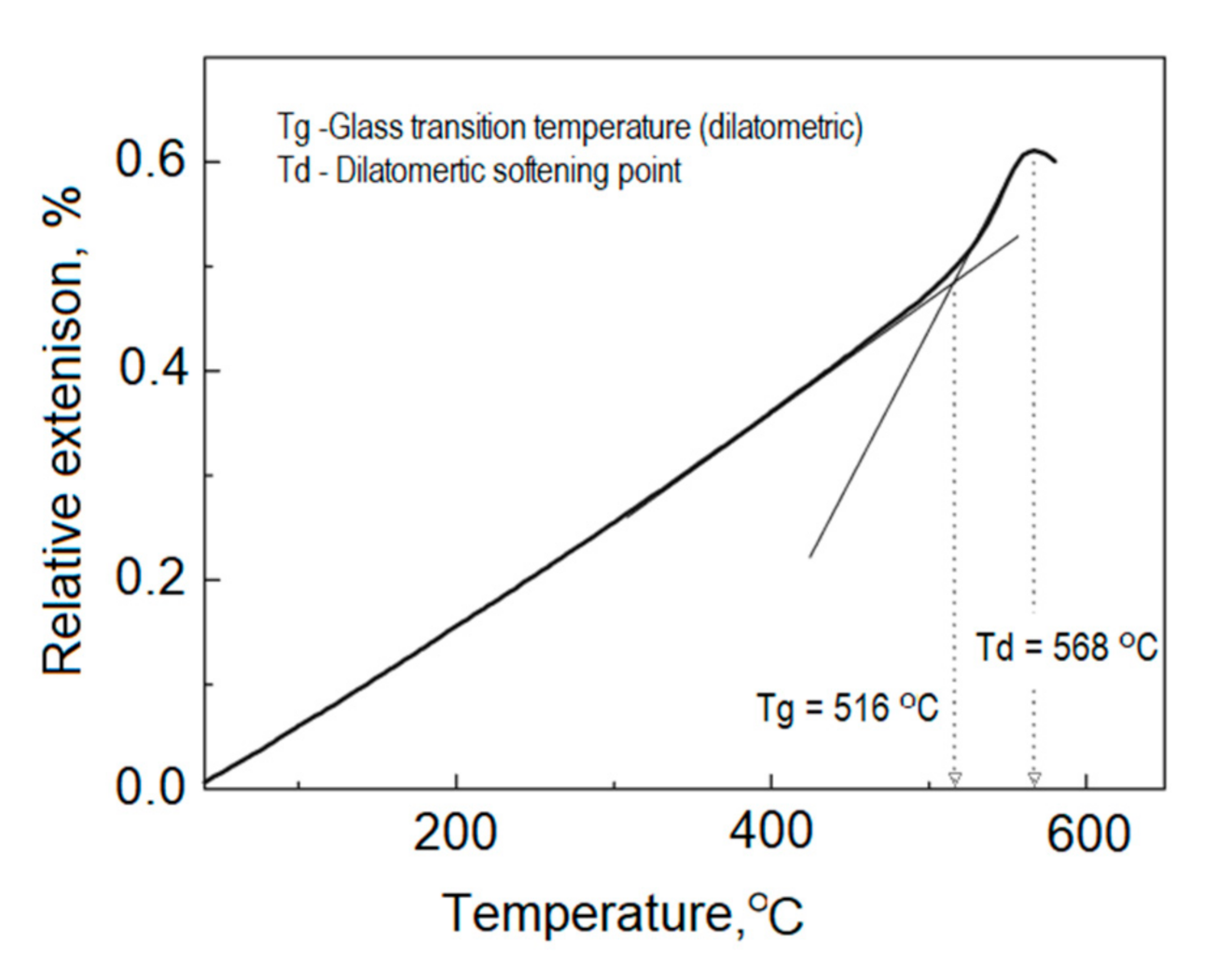
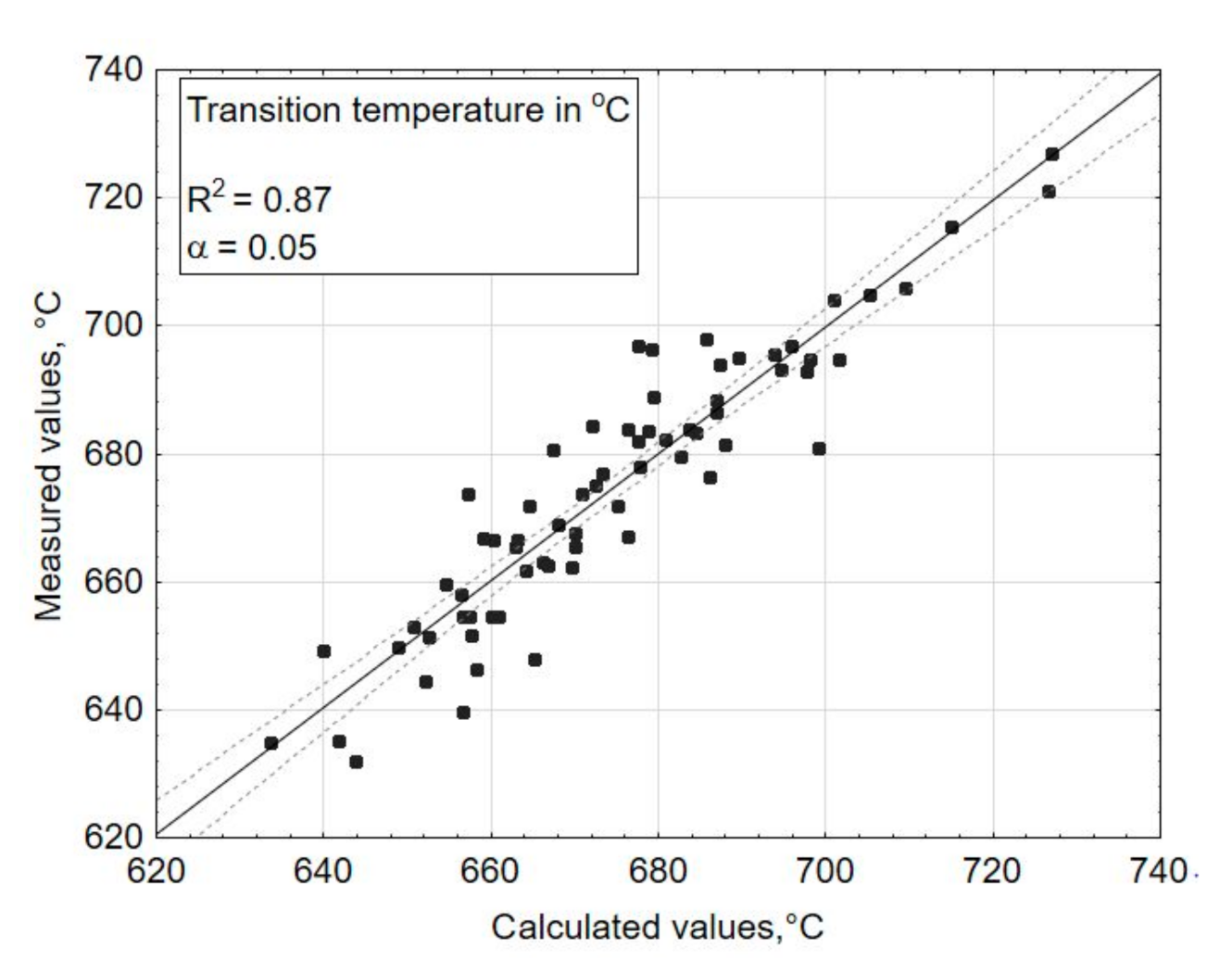
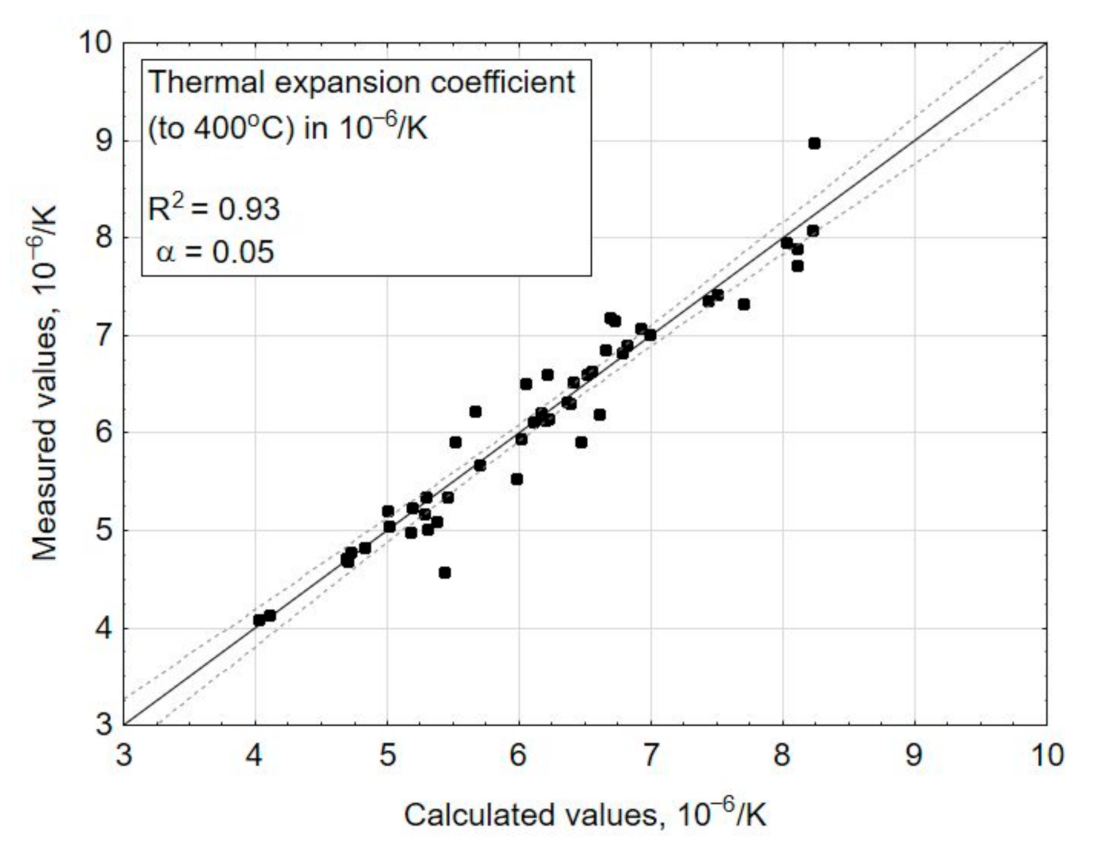
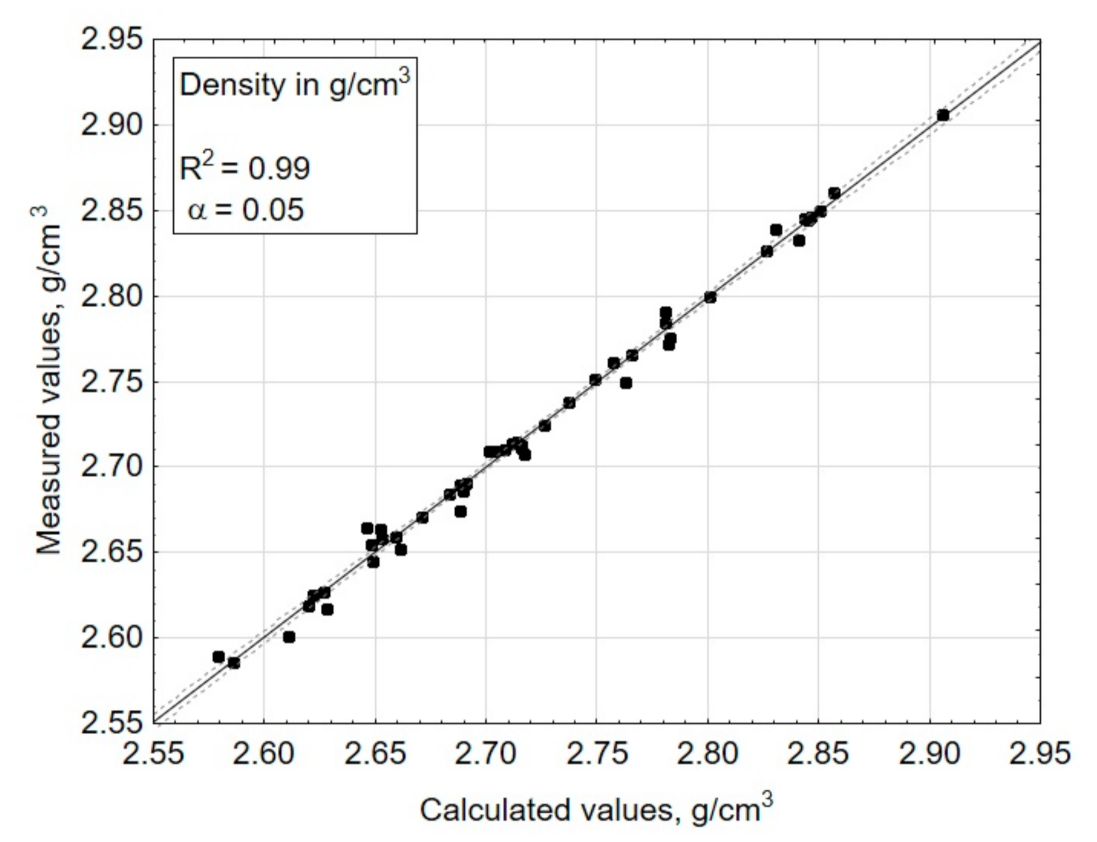
3. Results and Discussions
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.Q.; Tan, S.K.; Gersberg, R.M. Municipal Solid Waste Management in China: Status, Problems and Challenges. J. Environ. Manag. 2010, 91, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Hessenkemper, H.; Höhne, D.; Nadolny, A.; Bielecka, A.; Bieniarz, P.; Löbig, R. Herstellung Hochwertiger Dachdeckungsmaterialien aus Mineralischen Rohstoffen; Research Report; TU Bergakademie Freiberg: Freiberg, Germany, 2005. [Google Scholar]
- Hoehne, D.; Nadolny, A.; Loebig, R.; Bielecka, A.; Bieniarz, P.; Zawada, A. Herstellung von Glaserzeugnissen aus industriellen Reststoffen. Freib. Forschungshefte. Silikattechnik 2005, A 889, 4–25. [Google Scholar]
- Arena, U. Process and Technological Aspects of Municipal Solid Waste Gasification. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Iwaszko, J.; Zawada, A.; Przerada, I.; Lubas, M. Structural and Microstructural Aspects of Asbestos-cement Waste Vitrification. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 195, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Quesada, E.; Villaquirán-Caicedo, M.A.; Mejía de Gutiérrez, R. New Glass-ceramic from Ternary–quaternary Mixtures Based on Colombian Industrial Wastes. Blast Furnace Slag, Cupper Slag, Fly Ash and Glass Cullet. Boletín Soc. Española Cerámica Vidr. 2021. [Google Scholar] [CrossRef]
- Brandt, S. Analiza Danych; PWN: Warszawa, Poland, 1998. [Google Scholar]
- Nützenadel, P.; Zylla, S.; Nitzsche, A. Einsatzmöglichkeiten der Statistischen Versuchsplanung bei der Fritteentwicklung. Silikattechnik 1990, 41, 262–265. [Google Scholar]
- Zawada, A. The Influence of Redox State on the Location of Iron Ions in the Structure of Silicate Glass with High Iron Content. J. Mol. Struct. 2020, 1206, 127705. [Google Scholar] [CrossRef]
- Zawada, A.; Lubas, M.; Przerada, I.; Sitarz, M.; Adamczyk-Habrajska, M. The Effect of the Reducing Melting Atmosphere on Coordination Moieties in Aluminosilicate Glasses. J. Mol. Struct. 2020, 1218, 128474. [Google Scholar] [CrossRef]
- Matthes, S.D.; Ballani, F.; Stoyan, D. Modelling Post-crack Tension-softening Behavior of Fiber Reinforced Materials. Probabilistic Eng. Mech. 2016, 45, 157–163. [Google Scholar] [CrossRef]
- Höland, W.; Beall, G. Glass-Ceramic Technology, 2nd ed.; John Wileys: Hoboken, NJ, USA, 2012. [Google Scholar]
- Dávalos, J.; Bonilla, A.; Villaquirán-Caicedo, M.A.; Mejia de Gutiérrez, R. Preparation of Glass–Ceramic Materials from Coal Ash and Rice Husk Ash: Microstructural, Physical and Mechanical Properties. Boletín Soc. Española Cerámica Vidr 2020. [Google Scholar] [CrossRef]
- Saparuddin, D.I.; Hisham, N.A.N.; Aziz, S.A.; Matori, K.A.; Honda, S.; Iwamoto, Y.; Zaid, M.H.M. Effect of Sintering Temperature on the Crystal Growth, Microstructure and Mechanical Strength of Foam Glass-Ceramic from Waste Materials. J. Mater. Res. Technol. 2020, 9, 5640–5647. [Google Scholar] [CrossRef]
- Chinnam, R.K.; Francis, A.A.; Will, J.; Bernardo, E.; Boccaccini, A.R. Review. Functional Glasses and Glass-Ceramics Derived from Iron Rich Waste and Combination of Industrial Residues. J. Non Cryst. Solids 2013, 365, 63–74. [Google Scholar] [CrossRef]
- Schabbach, L.M.; Andreola, F.; Karamanova, E.; Lancellotti, I.; Karamanov, A.; Barbieri, L. Integrated Aproach to Establish the Sinter-crystallization Ability of Glasses from Secondary Raw Material. J. Non Cryst. Solids 2011, 357, 10–17. [Google Scholar] [CrossRef]
- Luan, J.; Li, A.; Su, T.; Cui, X. Synthesis of Nucleated Glass-ceramics Using Oil Shale Fly Ash. J. Hazard. Mater. 2010, 173, 427–432. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. 2017. Available online: http://statistica.com (accessed on 26 July 2020).
- Kessler, W. Multivariate Datenanalyse für die Pharma, Bio-und Prozessanalytik; Wiley-VCH Verlag: Weinheim, Germany, 2007; pp. 153–163. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Oehlert, G.W. A First Course in Design and Analysis of Experiments; University of Minnesota: Minneapolis, MN, USA, 2010. [Google Scholar]
- Harmon, L. Experiment Planning for Combinatorial Materials Discovery. J. Mater. Sci. 2003, 38, 4479–4485. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference and Prediction; Springer: New York, NY, USA, 2002. [Google Scholar]
- Liu, B.; Yang, Q.W.; Zhang, S.G. Integrated Utilization of Municipal Solid Waste Incineration Fly Ash and Bottom Ash for Preparation of Foam Glass–Ceramics. Rare Met. 2019, 38, 914–921. [Google Scholar] [CrossRef]
- Zhu, M.; Ji, R.; Li, Z.; Wang, H.; Liu, L.; Zhang, Z. Preparation of Glass Ceramic Foams for Thermal Insulation Applications from Coal Fly Ash and Waste Glass. Constr. Build. Mater. 2016, 112, 398–405. [Google Scholar] [CrossRef]
- Scheffler, E. Statistische Versuchsplanung und–Auswertung: Eine Einführung für Praktiker; 3 Aufl.; Deutscher Verlag für Grundstoffindustrie Stuttgart: Stuttgart, Germany, 1997; pp. 50–59. [Google Scholar]
- Polański, Z. Planowanie Doświadczeń w Technice; PWN: Warszawa, Poland, 1984. [Google Scholar]
- Mason, R.L.; Gunst, R.F.; Hess, J.L. Statistical Design and Analysis of Experiments; Wiley: Hoboken, NJ, USA, 1989; ISBN 0-471-85364-X. [Google Scholar]
- Elizabeth, A.; Peck, G.; Vining, G.; Montgomery, D.C. Introduction to Linear Regression Analysis; Wiley: Hoboken, NJ, USA, 1982. [Google Scholar]
- Draper, N.R.; Smith, H. Applied Regression Analysis; Wiley: Hoboken, NJ, USA, 1981; ISBN 0-471-2995-5. [Google Scholar]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology, Process and Product Optimization Using Designed Experiment; Wiley: Hoboken, NJ, USA, 1995; ISBN 0-471-58100-3. [Google Scholar]
- Konkol, J. Zastosowanie Analizy Struktury do Oceny Właściwości Betonów. Ph.D. Thesis, Rzeszów University of Technology, Rzeszów, Poland, March 2005. [Google Scholar]
- Strzałkowski, A.; Śliżyński, A. Matematyczne Metody Opracowywania Wyników Pomiarów; PWN: Warszawa, Poland, 1973. [Google Scholar]
- Konkol, J.; Prokopski, G. Optymalizacja wielokryterialna składu betonów zwykłych z uwzględnieniem parametrów wytrzymałościowych i strukturalnych. Przegląd Bud. 2006, 2, 23–28. [Google Scholar]
- Bieniarz, P.; Höhne, D.; Hessenkemper, H.; Zawada, A. Processing of Molten Solid Residues. Glass Sci. Technol. 2005, 78, 23–28. [Google Scholar]
- Zawada, A.; Bieniarz, P.; Kolan, C.; Hessenkemper, H. Modelling Selected Properties of Glasses Based on Slag from a Waste Incineration Plant. Glass Tech. Eur. J. Glass Sci. Tech. Part A 2013, 54, 72–76. [Google Scholar]
- DIN 52 324 Prüfung von Glas; Bestimmung der Transformationstemperatur; Deutsches Institut für Normung: Berlin, Germany, 1984.
- Mazurin, O.V.; Gankin, Y.V. Glass Transition Temperature: Problems with Measurement and Analysis of Existing Data. In Proceedings of the International Glass Congress, Strasbourg, France, 1–6 July 2007. [Google Scholar]
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Leonelli, C.; Manfredini, T. Recycling of Industrial Wastes in Ceramic Manufacturing: State of Art and Glass Case Studies. Ceram. Int. 2016, 42, 13333–13338. [Google Scholar] [CrossRef]


| Number of Components x | Regression Polynomial | ||
|---|---|---|---|
| 2nd Degree | 3rd Degree | 4th Degree | |
| Number of Coefficients b | |||
| 3 | 6 | 10 | 15 |
| 4 | 10 | 20 | 35 |
| 5 | 15 | 35 | 70 |
| 6 | 21 | 56 | 126 |
| 7 | 28 | 84 | 210 |
| Oxide | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | R2O * |
| wt% | 45–60 | 8–20 | 10–25 | 3–15 | 2–10 | 4–6 |
| Nr | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | Alkali |
|---|---|---|---|---|---|---|
| 1 | 46 | 21 | 23 | 2 | 2 | 5 |
| 2 | 44 | 13 | 21 | 8 | 9 | 4 |
| 3 | 59 | 9 | 10 | 13 | 2 | 5 |
| 4 | 46 | 10 | 22 | 14 | 2 | 5 |
| 5 | 60 | 19 | 11 | 3 | 4 | 4 |
| 6 | 45 | 21 | 23 | 2 | 3 | 5 |
| 7 | 58 | 14 | 19 | 2 | 3 | 4 |
| 8 | 45 | 23 | 18 | 3 | 6 | 4 |
| 9 | 46 | 19 | 10 | 10 | 7 | 5 |
| 10 | 43 | 21 | 18 | 3 | 10 | 4 |
| 11 | 44 | 8 | 21 | 4 | 4 | 6 |
| 12 | 46 | 9 | 19 | 3 | 4 | 7 |
| 13 | 45 | 9 | 19 | 4 | 4 | 6 |
| 11 | 43 | 8 | 21 | 4 | 3 | 6 |
| 12 | 44 | 9 | 19 | 4 | 4 | 6 |
| 13 | 45 | 8 | 19 | 4 | 4 | 7 |
| 14 | 54 | 8 | 16 | 3 | 3 | 6 |
| 15 | 46 | 9 | 17 | 3 | 4 | 7 |
| 16 | 49 | 8 | 18 | 3 | 3 | 7 |
| 17 | 48 | 8 | 18 | 3 | 4 | 7 |
| 18 | 48 | 8 | 18 | 3 | 4 | 7 |
| 19 | 48 | 8 | 19 | 3 | 4 | 7 |
| 20 | 47 | 8 | 18 | 3 | 4 | 7 |
| 14 | 50 | 8 | 18 | 3 | 4 | 7 |
| 15 | 46 | 9 | 20 | 4 | 4 | 7 |
| 16 | 47 | 9 | 20 | 4 | 4 | 7 |
| 21 | 47 | 9 | 20 | 4 | 4 | 7 |
| 22 | 47 | 8 | 19 | 4 | 4 | 7 |
| 23 | 48 | 8 | 19 | 3 | 4 | 7 |
| 24 | 46 | 8 | 19 | 3 | 4 | 7 |
| 25 | 51 | 8 | 18 | 3 | 4 | 7 |
| 26 | 51 | 8 | 18 | 3 | 4 | 6 |
| 27 | 48 | 8 | 20 | 3 | 4 | 6 |
| 28 | 45 | 8 | 19 | 4 | 4 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawada, A.; Przerada, I.; Lubas, M.; Sitarz, M.; Leśniak, M. Application of Statistical Methods in Predicting the Properties of Glass-Ceramic Materials Obtained from Inorganic Solid Waste. Materials 2021, 14, 2651. https://doi.org/10.3390/ma14102651
Zawada A, Przerada I, Lubas M, Sitarz M, Leśniak M. Application of Statistical Methods in Predicting the Properties of Glass-Ceramic Materials Obtained from Inorganic Solid Waste. Materials. 2021; 14(10):2651. https://doi.org/10.3390/ma14102651
Chicago/Turabian StyleZawada, Anna, Iwona Przerada, Małgorzata Lubas, Maciej Sitarz, and Magdalena Leśniak. 2021. "Application of Statistical Methods in Predicting the Properties of Glass-Ceramic Materials Obtained from Inorganic Solid Waste" Materials 14, no. 10: 2651. https://doi.org/10.3390/ma14102651
APA StyleZawada, A., Przerada, I., Lubas, M., Sitarz, M., & Leśniak, M. (2021). Application of Statistical Methods in Predicting the Properties of Glass-Ceramic Materials Obtained from Inorganic Solid Waste. Materials, 14(10), 2651. https://doi.org/10.3390/ma14102651







