Synthesis of Si-Based High-Efficiency and High-Durability Superhydrophilic-Underwater Superoleophobic Membrane of Oil–Water Separation
Abstract
1. Introduction
2. Experimental Section
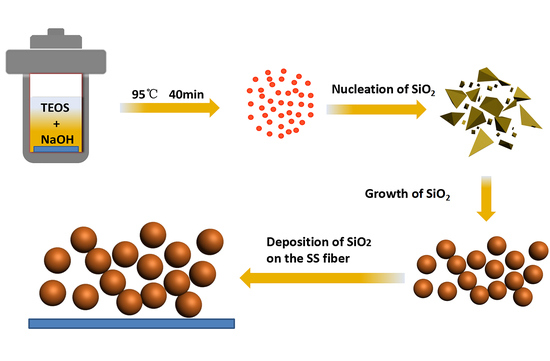
2.1. Materials and Synthesis
2.2. Characterization
2.3. Oil/Water Separation
2.4. Corrosion Resistance
2.5. Sandpaper Abrasion Test
2.6. Long-Term Stability
3. Results and Discussion
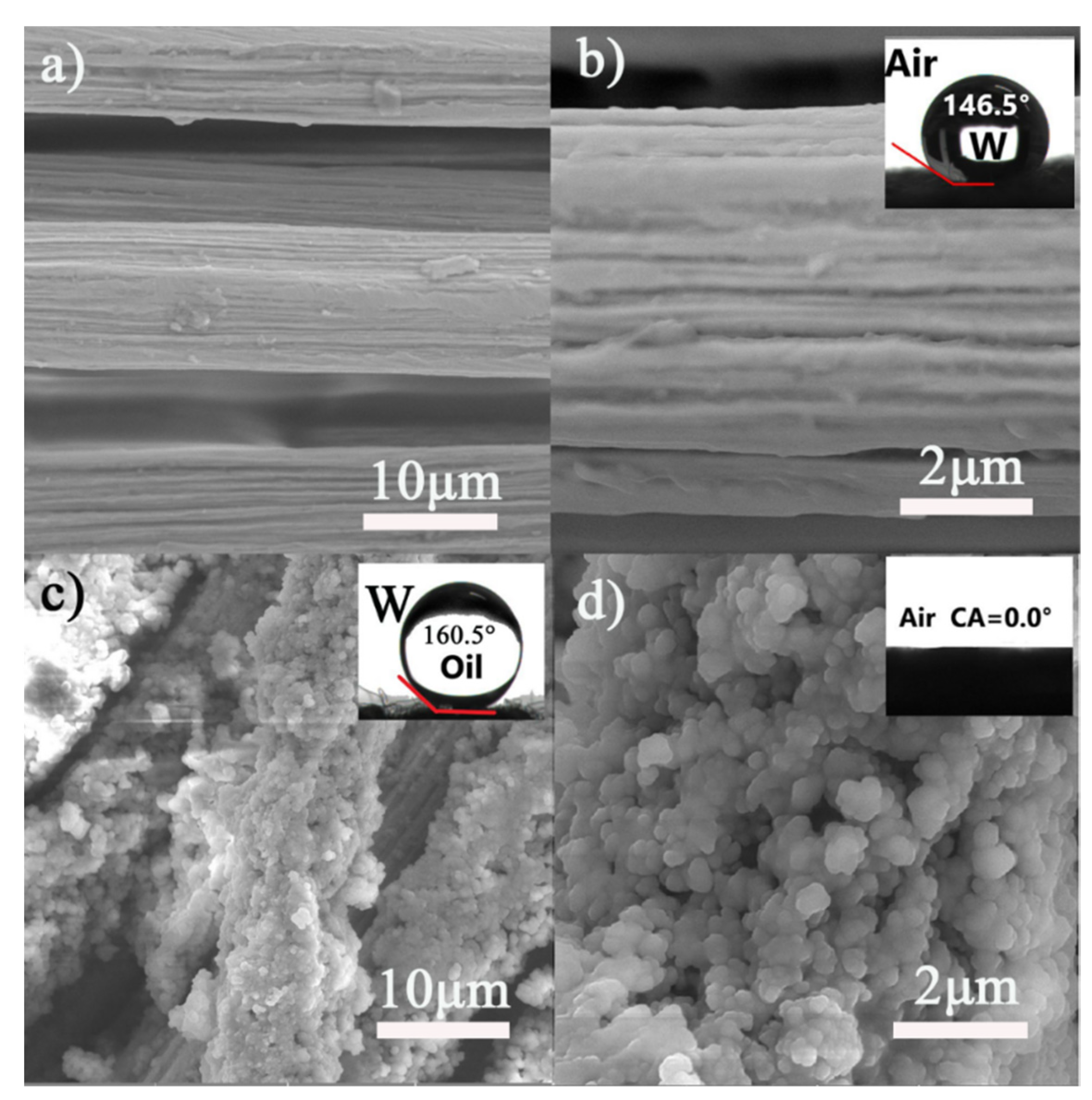
3.1. SEM Analysis
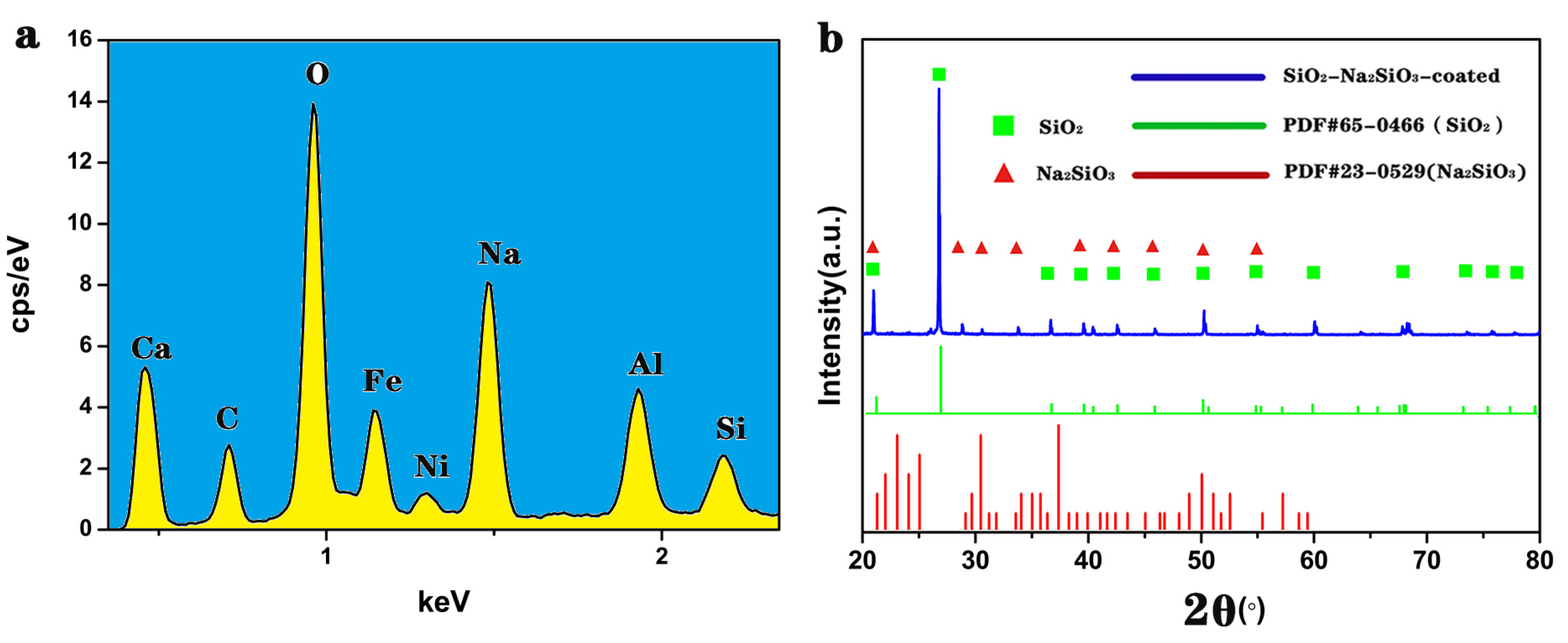
3.2. EDS and XRD Analysis
3.3. Surface Wettability
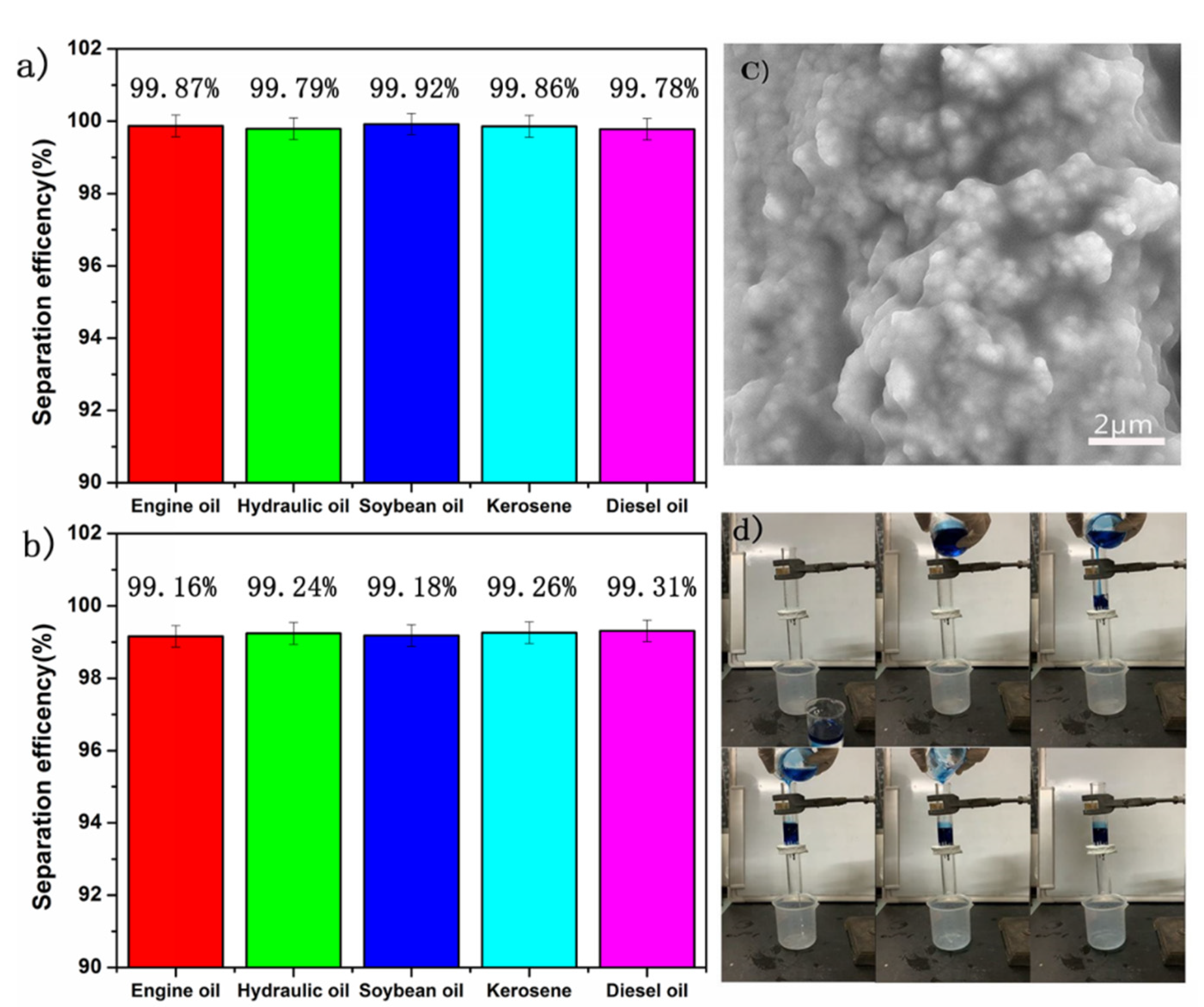
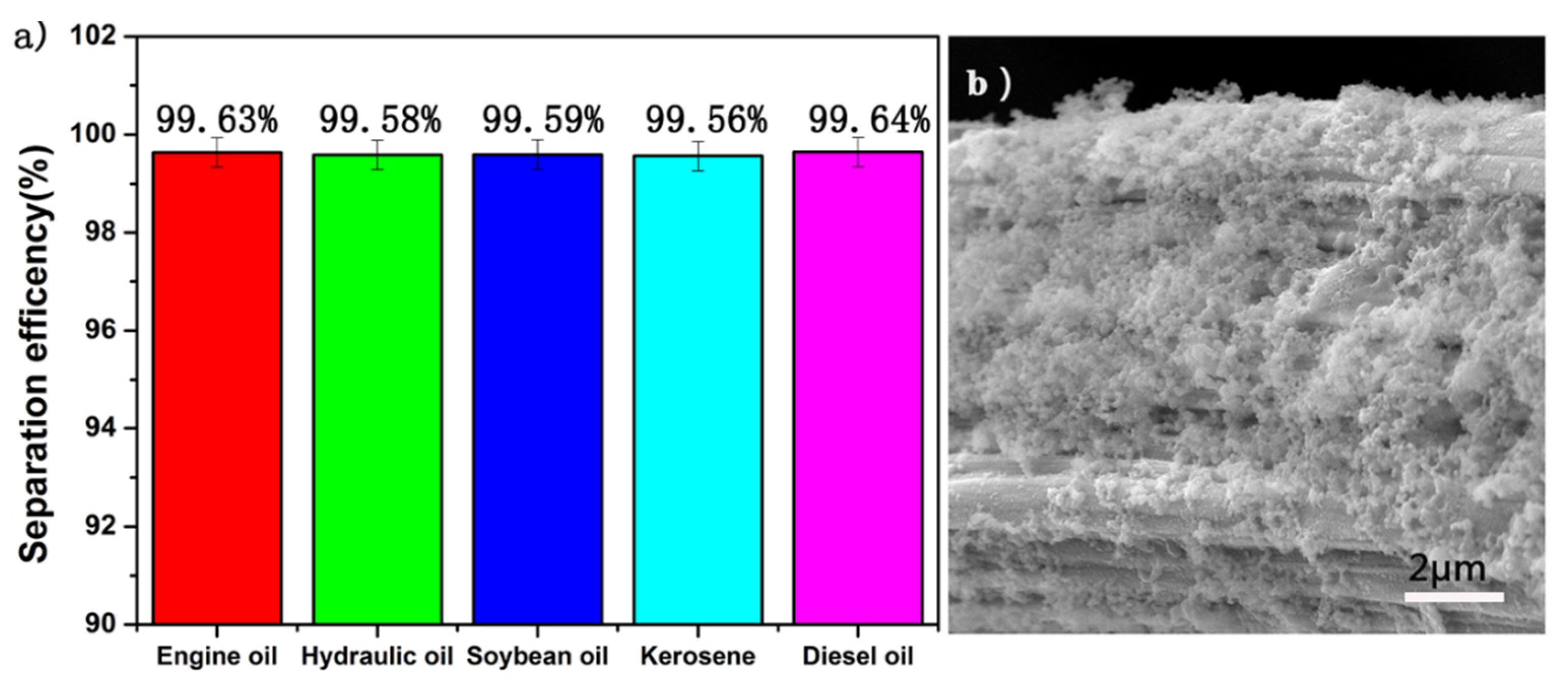
3.4. Oil and Water Separation Efficiency Test
3.5. The Water Flux Test
3.6. The Abrasion Test
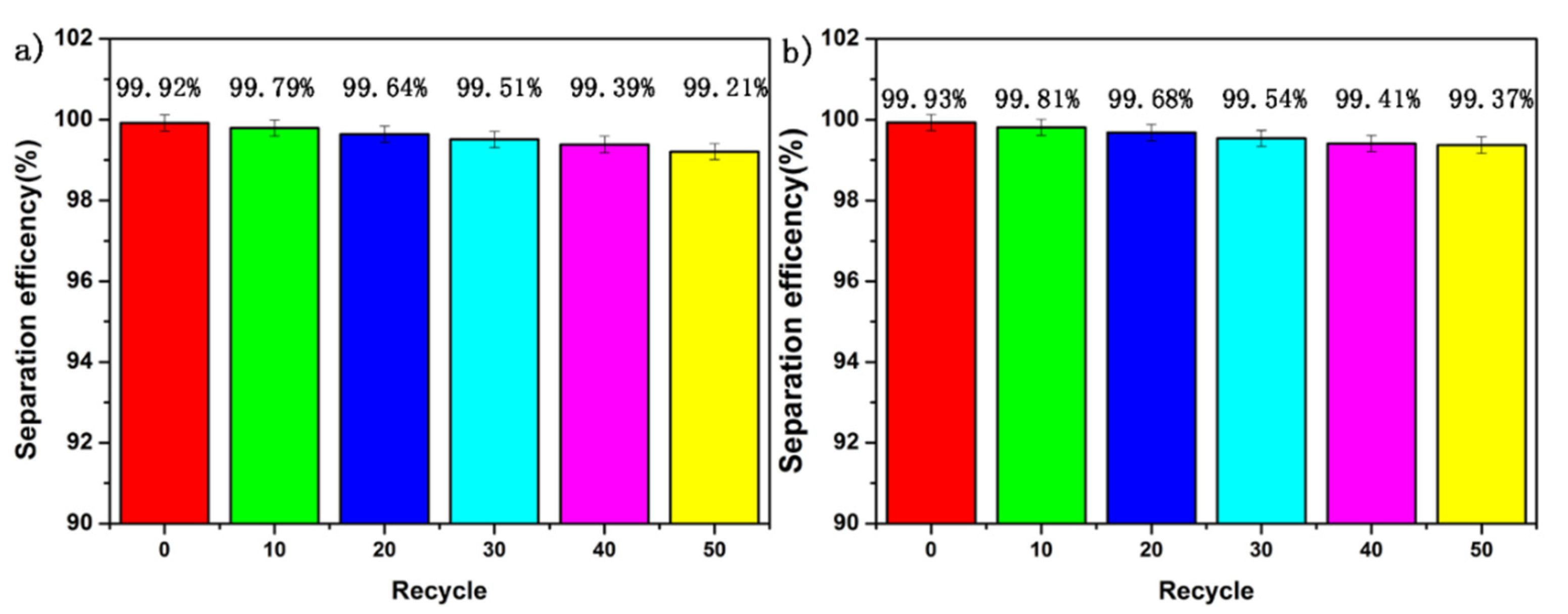
3.7. Long-Term Stability Test
3.8. Corrosion Resistance Test
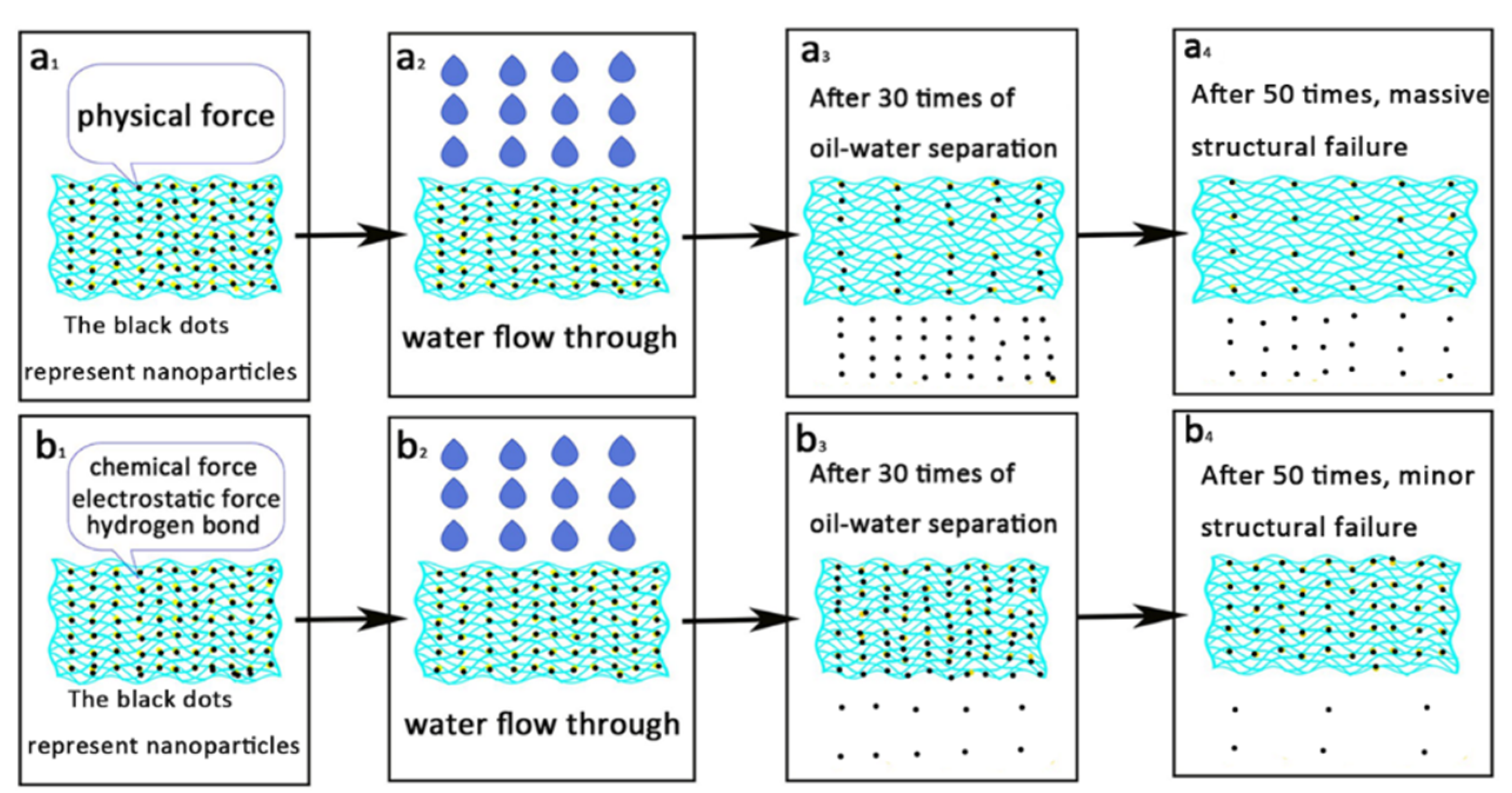
3.9. Principle of Strong Membrane Bonding
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, H.; Li, Z.; Ouyang, L.; Liang, Y.; Yuan, S. Hierarchical WO3@Cu(OH)2 nanorod arrays grown on copper mesh with superwetting and self-cleaning properties for high-performance oil/water separation. J. Alloys Compd. 2021, 855, 157421. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Easily enlarged and coating-free underwater superoleophobic fabric for oil/water and emulsion separation via a facile NaClO2 treatment. Sep. Purif. Technol. 2018, 195, 358–366. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.; Yang, T.; Bennett, P.; Zheng, Z.; Yang, Q.; Liu, D. Laser-structured superhydrophobic/superoleophilic aluminum surfaces for efficient oil/water separation. Environ. Sci. Pollut. Res. 2020, 27, 43138–43149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tao, Y.; Wang, J.; He, Y. Facile preparation of superhydrophobic porous wood for continuous oil-water separation. J. Water Process. Eng. 2020, 36, 101279. [Google Scholar] [CrossRef]
- Lai, H.; Yu, X.; Liu, M.; Cheng, Z. One-step solution immersion process for the fabrication of low adhesive underwater superoleophobic copper mesh film toward high-flux oil/water separation. Appl. Surf. Sci. 2018, 448, 241–247. [Google Scholar] [CrossRef]
- Nemade, P.R.; Ganjare, A.V.; Ramesh, K.; Rakte, D.M.; Vaishnavi, P.; Thapa, G. Low fouling sulphonated carbon soot-polysulphone membranes for rapid dehydration of stabilized oil-water emulsions. J. Water Process. Eng. 2020, 38, 101590. [Google Scholar] [CrossRef]
- Thakur, K.; Rajhans, A.; Kandasubramanian, B. Starch/PVA hydrogels for oil/water separation. Environ. Sci. Pollut. Res. 2019, 26, 32013–32028. [Google Scholar] [CrossRef] [PubMed]
- Elshorafa, R.; Saththasivam, J.; Liu, Z.; Ahzi, S. Efficient oil/saltwater separation using a highly permeable and fouling-resistant all-inorganic nanocomposite membrane. Environ. Sci. Pollut. Res. 2020, 27, 15488–15497. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, M.; Li, Y.; Yu, Y.; Yin, X.; Wu, J.; Chen, S.; Xu, J.; Wang, L.; Wang, H. Polyphenylene sulfide microfiber membrane with superhydrophobicity and superoleophilicity for oil/water separation. J. Mater. Sci. 2018, 53, 13243–13252. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, Z. Superhydrophilic and underwater superoleophobic MFI zeolite-coated film for oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 283–288. [Google Scholar] [CrossRef]
- Naik, N.S.; Padaki, M.; Déon, S.; Karunakaran, G.; Dizge, N.; Saxena, M. The efficient mixed matrix antifouling membrane for surfactant stabilized oil-in-water nanoemulsion separation. J. Water Process. Eng. 2019, 32, 100959. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Q.; Xie, A.; Lang, J.; Zhou, Z.; Yan, Y. Construction of superhydrophilic and underwater superoleophobic membranes via in situ oriented NiCo-LDH growth for gravity-driven oil/water emulsion separation. J. Taiwan Inst. Chem. Eng. 2019, 104, 240–249. [Google Scholar] [CrossRef]
- Tan, L.; Han, N.; Qian, Y.; Zhang, H.; Gao, H.; Zhang, L.; Zhang, X. Superhydrophilic and underwater superoleophobic poly (acrylonitrile-co-methyl acrylate) membrane for highly efficient separation of oil-in-water emulsions. J. Membr. Sci. 2018, 564, 712–721. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A simple and eco-friendly route for fabricating iron-based coating on metal mesh for efficient oil/water separation. Sep. Purif. Technol. 2019, 226, 31–38. [Google Scholar] [CrossRef]
- Sun, Y.; Zong, Y.; Yang, N.; Zhang, N.; Jiang, B.; Zhang, L.; Xiao, X. Surface hydrophilic modification of PVDF membranes based on tannin and zwitterionic substance towards effective oil-in-water emulsion separation. Sep. Purif. Technol. 2020, 234, 116015. [Google Scholar] [CrossRef]
- Saki, S.; Uzal, N. Preparation and characterization of PSF/PEI/CaCO3 nanocomposite membranes for oil/water separation. Environ. Sci. Pollut. Res. 2018, 25, 25315–25326. [Google Scholar] [CrossRef] [PubMed]
- Huaijie, C.; Ying, L. Facile design of a stable and inorganic underwater superoleophobic copper mesh modified by self-assembly sodium silicate and aluminum oxide for oil/water separation with high flux. J. Colloid Interface Sci. 2021, 598, 483–491. [Google Scholar]
- Deng, W.; Li, C.; Pan, F.; Li, Y. Efficient oil/water separation by a durable underwater superoleophobic mesh membrane with TiO2 coating via biomineralization. Sep. Purif. Technol. 2019, 222, 35–44. [Google Scholar] [CrossRef]
- Hou, Y.; Li, R.; Liang, J. Superhydrophilic nickel-coated meshes with controllable pore size prepared by electrodeposition from deep eutectic solvent for efficient oil/water separation. Sep. Purif. Technol. 2018, 192, 21–29. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Z.; Xie, A.; Meng, M.; Cui, Y.; Liu, S.; Lu, J.; Zhou, S.; Yan, Y.; Dong, H. Bio-inspired fabrication of superhydrophilic nanocomposite membrane based on surface modification of SiO2 anchored by polydopamine towards effective oil-water emulsions separation. Sep. Purif. Technol. 2019, 209, 434–442. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Gao, S.; Zhu, Y.; Li, J.; Jin, J. Micro/nano hierarchical poly(acrylic acid)-grafted-poly(vinylidene fluoride) layer coated foam membrane for temperature-controlled separation of heavy oil/water. Sep. Purif. Technol. 2015, 156, 207–214. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, T.; Huang, J.; Yan, T.; Li, C.; Liu, L.; Wang, L.; Jiao, F. Copper hydroxyphosphate nanosheets-covered robust membranes with superhydrophilicity and underwater ultralow adhesive superoleophobicity for oil/water separation and visible light photodegradation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124000. [Google Scholar] [CrossRef]
- Sunab, F.; Li, T.-T.; Zhanga, X.; Shiuc, B.-C.; Zhanga, Y.; Rena, H.-T.; Penga, H.-K.; Linabcdefg, J.-H.; Louabcfhi, C.-W. Facile fabrication of hydrophilic-underwater superoleophobic poly(N-isopropylacrylamide) coated PP/LPET nonwoven fabrics for highly efficient oil/water separation. Prog. Org. Coat. 2020, 148, 105780. [Google Scholar] [CrossRef]
- Yang, S.; Li, M.; Fang, G.; Xue, M.; Lu, Y. Flexible cement-sand coated cotton fabrics with superhydrophilic and underwater superoleophobic wettability for the separation of water/oil mixtures and oil-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125611. [Google Scholar] [CrossRef]
- Zhu, T.; Jiang, C.; Wu, J.; Wang, M.; Zhu, C.; Zhao, N.; Xu, J. Eco-friendly and one-step modification of poly(vinylidene fluoride) membrane with underwater superoleophobicity for effective emulsion separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125939. [Google Scholar] [CrossRef]
- Li, K.; Chen, W.; Wu, W.; Pan, Z.; Liang, Z.; Gan, J. Facile fabrication of superhydrophilic/underwater superoleophobic polyvinyl acetate/sodium silicate composite coating for the effective water/oil separation and the study on the anti-fouling property, durability and separation mechanism. Prog. Org. Coat. 2021, 150, 105979. [Google Scholar] [CrossRef]
- Gou, X.; Zhang, Y.; Long, L.; Liu, Y.; Tian, D.; Shen, F.; Yang, G.; Zhang, X.; Wang, L.; Deng, S. Superhydrophilic and underwater superoleophobic cement-coated mesh for oil/water separation by gravity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 605, 125338. [Google Scholar] [CrossRef]
- Zhou, D.-L.; Yang, D.; Han, D.; Zhang, Q.; Chen, F.; Fu, Q. Fabrication of superhydrophilic and underwater superoleophobic membranes for fast and effective oil/water separation with excellent durability. J. Membr. Sci. 2021, 620, 118898. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.; Chen, A.; Shang, C.; Peng, L.; Shao, J.; Liu, Z. Environmentally friendly kaolin-coated meshes with superhydrophilicity and underwater superoleophobicity for oil/water separation. Sep. Purif. Technol. 2020, 239, 116541. [Google Scholar] [CrossRef]
- Yanga, J.; Cuib, J.; Xieb, A.; Daia, J.; Lia, C.; Yana, Y. Facile preparation of superhydrophilic/underwater superoleophobic cellulose membrane with CaCO3 particles for oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125583. [Google Scholar] [CrossRef]
- Shang, B.; Zhan, Y.; Chen, M.; Wu, L. NIR triggered healable underwater superoleophobic coating with exceptional anti-biofouling performance. Appl. Surf. Sci. 2020, 528, 146805. [Google Scholar] [CrossRef]
- Wang, M.; Peng, M.; Zhu, J.; Li, Y.-D.; Zeng, J.-B. Mussel-inspired chitosan modified superhydrophilic and underwater superoleophobic cotton fabric for efficient oil/water separation. Carbohydr. Polym. 2020, 244, 116449. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Song, G.; Liu, Q.; Yang, C.; Qiu, J.; Zang, L.; Liu, H.; Chen, J. A facile route for the fabrication of a superhydrophilic and underwater superoleophobic phosphorylated PVA-coated mesh for both oil/water immiscible mixture and emulsion separation. Appl. Surf. Sci. 2021, 537, 147986. [Google Scholar] [CrossRef]
- Qing, W.; Li, X.; Wu, Y.; Shao, S.; Guo, H.; Yao, Z.; Chen, Y.; Zhang, W.; Tang, C.Y. In situ silica growth for superhydrophilic-underwater superoleophobic Silica/PVA nanofibrous membrane for gravity-driven oil-in-water emulsion separation. J. Membr. Sci. 2020, 612, 118476. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, C.; Liu, H.; Chen, M.; Xu, H.; Luo, W.; Zhang, F. Robust functionalization of underwater superoleophobic PVDF-HFP tubular nanofiber membranes and applications for continuous dye degradation and oil/water separation. J. Membr. Sci. 2020, 596, 117583. [Google Scholar] [CrossRef]
- Tian, L.; Li, W.; Ye, H.; Zhu, L.; Chen, H.; Liu, H. Environmentally benign development of superhydrophilic and underwater superoleophobic mesh for effective oil/water separation. Surf. Coat. Technol. 2019, 377, 124892. [Google Scholar] [CrossRef]
- Su, M.; Liu, Y.; Li, S.; Fang, Z.; He, B.; Zhang, Y.; Li, Y.; He, P. A rubber-like, underwater superoleophobic hydrogel for efficient oil/water separation. Chem. Eng. J. 2019, 361, 364–372. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Li, W.; Li, H.; She, H.; Zha, F. Facile fabrication of underwater superoleophobic SiO2 coated meshes for separation of polluted oils from corrosive and hot water. Sep. Purif. Technol. 2016, 168, 209–214. [Google Scholar] [CrossRef]
- Yu, H.; Lian, Z.; Xu, J.; Wan, Y.; Wang, Z.; Li, Y.; Yu, Z.; Weng, Z. Mechanically durable underwater superoleophobic surfaces based on hydrophilic bulk metals for oil/water separation. Appl. Surf. Sci. 2018, 437, 400–409. [Google Scholar] [CrossRef]
- Chen, X.; Zhai, Y.; Han, X.; Liu, H.; Hu, Y. Surface chemistry-dominated underwater superoleophobic mesh with mussel-inspired zwitterionic coatings for oil/water separation and self-cleaning. Appl. Surf. Sci. 2019, 483, 399–408. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, J.; Zhou, S. A facile immersion-curing approach to surface-tailored poly(vinyl alcohol)/silica underwater superoleophobic coatings with improved transparency and robustness. J. Mater. Chem. A 2017, 5, 10866–10875. [Google Scholar] [CrossRef]
- Caoab, X.; Zhangb, Q.; Zhangb, C.; Lib, Z.; Zhengb, W.; Liub, M.; Wangb, B.; Huangb, S.; Libc, L.; Huanga, X.; et al. A novel approach to coat silica on quantum dots: Forcing decomposition of tetraethyl orthosilicate in toluene at high temperature. J. Alloys Compd. 2020, 817, 152698. [Google Scholar] [CrossRef]
- Koohestani, B.; Mokhtari, P.; Yilmaz, E.; Mahdipour, F.; Darban, A.K. Geopolymerization mechanism of binder-free mine tailings by sodium silicate. Constr. Build. Mater. 2021, 268, 121217. [Google Scholar] [CrossRef]
- Hao, H.; Cao, Y.; Li, L.; Fan, G.; Liu, J. Dispersion and depression mechanism of sodium silicate on quartz: Combined molecular dynamics simulations and density functional theory calculations. Appl. Surf. Sci. 2021, 537, 147926. [Google Scholar] [CrossRef]
- Jolsterå, R.; Gunneriusson, L.; Forsling, W. Adsorption and surface complex modeling of silicates on maghemite in aqueous suspensions. J. Colloid Interface Sci. 2010, 342, 493–498. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.-H.; Chen, S.-H.; Yi, L.-L.; Yin, Z.-B.; Chen, Y.-J.; Jiang, H.; Li, C.-J. Synthesis of Si-Based High-Efficiency and High-Durability Superhydrophilic-Underwater Superoleophobic Membrane of Oil–Water Separation. Materials 2021, 14, 2628. https://doi.org/10.3390/ma14102628
Fang X-H, Chen S-H, Yi L-L, Yin Z-B, Chen Y-J, Jiang H, Li C-J. Synthesis of Si-Based High-Efficiency and High-Durability Superhydrophilic-Underwater Superoleophobic Membrane of Oil–Water Separation. Materials. 2021; 14(10):2628. https://doi.org/10.3390/ma14102628
Chicago/Turabian StyleFang, Xiao-Hui, Su-Hui Chen, Lan-Lin Yi, Zhong-Bin Yin, Yong-Jun Chen, Hong Jiang, and Chang-Jiu Li. 2021. "Synthesis of Si-Based High-Efficiency and High-Durability Superhydrophilic-Underwater Superoleophobic Membrane of Oil–Water Separation" Materials 14, no. 10: 2628. https://doi.org/10.3390/ma14102628
APA StyleFang, X.-H., Chen, S.-H., Yi, L.-L., Yin, Z.-B., Chen, Y.-J., Jiang, H., & Li, C.-J. (2021). Synthesis of Si-Based High-Efficiency and High-Durability Superhydrophilic-Underwater Superoleophobic Membrane of Oil–Water Separation. Materials, 14(10), 2628. https://doi.org/10.3390/ma14102628