Energetic-Materials-Driven Synthesis of Graphene-Encapsulated Tin Oxide Nanoparticles for Sodium-Ion Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Gr-SnO2 Nanoparticles
2.2. Characterization
2.3. Electrochemical Measurements
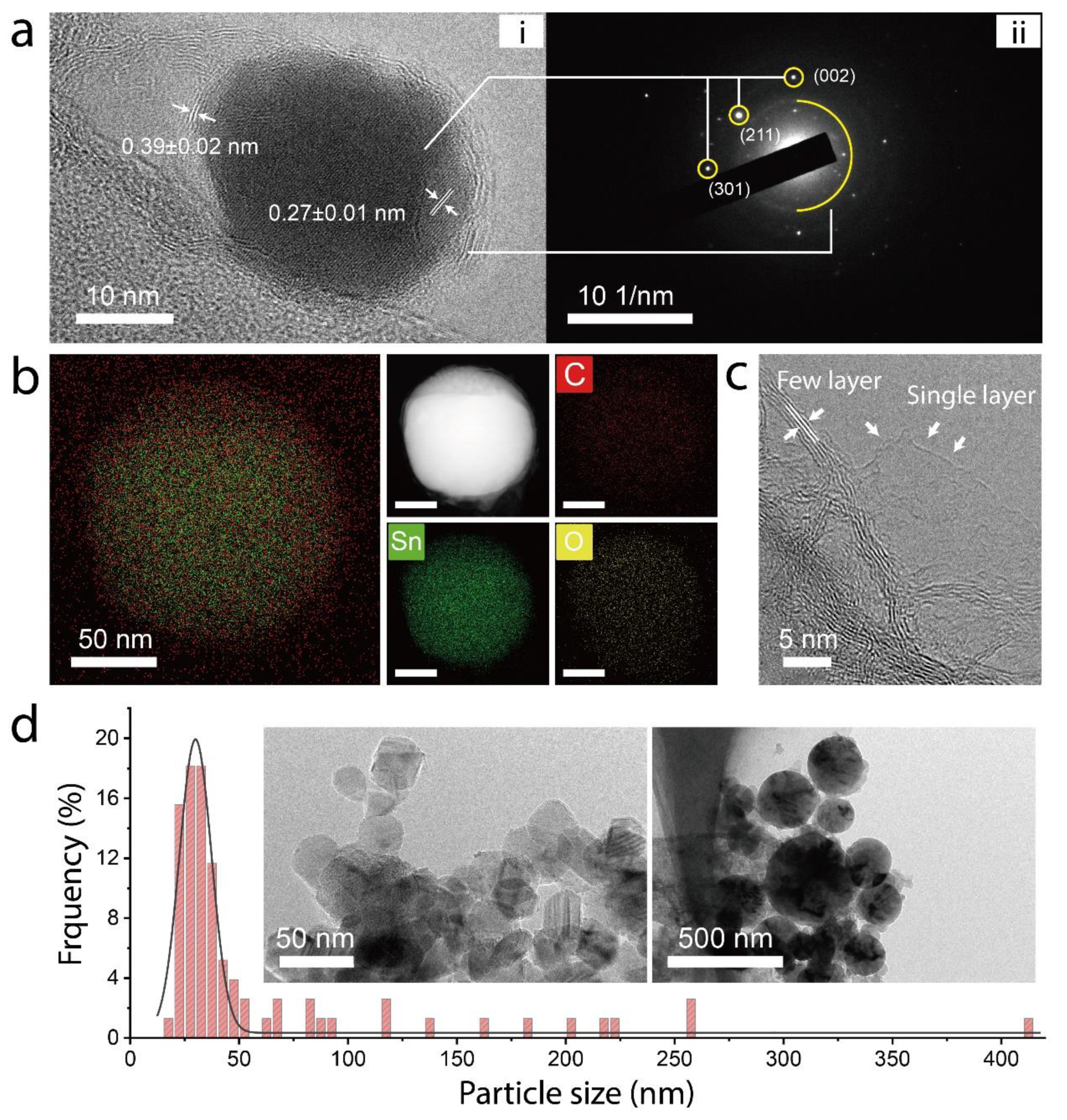
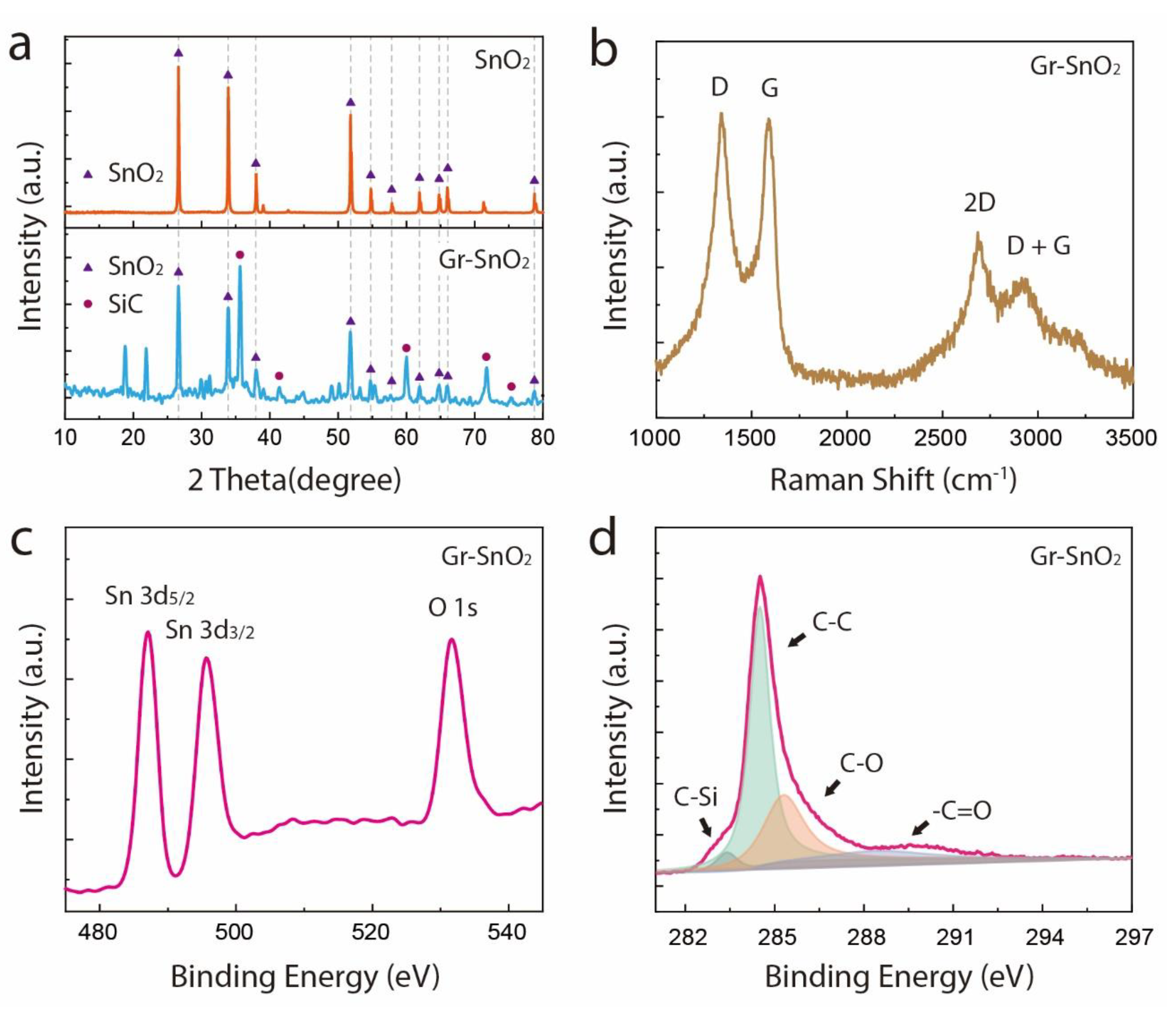
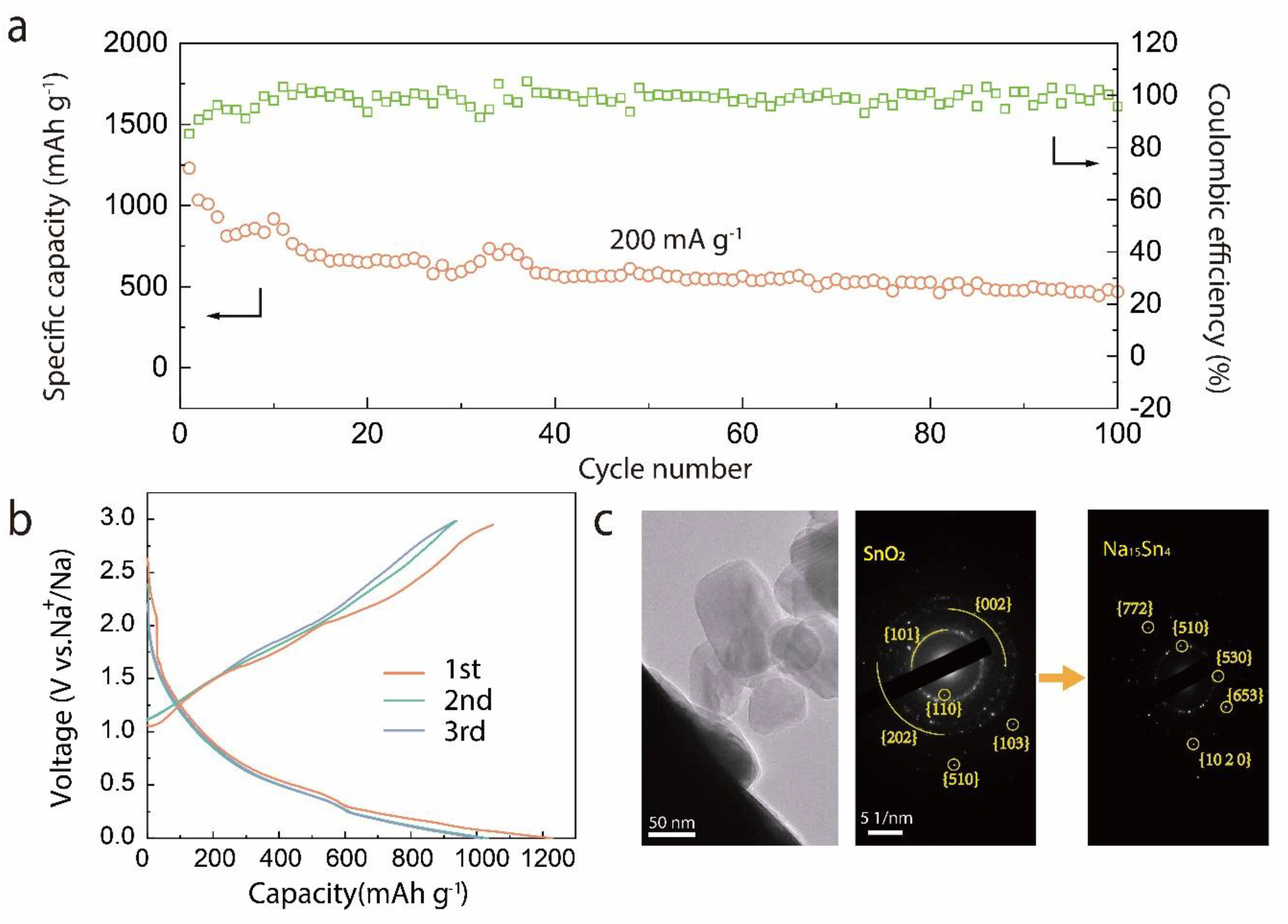
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morales-Narváez, E.; Sgobbi, L.F.; Machado, S.A.S.; Merkoçi, A. Graphene-encapsulated materials: Synthesis, applications and trends. Prog. Mater.Sci. 2017, 86, 1–24. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. SC-CO2-assisted process for a high energy density aerogel supercapacitor: The effect of GO loading. Nanotechnology 2017, 28, 204001. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.-H.; Wang, J.; Wexler, D.; Konstantinov, K.; Guo, Z.-P.; Liu, H.-K. Highly Reversible Lithium Storage in Spheroidal Carbon-Coated Silicon Nanocomposites as Anodes for Lithium-Ion Batteries. Angew. Chem. 2006, 118, 7050–7053. [Google Scholar] [CrossRef]
- Fan, X.; Shao, J.; Xiao, X.; Wang, X.; Li, S.; Ge, H.; Chen, L.; Wang, C. In situ synthesis of SnO2 nanoparticles encapsulated in micro/mesoporous carbon foam as a high-performance anode material for lithium ion batteries. J. Mater. Chem. A 2014, 2, 18367–18374. [Google Scholar] [CrossRef]
- Ma, C.; Xu, T.; Wang, Y. Advanced carbon nanostructures for future high performance sodium metal anodes. Energy Storage Mater. 2020, 25, 811–826. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, L.-J.; Guo, Y.-G. Binding SnO2 Nanocrystals in Nitrogen-Doped Graphene Sheets as Anode Materials for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, N.; Jiao, L.; Tao, Z.; Chen, J. Ultrasmall Sn Nanoparticles Embedded in Carbon as High-Performance Anode for Sodium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 214–220. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2007, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, K.; Lee, H.-W.; Lu, Z.; Liu, N.; Cui, Y. Growth of conformal graphene cages on micrometre-sized silicon particles as stable battery anodes. Nat. Energy 2016, 1, 15029. [Google Scholar] [CrossRef]
- Son, I.H.; Hwan Park, J.; Kwon, S.; Park, S.; Rümmeli, M.H.; Bachmatiuk, A.; Song, H.J.; Ku, J.; Choi, J.W.; Choi, J.-M.; et al. Silicon carbide-free graphene growth on silicon for lithium-ion battery with high volumetric energy density. Nat. Commun. 2015, 6, 7393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Self-Assembled Nanocomposite of Silicon Nanoparticles Encapsulated in Graphene through Electrostatic Attraction for Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 1086–1090. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Langrock, A.; Manivannan, A.; Ehrman, S.H.; Wang, C. Graphene-bonded and -encapsulated Si nanoparticles for lithium ion battery anodes. Small 2013, 9, 2810–2816. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A.; Stanford, M.G.; Kittrell, C.; Chen, W.; Salvatierra, R.V.; Ren, M.; McHugh, E.A.; Advincula, P.A.; et al. Gram-scale bottom-up flash graphene synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Liu, G.; Li, J.; Tian, H.; Li, J.; Ma, Y. Scalable Self-Propagating High-Temperature Synthesis of Graphene for Supercapacitors with Superior Power Density and Cyclic Stability. Adv. Mater. 2016, 29, 1604690. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Nersisyan, H.H.; Lee, J.H.; Ding, J.-R.; Kim, K.-S.; Manukyan, K.V.; Mukasyan, A.S. Combustion synthesis of zero-, one-, two- and three-dimensional nanostructures: Current trends and future perspectives. Prog. Energy Combust. Sci. 2017, 63, 79–118. [Google Scholar] [CrossRef]
- Yang, M.; Liu, J.; Li, S.; Zhang, S.; Wang, Y.; He, C. Ultrafast synthesis of graphene nanosheets encapsulated Si nanoparticles via deflagration of energetic materials for lithium-ion batteries. Nano Energy 2019, 65, 104028. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Lao, M.; Zhang, Y.; Luo, W.; Yan, Q.; Sun, W.; Dou, S.X. Alloy-Based Anode Materials toward Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700622. [Google Scholar] [CrossRef] [PubMed]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Yang, M.; Liu, J.; Li, S.; Zhang, S.; Zhuang, Z.; Wang, Y.; He, C. Self-sustained solid-state exothermic reaction for scalable graphene production. Mater. Des. 2020, 196, 109135. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Mishra, M.; Singh, A.P.; Singh, B.P.; Dhawan, S.K. Performance of a nanoarchitectured tin oxide@reduced graphene oxide composite as a shield against electromagnetic polluting radiation. RSC Adv. 2014, 4, 25904–25911. [Google Scholar] [CrossRef]
- Morgan, W.E.; Van Wazer, J.R. Binding energy shifts in the x-ray photoelectron spectra of a series of related Group IVa compounds. J. Phys. Chem. 1973, 77, 964–969. [Google Scholar] [CrossRef]
- Thomas, J.H. III X-ray photoelectron spectroscopy study of hydrogen plasma interactions with a tin oxide surface. Appl. Phys. Lett. 1983, 42, 794–796. [Google Scholar] [CrossRef]
- Themlin, J.-M.; Chtaïb, M.; Henrard, L.; Lambin, P.; Darville, J.; Gilles, J.-M. Characterization of tin oxides by x-ray-photoemission spectroscopy. Phys. Rev. B 1992, 46, 2460–2466. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, B.; Qiao, Z.; Hu, Y.; Zheng, W.; Zhang, L.; Zhou, Y.; Ji, G.; Yang, G. Gram-Scale Synthesis of Graphene Quantum Dots from Single Carbon Atoms Growth via Energetic Material Deflagration. Chem. Mater. 2015, 27, 4319–4327. [Google Scholar] [CrossRef]
- Terry, B.C.; Son, S.F.; Groven, L.J. Altering combustion of silicon/polytetrafluoroethylene with two-step mechanical activation. Combust. Flame 2015, 162, 1350–1357. [Google Scholar] [CrossRef]
- Lan, J.; Liu, J.; Zhang, S.; Xue, X.; Li, S.J.M. Design Influence of multi-oxidants on reaction characteristics of PTFE-Al-XmOY reactive material. Mater. Des. 2019, 186, 108325. [Google Scholar] [CrossRef]
- Gu, M.; Kushima, A.; Shao, Y.; Zhang, J.-G.; Liu, J.; Browning, N.D.; Li, J.; Wang, C. Probing the Failure Mechanism of SnO2 Nanowires for Sodium-Ion Batteries. Nano Lett. 2013, 13, 5203–5211. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Qu, J.; Legut, D.; Qin, J.; Li, X.; Zhang, Q. Unique Double-Interstitialcy Mechanism and Interfacial Storage Mechanism in the Graphene/Metal Oxide as the Anode for Sodium-Ion Batteries. Nano Lett. 2019, 19, 3122–3130. [Google Scholar] [CrossRef]
- Jiang, B.; He, Y.; Li, B.; Zhao, S.; Wang, S.; He, Y.B.; Lin, Z. Polymer-Templated Formation of Polydopamine-Coated SnO2 Nanocrystals: Anodes for Cyclable Lithium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2017, 56, 1869–1872. [Google Scholar] [CrossRef]
- Wang, L.; Yan, J.; Xu, Z.; Wang, W.; Wen, J.; Bai, X. Rate mechanism of vanadium oxide coated tin dioxide nanowire electrode for lithium ion battery. Nano Energy 2017, 42, 294–299. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and improving the initial Coulombic efficiency of high-capacity anode materials for practical sodium ion batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, S.; Zhou, L.; Chang, L.; Liu, W.; Yin, D.; Yi, Z.; Wang, L. SnO2 Quantum Dots: Rational Design to Achieve Highly Reversible Conversion Reaction and Stable Capacities for Lithium and Sodium Storage. Small 2020, 16, e2000681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, Y.; Zhao, C.; Pan, E.; Jia, M. 1D ultrafine SnO2 nanorods anchored on 3D graphene aerogels with hierarchical porous structures for high-performance lithium/sodium storage. J. Colloid Interface Sci. 2018, 532, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yuan, C.; Li, H.; Fan, K.; Wei, Z.; Sun, H.; Ma, J. Growth of SnO2 Nanoflowers on N-doped Carbon Nanofibers as Anode for Li- and Na-ion Batteries. Nanomicro Lett. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, X.; Gao, Z.; Hu, X.; Guo, J.; Cai, S.; Guo, R.; Ji, H.; Zheng, C.; Hu, W. Fabrication of porous carbon sphere@SnO2 @carbon layer coating composite as high performance anode for sodium-ion batteries. Appl. Surf. Sci. 2018, 433, 713–722. [Google Scholar] [CrossRef]
- Zhou, D.; Li, X.; Fan, L.-Z.; Deng, Y. Three-dimensional porous graphene-encapsulated CNT@SnO2 composite for high-performance lithium and sodium storage. Electr. Acta 2017, 230, 212–221. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Zhao, C.; Duan, Y.; He, X.; Jia, M. SnO2 quantum dots/graphene aerogel composite as high-performance anode material for sodium ion batteries. Mater. Lett. 2017, 191, 169–172. [Google Scholar] [CrossRef]
- Entwistle, J.; Rennie, A.; Patwardhan, S. A review of magnesiothermic reduction of silica to porous silicon for lithium-ion battery applications and beyond. J. Mater. Chem. A 2018, 6, 18344–18356. [Google Scholar] [CrossRef]


| Materials | Cycling Performance | Rate Capacity | Coulomb Efficiency | Ref. |
|---|---|---|---|---|
| SnO2 NRs@GA | 232 mAh·g−1 @50 mA·g−1 | 96 mAh·g−1 @1 A·g−1 | 58.4% | [42] |
| NC@SnO2 | 270 mAh·g−1 @100 mA·g−1 | 193 mAh·g−1 @1 A·g−1 | 38.2% | [43] |
| SnO2 QDs/GA | 319 mAh·g−1 @50 mA·g−1 | 150 mAh·g−1 @800 mA·g−1 | 54% | [46] |
| CNT@SnO2@G | 323 mAh·g−1 @25 mA·g−1 | 119 mAh·g−1 @1 A g−1 | 43% | [45] |
| PCS@SnO2@C | 326 mAh·g−1 @50 mA·g−1 | 82 mAh·g−1 @1.6 A·g−1 | 53.5% | [44] |
| SnO2/NC-2 | 342.2 mAh·g−1 @100 mA·g−1 | 212.6 mAh·g−1 @1 A·g−1 | 59.2% | [41] |
| C/SnO2/C | 370 mAh·g−1 @100 mA·g−1 | 105 mAh·g−1 @10 A·g−1 | - | [37] |
| Gr-SnO2 | 469 mAh·g−1@100 mA·g−1 | 209.5 mAh·g−1@1 A·g−1 | 85.3% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, J.; Yang, M.; Hou, L.; Xu, T.; Li, S.; Zhuang, Z.; He, C. Energetic-Materials-Driven Synthesis of Graphene-Encapsulated Tin Oxide Nanoparticles for Sodium-Ion Batteries. Materials 2021, 14, 2550. https://doi.org/10.3390/ma14102550
Wang Y, Liu J, Yang M, Hou L, Xu T, Li S, Zhuang Z, He C. Energetic-Materials-Driven Synthesis of Graphene-Encapsulated Tin Oxide Nanoparticles for Sodium-Ion Batteries. Materials. 2021; 14(10):2550. https://doi.org/10.3390/ma14102550
Chicago/Turabian StyleWang, Yingchun, Jinxu Liu, Min Yang, Lijuan Hou, Tingting Xu, Shukui Li, Zhihua Zhuang, and Chuan He. 2021. "Energetic-Materials-Driven Synthesis of Graphene-Encapsulated Tin Oxide Nanoparticles for Sodium-Ion Batteries" Materials 14, no. 10: 2550. https://doi.org/10.3390/ma14102550
APA StyleWang, Y., Liu, J., Yang, M., Hou, L., Xu, T., Li, S., Zhuang, Z., & He, C. (2021). Energetic-Materials-Driven Synthesis of Graphene-Encapsulated Tin Oxide Nanoparticles for Sodium-Ion Batteries. Materials, 14(10), 2550. https://doi.org/10.3390/ma14102550







