Al and Zr Porous Clay Heterostructures as Removal Agents of Basic Blue-41 Dye from an Artificially Polluted Solution: Regeneration Properties and Batch Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Intercalated Clay Precursors
2.3. Preparation of Metal Porous Clay Heterostructures (Al-PCH and Zr-PCH)
2.4. BAsic Blue-41 Removal Procedure
2.5. Characterization
3. Results and Discussion
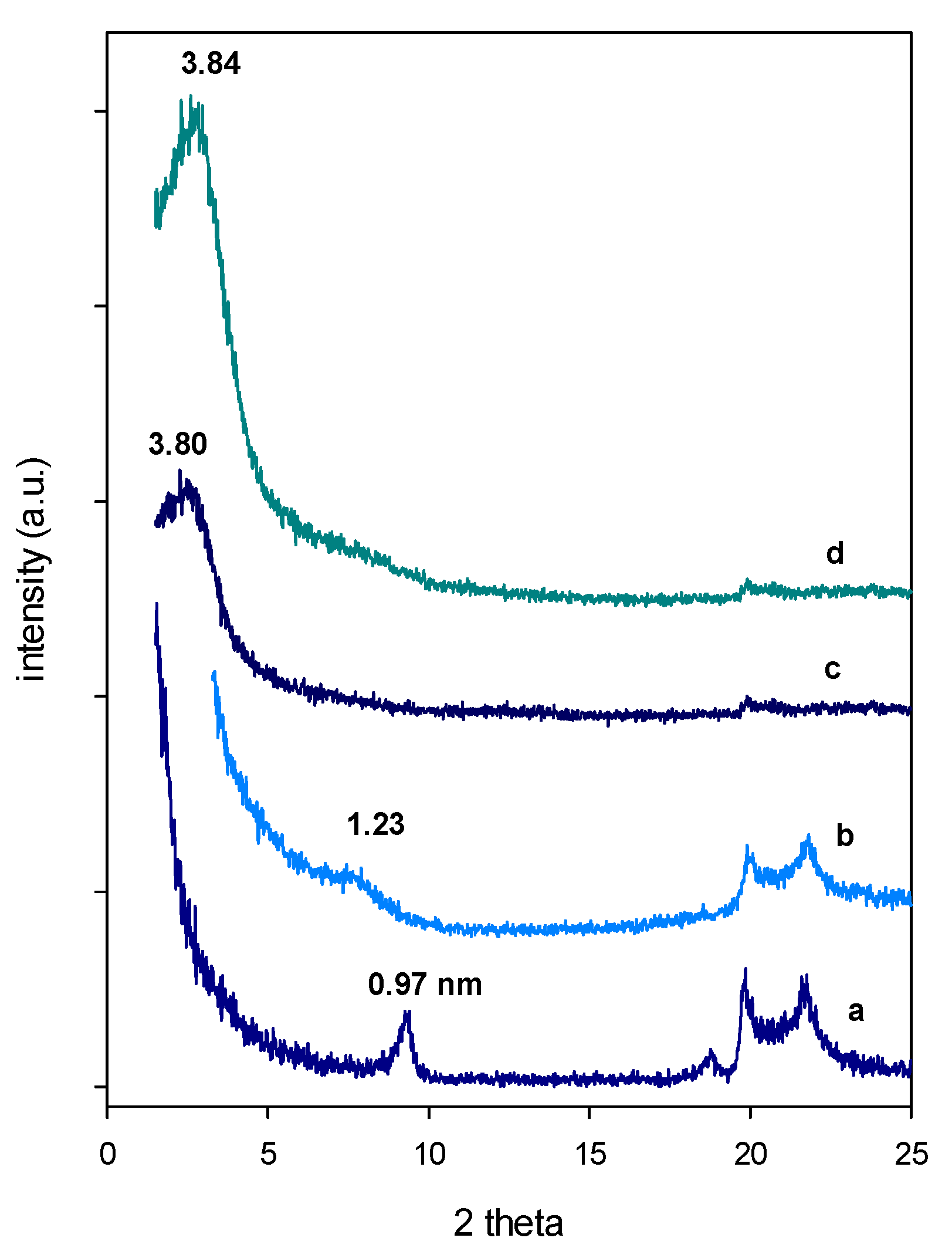
3.1. X-ray Diffraction (XRD)
3.2. XRF Data
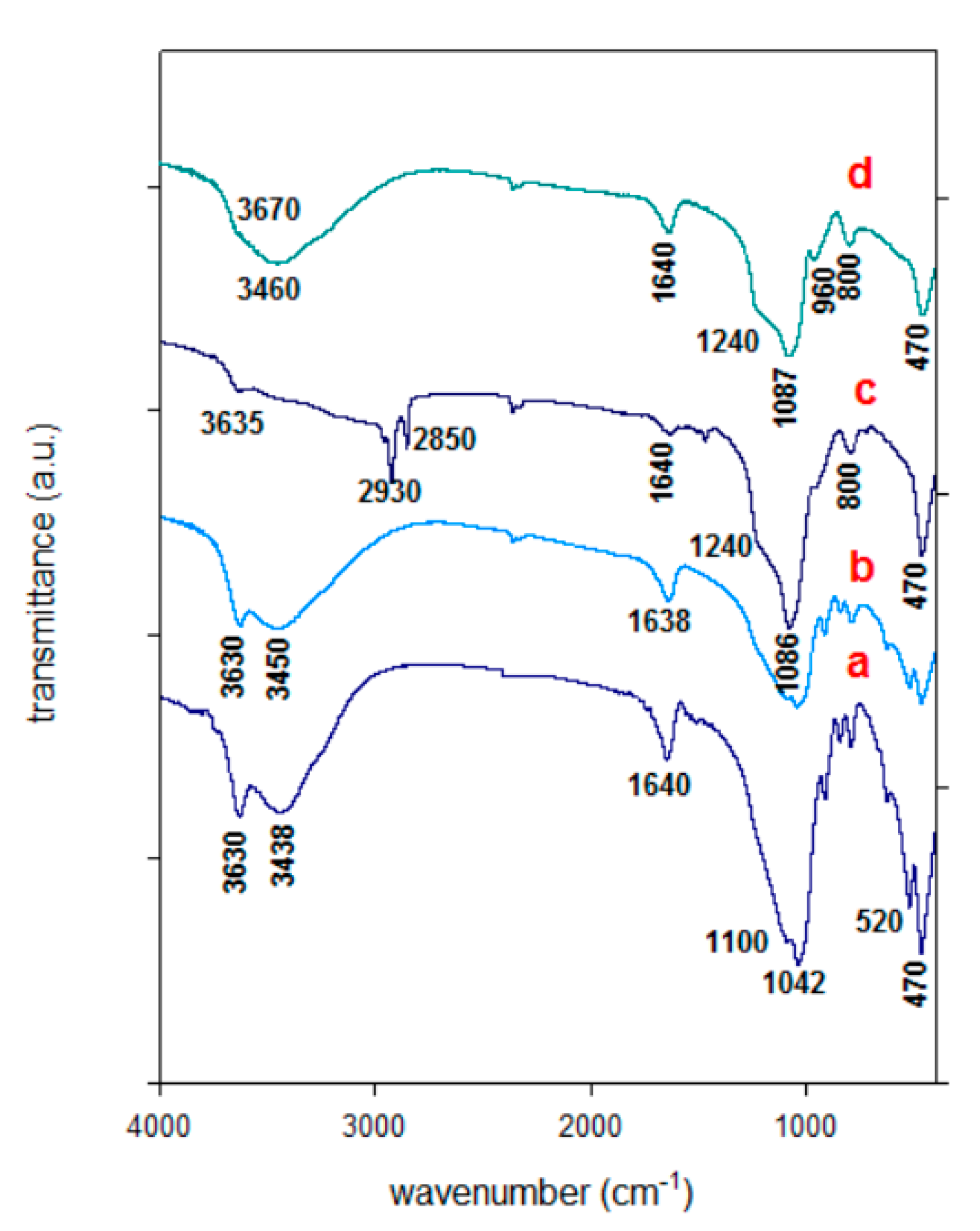
3.3. FTIR Spectra
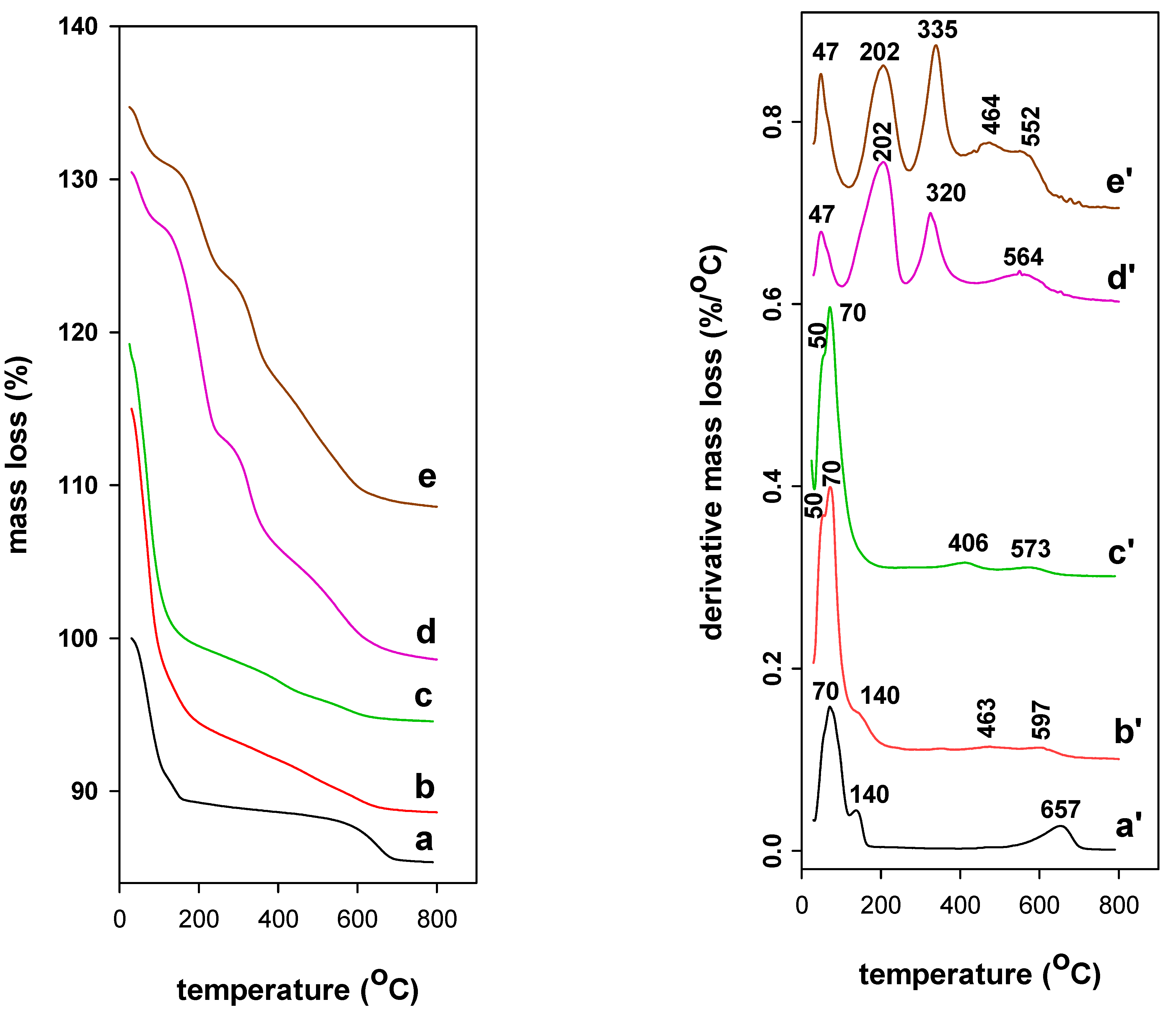
3.4. TGA Analysis
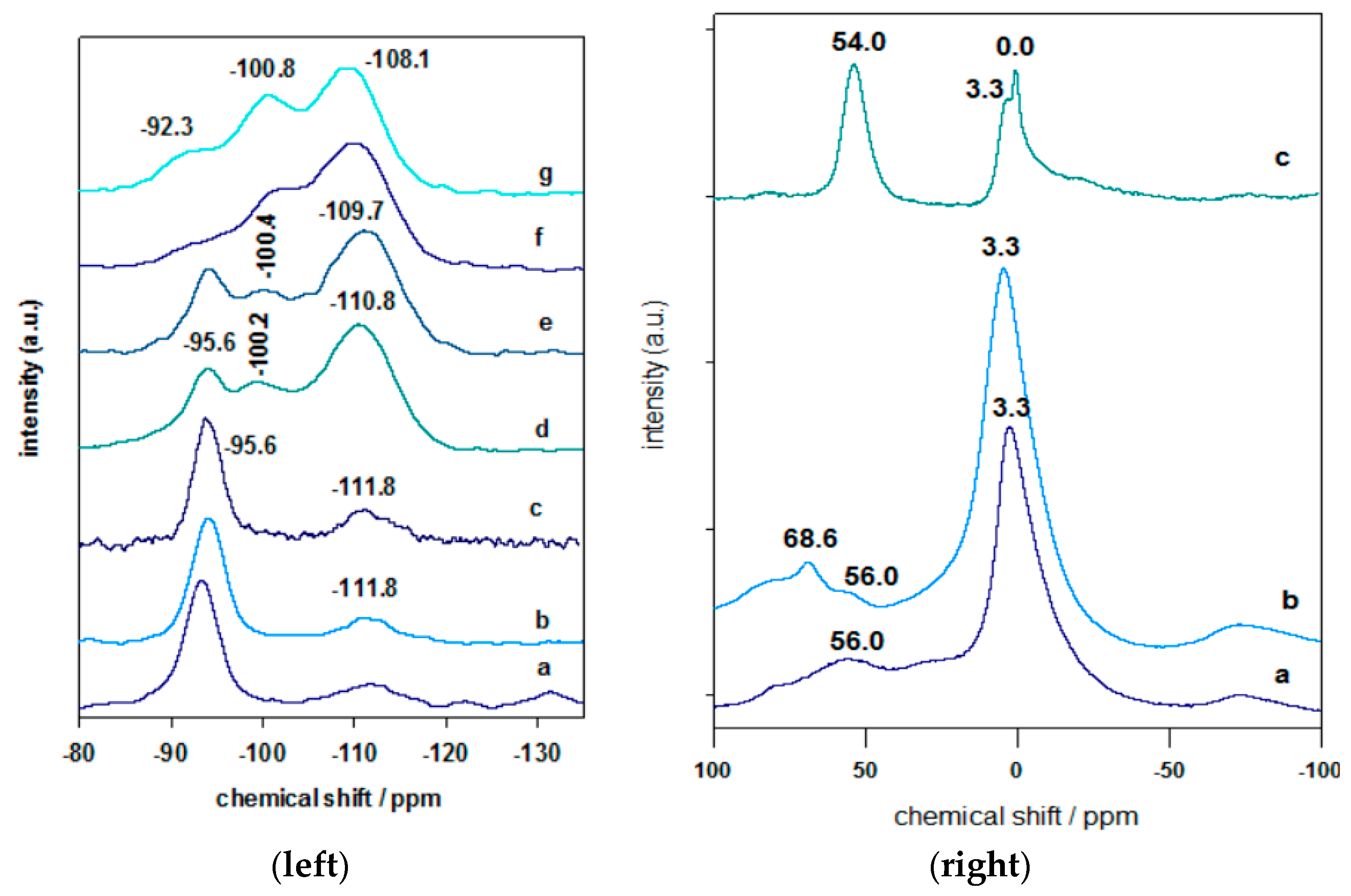
3.5. Solid 29Si and 27Al NMR
3.6. Microtextural Properties
4. Operational Effect on the Removal Properties of Basic Blue-41
4.1. Effect of BB-41 Initial Concentrations
4.2. Effect of Zr-PCH Dose
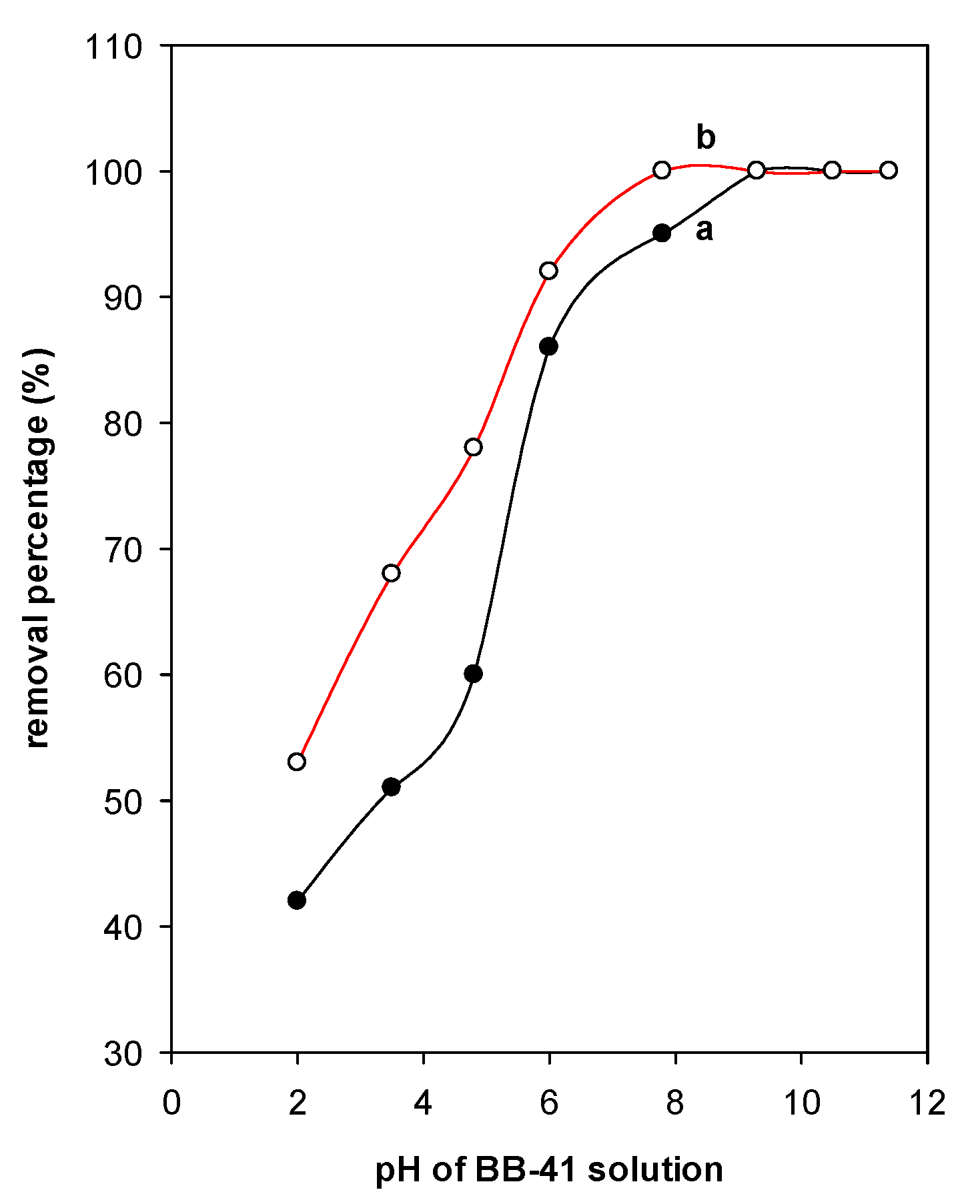
4.3. Effect of pH
4.4. Isotherms of Adsorption
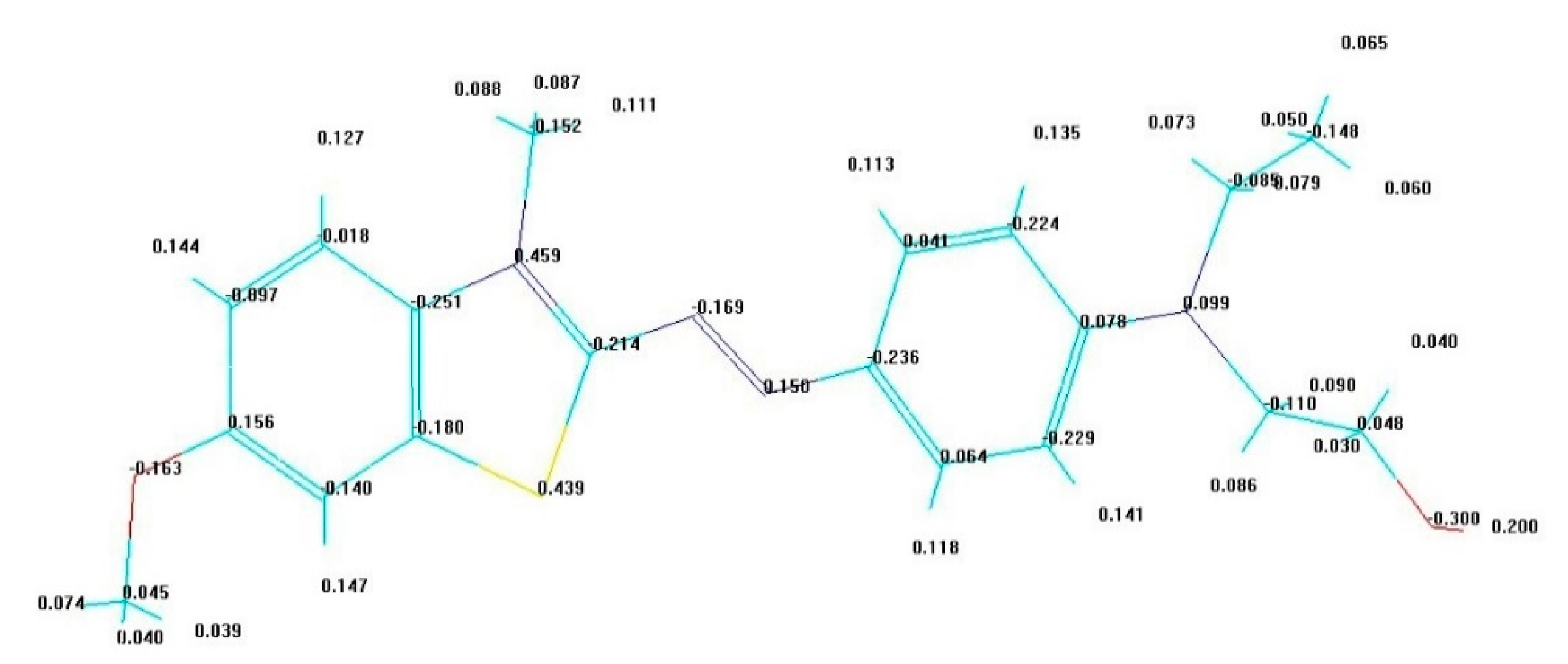
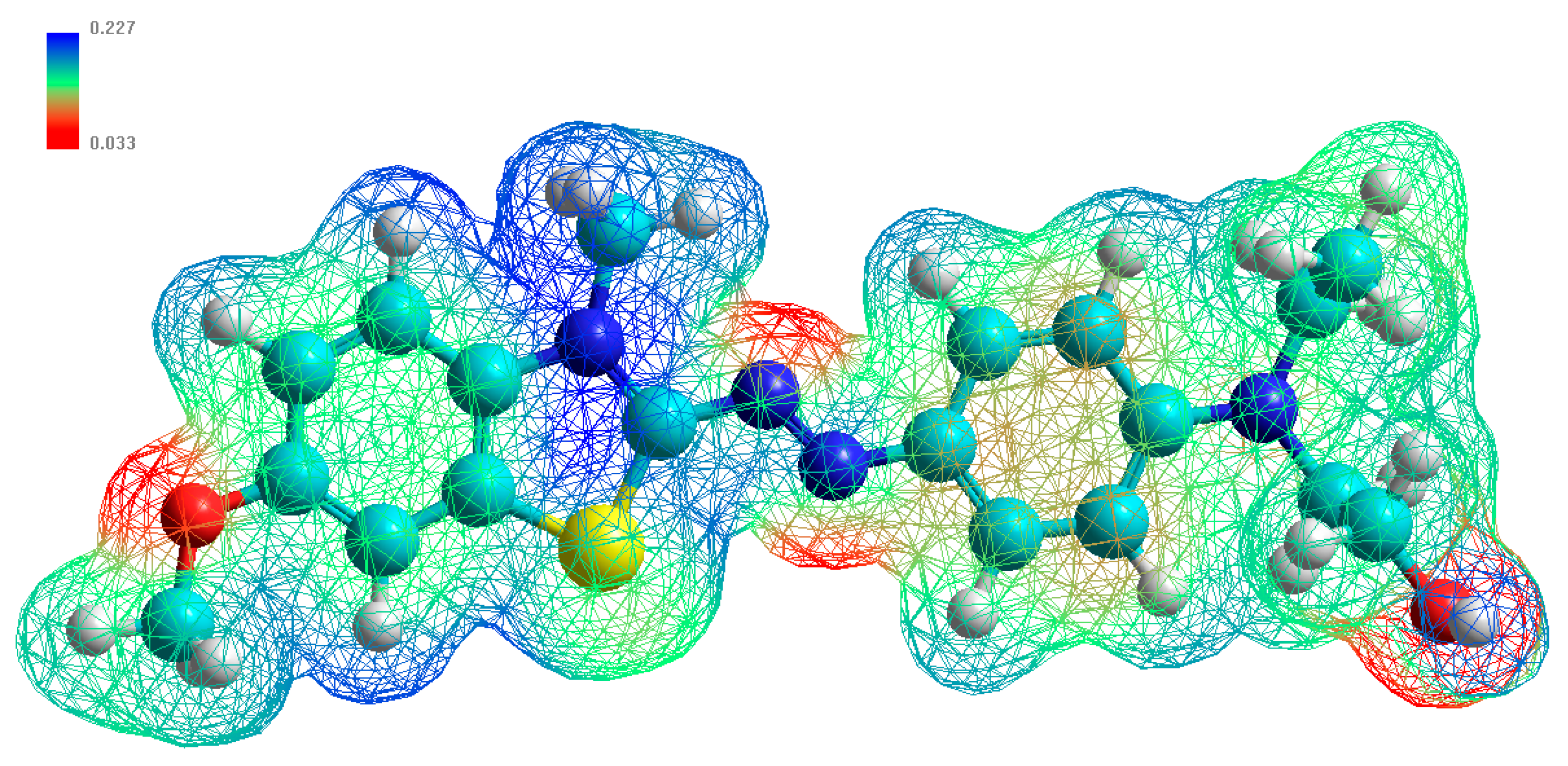
4.5. Theoretical Calculation
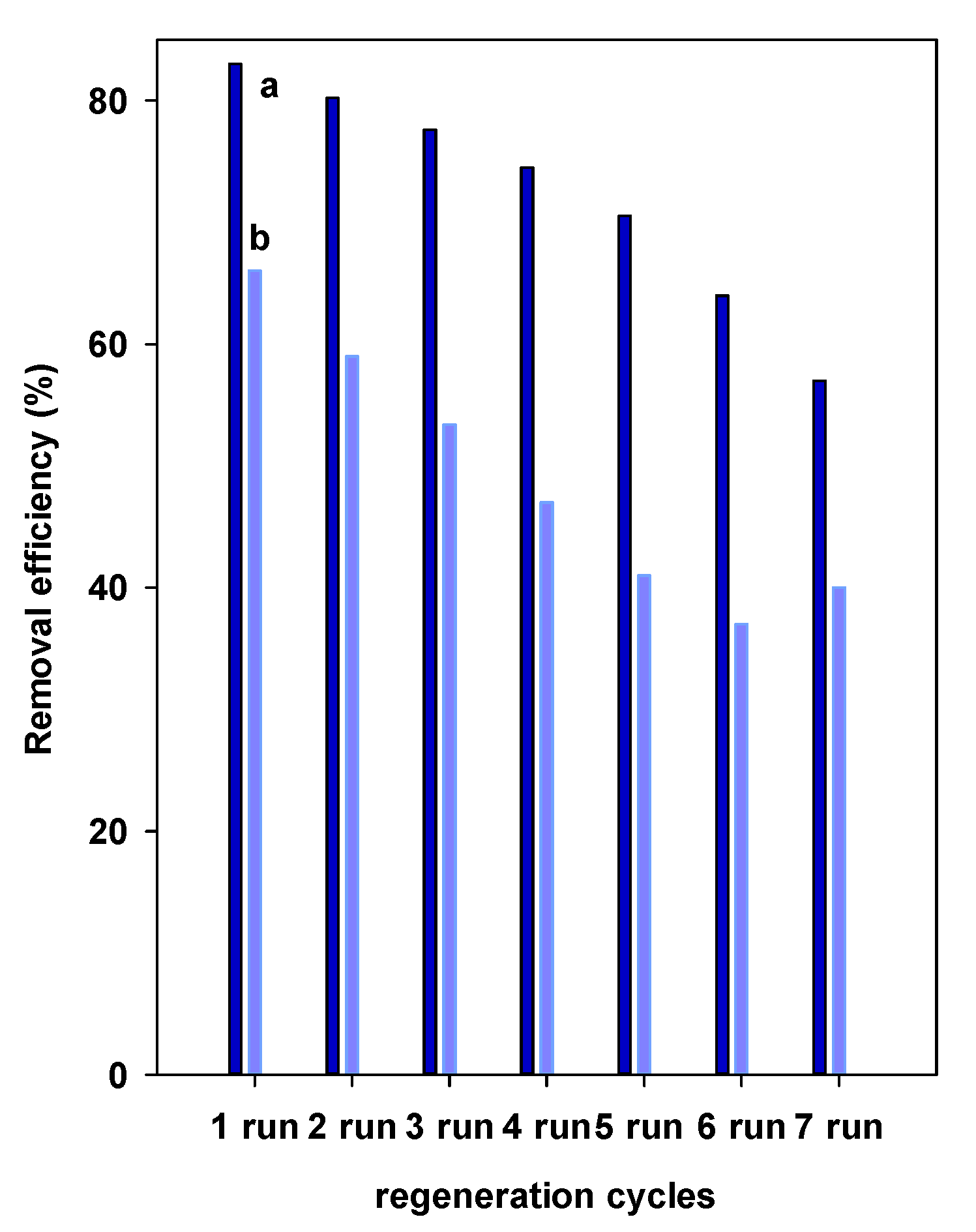
4.6. Regeneration Properties
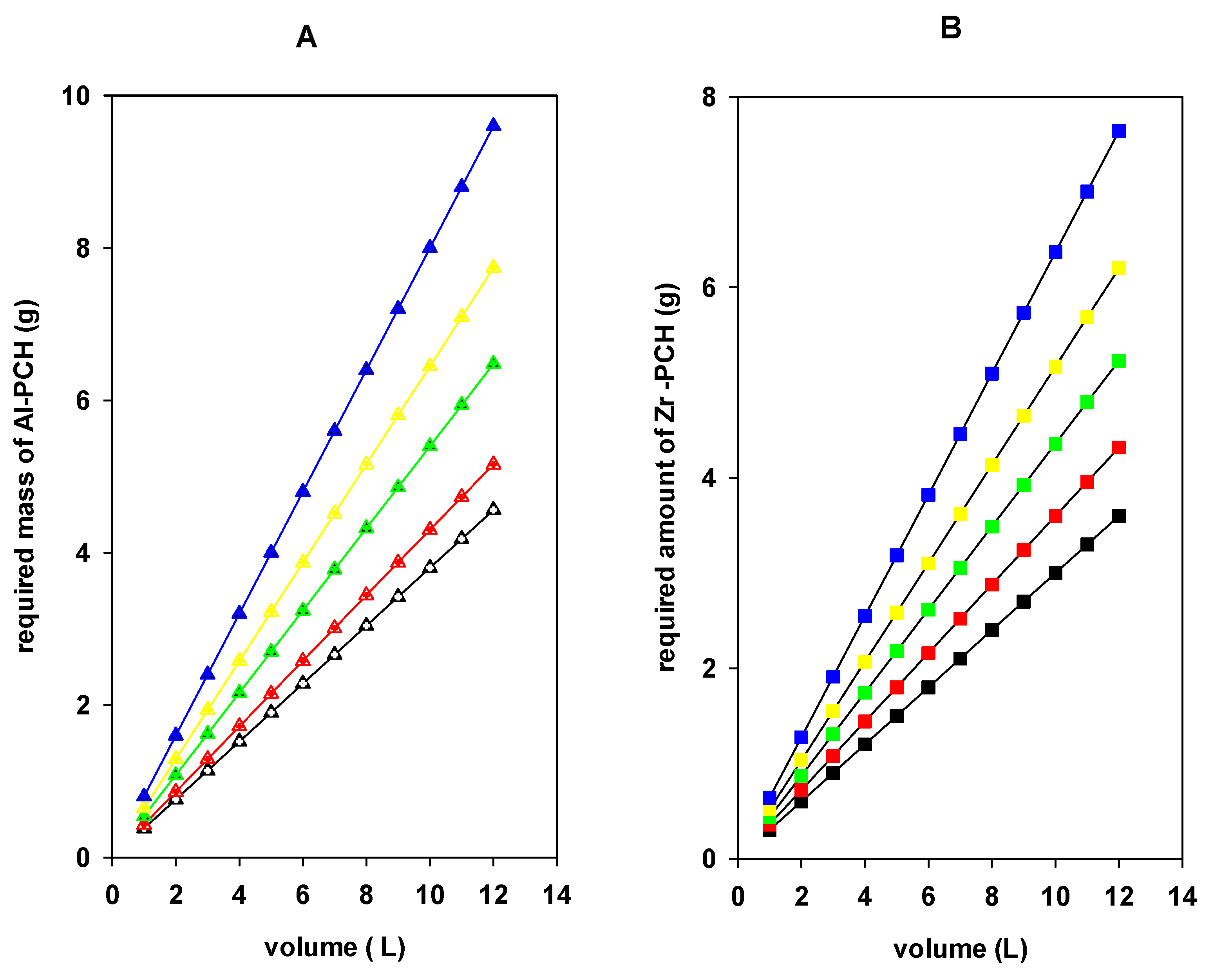
4.7. Single-Stage Batch Design Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Ghosh, A.; Dastidar, M.G.; Sreekrishnan, T.R. Response surface modeling of bioremediation of acid black 52 dye using Aspergillus flavus. Water Sci. Technol. 2017, 75, 2864–2874. [Google Scholar] [CrossRef]
- Christie, R.M. Environmental Aspects of Textile Dyeing; Woodhead: Boca Raton, FL, USA, 2007. [Google Scholar]
- O’Neil, C.; Hawkes, F.R.; Hawkes, D.L.; Lourenc, N.D.; Pinheiro, H.M.; Delee, W. Colour in textile effluents-sources, measurement, discharge consents and simulation: A review. J. Chem. Technol. Biotechnol. 1999, 74, 1009–1018. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Sudha, M.; Saranya, A.; Selvakumar, G.; Sivakumar, N. Microbial degradation of azo dyes: A review. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 670–690. [Google Scholar]
- Ayadi, I.; Souissi, Y.; Jlassi, I.; Peixoto, F.; Mnif, W. Chemical Synonyms, Molecular Structure and Toxicological Risk Assessment of Synthetic Textile Dyes: A Critical Review. J. Dev. Drugs 2016, 5, 151–155. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M. Dyes and Pigments: Their Structure and Properties. In Dyes and Pigments; Springer: Cham, Switzerland, 2016; pp. 13–29. [Google Scholar]
- Carmen, Z.; Daniel, S. Textile Organic Dyes—Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview; Puzyn, T., Mostrag-Szlichtyng, A., Eds.; Organic Pollutants Ten Years After the Stockholm Convention—Environmental and Analytical Update; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222–137241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar]
- Ngulube, T.; Gumbo, R.; Masindi, V.; Maity, A. An update on synthetic dyes adsorption onto clay based minerals: A state-of-art review. J. Environ. Manag. 2017, 191, 35–57. [Google Scholar] [CrossRef]
- Hassan, M.; Carr, C. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef]
- Barakan, S.; Aghazadeh, V. The advantages of clay mineral modification methods for enhancing adsorption efficiency in wastewater treatment: A review. Environ. Sci. Pollut. Res. 2021, 28, 2572–2599. [Google Scholar] [CrossRef]
- Pandey, S.; Ramontja, J. Natural bentonite clay and its composites for dye removal: Current state and future potential. Am. J. Chem. Appl. 2016, 3, 8–19. [Google Scholar]
- Okada, T.; Seki, Y.; Ogawa, M. Designed Nanostructures of Clay for Controlled Adsorption of Organic Compounds. J. Nanosci. 2014, 14, 2121–2134. [Google Scholar] [CrossRef]
- Guegan, R. Organoclay applications and limits in the environment. Comptes Rendus Chim. 2018, 22, 132–141. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Abboudi, M.; Rakass, S.; Hassani, H.; Ibrahim, S.; Al-Faze, R. Removal Properties of Anionic Dye Eosin by Cetyltrimethylammonium Organo-Clays: The Effect of Counter-Ions and Regeneration Studies. Molecules 2018, 23, 2364. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Yan, L.; Tan, S.X.; Zheng, J. Organoclays from alkaline-treated acid-activated clays: Properties and thermal stability. J. Therm. Anal. Calorim. 2014, 115, 1465–1475. [Google Scholar] [CrossRef]
- Eloussaief, M.; Benzina, M. Efficiency of natural and acid-activated clays in the removal of Pb(II) from aqueous solutions. J. Hazard. Mater. 2010, 178, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Kooli, F.; Liu, Y.; Al-Faze, R.; Al Suhaimi, A. Effect of acid activation of Saudi local clay mineral on removal properties of Basic Blue 41 from an aqueous solution. Appl. Clay Sci. 2015, 116, 23–30. [Google Scholar] [CrossRef]
- Kooli, F. Effect of C16TMA contents on the thermal stability of organo-bentonites: In situ X-ray diffraction analysis. Thermochim. Acta 2013, 551, 7–13. [Google Scholar] [CrossRef]
- Baloyi, S.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: A review. RSC Adv. 2018, 8, 5197–5211. [Google Scholar] [CrossRef]
- Gil, A.; Vicente, M. Progress and perspectives on pillared clays applied in energetic and environmental remediation processes. Curr. Opin. Green Sustain. Chem. 2020, 21, 56–63. [Google Scholar] [CrossRef]
- Cardona, Y.; Vicente, M.A.; Korili, S.A.; Gil, A. Progress and perspectives for the use of pillared clays as adsorbents for organic compounds in aqueous solution. De Gruyter 2020. [Google Scholar] [CrossRef]
- Galarneau, A.; Barodawalla, A.; Pinnavaia, T. Porous clay heterostructures formed by gallery-templated synthesis. Nature 1995, 374, 529–531. [Google Scholar] [CrossRef]
- Garea, S.A.; Mihal, A.; Vasile, E.; Voicu, G. Synthesis and characterization of porous clay heterostructures. Rev. Chim. 2014, 65, 649–656. [Google Scholar]
- Cecilia, J.A.; García-Sancho, C.; Franco, F. Montmorillonite based porous clay heterostructures: Influence of Zr in the structure and acidic properties. Microporous Mesoporous Mater. 2013, 176, 95–102. [Google Scholar] [CrossRef]
- Ahenach, J.; Cool, P.; Vansant, E.F. Enhanced Brönsted acidity created upon Al-grafting of porous clay heterostructures in aluminium acetylacetonate adsorption. Phys. Chem. Chem. Phys. 2000, 2, 5750–5755. [Google Scholar] [CrossRef]
- Kooli, F. Porous clay heterostructures (PCHs) from Al13-intercalated and Al13-pillared montmorillonites: Properties and heptane hydro-isomerization catalytic activity. Microporous Mesoporous Mater. 2014, 184, 184–192. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Hbaieb, K.; Al-Faze, R. Characterization and catalytic properties of porous clay heterostructures from zirconium intercalated clay and its pillared derivatives. Microporous Mesoporous Mater. 2016, 226, 482–492. [Google Scholar] [CrossRef]
- Zimowska, M.; Pálková, H.; Madejová, J.; Dula, R.; Pamin, K.; Olejniczak, Z.; Serwicka, B. Laponite-derived porous clay heterostructures: III. The effect of alumination. Microporous Mesoporous Mater. 2013, 175, 67–75. [Google Scholar] [CrossRef]
- Barakan, S.; Aghazadeh, V. Synthesis and characterization of hierarchical porous clay heterostructure from Al, Fe -pillared nano-bentonite using microwave and ultrasonic techniques. Microporous Mesoporous Mater. 2019, 278, 138–148. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kowalczyk, A.; Skoczek, M.; Rutkowska, M.; Gil, B.; Natkański, P.; Radko, M.; Motak, M.; Dębek, R.; Ryczkowski, J. Porous clay heterostructures intercalated with multicomponent pillars as catalysts for dehydration of alcohols. Appl. Clay Sci. 2018, 160, 116–125. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Vilarrasa-García, E.; Jiménez-Jiménez, J.; Rodriguez-Castellón, E. Synthesis, Characterization, Uses and Applications of Porous Clays Heterostructures: A Review. Chem. Rec. 2018, 18, 1085–1104. [Google Scholar] [CrossRef]
- Aguiar, J.; Cecilia, J.; Tavares, P.; Azevedo, D.; Rodríguez Castellón, E.; Lucena, J.; Silva Junior, J. Adsorption study of reactive dyes onto porous clay heterostructures. Appl. Clay Sci. 2017, 135, 35–44. [Google Scholar] [CrossRef]
- Tassanapayak, R.; Magaraphan, R.; Manuspiya, H. Functionalized Porous Clay Heterostructure for Heavy Metal Adsorption from Wastewater. Adv. Mater. Res. 2008, 55, 617–620. [Google Scholar] [CrossRef]
- Esfandiyari, S.; Takas, M. Synthesis and characterization of porous clay heterostructure intercalated with CuO nanoparticles as a visible light-driven photocatalyst. J. Iran Chem. Soc. 2019, 16, 45–55. [Google Scholar]
- Sanchis, R.; Cecilia, J.J.; Soriano, M.; Vázquez, M.; Dejoz, A.; Nieto, J. Porous clays heterostructures as supports of iron oxide for environmental catalysis. Chem. Eng. J. 2018, 334, 1159–1168. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Ben Haj Amara, A.; Ruiz-Hitzky, E. Organoclay hybrid materials as precursors of porous ZnO/silica-clay heterostructures for photocatalytic applications. Beilstein J. Nanotechnol. 2016, 7, 1971–1982. [Google Scholar] [CrossRef]
- Fatimah, I.; Ardianti, S.; Sahroni, I.; Purwiandono, G.; Sagadevan, S.; Doong, R.A. Visible light sensitized porous clay heterostructure photocatalyst of zinc-silica modified montmorillonite by using tris(2,2′-bipyridyl) dichlororuthenium. Appl. Clay Sci. 2021, 204, 106023. [Google Scholar] [CrossRef]
- Kass, L.; Harrison, G.L.; Lindheimer, C. A new stain for identification of avian leukocytes. Biotech. Histochem. 2002, 77, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kass, L. Basic Blue 41: A New Panoptic Stain for Blood and Bone Marrow Cells. J. Histotechnol. 1988, 11, 10–15. [Google Scholar] [CrossRef]
- Morodome, S.; Kawamura, K. Swelling behavior of Na- and Ca-montmorillonite up to 150 °C by in situ X-Ray diffraction experiments. Clays Clay Miner. 2009, 57, 150–160. [Google Scholar] [CrossRef]
- Kooli, F. Pillared montmorillontes from unusual antiperspirant aqueous solutions: Characterization and catalytic tests. Microporous Mesoporous Mater. 2013, 167, 228–236. [Google Scholar] [CrossRef]
- Chotiradsirikun, S.; Guo, R.; Bhalla, A.S.; Manuspiya, H. Novel synthesis route of porous clay heterostructures via mixed surfactant template and their dielectric behaviour. J. Porous Mater. 2021, 28, 117–128. [Google Scholar] [CrossRef]
- Son, Y.; Kim, T.-H.; Kim, D.; Hwang, Y. Porous Clay Heterostructure with Alginate Encapsulation for Toluene Removal. Nanomaterials 2021, 11, 388. [Google Scholar] [CrossRef]
- Kooli, F.; Jones, W. Al and Zr pillared acid-activated saponite clays: Characterization and properties. J. Mater. Chem. 1998, 8, 2119–2124. [Google Scholar] [CrossRef]
- Pichowicz, M.; Mokaya, R. Stability of Pillared Clays: Effect of compaction on the physicochemical properties of Al-Pillared Clays. Chem. Mater. 2004, 16, 263–269. [Google Scholar] [CrossRef]
- Cardona, Y.; Korili, Y.S.; Gil, A. Understanding the formation of Al13 and Al30 polycations to the development of microporous materials based on Al13-and Al30-PILC montmorillonites: A review. Appl. Clay Sci. 2021, 203. [Google Scholar] [CrossRef]
- Amari, A.; Gannouni, H.; Khan, M.I.; Almesfer, M.K.; Elkhaleefa, A.M.; Gannouni, A. Effect of Structure and Chemical Activation on the Adsorption Properties of Green Clay Minerals for the Removal of Cationic Dye. Appl. Sci. 2018, 8, 2302. [Google Scholar] [CrossRef]
- Gârea, S.A.; Mihai, A.I.; Vasile, E.; Nistor, C.; Sârbu, A.; Mitran, R. Synthesis of new porous clay heterostructures: The influence of co-surfactant type. Mat. Chem. Phys. 2016, 179, 17–26. [Google Scholar] [CrossRef]
- Kooli, F. Organo-bentonites with improved cetyltrimethylammonium contents. Clay Miner. 2014, 49, 683–692. [Google Scholar] [CrossRef]
- Jianxi, Z.; Ke, W.; Ping, Z.; Yuebo, W.; Lingya, M.; Yunfei, X.; Runliang, Z.; Hongmei, L.; Hongping, H. Keggin-Al 30 pillared montmorillonite. Microporous Mesoporous Mater. 2017, 242, 256–263. [Google Scholar]
- Trigueiro, P.; Pereira, F.R.; Guillemin, D.; Rigaud, B.; Balme, S.; Janot, J.M.; dos Santos, I.M.G.; Fonseca, M.G.; Walter, P.; Jaber, M. When anthraquinone dyes meet pillared montmorillonite: 1 stability or fading upon exposure to light? Dyes Pigm. 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Krupskaya, V.; Zakusin, V.; Tyupina, E.; Dorzhieva, O.; Zhukhlistov, A.; Belousov, P.; Timofeeva, M. Experimental Study of Montmorillonite Structure and Transformation of Its Properties under Treatment with Inorganic Acid Solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Belver, C.; Aranda, P.; Martin-Luengo, M.A.; Ruiz-Hitzky, E. New silica/alumina–clay heterostructures: Properties as acid catalysts. Microporous Mesoporous Mater. 2012, 147, 157–166. [Google Scholar] [CrossRef]
- Schwanke, A.; Pergher, B. Porous heterostructured clays—Recent advances and challenges—Review. Cerâmica 2013, 59, 576–587. [Google Scholar] [CrossRef]
- Enotiadis, A.; Tsokaridou, M.; Chalmpes, N.; Sakavitsi, V.; Spyrou, K.; Gournis, D. Synthesis and characterization of porous clay-organic heterostructures. J. Sol-Gel Sci. Technol. 2019, 91, 295–301. [Google Scholar] [CrossRef]
- Alipour, M.; Vosoughi, M.; Mokhtari, S.; Sadeghi, H.; Rashtbari, Y.; Shirmardi, M.; Azad, R. Optimising the basic violet 16 adsorption from aqueous solutions by magnetic graphene oxide using the response surface model based on the Box-Behnken design. Int. J. Environ. Anal. Chem. 2019, 101, 758–777. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arabian J. Chem. 2017, 10, 1629–1638. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L.; Al-Faze, R.; Al-Sehimi, A. Removal enhancement of Basic Blue 41 by waste brick from an aqueous solution. Arabian J. Chem. 2015, 8, 333–342. [Google Scholar] [CrossRef]
- Bembli, M.; Kooli, F.; Boughzala, K. Investigation of Basic Blue 41 removal by waste product from the phosphate industry: Batch design and regeneration. Arct. J. 2021, 74, 41–79. [Google Scholar]
- Humelnicu, I.; Baiceanu, A.; Ignat, M.; Dulman, V. The removal of Basic Blue 41 textile dye from aqueous solution by adsorption onto natural zeolitic tuff: Kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 105, 274–287. [Google Scholar] [CrossRef]
- Roulia, M.; Vassiliadis, A.A. Interactions between C.I. Basic Blue 41 and aluminosilicate sorbents. J. Colloid Interface Sci. 2005, 291, 37–44. [Google Scholar] [CrossRef]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252, 428–461. [Google Scholar] [CrossRef] [PubMed]
- Mnasri Ghnimia, S.; Frini Strasra, N. A comparison of single and mixed pillared clays for zinc and chromium cations removal. Appl. Clay Sci. 2018, 158, 150–157. [Google Scholar] [CrossRef]
- Gil, A.; Assis, F.; Albeniz, S.; Korili, S. Removal of dyes from wastewaters by adsorption on pillared clays. Chem. Eng. Technol. 2011, 168, 1032–1040. [Google Scholar] [CrossRef]
- Goyne, K.W.; Zimmerman, A.R.; Newalkar, B.L.; Komarneni, S.; Brantley, S.L.; Chorover, J. Surface charge of variable porosity Al2O3(s) and SiO2(s) adsorbents. J. Porous Mater. 2002, 9, 243–256. [Google Scholar] [CrossRef]
- Arellano-Cárdenas, S.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; López-Cortez, M.S. Study of the Surface Charge of a Porous Clay Heterostructure (PCH) and Its Adsorption Capacity of Alkaline Metals. J. Mex. Chem. Soc. 2010, 54, 92–97. [Google Scholar] [CrossRef]
- Adiram-Filiba, N.; Schremer, A.; Ohaion, E.; Nadav-Tsubery, M.; Lublin-Tennenbaum, T.; Keinan-Adamsky, K.; Goobes, G. Ubiquitin immobilized on mesoporous MCM41 silica surfaces—Analysis by solid-state NMR with biophysical and surface characterization. Biointerphases 2017, 12, 02D414. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Daana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383–122405. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Muñoz, H.; Blanco, C.; Gil, A.; Vicente, M.; Galeano, L. Preparation of Al/Fe-Pillared Clays: Effect of the Starting Mineral. Materials 2017, 10, 1364. [Google Scholar] [CrossRef]
- Aguiar, J.E.; Bezerra, B.T.C.; Siqueira, A.C.A.; Barrera, D.; Sapag, K.; Azevedo, D.C.S.; Lucena, S.M.P.; Silva, I.J. Improvement in the Adsorption of Anionic and Cationic Dyes from Aqueous Solutions: A Comparative Study using Aluminium Pillared Clays and Activated Carbon. Sep. Sci. Technol. 2014, 49, 741–751. [Google Scholar] [CrossRef]
- Gougazeh, M.; Kooli, F.; Buhl, J.C. Removal efficiency of basicblue41 by three zeolites prepared from natural Jordanian kaolinite. Clays Clay Miner. 2019, 67, 143–153. [Google Scholar] [CrossRef]
- Selim, M.M.; El-Makkawi, D.M.; Ibrahim, F.A. Innovative synthesis of black zeolites-based kaolin and their adsorption behavior in the removal of methylene blue from water. J. Mater. Sci. 2018, 53, 3323–3331. [Google Scholar] [CrossRef]
- Zarezadeh-Mehrizi, M.; Badiei, A.R. Highly efficient removal of Basic Blue 41 with nanoporous silica. Water Resour. Ind. 2014, 5, 49–57. [Google Scholar] [CrossRef]
- Saad, N.; Al-Mawla, M.; Moubarak, E.; Al-Ghoul, M.; El-Rassy, H. Surface functionalized silica aerogels and alcogels for methylene blue adsorption. RSC Adv. 2015, 5, 6111–6122. [Google Scholar] [CrossRef]
- Han, H.; Wei, W.; Jiang, Z.; Lu, L.; Zhu, J.; Xie, J. Removal of cationic dyes from aqueous solution by adsorption ontohydrophobic/hydrophilic silica aerogel, Colloids and Surfaces A: Physicochem. Eng. Asp. 2016, 509, 539–549. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. 2002, 108, 134–142. [Google Scholar] [CrossRef]
- Li, P.; Quinlivan, P.A.; Knappe, D.R.U. Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon 2002, 40, 2085–2100. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Habibi-Yangjeh, A.; Eftekhari, S. Nanodimensional AlMCM-41 material for adsorption of dyes: Thermodynamic and kinetic studies. Int. J. Nano Dimens. 2013, 4, 91–104. [Google Scholar]
- Shahadat, M.; Isamil, S. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: A review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar]
- Kulkarni, S.; Kaware, J. Regeneration and Recovery in Adsorption—A Review. Int. J. Innov. Sci. Eng. Technol. 2014, 1, 61–64. [Google Scholar]
- Mansour, R.A.; Simeda, M.G.; Zaatout, A.A. Removal of brilliant green dye from synthetic wastewater under batch mode using chemically activated date pit carbon. RSC Adv. 2021, 11, 7851–7861. [Google Scholar] [CrossRef]
- Pirozzi, D.; Sannino, F. Design of a multi-stage stirred adsorber using mesoporous metal oxides for herbicide from wastewaters. J. Environ. Chem. Eng. 2014, 2, 211–219. [Google Scholar] [CrossRef]
- Boughzala, K.; Kooli, F.; Meksi, N.; Bechrifa, A.; Bouzouita, K. Waste products from the phosphate industry as efficient removal of Acid Red 88 dye from aqueous solution: Their regeneration uses and batch design adsorber. Desalination Water Treat. 2020, 202, 410–419. [Google Scholar] [CrossRef]




| Samples | SiO2 | Al2O3 | MgO | Fe2O3 | CaO | TiO2 | K2O | MO * |
|---|---|---|---|---|---|---|---|---|
| Ca-Mt | 72.2 | 14.5 | 3.09 | 0.884 | 1.77 | 0.236 | 0.108 | 0.00 |
| Al-Mt | 55.0 | 20.2 | 2.43 | 0.489 | 0.05 | 0.019 | 0.062 | 12.01 |
| Al-PCH | 80.1 | 6.43 | 0.61 | 0.141 | 0.02 | 0.032 | 0.030 | 10.26 |
| Zr-Mt | 49.10 | 8.65 | 2.27 | 0.663 | 0.04 | 0.021 | 0.062 | 11.00 |
| Zr-PCH | 79.9 | 5.54 | 0.66 | 0.130 | 0.014 | 0.002 | 0.030 | 7.83 |
| Samples | SBET (m2/g) | µpore Volume * | TPV (cc/g) | APD (nm) |
|---|---|---|---|---|
| Ca-Mt | 90 | 0.00 | 0.051 | 5.81 |
| Al-Mt | 281 | 0.083 | 0.262 | 3.66 |
| Al-PCH | 850 | 0.00 | 0.851 | 3.82 |
| Zr-Mt | 277 | 0.125 | 0.224 | 2.62 |
| Zr-PCH | 918 | 0.082 | 0.801 | 3.24 |
| Samples | qm (mg/g) | KL | R2 | KF | 1/n | R2 |
|---|---|---|---|---|---|---|
| Ca-Mt | 57 | 0.0289 | 0.9854 | 5.44 | 0.5203 | 0.7120 |
| Al-Mt | 88 | 0.0564 | 0.9986 | 7.53 | 0.412 | 0.7531 |
| Al-PCH | 274 | 0.2280 | 0.9996 | 63.34 | 0.37 | 0.7635 |
| Zr-Mt | 114 | 0.07720 | 0.9991 | 13.68 | 0.433 | 0.7641 |
| Zr-PCH | 346 | 0.2122 | 0.9996 | 188.85 | 0.15 | 0.7603 |
| Materials | qm (mg/g) | Reference |
|---|---|---|
| Saudi Local clay | 50–70 | [23] |
| Natural zeolite | 77 | [66] |
| Sodalite zeolite | 39 | [79] |
| Zeolite X | 27 | [80] |
| Al-Pillared clays | 88 | This study |
| Zr- Pillared clays | 114 | This study |
| Al-PCH material | 274 | This study |
| Zr-PCH material | 346 | This study |
| Brick waste materials | 60–70 | [64] |
| nanoporous silica | 345 | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Dmour, H.; Kooli, F.; Mohmoud, A.; Liu, Y.; Popoola, S.A. Al and Zr Porous Clay Heterostructures as Removal Agents of Basic Blue-41 Dye from an Artificially Polluted Solution: Regeneration Properties and Batch Design. Materials 2021, 14, 2528. https://doi.org/10.3390/ma14102528
Al Dmour H, Kooli F, Mohmoud A, Liu Y, Popoola SA. Al and Zr Porous Clay Heterostructures as Removal Agents of Basic Blue-41 Dye from an Artificially Polluted Solution: Regeneration Properties and Batch Design. Materials. 2021; 14(10):2528. https://doi.org/10.3390/ma14102528
Chicago/Turabian StyleAl Dmour, Hmoud, Fethi Kooli, Ahmed Mohmoud, Yan Liu, and Saheed A. Popoola. 2021. "Al and Zr Porous Clay Heterostructures as Removal Agents of Basic Blue-41 Dye from an Artificially Polluted Solution: Regeneration Properties and Batch Design" Materials 14, no. 10: 2528. https://doi.org/10.3390/ma14102528
APA StyleAl Dmour, H., Kooli, F., Mohmoud, A., Liu, Y., & Popoola, S. A. (2021). Al and Zr Porous Clay Heterostructures as Removal Agents of Basic Blue-41 Dye from an Artificially Polluted Solution: Regeneration Properties and Batch Design. Materials, 14(10), 2528. https://doi.org/10.3390/ma14102528