Characteristics of Waste Iron Powder as a Fine Filler in a High-Calcium Fly Ash Geopolymer
Abstract
1. Introduction
2. Materials and Methodology
2.1. Materials
2.1.1. Fly Ash
2.1.2. Waste Iron Powder
2.1.3. Alkaline Activators
2.2. Experimental Procedures
2.2.1. Manufacturing Process
2.2.2. Mixing Proportion
- Controlled geopolymer paste (CGP).
- WIP mixed geopolymer mortar (WGm).
2.2.3. Analytical Methods
- Physical properties.
- Mechanical properties.
2.2.4. Microstructure Analysis
2.2.5. Temperature Monitoring
3. Results and Discussion
3.1. Physical Properties
3.1.1. Bulk Density of Hardened Geopolymer Paste
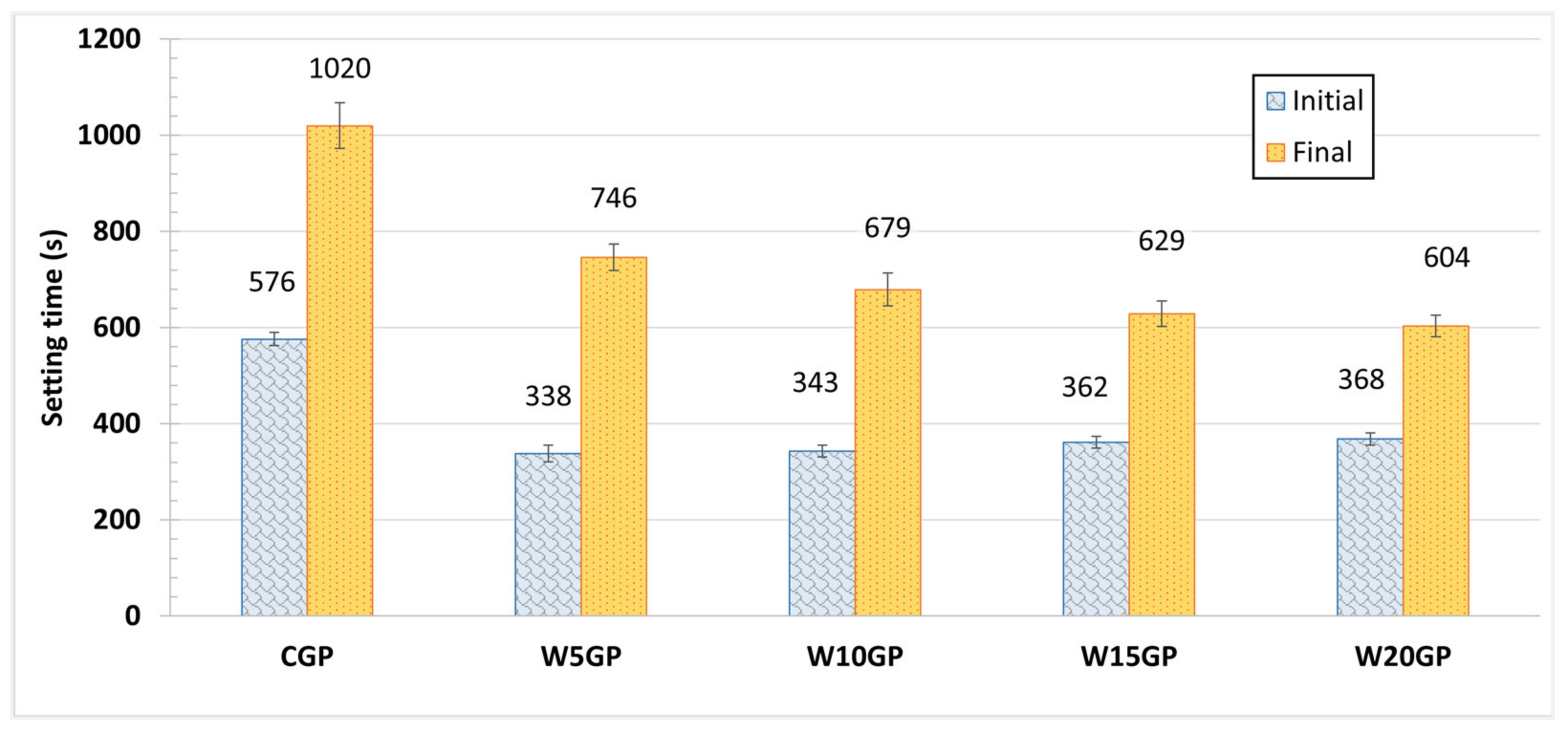
3.1.2. Setting Time Test
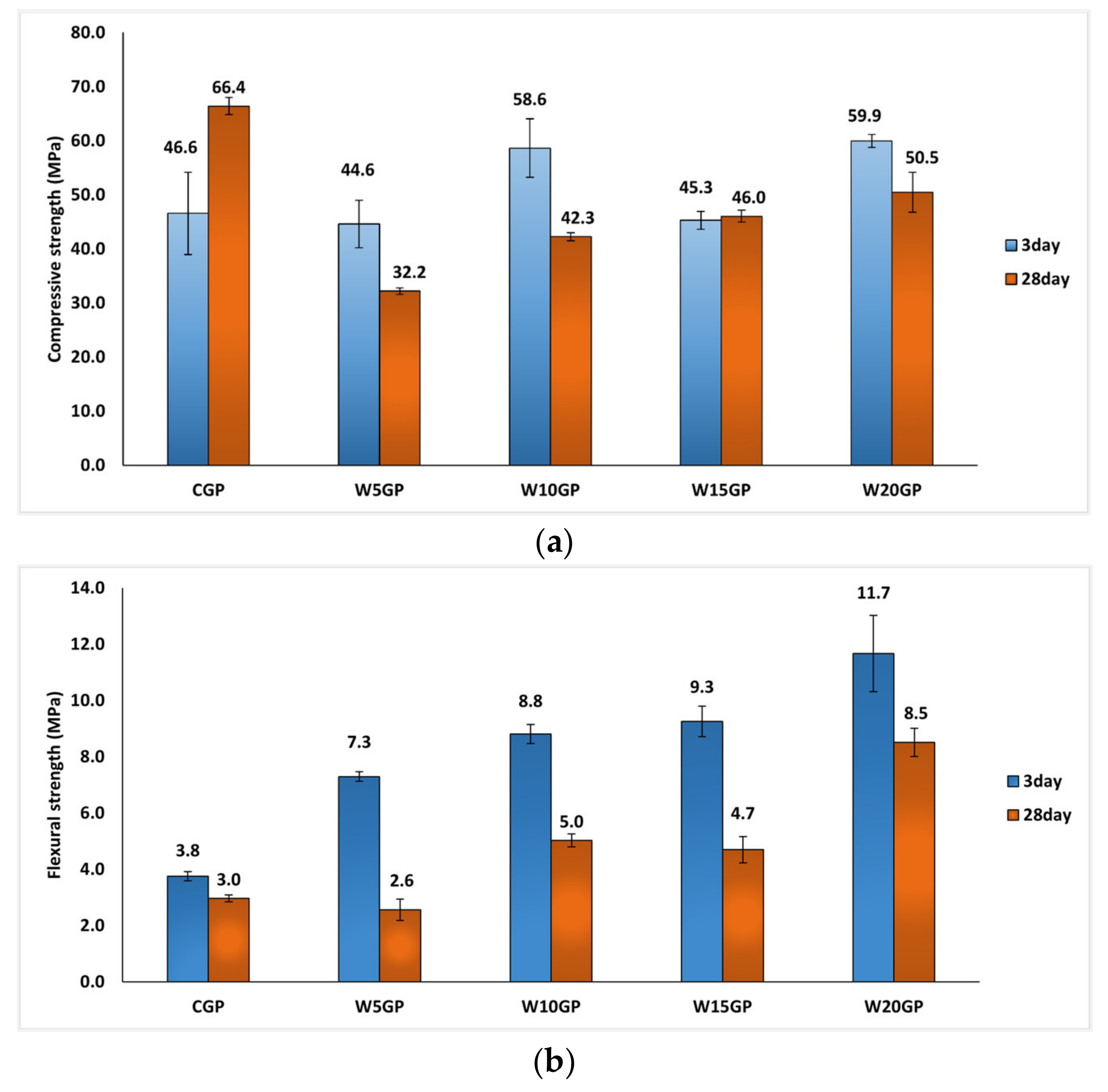
3.2. Mechanical Properties
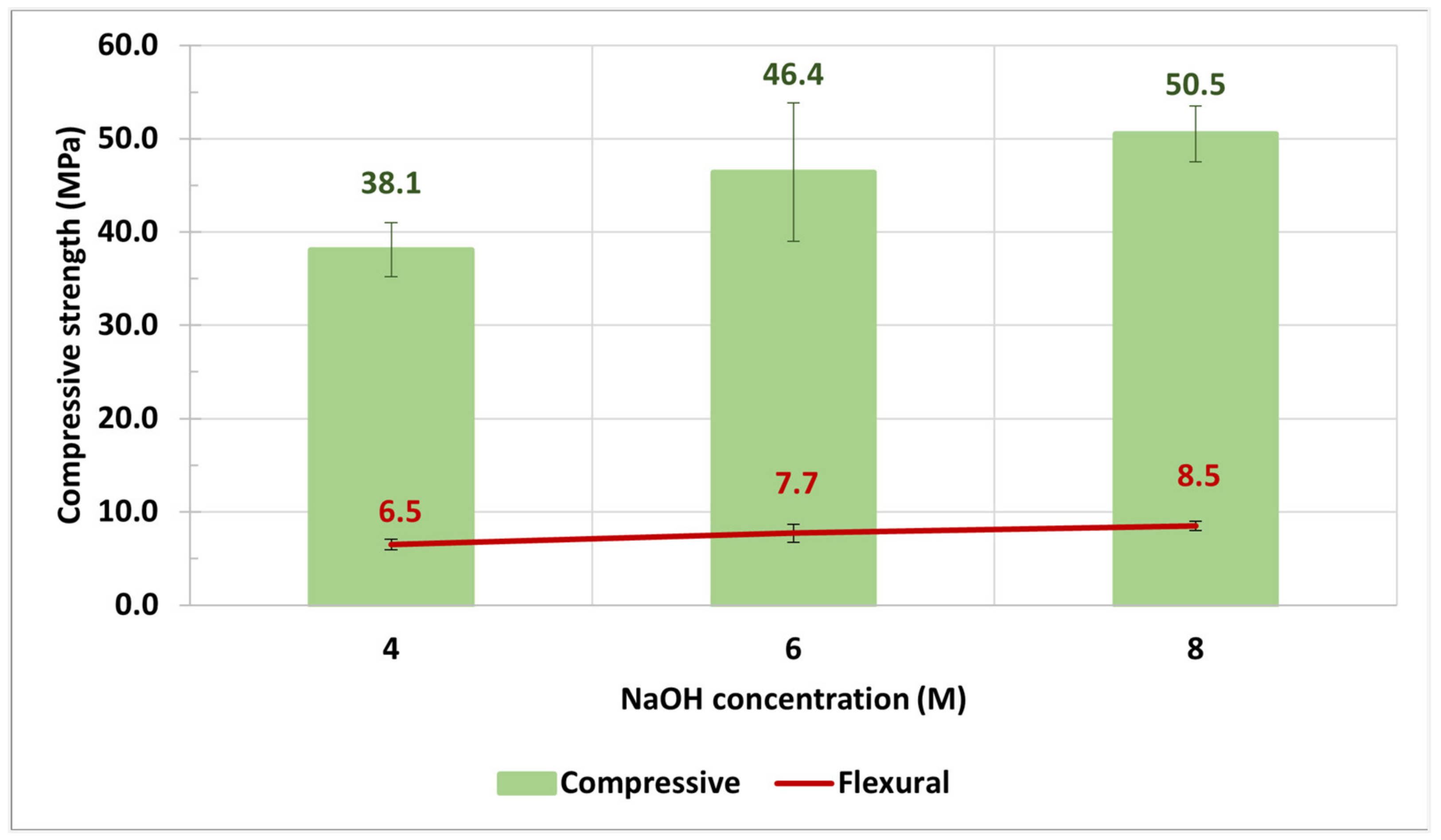
3.3. NaOH Concentration and SS/SH
3.4. Heat Curing Temperature
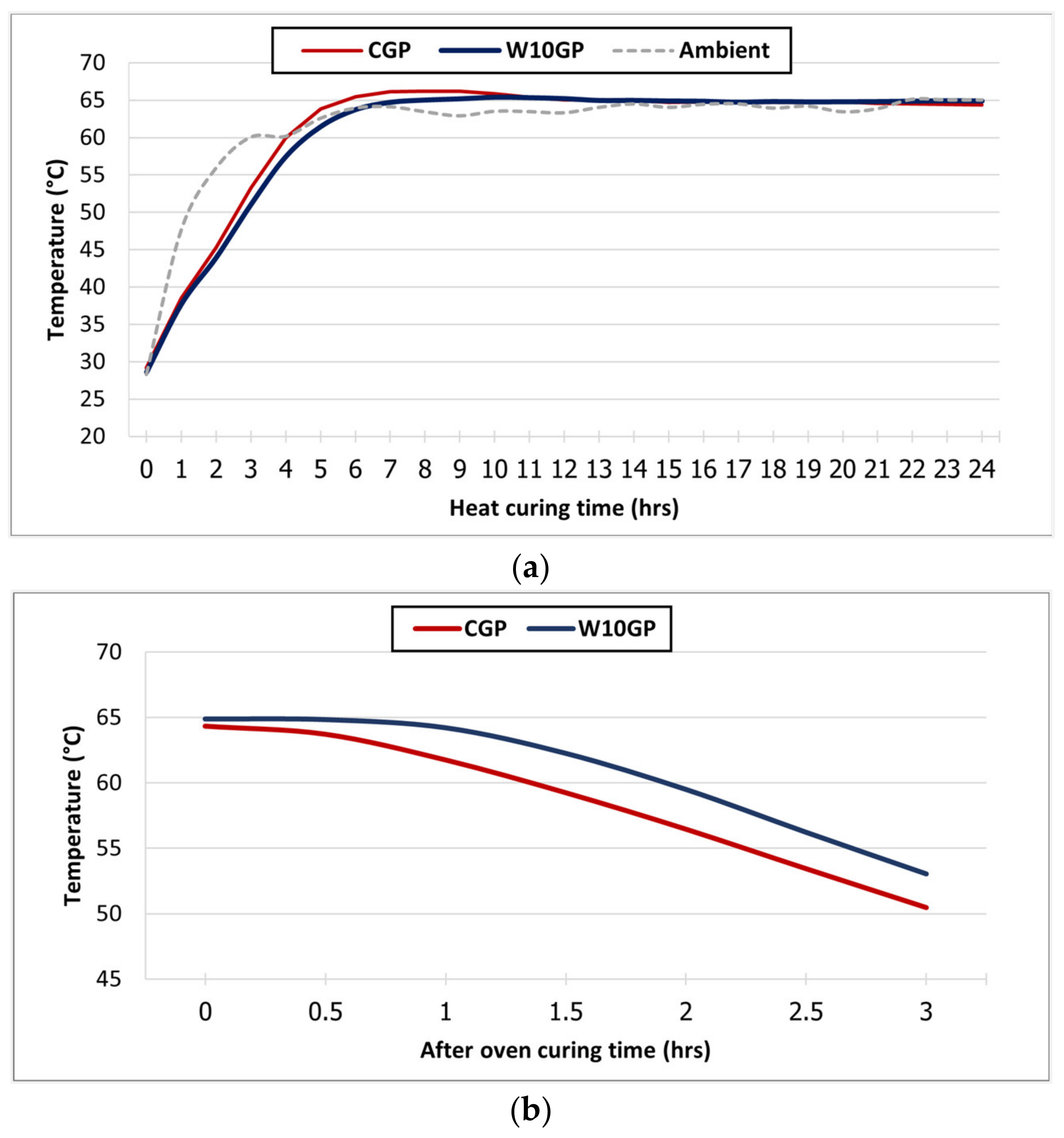
3.5. Temperature Monitoring
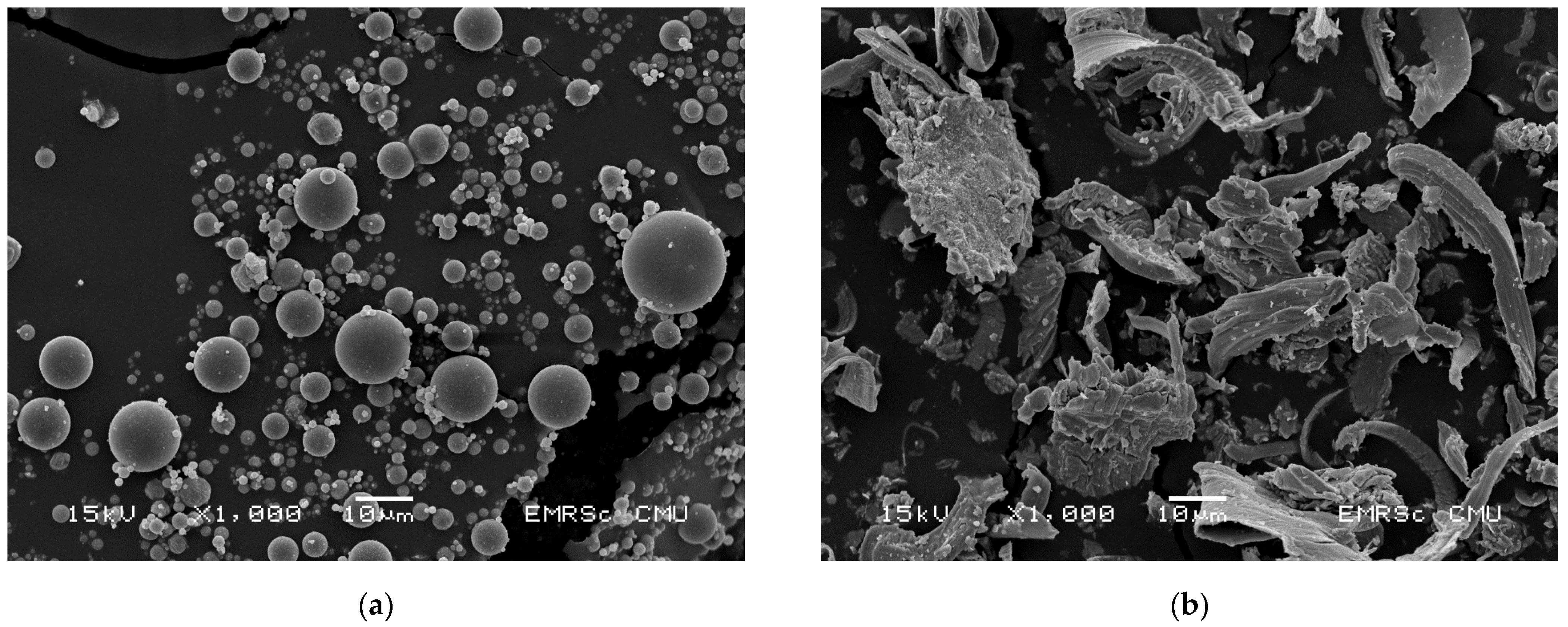
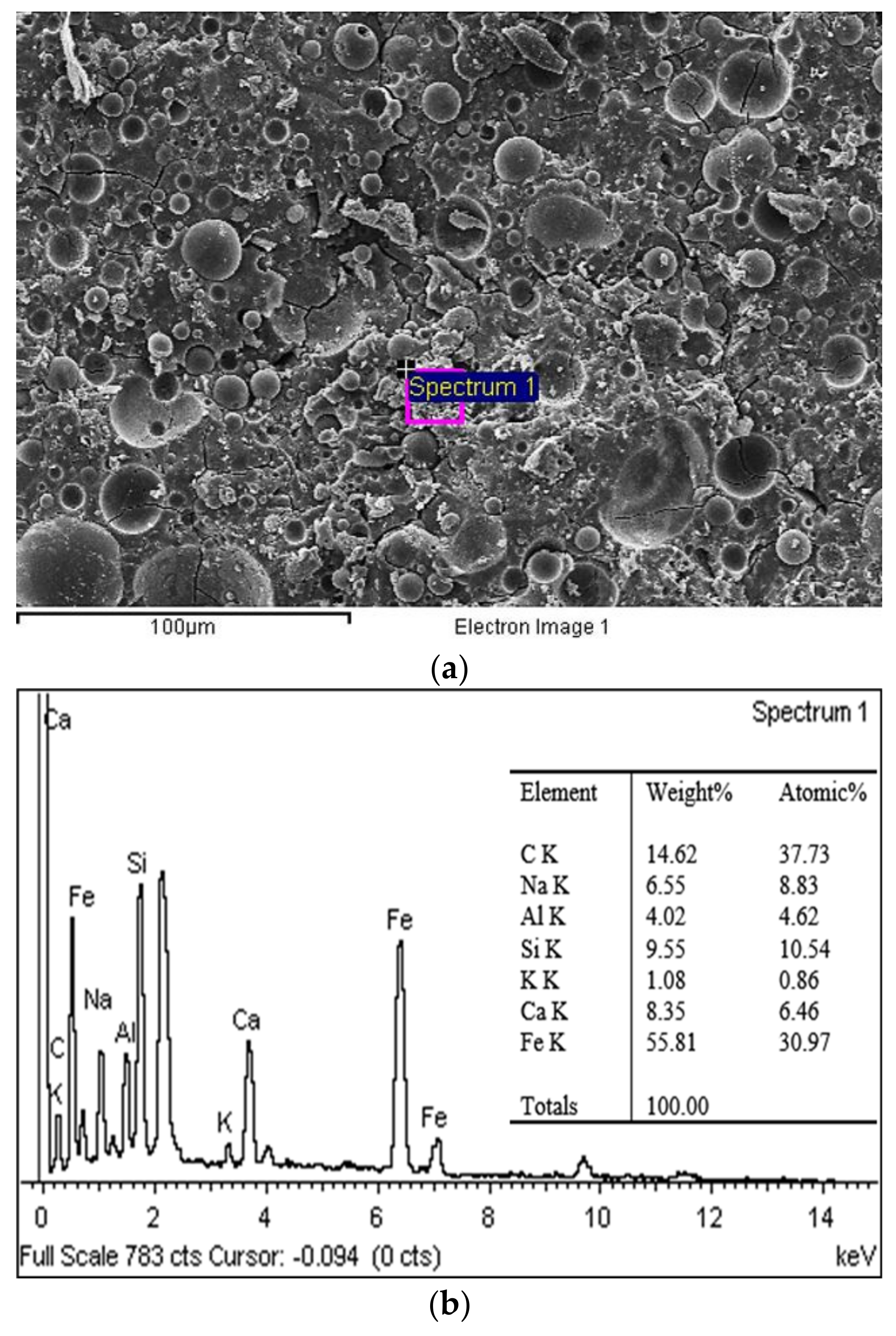
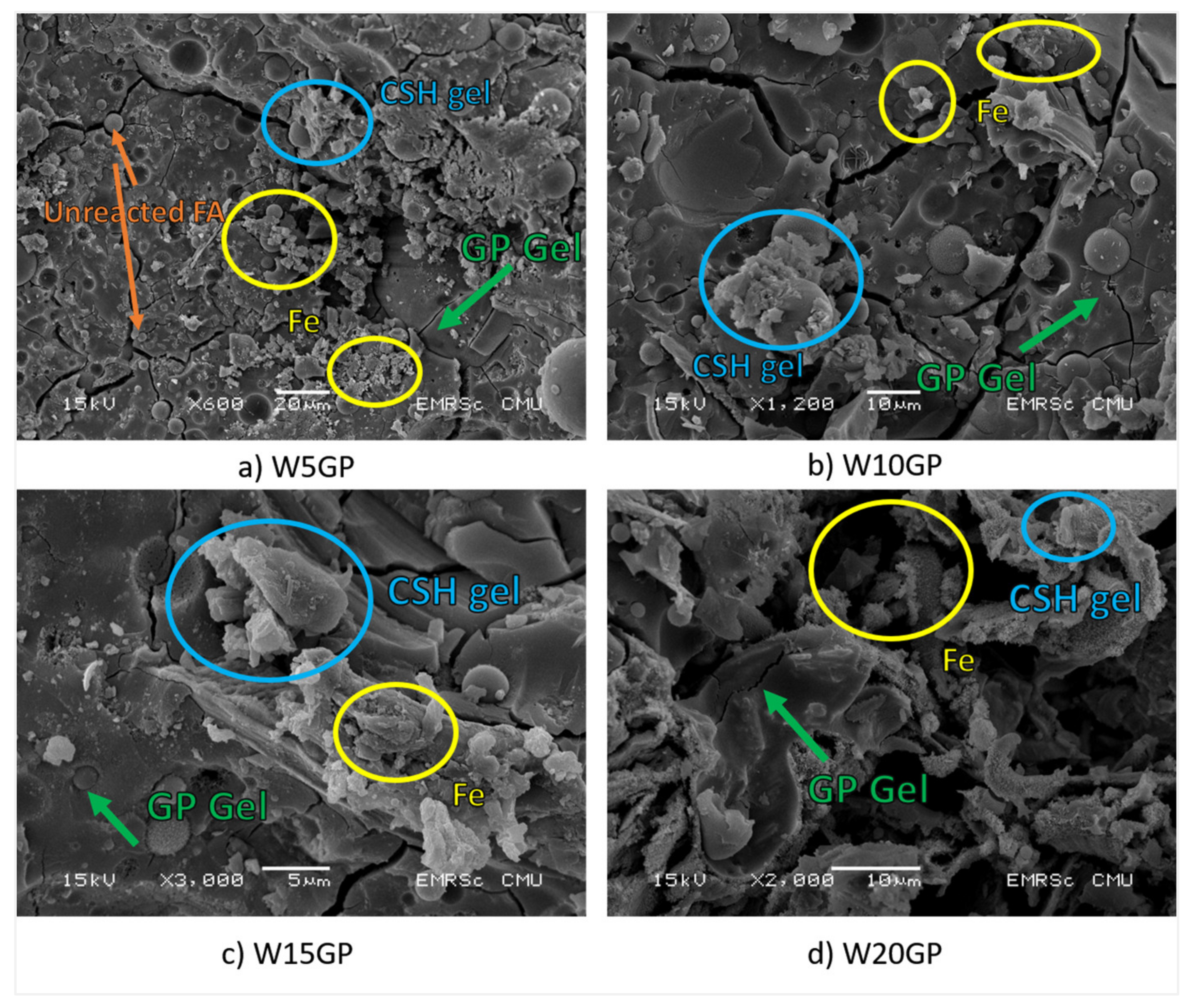
3.6. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rattanashotinunt, C.; Tangchirapat, W.; Jaturapitakkul, C.; Cheewaket, T.; Chindaprasirt, P. Investigation on the Strength, Chloride Migration, and Water Permeability of Eco-Friendly Concretes from Industrial by-Product Materials. J. Clean. Prod. 2018, 172, 1691–1698. [Google Scholar] [CrossRef]
- Abdulmatin, A.; Khongpermgoson, P.; Jaturapitakkul, C.; Tangchirapat, W. Use of Eco-Friendly Cementing Material in Concrete Made from Bottom Ash and Calcium Carbide Residue. Arab. J. Sci. Eng. 2018, 43, 1617–1626. [Google Scholar] [CrossRef]
- Rattanachu, P.; Toolkasikorn, P.; Tangchirapat, W.; Chindaprasirt, P.; Jaturapitakkul, C. Performance of Recycled Aggregate Concrete with Rice Husk Ash as Cement Binder. Cem. Concr. Res. 2020, 108, 103533. [Google Scholar] [CrossRef]
- Peerapong, J.; Kornkanok, B.; Tanapon, P.; Suphat, C.; Prinya, C.; Hamid, N. Recycled Concrete Aggregates in Roadways: Laboratory Examination of Self-Cementing Characteristics. J. Mater. Civ. Eng. 2015, 27, 04014270. [Google Scholar] [CrossRef]
- Huan, Y.; Siripun, K.; Jitsangiam, P.; Nikraz, H. A Preliminary Study on Foamed Bitumen Stabilisation for Western Australian Pavements. Sci. Res. Essays 2010, 5, 23. [Google Scholar]
- Sounthararajah, A.; Bui, H.; Nguyen, N.; Jitsangiam, P.; Kodikara, J. Early-Age Fatigue Damage Assessment of Cement-Treated Bases Under Repetitive Heavy Traffic Loading. J. Mater. Civ. Eng. 2018, 30, 04018079. [Google Scholar] [CrossRef]
- Rattanasak, U. Geopolymer, 1st ed.; Thailand Concrete Association: Bangkok, Thailand, 2017; Volume 167. [Google Scholar]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-Activated Fly Ashes: A Cement for the Future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative Study on the Characteristics of FA and Bottom Ash Geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jenjirapanya, S.; Rattanasak, U. Characterizations of FBC/PCC Fly Ash Geopolymeric Composites. Constr. Build. Mater. 2014, 66, 72–78. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U.; Vongvoradit, P.; Jenjirapanya, S. Thermal Treatment and Utilization of Al-Rich Waste in High Calcium FA Geopolymeric Materials. Int. J. Min. Met. Mater. 2012, 19, 872–878. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Sustainable Development. In Proceedings of the World Congress Geopolymer, Saint-Quentin, France, 1 July 2005; pp. 9–15. [Google Scholar]
- Jitsangiam, P.; Suwan, T.; Pimraksa, K.; Sukontasukkul, P.; Chindaprasirt, P. Challenge of Adopting Relatively Low Strength and Self-Cured Geopolymer for Road Construction Application: A Review and Primary Laboratory Study. Int. J. Pavement Eng. 2019. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnanon, V. Workability and Strength of Coarse High Calcium FA Geopolymer. Cem. Concr. Res. 2007, 29, 224–229. [Google Scholar] [CrossRef]
- Suwan, T.; Fan, M.; Braimah, N. Internal Heat Liberation and Strength Development of Self-Cured Geopolymers in Ambient Curing Conditions. Constr. Build. Mater. 2016, 114, 297–306. [Google Scholar] [CrossRef]
- Carabba, L.; Santandrea, M.; Carloni, C.; Manzi, S.; Bignozzi, M.C. Steel Fiber Reinforced Geopolymer Matrix (S-FRGM) Composites Applied to Reinforced Concrete Structures for Strengthening Applications: A Preliminary Study. Compos. Part B-Eng. 2017, 128, 83–90. [Google Scholar] [CrossRef]
- Tanyildizi, H.; Yonar, Y. Mechanical Properties of Geopolymer Concrete Containing Polyvinyl Alcohol Fiber Exposed to High Temperature. Constr. Build. Mater. 2016, 126, 381–387. [Google Scholar] [CrossRef]
- Maichin, P.; Suwan, T.; Jitsangiam, P.; Chindaprasirt, P.; Fan, M. Effect of Self-Treatment Process on Properties of Natural Fiber-Reinforced Geopolymer Composites. Mater. Manuf. Process. 2020, 35, 1120–1128. [Google Scholar] [CrossRef]
- Kumar, S.; Yankwa Djobo, J.N.; Kumar, A.; Kumar, S. Geopolymerization Behavior of Fine Iron-Rich Fraction of Brown Fly Ash. J. Build. Eng. 2016, 8, 172–178. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R. Ferro-Sialate Geopolymers (-Fe-O-Si-O-Al-O-). Geopolymer Inst. Libr. 2020. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Jameson, G.N.L.; Rahier, H.; Chinje Melo, U.F. The Role of Iron in the Formation of Inorganic Polymers (Geopolymers) from Volcanic Ash: A 57Fe Mössbauer Spectroscopy Study. J. Mater. Sci. 2013, 48, 5280–5286. [Google Scholar] [CrossRef]
- Center of Excellence on Hazardous Substance Management. 100 Types of Industrial Waste Quantities (2008–2011) in Thailand. 2012. Available online: http://recycle.dpim.go.th/wastelist/download_files/G/waste_quantity.pdf (accessed on 11 April 2021).
- Ghannam, S.; Najm, H.; Vasconez, R. Experimental Study of Concrete Made with Granite and Iron Powders as Partial Replacement of Sand. Sustain. Mater. Technol. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Arabani, M.; Mirabdolazimi, S.M. Experimental Investigation of the Fatigue Behaviour of Asphalt Concrete Mixtures Containing Waste Iron Powder. Mater. Sci. Eng. A 2011, 528, 3866–3870. [Google Scholar] [CrossRef]
- Liu, K.; Fu, C.; Dai, D.; Jin, C.; Li, W.; Li, S.; Xu, X. Induction Heating Performance of Asphalt Pavements Incorporating Electrically Conductive and Magnetically Absorbing Layers. Constr. Build. Mater. 2019, 229, 116805. [Google Scholar] [CrossRef]
- Suwan, T.; Fan, M.; Braimah, N. Micro-Mechanisms and Compressive Strength of Geopolymer-Portland Cementitious System under Various Curing Temperatures. Mater. Chem. Phys. 2016, 180, 219–225. [Google Scholar] [CrossRef]
- ASTM C618-19 Standard Specification for Coal FA and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2019.
- Chindaprasirt, P.; Rattanasak, U. Characterization of the High-Calcium FA Geopolymer Mortar with Hot-Weather Curing Systems for Sustainable Application. Adv. Powder Technol. 2017, 28, 2317–2324. [Google Scholar] [CrossRef]
- ASTM C191-19 Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle; ASTM International: West Conshohocken, PA, USA, 2019.
- BS EN 196-1 Methods of Testing Cement. Determination of Strength; British Standards Institution: London, UK, 2016. [Google Scholar]
- Suwan, T.; Fan, M. Influence of OPC Replacement and Manufacturing Procedures on the Properties of Self-Cured Geopolymer. Constr. Build. Mater. 2014, 73, 551–561. [Google Scholar] [CrossRef]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH Solution on the Synthesis of FA Geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Mustafa Al Bakria, A.M.; Kamarudin, H.; BinHussain, M.; Khairul Nizar, I.; Zarina, Y.; Rafiza, A.R. The Effect of Curing Temperature on Physical and Chemical Properties of Geopolymers. In Proceedings of the Physics Procedia; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 22, pp. 286–291. [Google Scholar]
- Satpute Manesh, B.; Wakchaure Madhukar, R.; Patankar Subhash, V. Effect of Duration and Temperature of Curing on Compressive Strength of Geopolymer Concrete. Int. J. Eng. Innov. Technol. 2012, 1, 152–155. [Google Scholar]
- Song, W.; Zhu, Z.; Peng, Y.; Wan, Y.; Xu, X.; Pu, S.; Song, S.; Wei, Y. Effect of Steel Slag on Fresh, Hardened and Microstructural Properties of High-Calcium FA Based Geopolymers at Standard Curing Condition. Constr. Build. Mater. 2019, 229, 116933. [Google Scholar] [CrossRef]
- Yip, C.K.; van Deventer, J.S.J. Microanalysis of Calcium Silicate Hydrate Gel Formed within a Geopolymeric Binder. J. Mater. Sci. 2003, 38, 3851–3860. [Google Scholar] [CrossRef]
- Jaśniok, T.; Słomka-Słupik, B.; Zybura, A. The Concrete Reinforcement Chloride Corrosion, Immediately after Its Initiation. Cem. Wapno Beton 2014, 3, 158–165. [Google Scholar]
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent Durability Studies on Concrete Structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, J.; Hu, B.; Jin, W. Crack Shape and Rust Distribution in Corrosion-Induced Cracking Concrete. Corros. Sci. 2012, 55, 385–393. [Google Scholar] [CrossRef]
- Eiamwijit, M.; Pachana, K.; Kaewpirom, S.; Rattanasak, U.; Chindaprasirt, P. Comparative Study on Morphology of Ground Sub-Bituminus FBC FA Geopolymeric Material. Adv. Powder Technol. 2015, 26, 1053–1057. [Google Scholar] [CrossRef]




| Component | Fly Ash | WIP |
|---|---|---|
| Al2O3 | 8.52 | - |
| SiO2 | 18.17 | 1.96 |
| Fe2O3 | 29.85 | 95.95 |
| CaO | 31.41 | - |
| SO3 | 8.43 | - |
| K2O | 2.47 | - |
| TiO2 | 0.58 | - |
| SrO | 0.29 | - |
| MnO | 0.28 | 0.53 |
| Cr2O3 | - | 1.56 |
| Mixture | L/B | %FA | %WIP | FA (g) | WIP (g) | NaOH (M) | SS/SH Mass Ratio | Heat Curing Temp. (°C) | Temp. Monitoring | Testing Age (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| CGP | 0.45 | 100 | 0 | 1500 | 0 | 4, 6, 8 | 0.67, 1.00 | 30, 40, 50, 60 | ✓ | 3, 28 |
| W5GP | 0.45 | 100 | 5 | 1500 | 75 | 4, 6, 8 | 0.67, 1.00 | 60 | - | 3, 28 |
| W10GP | 0.45 | 100 | 10 | 1500 | 150 | 4, 6, 8 | 0.67, 1.00 | 60 | ✓ | 3, 28 |
| W15GP | 0.45 | 100 | 15 | 1500 | 225 | 4, 6, 8 | 0.67, 1.00 | 60 | - | 3, 28 |
| W20GP | 0.45 | 100 | 20 | 1500 | 300 | 4, 6, 8 | 0.67, 1.00 | 30, 40, 50, 60 | - | 3, 28 |
| Samples | Area (a.u.) |
|---|---|
| CGP | 4110 |
| W5GP | 4032 |
| W10GP | 3644 |
| W15GP | 3346 |
| W20GP | 3005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nongnuang, T.; Jitsangiam, P.; Rattanasak, U.; Tangchirapat, W.; Suwan, T.; Thongmunee, S. Characteristics of Waste Iron Powder as a Fine Filler in a High-Calcium Fly Ash Geopolymer. Materials 2021, 14, 2515. https://doi.org/10.3390/ma14102515
Nongnuang T, Jitsangiam P, Rattanasak U, Tangchirapat W, Suwan T, Thongmunee S. Characteristics of Waste Iron Powder as a Fine Filler in a High-Calcium Fly Ash Geopolymer. Materials. 2021; 14(10):2515. https://doi.org/10.3390/ma14102515
Chicago/Turabian StyleNongnuang, Toon, Peerapong Jitsangiam, Ubolluk Rattanasak, Weerachart Tangchirapat, Teewara Suwan, and Suriyah Thongmunee. 2021. "Characteristics of Waste Iron Powder as a Fine Filler in a High-Calcium Fly Ash Geopolymer" Materials 14, no. 10: 2515. https://doi.org/10.3390/ma14102515
APA StyleNongnuang, T., Jitsangiam, P., Rattanasak, U., Tangchirapat, W., Suwan, T., & Thongmunee, S. (2021). Characteristics of Waste Iron Powder as a Fine Filler in a High-Calcium Fly Ash Geopolymer. Materials, 14(10), 2515. https://doi.org/10.3390/ma14102515







