Comparative Analysis of Pelletized and Unpelletized Sunflower Husks Combustion Process in a Batch-Type Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Combustion Procedure
3. Results and Discussion
3.1. Temperature Characteristics
3.2. Combustion Kinetics
3.2.1. Temperature Front Velocity and Combustion Front Velocity
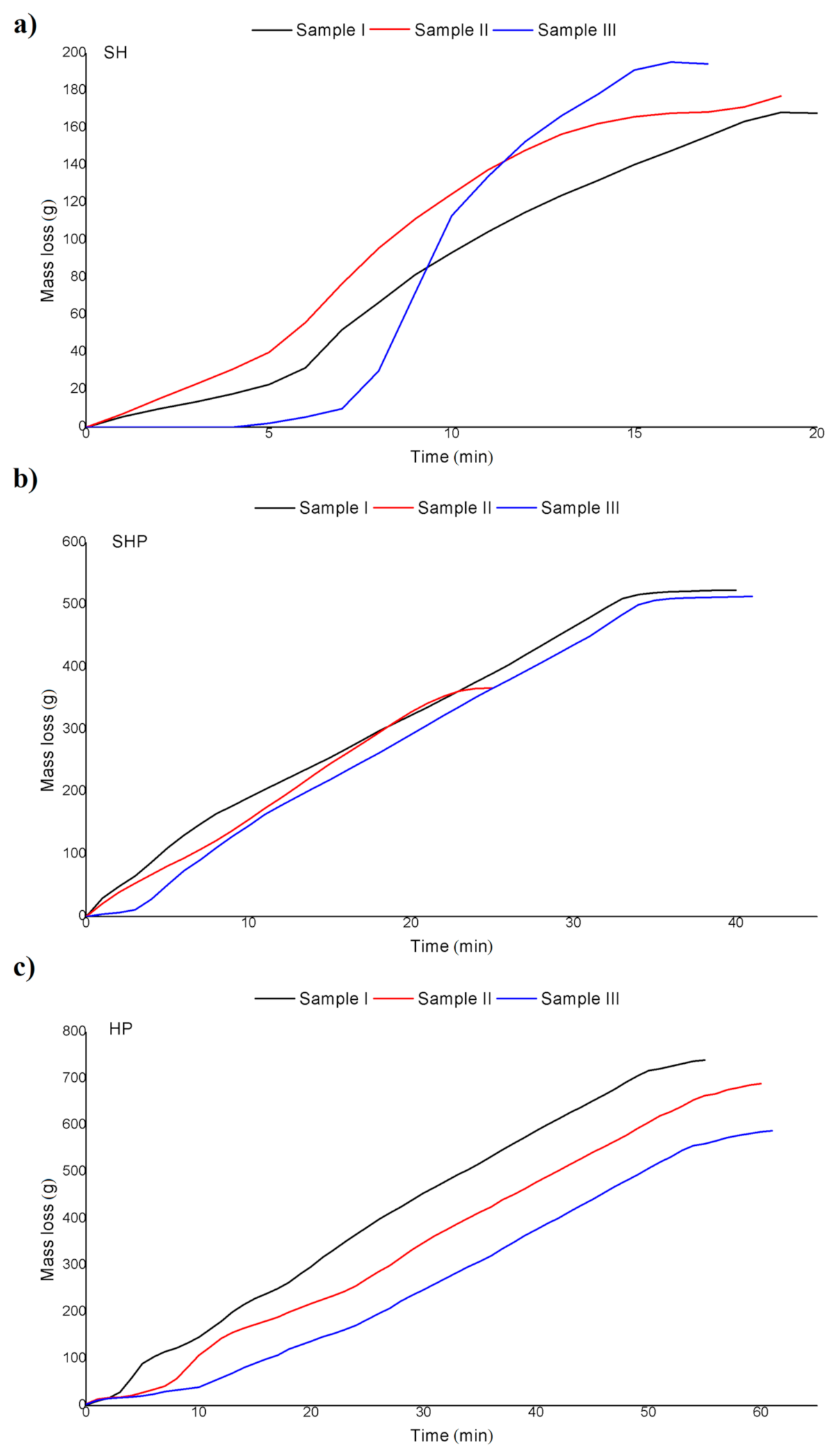
3.2.2. Mass Loss
3.3. Energy Balance
3.4. Analysis of Bottom Ash
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| SH | Sunflower husk |
| SHP | Sunflower husk pellets |
| HP | Hardwood pellets |
| HHV | Higher heating value of the fuel (MJ/kg) |
| Tflame | Averaged flame temperature (°C) |
| TMAX | Maximum combustion temperature (°C) |
| vt | Temperature front velocity (mm/min) |
| vc | Combustion front velocity (mm/min) |
| vm | Mass loss rate (g/min) |
| Fout | Fuel consumption density (kg/s·m2) |
| Pρ | Power output density (MW/m2) |
| T1–T4 | Thermocouple 1–4 |
| Ag | Surface area of the grate (m2) |
| ρbulk | Bulk density of the fuel bed (kg/m3) |
References
- Yanik, J.; Duman, G.; Karlström, O.; Brink, A. NO and SO2 emissions from combustion of raw and torrefied biomasses and their blends with lignite. J. Environ. Manag. 2018, 227, 155–161. [Google Scholar] [CrossRef]
- Lynch, D.; Henihan, A.M.; Bowen, B.; Lynch, D.; McDonnell, K.; Kwapinski, W.; Leahy, J.J. Utilisation of poultry litter as an energy feedstock. Biomass Bioenergy 2013, 49, 197–204. [Google Scholar] [CrossRef]
- Lundgren, J.; Pettersson, E. Combustion of horse manure for heat production. Bioresour. Technol. 2009, 100, 3121–3126. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Yang, B.; Jahng, D. Combustion characteristics of biodried sewage sludge. Waste Manag. 2018, 72, 296–305. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska-Golachowska, M.; Musiał, T.; Wolski, K. Combustion of pelleted sewage sludge with reference to coal and biomass. Fuel 2016, 170, 141–160. [Google Scholar] [CrossRef]
- Ronda, A.; Gomez-Barea, A.; Haro, P.; de Almeida, V.F.; Salinero, J. Elements partitioning during thermal conversion of sewage sludge. Fuel Process. Technol. 2019, 186, 156–166. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S. Combustion behavior of biomass fuels and their blends with lignite. Thermochem. Acta 2011, 192–199. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, Y.; Li, X.; Liu, H.; Yan, W.; Sui, R.; Lu, Q. Temperature and emissivity measurements from combustion of pine wood, rice husk and fire wood using flame emission spectrum. Fuel Process. Technol. 2020, 204, 106423. [Google Scholar] [CrossRef]
- Zeng, T.; Pollex, A.; Weller, N.; Lenz, V.; Nelles, M. Blended biomass pellets as fuel for small scale combustion appliances: Effect of blending on slag formation in the bottom ash and pre-evaluation options. Fuel 2018, 212, 108–116. [Google Scholar] [CrossRef]
- Van, L.S.; Koppejan, J. The Handbook of Biomass Combustion and Co-Firing; Earthscan: London, UK, 2008. [Google Scholar]
- Ríos-Badrán, I.M.; Luzardo-Ocampo, I.; García-Trejo, J.F.; Santos-Cruz, J.; Gutierrez-Antonio, C. Production and characterization of fuel pellets from rice husk and wheat straw. Renew. Energy 2020, 145, 500–507. [Google Scholar] [CrossRef]
- Pradhan, P.; Mahajani, S.M.; Arora, A. Production and utilization of fuel pellets from biomass: A review. Fuel Process. Technol. 2018, 181, 215–232. [Google Scholar] [CrossRef]
- Labbé, R.; Paczkowski, S.; Knappe, V.; Russ, M.; Wöhler, M.; Pelz, S. Effect of feedstock particle size distribution and feedstock moisture content on pellet production efficiency, pellet quality, transport and combustion emissions. Fuel 2020, 263, 116662. [Google Scholar] [CrossRef]
- Monedero, E.; Portero, H.; Lapuerta, M. Pellet blends of poplar and pine sawdust: Effects of material composition, additive, moisture content and compression die on pellet quality. Fuel Process. Technol. 2015, 132, 5–23. [Google Scholar] [CrossRef]
- Nielsen, S.K.; Mandø, M.; Rosenørn, A.B. Review of die design and process parameters in the biomass pelleting proces. Powder Technol. 2020, 364, 971–985. [Google Scholar] [CrossRef]
- Perea-Moreno, M.A.; Manzano-Agugliaro, F.; Perea-Moreno, A.J. Sustainable Energy Based on Sunflower Seed Husk Boiler for Residential Buildings. Sustainability 2018, 10, 3407. [Google Scholar] [CrossRef]
- Havrysh, V.; Kalinichenko, A.; Mentel, G.; Mentel, U.; Vasbieva, D.G. Husk Energy Supply Systems for Sunflower Oil Mills. Energies 2020, 13, 361. [Google Scholar] [CrossRef]
- Bilandzija, N.; Voca, N.; Jelcic, B.; Jurisic, V.; Matin, A.; Grubor, M.; Kricka, T. Evaluation of Croatian agricultural solid biomass energy potential. Renew. Sustain. Energy Rev. 2018, 93, 225–230. [Google Scholar] [CrossRef]
- Quaranta, N.; Unsen, M.; López, H.; Giansiracusa, C.; Roether, J.A.; Boccaccini, A.R. Ash from sunflower husk as raw material for ceramic products. Ceram. Int. 2011, 37, 377–385. [Google Scholar] [CrossRef]
- Ooi, T.C.; Aries, E.; Ewan, B.C.R.; Thompson, D.; Anderson, D.R.; Fisher, R.; Fray, T.; Tognarelli, D. The study of sunflower seed husks as a fuel in the iron ore sintering process. Miner. Eng. 2008, 21, 167–177. [Google Scholar] [CrossRef]
- Barczewski, M.; Sałasińska, K.; Szulc, J. Application of sunflower husk, hazelnut shell and walnut shell as waste agricultural fillers for epoxy-based composites: A study into mechanical behavior related to structural and rheological properties. Polym. Test. 2019, 75, 1–11. [Google Scholar] [CrossRef]
- Kułażyński, M.; Jabłoński, S.; Kaczmarczyk, J.; Świątek, Ł.; Pstrowska, K.; Łukaszewicz, M. Technological aspects of sunflower biomass and brown coal co-firing. J. Energy Inst. 2017, 91, 668–675. [Google Scholar] [CrossRef]
- Bala-Latwiniak, A.; Zajemska, M. Computational and experimental study of pine and sunflower huskpellet combustion and co-combustion with oats in domestic boiler. Renew. Energy 2020, 62, 151–159. [Google Scholar] [CrossRef]
- López, R.; Fernández, C.; Fierro, J.; Cara, J.; Martínez, O.; Sánchez, M.E. Oxy-combustion of corn, sunflower, rape and microalgae bioresidues and their blends from the perspective of thermogravimetric analysis. Energy 2014, 74, 845–854. [Google Scholar] [CrossRef]
- Kluska, J.; Turzyński, T.; Kardaś, D. Experimental tests of co-combustion of pelletized leather tannery wastes and hardwood pellets. Waste Manag. 2018, 79, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.K.; Bram, S.; Delattin, F.; Laha, P.; Vandendael, I.; Hubin, A.; de Ruyck, J. Agro-pellets for domestic heating boilers: Standard laboratory and real life performance. Appl. Energy 2012, 90, 17–23. [Google Scholar] [CrossRef]
- Cardozo, E.; Erlich, C.; Alejo, L.; Fransson, T.H. Combustion of agricultural residues: An experimental study for small-scale applications. Fuel 2014, 115, 778–787. [Google Scholar] [CrossRef]
- Kougioumtzis, M.A.; Kanaveli, I.P.; Karampinis, E.; Grammelis, P.; Kakaras, E. Combustion of olive tree pruning pellets versus sunflower husk pellets at industrial boiler. Monit. Emiss. Combust. Effic. Renew. Energy 2021, 171, 516–525. [Google Scholar] [CrossRef]
- Llorente, M.J.F.; Garcia, J.E.C. Comparing methods for predicting the sintering of biomass ash in combustion. Fuel 2005, 84, 1893–1900. [Google Scholar] [CrossRef]
- Svoboda, K.; Pohořelý, M.; Hartman, M.; Martinec, J. Pretreatment and feeding of biomass for pressurized entrained flow gasification. Fuel Process. Technol. 2009, 90, 629–635. [Google Scholar] [CrossRef]
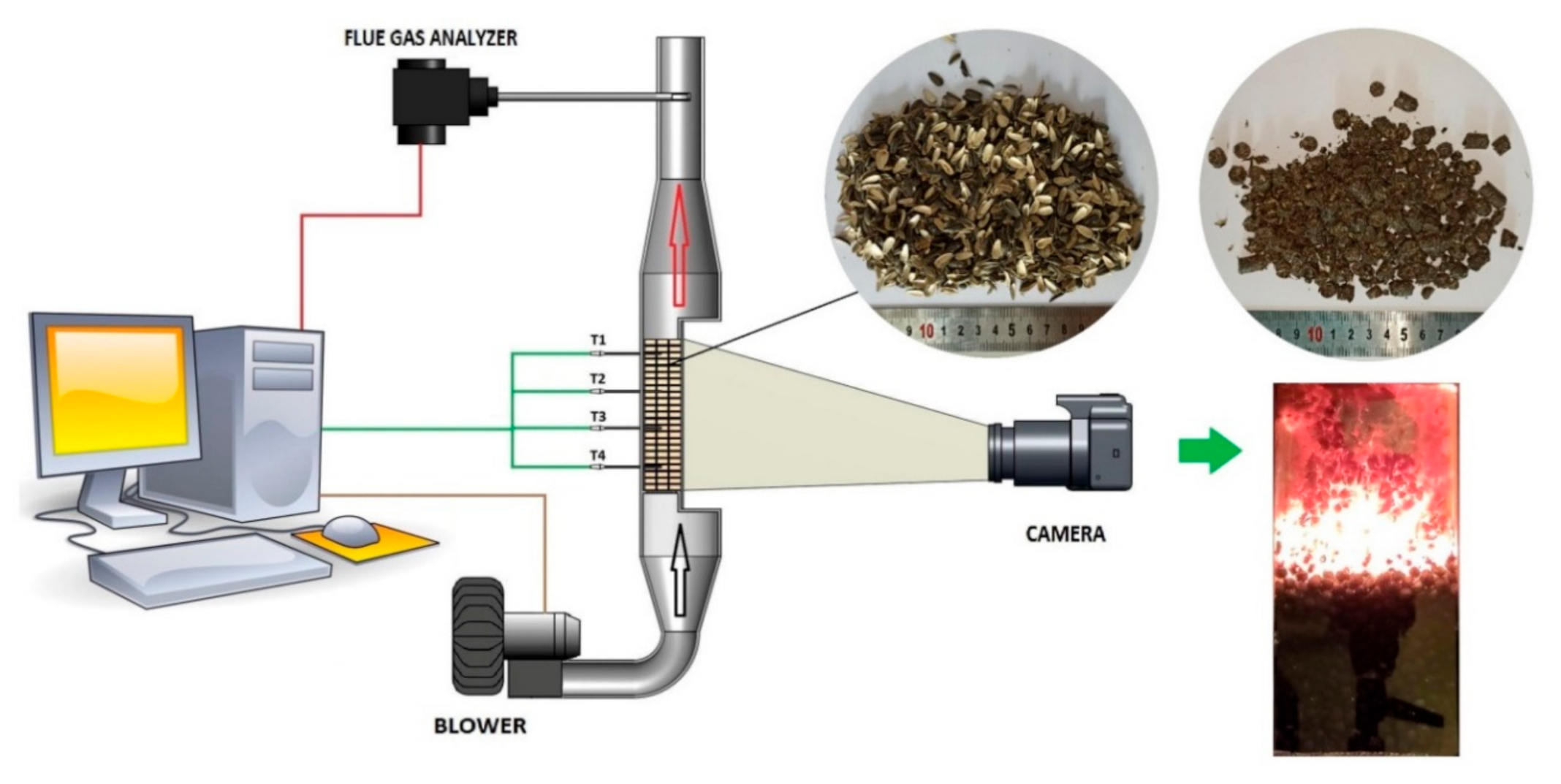

| Parameter | Sunflower Husk | Sunflower Husk Pellets | Hardwood Pellets |
|---|---|---|---|
| HHV (MJ/kg) | 18.11 | 19.18 | 19.60 |
| Moisture (wt%), as delivered | 9.61 | 10.71 | 6.1 |
| Proximate (wt.%db) a | |||
| Volatiles | 82.7 | 83.59 | 76.3 |
| Fixed carbon | 16.1 | 14.51 | 21.4 |
| Ash | 1.2 | 1.9 | 2.3 |
| Ultimate (wt.%db) a | |||
| C | 46.21 | 43.38 | 48.50 |
| H | 6.06 | 6.62 | 5.30 |
| O | 46.58 | 48.81 | 45.56 |
| N | 0.88 | 1.19 | 0.40 |
| Parameter | Sunflower Husk | Sunflower Husk Pellets | Wood Pellets |
|---|---|---|---|
| HHVavr (MJ/kg) | 18.11 | 19.18 | 19.60 |
| vc (mm/min) | 19.0 | 8.0 | 5.2 |
| vm (g/min) | 13.2 | 14.7 | 13.1 |
| ρbulk (kg/m3) | 139 | 478 | 669 |
| TMAX (°C) | 840 | 1110 | 1090 |
| Tflame (°C) | - | 800 | 940 |
| Fout (kg/s·m2) | 0.072 | 0.083 | 0.077 |
| Pρ (MW/m2) | 1.30 | 1.59 | 1.50 |
| Compound | Sunflower Husk | Sunflower Husk Pellets |
|---|---|---|
| K2O | 33.97 | 35.14 |
| CaO | 25.50 | 37.40 |
| P2O5 | 14.58 | 5.38 |
| MgO | 13.92 | 11.73 |
| SO3 | 5.14 | 5.66 |
| SiO2 | 3.94 | 1.92 |
| Fe2O3 | 1.49 | 0.99 |
| Al2O3 | 0.77 | 0.21 |
| Cl | 0.21 | 0.85 |
| MnO | 0.20 | 0.20 |
| SrO | 0.1 | 0.09 |
| CuO | 0.08 | 0.07 |
| ZnO | 0.08 | 0.11 |
| Ash | Initial Deformation Temperature (°C) | Spherical Temperature (°C) | Hemispherical Temperature (°C) | Fluid Temperature (°C) |
|---|---|---|---|---|
| Sunflower husk | 970 | >1500 | >1500 | >1500 |
| Sunflower husk pellets | 1100 | 1490 | >1500 | >1500 |
| Pine wood | 1190 | 1200 | 1220 | 1280 |
| Wheat straw | 850 | 1040 | 1120 | 1320 |
| Rice straw | 860 | 980 | 1100 | 1220 |
| Willow wood | 1380 | 1540 | 1550 | 1560 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turzyński, T.; Kluska, J.; Ochnio, M.; Kardaś, D. Comparative Analysis of Pelletized and Unpelletized Sunflower Husks Combustion Process in a Batch-Type Reactor. Materials 2021, 14, 2484. https://doi.org/10.3390/ma14102484
Turzyński T, Kluska J, Ochnio M, Kardaś D. Comparative Analysis of Pelletized and Unpelletized Sunflower Husks Combustion Process in a Batch-Type Reactor. Materials. 2021; 14(10):2484. https://doi.org/10.3390/ma14102484
Chicago/Turabian StyleTurzyński, Tomasz, Jacek Kluska, Mateusz Ochnio, and Dariusz Kardaś. 2021. "Comparative Analysis of Pelletized and Unpelletized Sunflower Husks Combustion Process in a Batch-Type Reactor" Materials 14, no. 10: 2484. https://doi.org/10.3390/ma14102484
APA StyleTurzyński, T., Kluska, J., Ochnio, M., & Kardaś, D. (2021). Comparative Analysis of Pelletized and Unpelletized Sunflower Husks Combustion Process in a Batch-Type Reactor. Materials, 14(10), 2484. https://doi.org/10.3390/ma14102484






