Ultrasound-Assisted Surface Modification of MWCNT Using Organic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
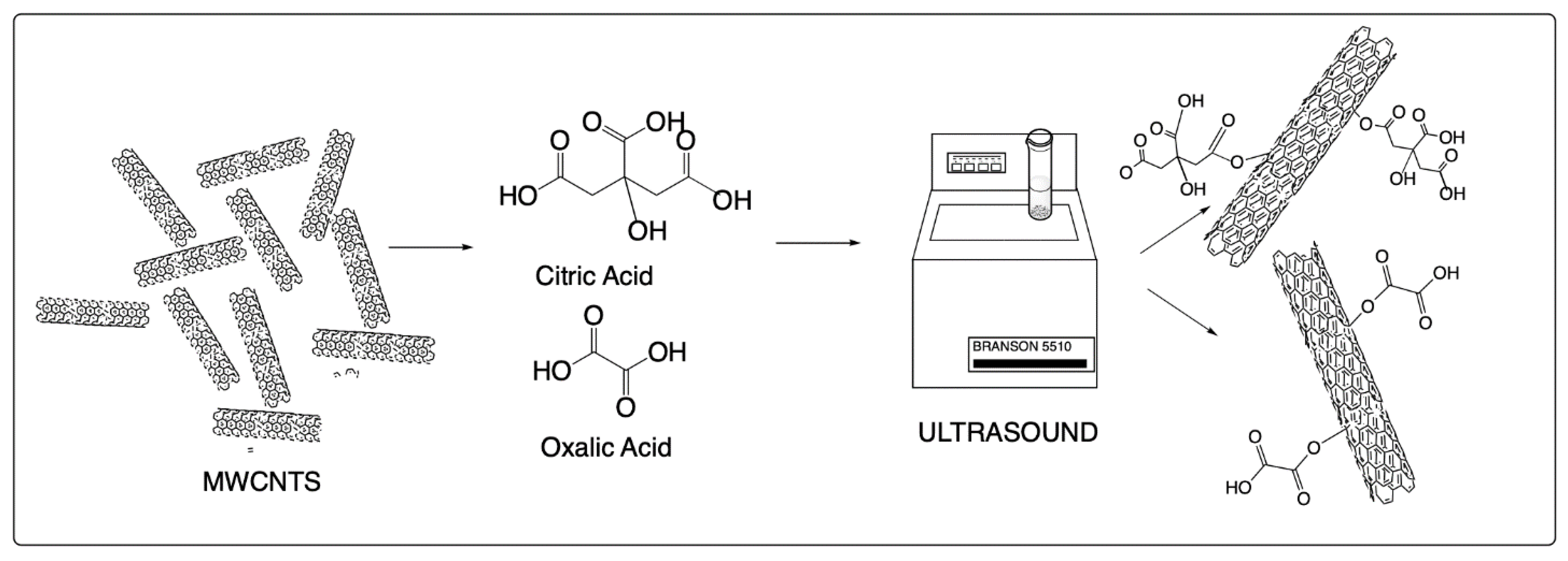
2.2. Surface Modification of MWCNT
2.3. Characterization Techniques
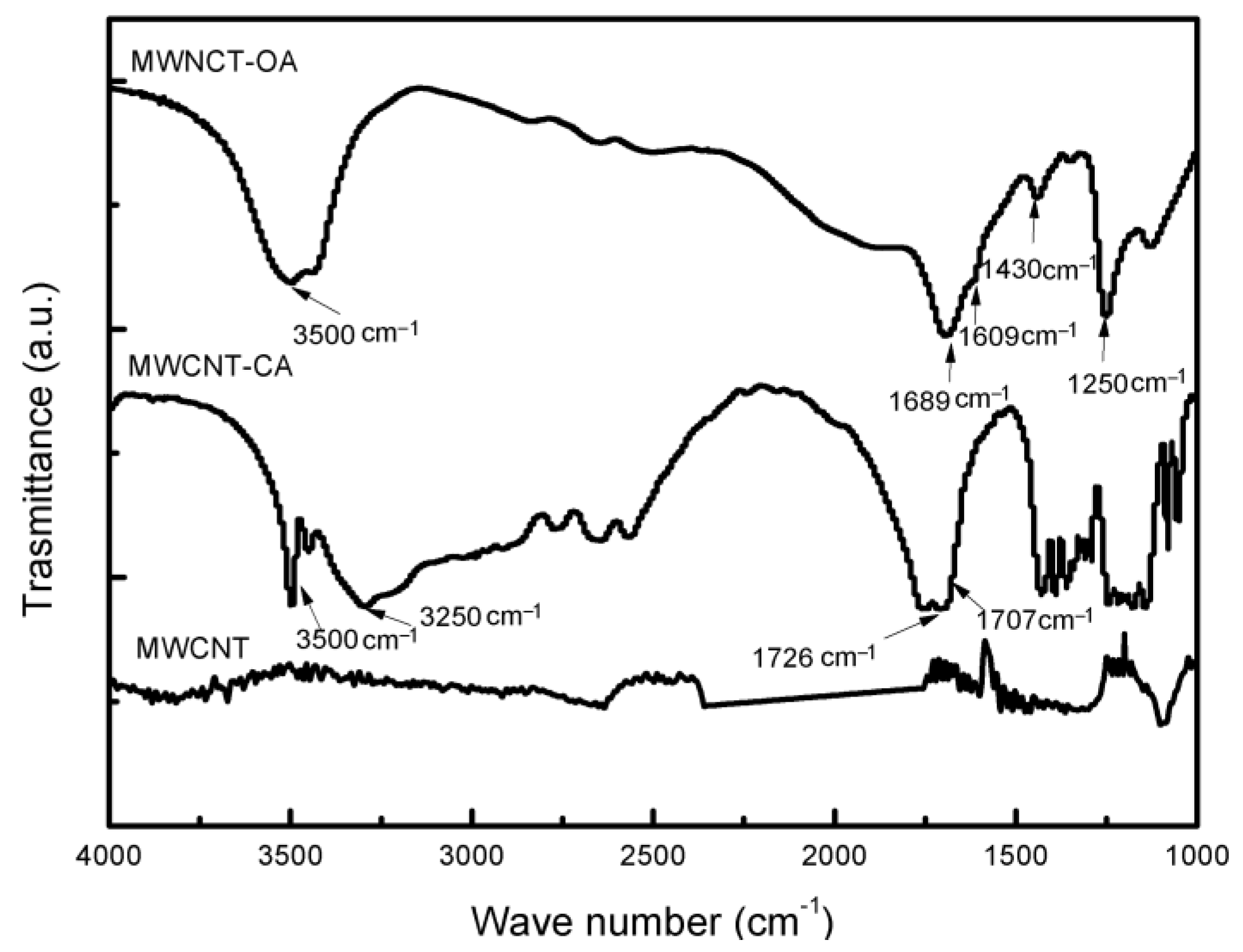
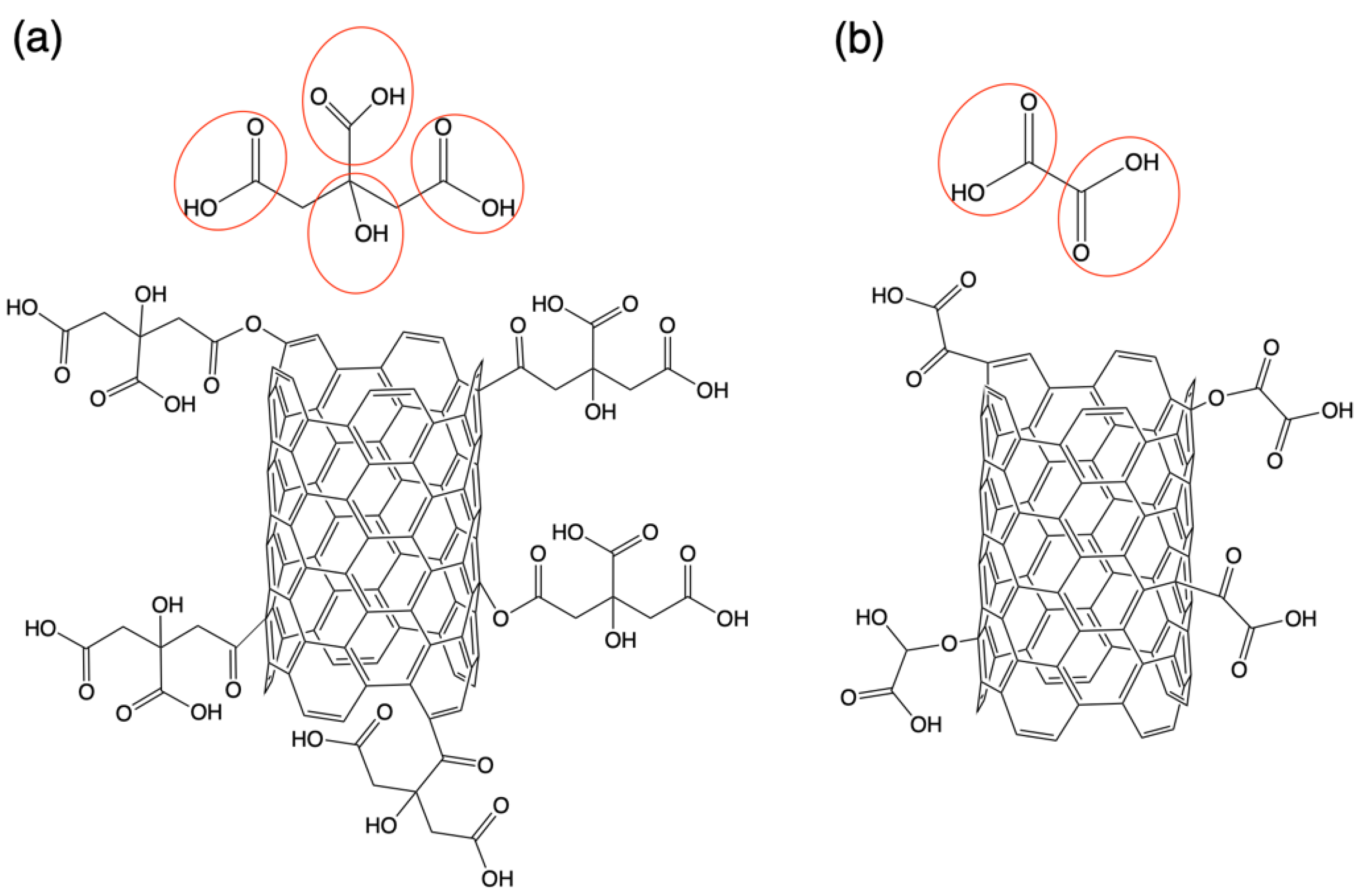
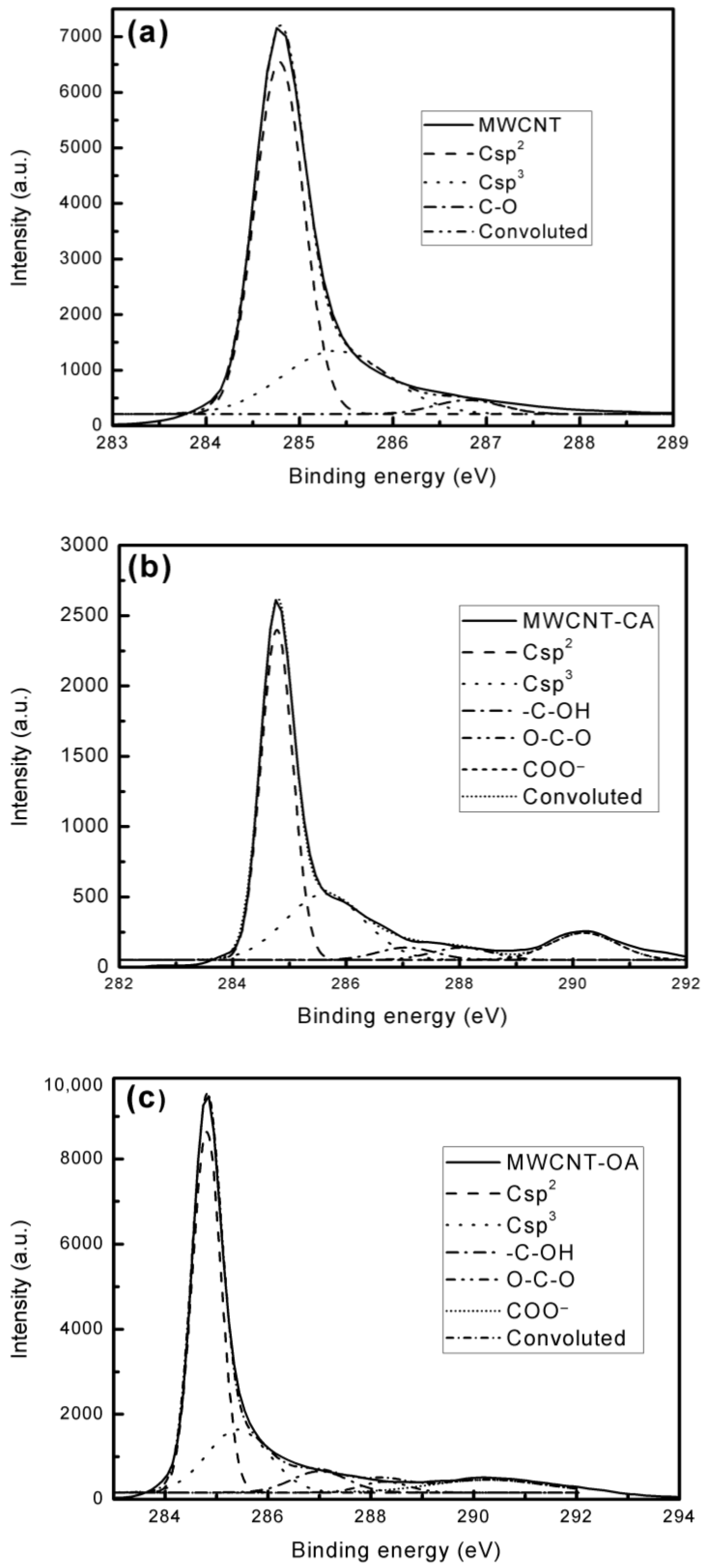
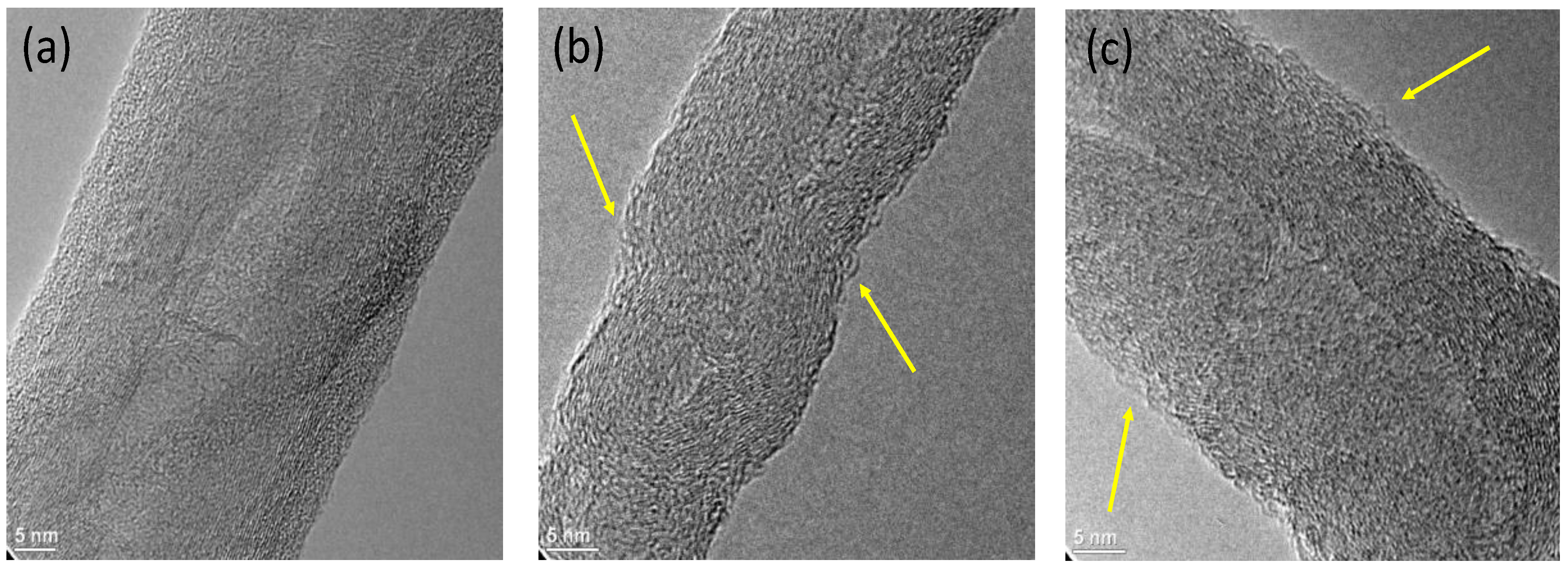
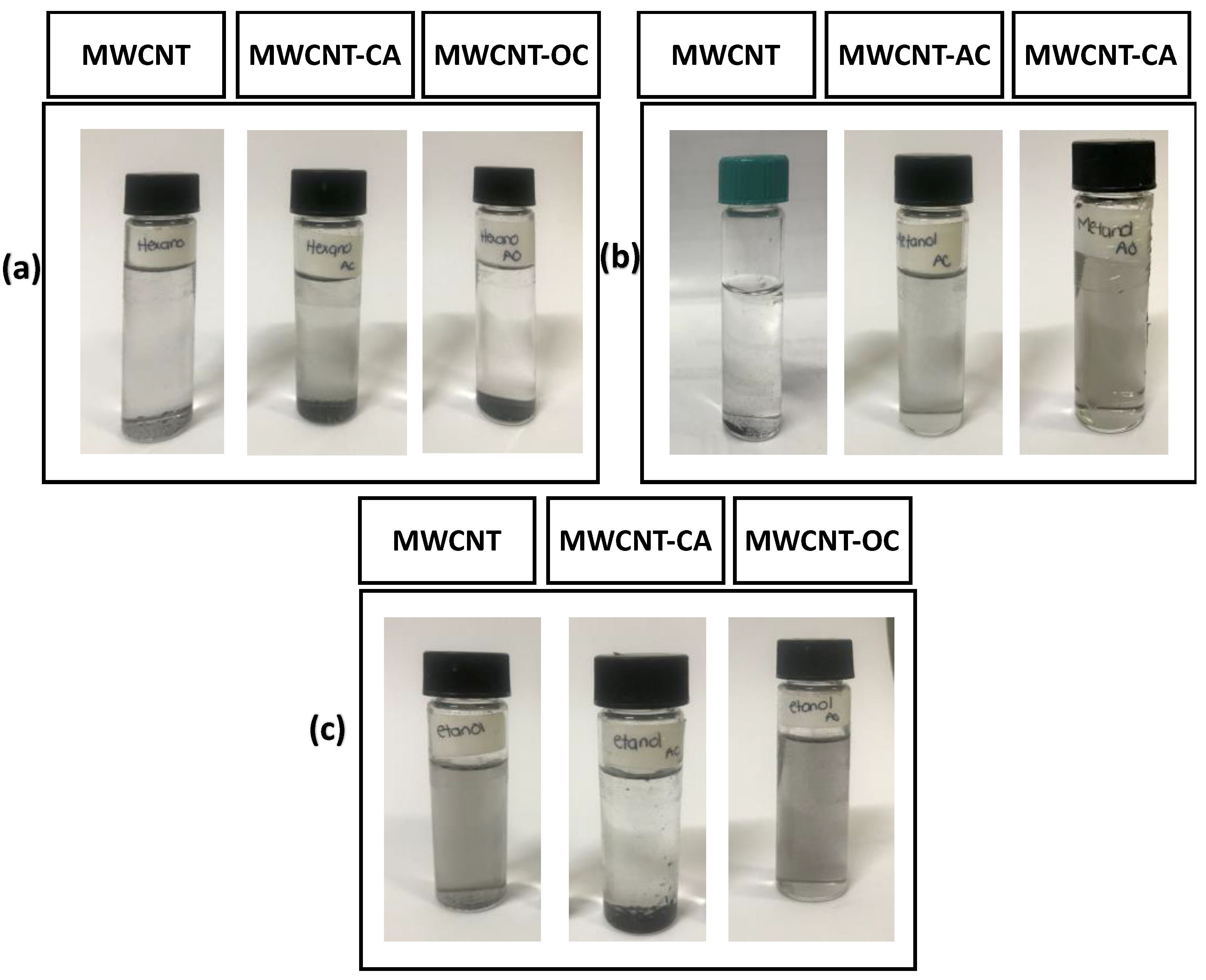
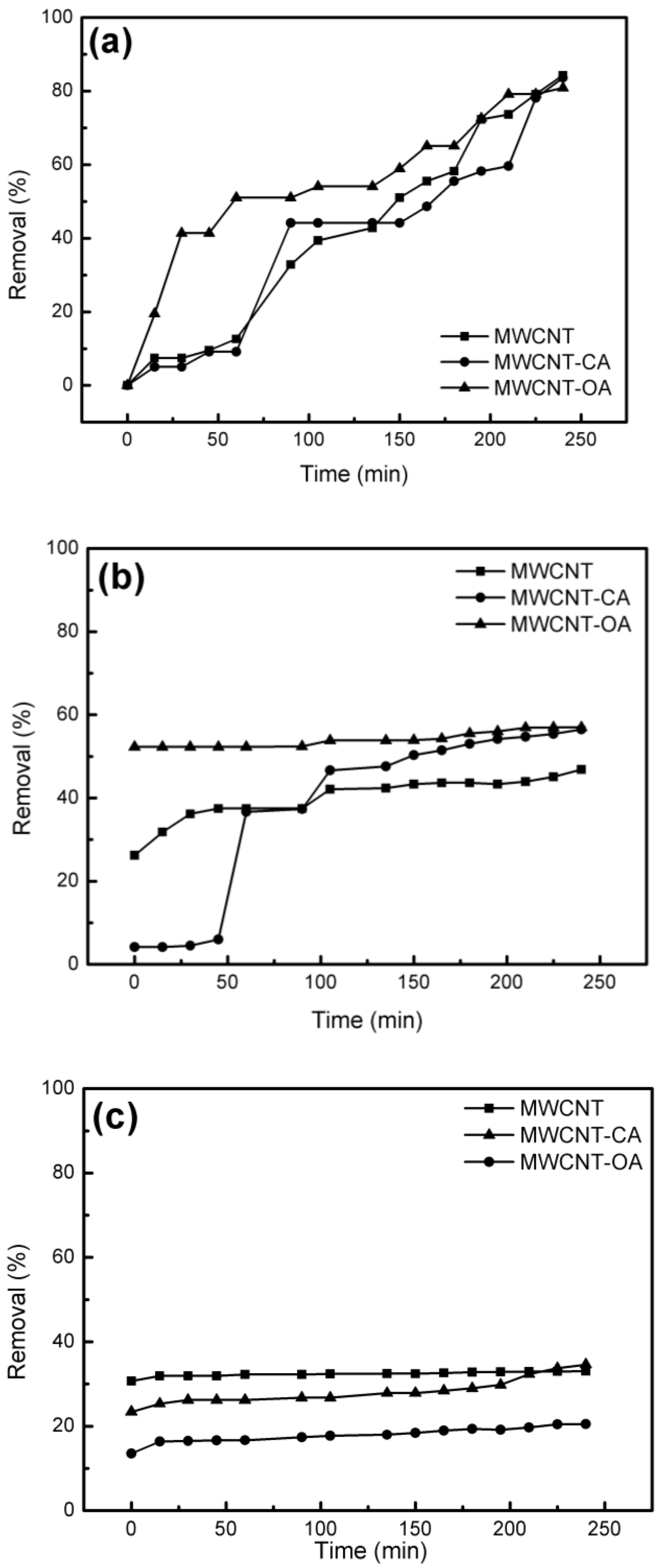
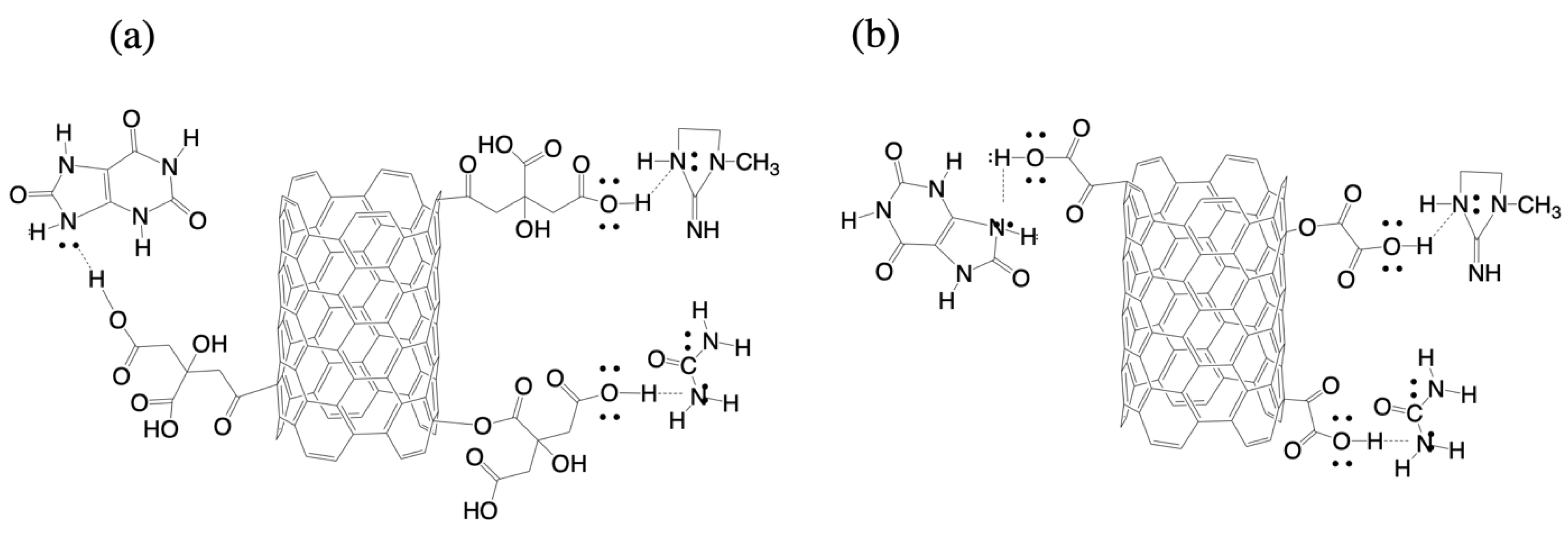
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bibi, S.; Yasin, T.; Nawaz, M.; Price, G.J. Comparative study of the modification of multi-wall carbon nanotubes by gamma irradiation and sonochemically assisted acid etching. Mater. Chem. Phys. 2018, 207, 23–29. [Google Scholar] [CrossRef]
- Mohmood, I.; Lopes, C.B.; Lopes, I.; Ahmad, I.; Duarte, A.C.; Pereira, E. Nanoscale materials and their use in water contaminants removal—A review. Environ. Sci. Pollut. Res. 2013, 20, 1239–1260. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-J.; Rosa, A.; Liu, X.; Su, D.S. Characterization and use of functionalized carbon nanotubes for the adsorption of heavy metal anions. New Carbon Mater. 2011, 26, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Carmalin, S.A.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Enviromental application of cacbon- based nanomaterial. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Abidin, M.N.Z.; Goh, P.; Ismail, A.F.; Othman, M.H.D.; Hasbullah, H.; Said, N.; Kadir, S.H.S.A.; Kamal, F.; Abdullah, M.S.; Ng, B.C. Antifouling polyethersulfone hemodialysis membranes incorporated with poly (citric acid) polymerized multi-walled carbon nanotubes. Mater. Sci. Eng. C 2016, 68, 540–550. [Google Scholar] [CrossRef]
- Irfan, M.; Idris, A.; Yusof, N.M.; Khairuddin, N.F.M.; Akhmal, H. Surface modification and performance enhancement of nano-hybrid f-MWCNT/PVP90/PES hemodialysis membranes. J. Membr. Sci. 2014, 467, 73–84. [Google Scholar] [CrossRef]
- Price, G.J.; Nawaz, M.; Yasin, T.; Bibi, S. Sonochemical modification of carbon nanotubes for enhanced nanocomposite performance. Ultrason. Sonochem. 2018, 40, 123–130. [Google Scholar] [CrossRef]
- Gao, X.; Isayev, A.I.; Yi, C. Ultrasonic treatment of polycarbonate/carbon nanotubes composites. Polymer 2016, 84, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.T.R.; Rasheed, T.; Hussain, D.; Haq, M.N.U.; Majeeda, S.; Shafi, S.; Ahmed, N.; Nawaz, R. Modification strategies for improving the solubility/dispersion of carbon nanotubes. J. Mol. Liq. 2020, 297, 111919. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Rehman, A.; Park, M.; Park, S.-J. Current Progress on the Surface Chemical Modification of Carbonaceous Materials. Coatings 2019, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef]
- Maleki, A. Green oxidation protocol: Selective conversions of alcohols and alkenes to aldehydes, ketones and epoxides by using a new multiwall carbon nanotube-based hybrid nanocatalyst via ultrasound irradiation. Ultrason. Sonochem. 2018, 40, 460–464. [Google Scholar] [CrossRef]
- Mallakpour, S.; Abdolmaleki, A.; Azimi, F. Ultrasonic-assisted biosurface modification of multi-walled carbon nanotubes with Thiamine and its influence on the properties of PVC/Tm-MWCNTs nanocomposite films. Ultrason. Sonochem. 2017, 39, 589–596. [Google Scholar] [CrossRef]
- Xie, X.; Mai, Y.; Zhou, X. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Cabello, C.; Rincón, S.; Bartolo, P.; Ruiz-Espinoza, J.; Zepeda, A. Incorporation of organic groups on the surface of multi-walled carbon nanotubes using an ultrasonic tip. Full. Nanotub. Carbon Nanostruct. 2018, 26, 502–509. [Google Scholar] [CrossRef]
- Sáenz-Galindo, A.; Rodríguez-Ramírez, K.F.; Rubio-González, W.M.; Barajas-Bermúdez, L.; Ramírez-Mendoza, L.A.; Ávila-Orta, C.A.; Jiménez-Barrera, R.M. Modificación superficial asistida con energía ultrasónica de nanotubos de carbono con ácido maléico, ácido malónico y ácido tartárico. Av. Quim. 2016, 11, 47–52. [Google Scholar]
- Andrade-Guel, M.; Cabello-Alvarado, C.J.; Cruz-Delgado, V.J.; Bartolo-Perez, P.; De León-Martínez, P.A.; Sáenz-Galindo, A.; Cadenas-Pliego, G.; Ávila-Orta, C. Surface Modification of Graphene Nanoplatelets by Organic Acids and Ultrasonic Radiation for Enhance Uremic Toxins Adsorption. Materials 2019, 12, 715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Gusain, A.; Saxena, V.; Chauhan, A.K.; Veerender, P.; Koiry, S.P.; Jha, P.; Jain, A.; Aswal, D.K.; Gupta, S.K. XPS, UV–Vis, FTIR, and EXAFS Studies to Investigate the Binding Mechanism of N719 Dye onto Oxalic Acid Treated TiO2 and Its Implication on Photovoltaic Properties. J. Phys. Chem. C 2013, 117, 21096–21104. [Google Scholar] [CrossRef] [Green Version]
- Mendive, C.B.; Bredow, T.; Blesa, M.A.; Bahnemann, D.W. ATR-FTIR measurements and quantum chemical calculations concerning the adsorption and photoreaction of oxalic acid on TiO2. Phys. Chem. Chem. Phys. 2006, 8, 3232. [Google Scholar] [CrossRef] [PubMed]
- Bokobza, L.; Zhang, J. Raman spectroscopic characterization of multiwall carbon nanotubes and of composites. Express Polym. Lett. 2012, 6, 601–608. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Kövér, L.; Tóth, J.; Biniak, S.; Trykowski, G.; Judek, J. Multiwall carbon nanotubes purification and oxidation by nitric acid studied by the FTIR and electron spectroscopy methods. J. Alloys Compd. 2010, 501, 77–84. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Liu, J.; Ebert, A.; Variava, M.F.; Dehghani, F.; Harris, A.T. Surface modification and Pt functionalisation of multi-walled carbon nanotubes in methanol expanded with supercritical CO2. Chem. Eng. J. 2010, 165, 974–979. [Google Scholar] [CrossRef]
- Hamouma, O.; Oukil, D.; Omastová, M.; Chehimi, M.M. Flexible paper@carbon nanotube@polypyrrole composites: The combined pivotal roles of diazonium chemistry and sonochemical polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 350–360. [Google Scholar] [CrossRef]
- Jeon, K.S.; Nirmala, R.; Navamathavan, R.; Kim, H.Y. Mechanical behavior of electrospun Nylon66 fibers reinforced with pristine and treated multi-walled carbon nanotube fillers. Ceram. Int. 2013, 39, 8199–8206. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Zhang, W.; Lee, H.-R.; Kim, J.-H. Surface Modification of Multi-walled Carbon Nanotubes for Enhancement of Dispersion and Electrochemical Properties. J. Korean Inst. Surf. Eng. 2008, 41, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, C.; Su, W.; Lv, W.; Zhu, S.; Wang, F.; Fu, Q. Water dispersed multi-walled carbon nanotubes modified by tannin acid. Mater. Lett. 2014, 123, 44–47. [Google Scholar] [CrossRef]
- Avilés, F.; Cauich-Rodríguez, J.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Hong, C.K.; Shim, S.E. Measurement of the dispersion stability of pristine and surface-modified multiwalled carbon nanotubes in various nonpolar and polar solvents. Meas. Sci. Technol. 2007, 18, 3707–3712. [Google Scholar] [CrossRef]
- Farbod, M.; Tadavani, S.K.; Kiasat, A. Surface oxidation and effect of electric field on dispersion and colloids stability of multiwalled carbon nanotubes. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 384, 685–690. [Google Scholar] [CrossRef]
- Eknoyan, G.; Beck, G.J.; Cheung, A.K.; Daugirdas, J.T.; Greene, T.; Kusek, J.W.; Allon, M.; Bailey, J.; Delmez, J.A.; Depner, T.A.; et al. Effect of dialysis dose and membrane flux in maintenace hemodialysis. N. Engl. J. Med. Eff. 2002, 347, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Alvarado, C.J.; Andrade-Guel, M.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Cortés-Hernández, D.A.; Bartolo-Pérez, P.; Ávila-Orta, C.; Cruz-Delgado, V.J.; Zepeda-Pedreguera, A. Graphene Nanoplatelets Modified with Amino-Groups by Ultrasonic Radiation of Variable Frequency for Potential Adsorption of Uremic Toxins. Nanomaterials 2019, 9, 1261. [Google Scholar] [CrossRef] [Green Version]
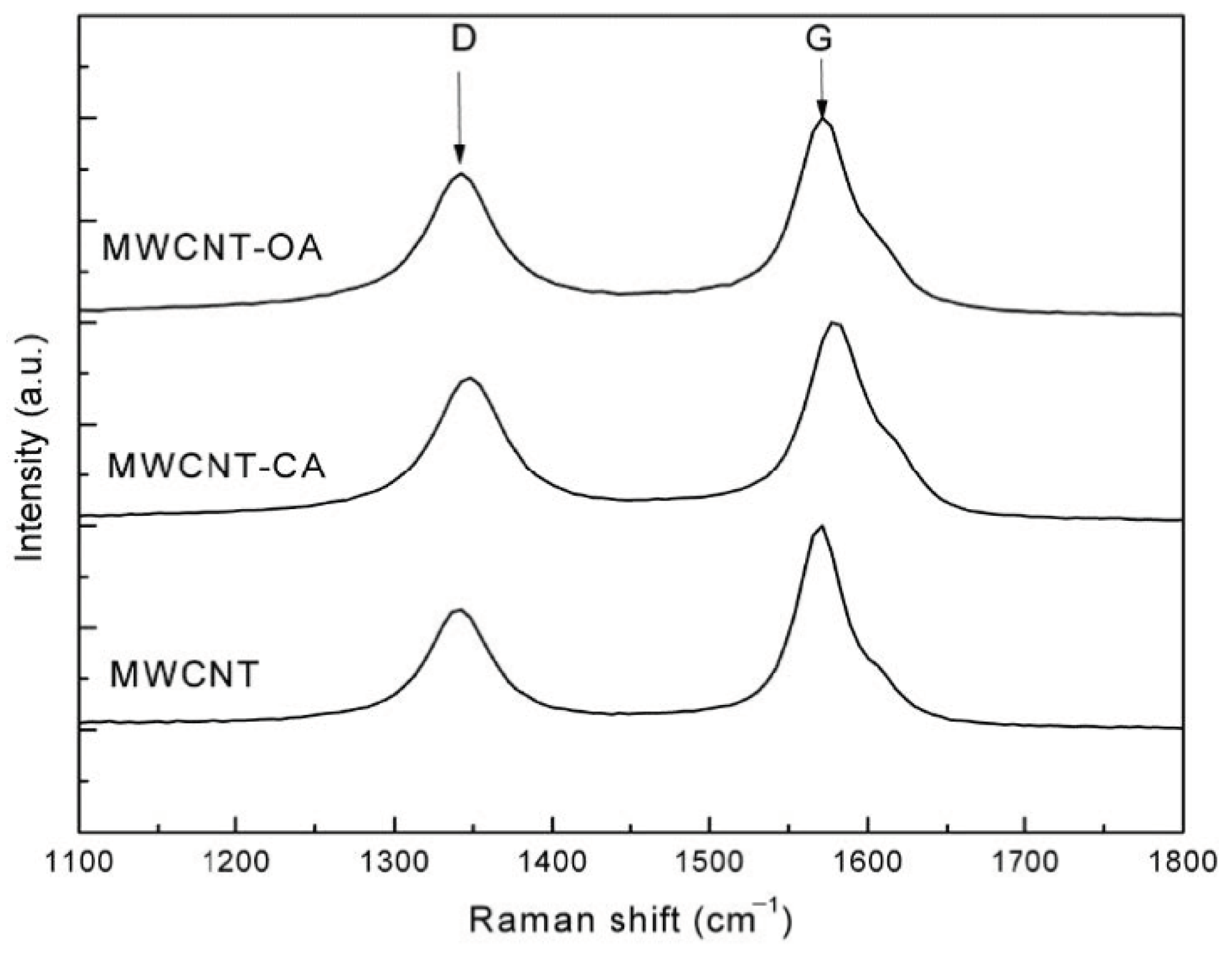
| Sample | ID | IG | ID/IG |
|---|---|---|---|
| MWCNT | 0.5859 | 0.9960 | 0.5882 |
| MWCNT-CAMWCNT-CA | 0.7286 | 1.0002 | 0.7284 |
| MWCNT-OA | 0.7261 | 0.9960 | 0.7290 |
| Sample | C/O | Csp2 (%) | Csp3 (%) | C–O (%) | O–C–O (%) | COO− (%) |
|---|---|---|---|---|---|---|
| MWCNT | 54.55 | 69.136 | 27.068 | 3.796 | - | - |
| MWCNT-CA | 2.62 | 55.357 | 27.367 | 3.804 | 3.901 | 9.570 |
| MWCNT-OA | 8.49 | 59.412 | 22.469 | 6.852 | 2.877 | 8.390 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de León-Martínez, P.A.; Sáenz-Galindo, A.; Ávila-Orta, C.A.; Castañeda-Facio, A.O.; Andrade-Guel, M.L.; Sierra, U.; Alvarado-Tenorio, G.; Bernal-Martínez, J. Ultrasound-Assisted Surface Modification of MWCNT Using Organic Acids. Materials 2021, 14, 72. https://doi.org/10.3390/ma14010072
de León-Martínez PA, Sáenz-Galindo A, Ávila-Orta CA, Castañeda-Facio AO, Andrade-Guel ML, Sierra U, Alvarado-Tenorio G, Bernal-Martínez J. Ultrasound-Assisted Surface Modification of MWCNT Using Organic Acids. Materials. 2021; 14(1):72. https://doi.org/10.3390/ma14010072
Chicago/Turabian Stylede León-Martínez, Patricia A., Aidé Sáenz-Galindo, Carlos A. Ávila-Orta, Adalí O. Castañeda-Facio, Marlene L. Andrade-Guel, Uriel Sierra, German Alvarado-Tenorio, and Juan Bernal-Martínez. 2021. "Ultrasound-Assisted Surface Modification of MWCNT Using Organic Acids" Materials 14, no. 1: 72. https://doi.org/10.3390/ma14010072
APA Stylede León-Martínez, P. A., Sáenz-Galindo, A., Ávila-Orta, C. A., Castañeda-Facio, A. O., Andrade-Guel, M. L., Sierra, U., Alvarado-Tenorio, G., & Bernal-Martínez, J. (2021). Ultrasound-Assisted Surface Modification of MWCNT Using Organic Acids. Materials, 14(1), 72. https://doi.org/10.3390/ma14010072








