Biodiesel Production on Monometallic Pt, Pd, Ru, and Ag Catalysts Supported on Natural Zeolite
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Catalytic Materials
2.2. Characterization of the Catalytic Material
2.3. Catalytic Activity Measurements in Transesterification of the Vegetable Oil with Methanol
3. Results and Discussion
3.1. Transesteryfication of Vegetable Oil with Methanol Reaction
3.2. The Characterization of the Physicochemical Properties of the Investigated Catalysts
3.2.1. Specific Surface Area, Porosity, and Characterization of the Natural Zeolite
3.2.2. The Acidity Properties of the Synthesized Catalysts Systems
3.2.3. Basic Properties of the Synthesized Catalyst Systems
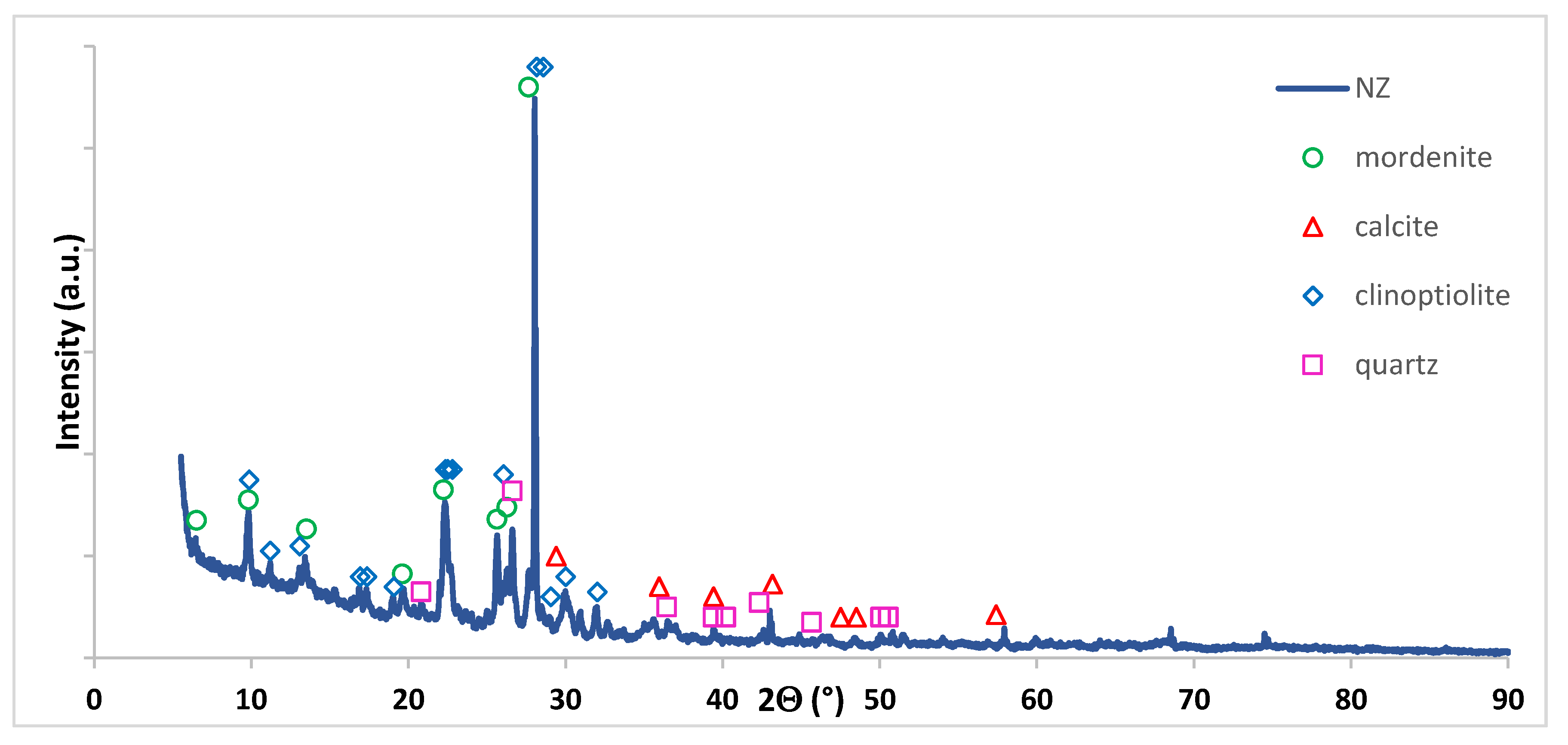
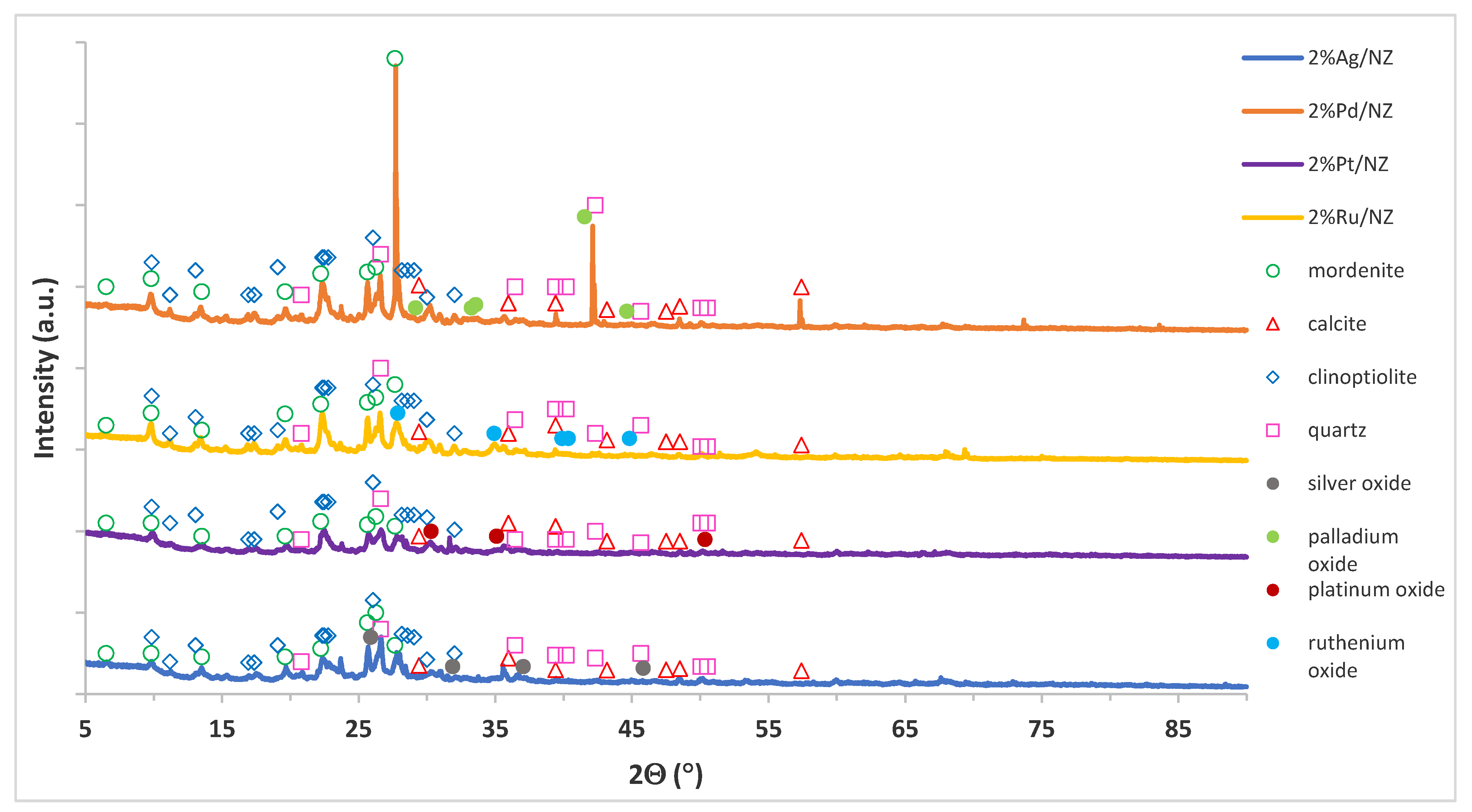
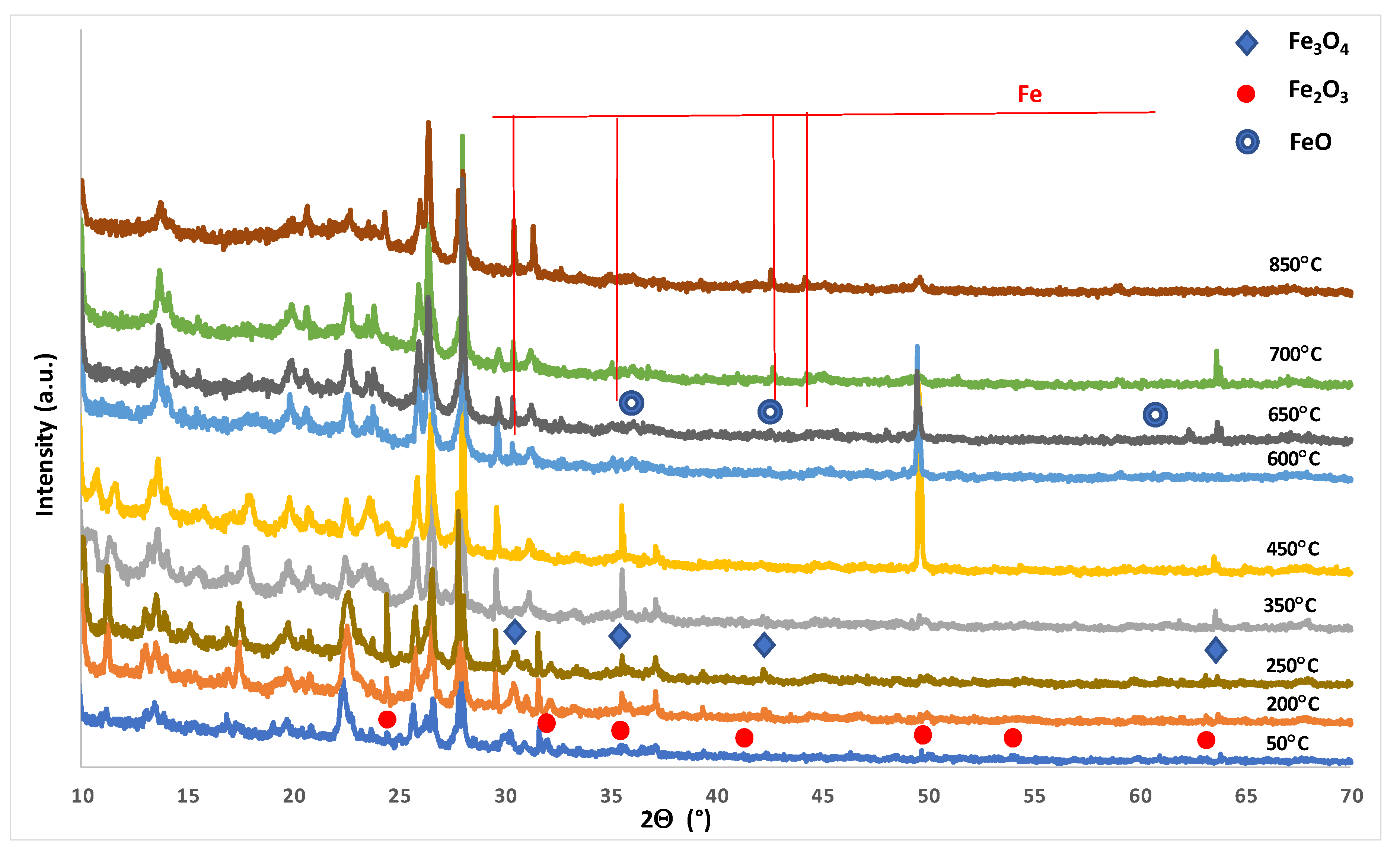
3.2.4. Phase Composition Studies of Monometallic Catalysts
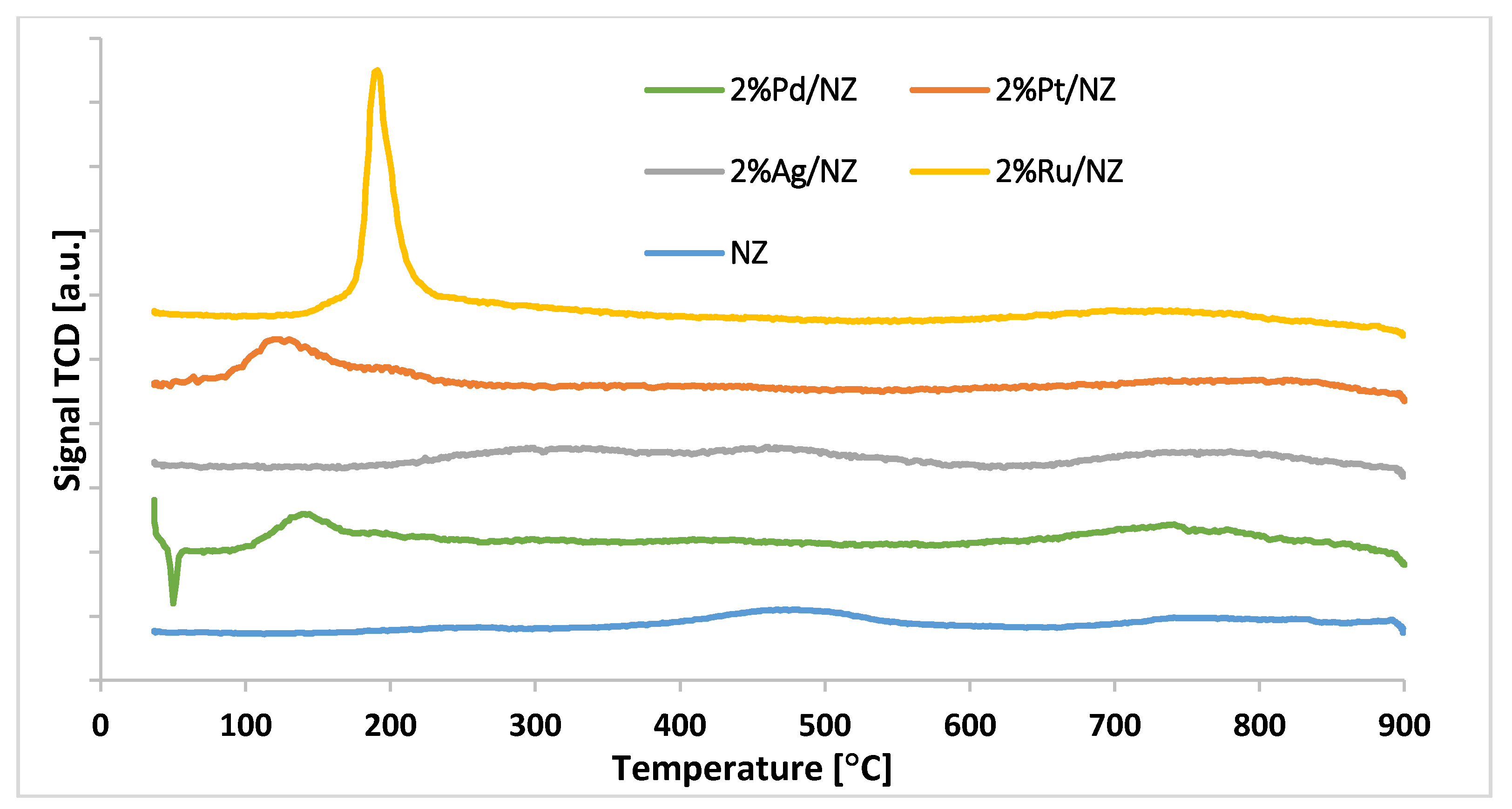
3.2.5. Reduction Behavior of the Catalytic Materials
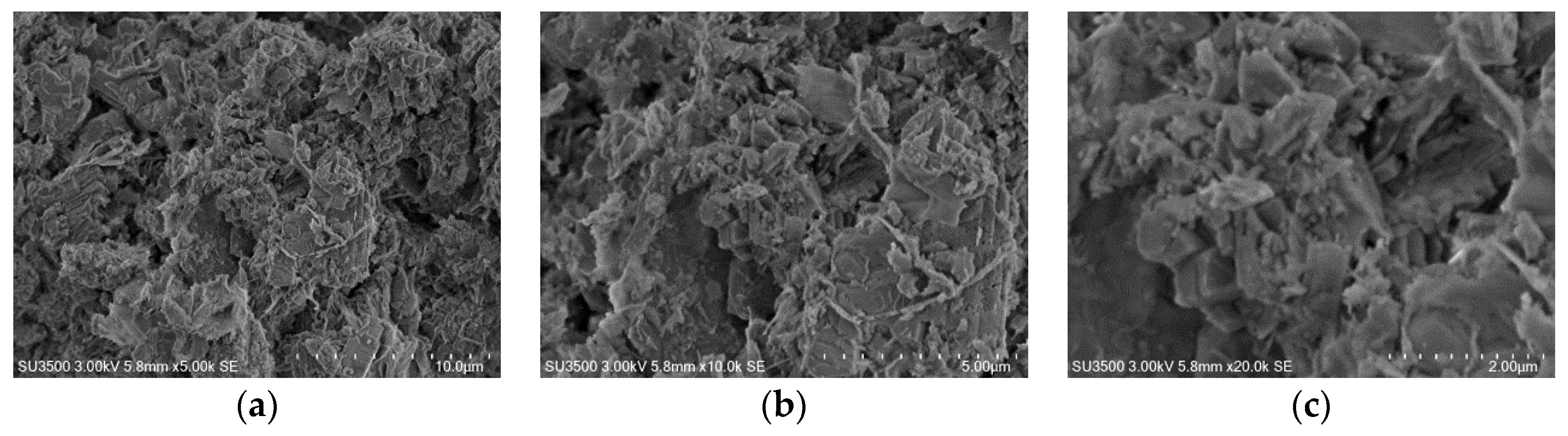
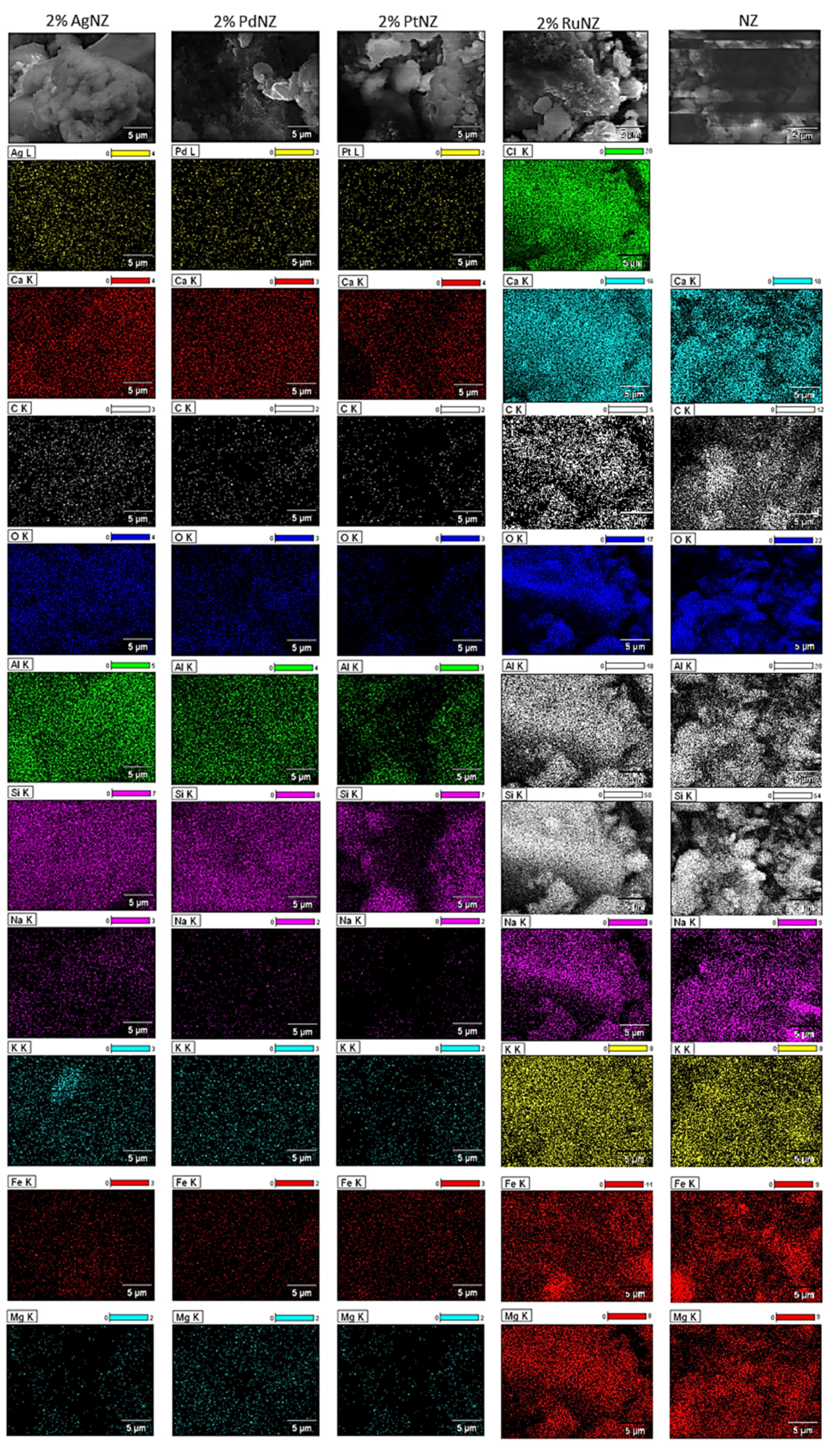
3.2.6. The Morphology of the Investigated Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Maleki, H.; Kazemeini, M.; Larimi, A.S.; Khorasheh, F. Transesterification of Canola Oil and Methanol by Lithium Impregnated CaO-La2O3 Mixed Oxide for Biodiesel Synthesis. J. Ind. Eng. Chem. 2017, 47, 399–404. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in Solid Acid Catalysts for Biodiesel Production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Ruhul, A.M.; Kalam, M.A.; Masjuki, H.H.; Fattah, I.M.R.; Reham, S.S.; Rashed, M.M. State of the Art of Biodiesel Production Processes: A Review of the Heterogeneous Catalyst. RSC Adv. 2015, 5, 101023–101044. [Google Scholar] [CrossRef]
- Solis, J.L.; Berkemar, A.L.; Alejo, L.; Kiros, Y. Biodiesel from Rapeseed Oil (Brassica Napus) by Supported Li2O and MgO. Int. J. Energy Environ. Eng. 2017, 8, 9–23. [Google Scholar] [CrossRef]
- Kwong, T.-L.; Yung, K.-F. Heterogeneous Alkaline Earth Metal–Transition Metal Bimetallic Catalysts for Synthesis of Biodiesel from Low Grade Unrefined Feedstock. RSC Adv. 2015, 5, 83748–83756. [Google Scholar] [CrossRef]
- Nabgan, W.; Tuan Abdullah, T.A.; Mat, R.; Nabgan, B.; Triwahyono, S.; Ripin, A. Hydrogen Production from Catalytic Steam Reforming of Phenol with Bimetallic Nickel-Cobalt Catalyst on Various Supports. Appl. Catal. Gen. 2016, 527, 161–170. [Google Scholar] [CrossRef]
- AlSharifi, M.; Znad, H. Transesterification of Waste Canola Oil by Lithium/Zinc Composite Supported on Waste Chicken Bone as an Effective Catalyst. Renew. Energy 2020, 151, 740–749. [Google Scholar] [CrossRef]
- Keskin, A.; Yaşar, A.; Yıldızhan, Ş.; Uludamar, E.; Emen, F.M.; Külcü, N. Evaluation of Diesel Fuel-Biodiesel Blends with Palladium and Acetylferrocene Based Additives in a Diesel Engine. Fuel 2018, 216, 349–355. [Google Scholar] [CrossRef]
- Kesieme, U.; Pazouki, K.; Murphy, A.; Chrysanthou, A. Biofuel as an Alternative Shipping Fuel: Technological, Environmental and Economic Assessment. Sustain. Energy Fuels 2019, 3, 899–909. [Google Scholar] [CrossRef]
- Rahimi, A.; Moradi, G.; Abolhasan Alavi, S.; Ardjmand, M. Simultaneous Extraction of Rapeseed Oil and Conversion to Biodiesel Using Heterogeneous and Homogeneous Catalysts. Environ. Prog. Sustain. Energy 2018, 37, 518–523. [Google Scholar] [CrossRef]
- Yunus Khan, T.M.; Badruddin, I.A.; Ankalgi, R.F.; Badarudin, A.; Hungund, B.S.; Ankalgi, F.R. Biodiesel Production by Direct Transesterification Process via Sequential Use of Acid–Base Catalysis. Arab. J. Sci. Eng. 2018, 43, 5929–5936. [Google Scholar] [CrossRef]
- Kwon, K.; Vahdat, N.; Mbah, J. Fatty Acid Methyl Ester Biofuels Produced from Canola Oil with Honeycomb Monolithic Catalysts. Fuel 2015, 145, 116–126. [Google Scholar] [CrossRef]
- Kant Bhatia, S.; Kant Bhatia, R.; Jeon, J.-M.; Pugazhendhi, A.; Kumar Awasthi, M.; Kumar, D.; Kumar, G.; Yoon, J.-J.; Yang, Y.-H. An Overview on Advancements in Biobased Transesterification Methods for Biodiesel Production: Oil Resources, Extraction, Biocatalysts, and Process Intensification Technologies. Fuel 2021, 285, 119117. [Google Scholar] [CrossRef]
- Nowicki, J.; Lach, J.; Organek, M.; Sabura, E. Transesterification of Rapeseed Oil to Biodiesel over ZR-Dopped MgAl Hydrotalcites. Appl. Catal. Gen. 2016, 524, 17–24. [Google Scholar] [CrossRef]
- Ataya, F.; Dubé, M.A.; Ternan, M. Acid-Catalyzed Transesterification of Canola Oil to Biodiesel under Single- and Two-Phase Reaction Conditions. Energy Fuels 2007, 21, 2450–2459. [Google Scholar] [CrossRef]
- Esipovich, A.L.; Rogozhin, A.E.; Belousov, A.S.; Kanakov, E.A.; Danov, S.M. A Comparative Study of the Separation Stage of Rapeseed Oil Transesterification Products Obtained Using Various Catalysts. Fuel Process. Technol. 2018, 173, 153–164. [Google Scholar] [CrossRef]
- Hájek, M.; Vávra, A.; Mück, J. Butanol as a Co-Solvent for Transesterification of Rapeseed Oil by Methanol under Homogeneous and Heterogeneous Catalyst. Fuel 2020, 277, 118239. [Google Scholar] [CrossRef]
- Hájek, M.; Kutálek, P.; Smoláková, L.; Troppová, I.; Čapek, L.; Kubička, D.; Kocík, J.; Thanh, D.N. Transesterification of Rapeseed Oil by Mg-Al Mixed Oxides with Various Mg/Al Molar Ratio. Chem. Eng. J. 2015, 263, 160–167. [Google Scholar] [CrossRef]
- Salinas, D.; Escalona, N.; Pecchi, G.; Fierro, J.L.G. Lanthanum Oxide Behavior in La2O3-Al2O3 and La2O3-ZrO2 Catalysts with Application in FAME Production. Fuel 2019, 253, 400–408. [Google Scholar] [CrossRef]
- Alsharifi, M.; Znad, H.; Hena, S.; Ang, M. Biodiesel Production from Canola Oil Using Novel LI/tio2 as a Heterogeneous Catalyst Prepared via Impregnation Method. Renew. Energy 2017, 114, 1077–1089. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.M.A.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Smoláková, L.; Pöpperle, L.; Kocík, J.; Dubnová, L.; Horáček, J.; Čapek, L. Catalytic Behavior of Mg–Al and Zn–Al Mixed Oxides in the Transesterification of Rapeseed Oil: Comparison of Batch and Fixed Bed Reactors. React. Kinet. Mech. Catal. 2017, 121, 209–224. [Google Scholar] [CrossRef]
- Al-Saadi, A.; Mathan, B.; He, Y. Esterification and Transesterification over SrO-ZnO/Al2O3 as a Novel Bifunctional Catalyst for Biodiesel Production. Renew. Energy 2020, 158, 388–399. [Google Scholar] [CrossRef]
- Khatibi, M.; Khorasheh, F.; Larimi, A. Biodiesel Production via Transesterification of Canola Oil in the Presence of Na-K Doped CaO Derived from Calcined Eggshell. Renew. Energy 2021, 163, 1626–1636. [Google Scholar] [CrossRef]
- Marwaha, A.; Dhir, A.; Mahla, S.K.; Mohapatra, S.K. An Overview of Solid Base Heterogeneous Catalysts for Biodiesel Production. Catal. Rev. 2018, 60, 594–628. [Google Scholar] [CrossRef]
- Lourinho, G.; Brito, P. Advanced Biodiesel Production Technologies: Novel Developments. Rev. Environ. Sci. Biotechnol. 2015, 14, 287–316. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Chakravarthy, M.; Kumar, R.R.; Yuvaraj, D.; Jayamuthunagai, J.; Kumar, R.P.; Palani, S. Biodiesel Production Using Chemical and Biological Methods—A Review of Process, Catalyst, Acyl Acceptor, Source and Process Variables. Renew. Sustain. Energy Rev. 2014, 38, 368–382. [Google Scholar] [CrossRef]
- Sahu, G.; Gupta, N.K.; Kotha, A.; Saha, S.; Datta, S.; Chavan, P.; Kumari, N.; Dutta, P. A Review on Biodiesel Production through Heterogeneous Catalysis Route. Chem. Bio. Eng. Rev. 2018, 5, 231–252. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous Catalysts for Biodiesel Production. Energy Fuels 2007, 22, 207–217. [Google Scholar] [CrossRef]
- Mierczynski, P.; Chalupka, K.A.; Maniukiewicz, W.; Kubicki, J.; Szynkowska, M.I.; Maniecki, T.P. SrAl2O4 Spinel Phase as Active Phase of Transesterification of Rapeseed Oil. Appl. Catal. Environ. 2015, 164, 176–183. [Google Scholar] [CrossRef]
- Lawan, I.; Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. Synergies between the Microwave Reactor and CaO/Zeolite Catalyst in Waste Lard Biodiesel Production. Renew. Energy 2020, 145, 2550–2560. [Google Scholar] [CrossRef]
- Chen, C.; Cai, L.; Zhang, L.; Fu, W.; Hong, Y.; Gao, X.; Jiang, Y.; Li, L.; Yan, X.; Wu, G. Transesterification of Rice Bran Oil to Biodiesel Using Mesoporous NaBeta Zeolite-Supported Molybdenum Catalyst: Experimental and Kinetic Studies. Chem. Eng. J. 2020, 382, 122839. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Modern Heterogeneous Catalysts for Biodiesel Production: A Comprehensive Review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Mierczynski, P.; Ciesielski, R.; Kedziora, A.; Maniukiewicz, W.; Shtyka, O.; Kubicki, J.; Albinska, J.; Maniecki, T.P. Biodiesel Production on MgO, CaO, SrO and BaO Oxides Supported on (SrO)(Al2O3) Mixed Oxide. Catal. Lett. 2015, 145, 1196–1205. [Google Scholar] [CrossRef]
- Macala, G.; Robertson, A.; Johnson, C.; Day, Z.; Lewis, R.; White, M.; Iretskii, A.; Ford, P. Transesterification Catalysts from Iron Doped Hydrotalcite-like Precursors: Solid Bases for Biodiesel Production. Catal. Lett. 2008, 122, 205–209. [Google Scholar] [CrossRef]
- Suzuta, T.; Toba, M.; Abe, Y.; Yoshimura, Y. Iron Oxide Catalysts Supported on Porous Silica for the Production of Biodiesel from Crude Jatropha Oil. J. Am. Oil Chem. Soc. 2012, 89, 1981–1989. [Google Scholar] [CrossRef]
- Jozwiak, W.; Maniecki, T.; Mierczynski, P.; Bawolak, K.; Maniukiewicz, W. Reduction Study of Iron-Alumina Binary Oxide Fe2-xAlxO3. Pol. J. Chem. 2009, 83, 2153–2162. [Google Scholar]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction Behavior of Iron Oxides in Hydrogen and Carbon Monoxide Atmospheres. Appl. Catal. Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Naresh, D.; Kumar, V.P.; Harisekhar, M.; Nagaraju, N.; Putrakumar, B.; Chary, K.V.R. Characterization and Functionalities of Pd/Hydrotalcite Catalysts. Appl. Surf. Sci. 2014, 314, 199–207. [Google Scholar] [CrossRef]
- Pergher, S.B.C.; Dallago, R.M.; Veses, R.C.; Gigola, C.E.; Baibich, I.M. Pd/NaY-Zeolite and Pd-W/NaY-Zeolite Catalysts: Preparation, Characterization and NO Decomposition Activity. J. Mol. Catal. Chem. 2004, 209, 107–115. [Google Scholar] [CrossRef]
- Mierczynski, P.; Maniukiewicz, W.; Maniecki, T. Comparative Studies of Pd, Ru, Ni, Cu/ZnAl2O4 Catalysts for the Water Gas Shift Reaction. Cent. Eur. J. Chem. 2013, 11, 912–919. [Google Scholar] [CrossRef]
- Pérez-Bustos, H.F.; Lucio-Ortiz, C.J.; de la Rosa, J.R.; de Haro del Río, D.A.; Sandoval-Rangel, L.; Martínez-Vargas, D.X.; Maldonado, C.S.; Rodriguez-González, V.; Garza-Navarro, M.A.; Morales-Leal, F.J. Synthesis and Characterization of Bimetallic Catalysts Pd-Ru and Pt-Ru Supported on γ-Alumina and Zeolite FAU for the Catalytic Transformation of HMF. Fuel 2019, 239, 191–201. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Millot, Y.; Krafft, J.-M.; Popovych, N.; Kyriienko, P. Incorporation of Silver Atoms into the Vacant T-Atom Sites of the Framework of SiBEA Zeolite as Mononuclear Ag(I) Evidenced by XRD, FTIR, NMR, DR UV–vis, XPS, and TPR. J. Phys. Chem. 2013, 117, 12552–12559. [Google Scholar] [CrossRef]
- Bartolomeu, R.; Bértolo, R.; Casale, S.; Fernandes, A.; Henriques, C.; da Costa, P.; Ribeiro, F. Particular Characteristics of Silver Species on Ag-Exchanged LTL Zeolite in K and H Form. Microporous Mesoporous Mater. 2013, 169, 137–147. [Google Scholar] [CrossRef]
- Checa, M.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Deactivation Study of Supported Pt Catalyst on Glycerol Hydrogenolysis. Appl. Catal. Gen. 2015, 507, 34–43. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Li, S.; Chen, M.; Guo, L.; Zhou, J. Ru/hierarchical HZSM-5 Zeolite as Efficient BI-Functional Adsorbent/Catalyst for Bulky Aromatic VOCs Elimination. Microporous Mesoporous Mater. 2018, 258, 17–25. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, L.; Wang, G.; Deng, W.; Guo, L. Total Catalytic Oxidation of Chlorinated Aromatics over Bimetallic Pt–Ru Supported on Hierarchical HZSM-5 Zeolite. Microporous Mesoporous Mater. 2020, 308, 110538. [Google Scholar] [CrossRef]
- Gao, D.; Li, S.; Wang, X.; Xi, L.; Lange, K.M.; Ma, X.; Lv, Y.; Yang, S.; Zhao, K.; Loussala, H.M.; et al. Ultrafine PtRu Nanoparticles Confined in Hierarchically Porous Carbon Derived from Micro-Mesoporous Zeolite for Enhanced Nitroarenes Reduction Performance. J. Catal. 2019, 370, 385–403. [Google Scholar] [CrossRef]

| Mobile Phase Gradient. | Flow Rate mL·min−1 | ||
|---|---|---|---|
| Time (min) | Solvent A (%) | Solvent B (%) | |
| 0.0 | 100 | 0 | 1 |
| 20.0 | 100 | 0 | 1 |
| 45.0 | 0 | 100 | 1 |
| 70.0 | 0 | 100 | 1 |
| 75 | 100 | 0 | 1 |
| Catalyst |
Reaction Temperature (°C) |
Reaction Time (h) |
Molar Ratio Oil: Methanol | Calcination Temperature (°C) |
Catalyst Weight (g) |
Triglycerides Conversion (%) | FAME Yield (%) |
|---|---|---|---|---|---|---|---|
| NZ | 260 | 2 | 1:9 | 400 | 0.5 | 90.5 | 67.2 |
| 0.5%Ru/NZ | 260 | 2 | 1:9 | 400 | 0.5 | 90.2 | 48.0 |
| 0.5%Pd/NZ | 260 | 2 | 1:9 | 400 | 0.5 | 79.9 | 62.4 |
| 0.5%Pt/NZ | 260 | 2 | 1:9 | 400 | 0.5 | 89.6 | 47.4 |
| 0.5%Ag/NZ | 260 | 2 | 1:9 | 400 | 0.5 | 81.0 | 47.9 |
| 2%Pt/NZ | 260 | 2 | 1:9 | 400 | 0.5 | 98.9 | 94.6 |
| 2%Pd/NZ | 260 | 2 | 1:9 | 400 | 0.5 | 98.9 | 73.8 |
| 2%Pd/NZ (cal.) | 260 | 2 | 1:9 | 400 | 0.5 | 94.6 | 64.8 |
| 2%Ru/NZ | 260 | 2 | 1:9 | 400 | 0.5 | 95.3 | 71.0 |
| 2%Ag/NZ | 260 | 2 | 1:9 | 400 | 0.5 | 96.3 | 71.6 |
| Material | BET Surface Area (m2/g) | Monolayer Capacity (cm3/g) | Average Pore Radius (nm) |
|---|---|---|---|
| NZ | 25.95 | - | 4.09 |
| 2%Ag/NZ | 21.32 | 0.076 | 6.20 |
| 2%Pd/NZ | 21.35 | 0.076 | 6.38 |
| 2%Pt/NZ | 16.11 | 0.067 | 6.67 |
| 2%Ru/NZ | 26.37 | 0.075 | 5.84 |
| Element | EDX | XPS |
|---|---|---|
| Ca | 3.55 | 6.98 |
| C | - | 7.61 |
| O | 52.02 | 55.38 |
| Al | 7.95 | 5.84 |
| Si | 31.05 | 19.27 |
| Na | 1.75 | - |
| K | 1.12 | - |
| Fe | 2.55 | 4.42 |
| Mg | - | 0.51 |
| SiO2/Al2O3 | 4.42 | 3.85 |
| Catalytic Systems | Total Acidity (mmol/g) 100–600 °C | Weak Centers (mmol/g) 100–300 °C | Medium Centers (mmol/g) 300–450 °C | Strong Centers (mmol/g) 450–600 °C |
|---|---|---|---|---|
| NZ | 1.0 | 0.6 | 0.3 | 0.1 |
| 0.5%Ag/NZ | 1.2 | 0.7 | 0.5 | 0.1 |
| 2%Ag/NZ | 1.9 | 0.7 | 0.8 | 0.3 |
| 0.5%Pt/NZ | 2.0 | 1.1 | 0.7 | 0.1 |
| 2%Pt/NZ | 2.1 | 1.2 | 0.7 | 0.2 |
| 0.5%Pd/NZ | 1.2 | 0.6 | 0.5 | 0.1 |
| 2%Pd/NZ | 1.9 | 0.9 | 0.9 | 0.1 |
| 0.5%Ru/NZ | 2.0 | 1.0 | 0.6 | 0.2 |
| 2%Ru/NZ | 2.3 | 1.3 | 0.9 | 0.1 |
| Catalytic Systems | Total Basicity (mmol/g) 100–600 °C | Weak Centers (mmol/g) 100–300 °C | Medium Centers (mmol/g) 300–450 °C | Strong Centers (mmol/g) 450–600 °C |
|---|---|---|---|---|
| NZ | 0.8 | 0.4 | 0.0 | 0.4 |
| 2%Ag/NZ | 0.5 | 0.3 | 0.1 | 0.1 |
| 2%Pt/NZ | 0.6 | 0.4 | 0.1 | 0.1 |
| 2%Pd/NZ | 0.5 | 0.4 | 0.05 | 0.05 |
| 2%Ru/NZ | 0.6 | 0.4 | 0.1 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierczynski, P.; Mosińska, M.; Szkudlarek, L.; Chalupka, K.; Tatsuzawa, M.; Al Maskari, M.; Maniukiewicz, W.; Wahono, S.K.; Vasilev, K.; Szynkowska-Jozwik, M.I. Biodiesel Production on Monometallic Pt, Pd, Ru, and Ag Catalysts Supported on Natural Zeolite. Materials 2021, 14, 48. https://doi.org/10.3390/ma14010048
Mierczynski P, Mosińska M, Szkudlarek L, Chalupka K, Tatsuzawa M, Al Maskari M, Maniukiewicz W, Wahono SK, Vasilev K, Szynkowska-Jozwik MI. Biodiesel Production on Monometallic Pt, Pd, Ru, and Ag Catalysts Supported on Natural Zeolite. Materials. 2021; 14(1):48. https://doi.org/10.3390/ma14010048
Chicago/Turabian StyleMierczynski, Pawel, Magdalena Mosińska, Lukasz Szkudlarek, Karolina Chalupka, Misa Tatsuzawa, Marwa Al Maskari, Waldemar Maniukiewicz, Satriyo K. Wahono, Krasimir Vasilev, and Malgorzata I. Szynkowska-Jozwik. 2021. "Biodiesel Production on Monometallic Pt, Pd, Ru, and Ag Catalysts Supported on Natural Zeolite" Materials 14, no. 1: 48. https://doi.org/10.3390/ma14010048
APA StyleMierczynski, P., Mosińska, M., Szkudlarek, L., Chalupka, K., Tatsuzawa, M., Al Maskari, M., Maniukiewicz, W., Wahono, S. K., Vasilev, K., & Szynkowska-Jozwik, M. I. (2021). Biodiesel Production on Monometallic Pt, Pd, Ru, and Ag Catalysts Supported on Natural Zeolite. Materials, 14(1), 48. https://doi.org/10.3390/ma14010048







