Histological and Histomorphometric Analyses of Bone Regeneration in Osteoporotic Rats Using a Xenograft Material
Abstract
1. Introduction
2. Materials and Methods
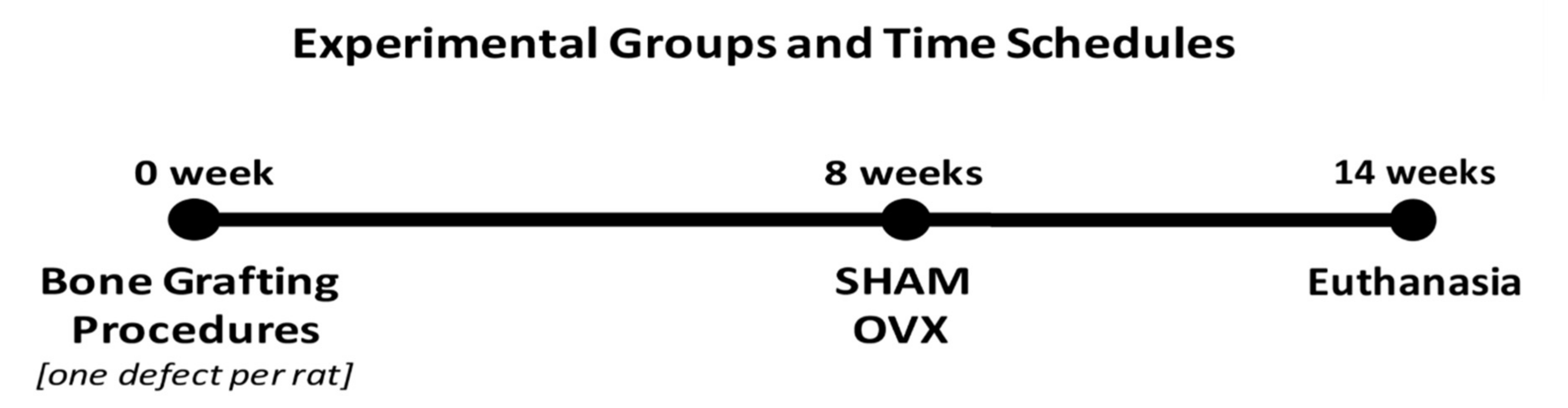
2.1. Experimental Animal Model
2.2. Sample Size Calculation
2.3. Experimental Surgical Procedures
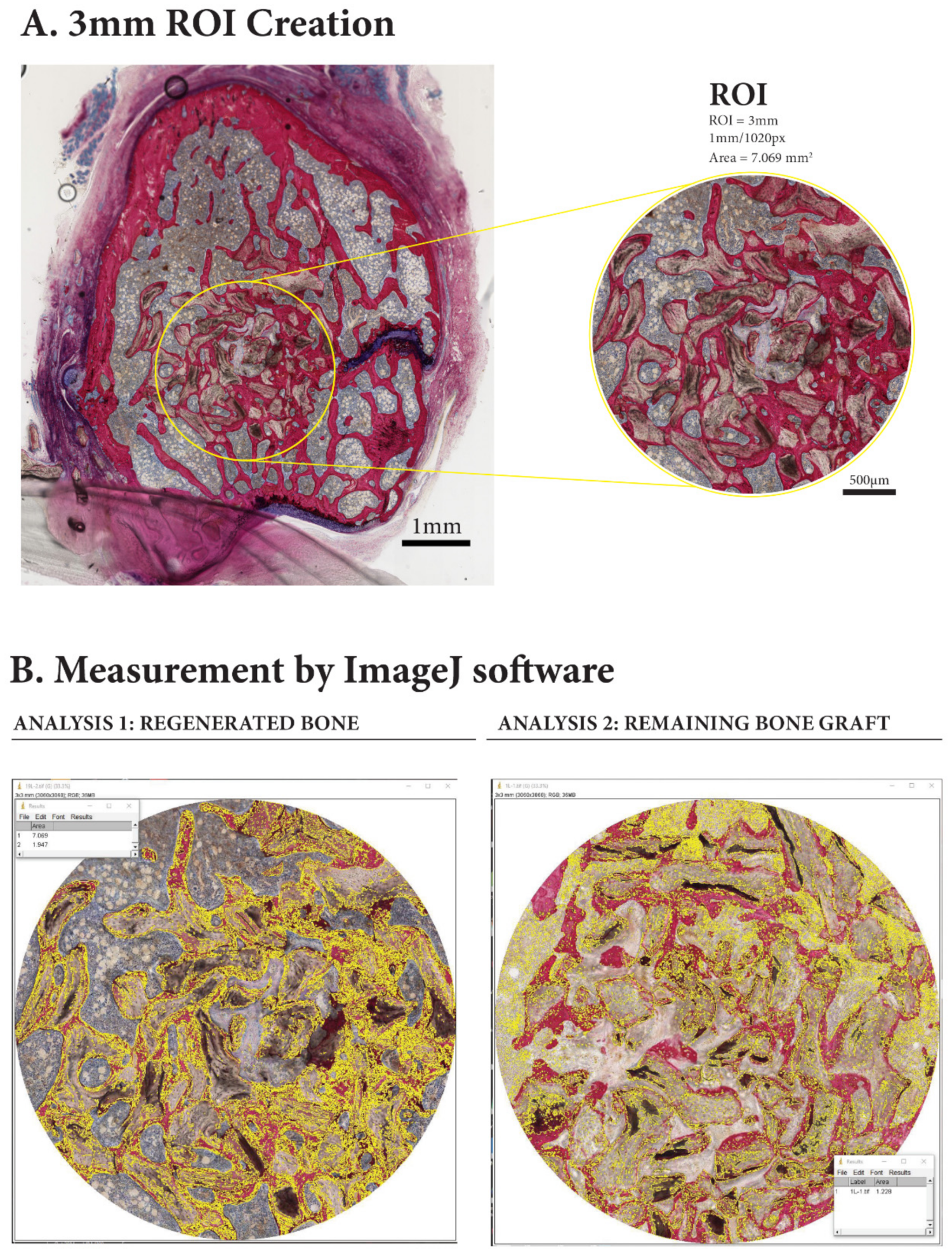
2.4. Histological Specimen Preparation and Evaluation
2.5. Statistical Analysis
3. Results
3.1. Animal Observations
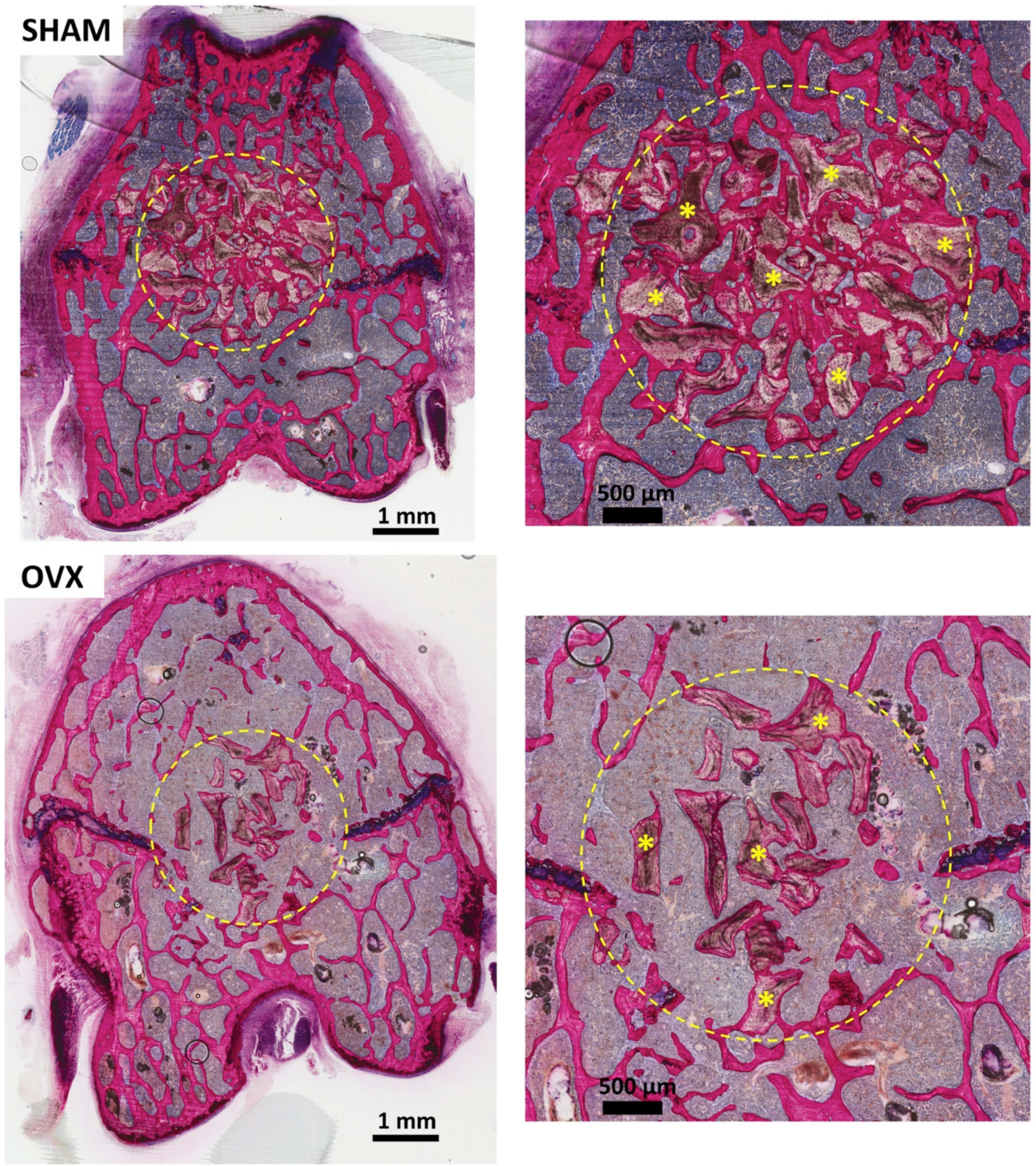
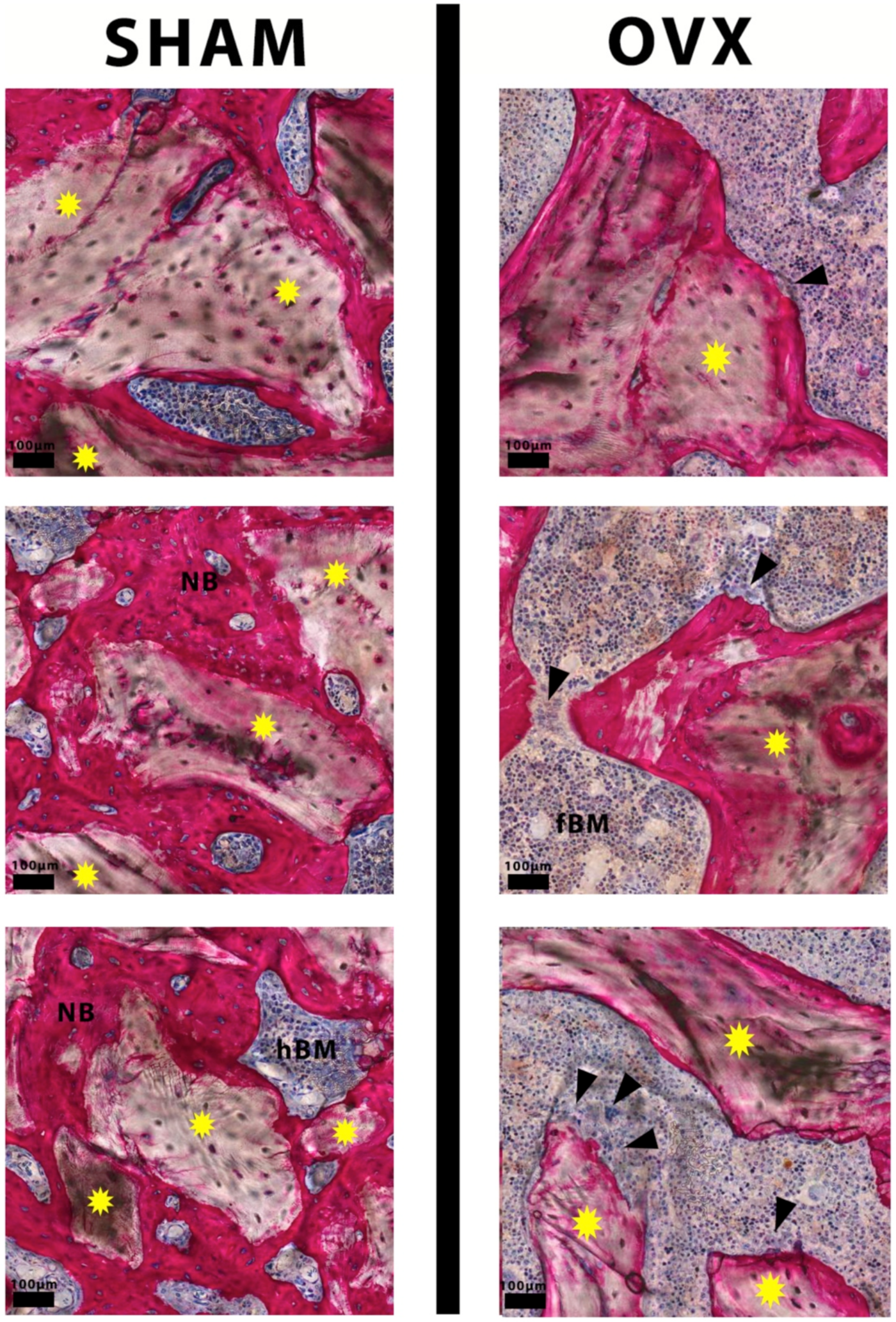
3.2. Descriptive Histological Evaluation
3.3. Histomorphometric Evaluation
4. Discussion
5. Conclusions
6. Impact Statement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunsvold, M.A.; Mellonig, J.T. Bone grafts and periodontal regeneration. Periodontol. 2000 1993, 1, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Nasr, H.F.; Aichelmann-Reidy, M.E.; Yukna, R.A. Bone and bone substitutes. Periodontol. 2000 1999, 19, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Cypher, T.J.; Grossman, J.P. Biological principles of bone graft healing. J. Foot Ankle Surg. 1996, 35, 413–417. [Google Scholar] [CrossRef]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M.L. Autogenous iliac crest bone graft. Complications and functional assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef]
- Allegrini, S., Jr.; Koening, B., Jr.; Allegrini, M.R.; Yoshimoto, M.; Gedrange, T.; Fanghaenel, J.; Lipski, M. Alveolar ridge sockets preservation with bone grafting-review. Ann. Acad. Med. Stetin 2008, 54, 70–81. [Google Scholar]
- Piattelli, M.; Favero, G.A.; Scarano, A.; Orsini, G.; Piattelli, A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: A histologic long-term report of 20 cases in humans. Int. J. Oral. Maxillofac Implant. 1999, 14, 835–840. [Google Scholar]
- Gazdag, A.R.; Lane, J.M.; Glaser, D.; Forster, R.A. Alternatives to autogenous bone graft: Efficacy and indications. Jaaos-J. Am. Acad. Orthop. Surg. 1995, 3, 1–8. [Google Scholar] [CrossRef]
- Masaki, C.; Nakamoto, T.; Mukaibo, T.; Kondo, Y.; Hosokawa, R. Strategies for alveolar ridge reconstruction and preservation for implant therapy. J. Prosthodont. Res. 2015, 59, 220–228. [Google Scholar] [CrossRef]
- Khojasteh, A.; Soheilifar, S.; Mohajerani, H.; Nowzari, H. The effectiveness of barrier membranes on bone regeneration in localized bony defects: A systematic review. Int. J. Oral. Maxillofac. Implant. 2013, 28, 1076–7089. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants-a Cochrane systematic review. Eur. J. Oral Implant. 2009, 2, 167–184. [Google Scholar]
- Aytekin, M.; Arisan, V. Alveolar Ridge Augmentation Techniques in Implant Dentistry. In Oral and Maxillofacial Surgery; IntechOpen: London, UK, 2020. [Google Scholar]
- Cicciù, M.; Fiorillo, L.; Cervino, G.; Habal, M.B. BMP Application as Grafting Materials for Bone Regeneration in the Craniofacial Surgery: Current Application and Future Directions by an RCT Analysis. J. Craniofacial Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dervis, E. Oral implications of osteoporosis. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2005, 100, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Calciolari, E.; Mardas, N.; Dereka, X.; Kostomitsopoulos, N.; Petrie, A.; Donos, N. The effect of experimental osteoporosis on bone regeneration: Part 1, histology findings. Clin. Oral. Implant. Res. 2017, 28, e101–e110. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Simmons, H.; Pirie, C.; Ke, H. FDA Guidelines and animal models for osteoporosis. Bone 1995, 17, S125–S133. [Google Scholar] [CrossRef]
- Jiupeng, D.; Mengchun, Q.; Jianping, L.; Ming, Z.; Jinyuan, L.; Yongqiang, L.; Wei, D. Effect of local treatment with Zoledronate Acid on bone healing of Bio-oss bone graft and osseointegration of dental implants in osteoporotic rats. In Proceedings of the 2011 International Conference on Human Health and Biomedical Engineering, Jilin, China, 19–22 August 2011; pp. 973–976. [Google Scholar]
- Benisch, P.; Schilling, T.; Klein-Hitpass, L.; Frey, S.N.P.; Seefried, L.; Raaijmakers, N.; Krug, M.; Regensburger, M.; Zeck, S.; Schinke, T. The transcriptional profile of mesenchymal stem cell populations in primary osteoporosis is distinct and shows overexpression of osteogenic inhibitors. PLoS ONE 2012, 7, e45142. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Osteoclasts, integrins, and osteoporosis. J. Bone Miner. Metab. 2000, 18, 344. [Google Scholar] [CrossRef]
- Jeffcoat, M. The association between osteoporosis and oral bone loss. J. Periodontol. 2005, 76, 2125–2132. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; van den Beucken, J.J.; Jansen, J.A. Osteoporotic rat models for evaluation of osseointegration of bone implants. Tissue Eng. Part. C Methods 2014, 20, 493–505. [Google Scholar] [CrossRef]
- Shaheen, M.Y.; Basudan, A.M.; de Vries, R.B.; van den Beucken, J.J.; Jansen, J.A.; Alghamdi, H.S. Bone Regeneration Using Antiosteoporotic Drugs in Adjunction with Bone Grafting: A Meta-Analysis. Tissue Eng. Part. B Rev. 2019, 25, 500–509. [Google Scholar] [CrossRef]
- Vesterinen, H.; Sena, E.; Egan, K.; Hirst, T.; Churolov, L.; Currie, G.; Antonic, A.; Howells, D.; Macleod, M. Meta-analysis of data from animal studies: A practical guide. J. Neurosci. Methods 2014, 221, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, C.I.A.; Ulrich, D.J.O.; Jansen, J.A.; van den Beucken, J. The performance of CPC/PLGA and Bio-Oss® for bone regeneration in healthy and osteoporotic rats. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Pecora, G.; Piattelli, M.; Piattelli, A. Osseointegration in a sinus augmented with bovine porous bone mineral: Histological results in an implant retrieved 4 years after insertion. A case report. J. Periodontol. 2004, 75, 1161–1166. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Karring, T. Guided tissue regeneration combined with a deproteinized bovine bone mineral (Bio-Oss®) in the treatment of intrabony periodontal defects: 6-year results from a randomized-controlled clinical trial. J. Clin. Periodontol. 2010, 37, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar]
- Durão, S.; Gomes, P.S.; Colaço, B.; Silva, J.; Fonseca, H.; Duarte, J.; Felino, A.; Fernandes, M.H. The biomaterial-mediated healing of critical size bone defects in the ovariectomized rat. Osteoporos. Int. 2014, 25, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.G.; Awartani, F.A.; Niazy, A.A.; Jansen, J.A.; Alghamdi, H.S. A Combination of Biphasic Calcium Phosphate (Maxresorb®) and Hyaluronic Acid Gel (Hyadent®) for Repairing Osseous Defects in a Rat Model. Appl. Sci. 2020, 10, 1651. [Google Scholar] [CrossRef]
- Van Houdt, C.I.; Tim, C.R.; Crovace, M.C.; Zanotto, E.D.; Peitl, O.; Ulrich, D.J.; Jansen, J.A.; Parizotto, N.A.; Renno, A.C.; van den Beucken, J.J. Bone regeneration and gene expression in bone defects under healthy and osteoporotic bone conditions using two commercially available bone graft substitutes. Biomed. Mater. 2015, 10, 035003. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Aaboe, M.; Pinholt, E.M.; Hjorting-Hansen, E.; Melsen, F.; Ruyter, I.E. Tissue reaction and material characteristics of four bone substitutes. Int. J. Oral Maxillofac Implant. 1996, 11, 55–66. [Google Scholar]
- Berglundh, T.; Lindhe, J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin. Oral Implant. Res. 1997, 8, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Pinholt, E.M.; Bang, G.; Haanaes, H.R. Alveolar ridge augmentation in rats by Bio-Oss. Eur. J. Oral Sci. 1991, 99, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Tapety, F.I.; Amizuka, N.; Uoshima, K.; Nomura, S.; Maeda, T. A histological evaluation of the involvement of Bio-Oss® in osteoblastic differentiation and matrix synthesis. Clin. Oral Implant. Res. 2004, 15, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.S. Animal models of osteoporosis-necessity and limitations. Eur Cell Mater. 2001, 1, 13. [Google Scholar]
- Sanfilippo, F.; Bianchi, A.E. Osteoporosis: The effect on maxillary bone resorption and therapeutic possibilities by means of implant prostheses-a literature review and clinical considerations. Int. J. Periodontics Restor. Dent. 2003, 23, 447–457. [Google Scholar] [CrossRef]
- Blomqvist, J.E.; Alberius, P.; Isaksson, S.; Linde, A.; Hansson, B.-G.R. Factors in implant integration failure after bone grafting: An osteometric and endocrinologic matched analysis. Int. J. Oral Maxillofac. Surg. 1996, 25, 63–68. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, H.K.; Härkönen, P.L. Estrogen and bone metabolism. Maturitas 1996, 23, S65–S69. [Google Scholar] [CrossRef]
- Teófilo, J.M.; Brentegani, L.G.; Lamano-Carvalho, T.L. Bone healing in osteoporotic female rats following intra-alveolar grafting of bioactive glass. Arch. Oral Biol. 2004, 49, 755–762. [Google Scholar] [CrossRef]
- Luize, D.S.; Bosco, A.F.; Bonfante, S.; de Almeida, J.M. Influence of ovariectomy on healing of autogenous bone block grafts in the mandible: A histomorphometric study in an aged rat model. Int. J. Oral. Maxillofac. Implant. 2008, 23, 207–214. [Google Scholar]
- Hayashi, K.; Uenoyama, K.; Matsuguchi, N.; Nakagawa, S.; Sugioka, Y. The affinity of bone to hydroxyapatite and alumina in experimentally induced osteoporosis. J. Arthroplast. 1989, 4, 257–262. [Google Scholar] [CrossRef]
- Hayashi, K.; Uenoyama, K.; Mashima, T.; Sugioka, Y. Remodelling of bone around hydroxyapatite and titanium in experimental osteoporosis. Biomaterials 1994, 15, 11–16. [Google Scholar] [CrossRef]
- Fuegl, A.; Tangl, S.; Keibl, C.; Watzek, G.; Redl, H.; Gruber, R. The impact of ovariectomy and hyperglycemia on graft consolidation in rat calvaria. Clin. Oral. Implant. Res. 2011, 22, 524–529. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-X.; Zhang, G.; Pan, X.-H.; Liu, Z.; Zheng, L.-Z.; Chan, C.-W.; Lee, K.-M.; Cao, Y.-P.; Li, G.; Wei, L. Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: A drill-hole defect model. Bone 2011, 48, 1388–1400. [Google Scholar] [CrossRef]
- Rolvien, T.; Barbeck, M.; Wenisch, S.; Amling, M.; Krause, M. Cellular mechanisms responsible for success and failure of bone substitute materials. Int. J. Mol. Sci. 2018, 19, 2893. [Google Scholar] [CrossRef] [PubMed]
- Shiwaku, Y.; Neff, L.; Nagano, K.; Takeyama, K.-I.; de Bruijn, J.; Dard, M.; Gori, F.; Baron, R. The crosstalk between osteoclasts and osteoblasts is dependent upon the composition and structure of biphasic calcium phosphates. PLoS ONE 2015, 10, e0132903. [Google Scholar] [CrossRef]
- Westhauser, F.; Essers, C.; Karadjian, M.; Reible, B.; Schmidmaier, G.; Hagmann, S.b.; Moghaddam, A. Supplementation with 45S5 Bioactive Glass Reduces In Vivo Resorption of the β-Tricalcium-Phosphate-Based Bone Substitute Material Vitoss. Int. J. Mol. Sci. 2019, 20, 4253. [Google Scholar] [CrossRef] [PubMed]
- Oddie, G.; Schenk, G.; Angel, N.; Walsh, N.; Guddat, L.; De Jersey, J.; Cassady, A.; Hamilton, S.; Hume, D. Structure, function, and regulation of tartrate-resistant acid phosphatase. Bone 2000, 27, 575–584. [Google Scholar] [CrossRef]
- Simon, F.; Oberhuber, A.; Schelzig, H. Advantages and Disadvantages of Different Animal Models for Studying Ischemia/Reperfusion Injury of the Spinal Cord. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 744. [Google Scholar] [CrossRef]

| Study Groups | Material | Number of Animals (n) |
|---|---|---|
| SHAM | xenograft material (anorganic cancellous bone graft granules) InterOss® | n = 7 |
| OVX | xenograft material (anorganic cancellous bone graft granules) InterOss® | n = 8 |
| SHAM (n = 7) | OVX (n = 8) | |
|---|---|---|
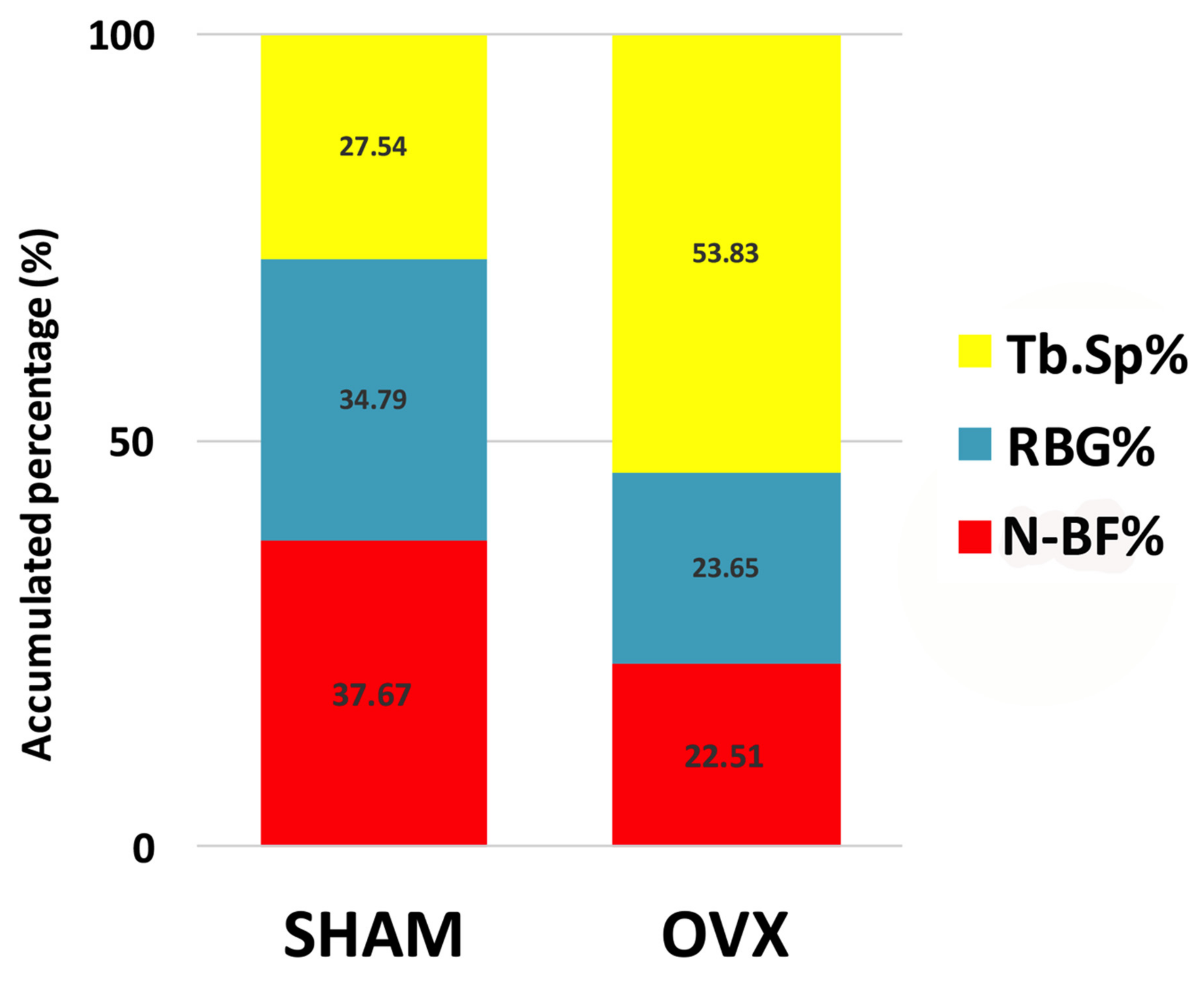
| New bone formation (N-BF%) | 37.7 ± 7.9 * | 22.5 ± 3.0 |
| Remaining bone graft (RBG%) | 34.8 ± 9.6 * | 23.7 ± 5.8 |
| Trabecular bone space (Tb.Sp%) | 27.5 ± 14.3 * | 53.8 ± 7.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaheen, M.Y.; Basudan, A.M.; Niazy, A.A.; van den Beucken, J.J.J.P.; Jansen, J.A.; Alghamdi, H.S. Histological and Histomorphometric Analyses of Bone Regeneration in Osteoporotic Rats Using a Xenograft Material. Materials 2021, 14, 222. https://doi.org/10.3390/ma14010222
Shaheen MY, Basudan AM, Niazy AA, van den Beucken JJJP, Jansen JA, Alghamdi HS. Histological and Histomorphometric Analyses of Bone Regeneration in Osteoporotic Rats Using a Xenograft Material. Materials. 2021; 14(1):222. https://doi.org/10.3390/ma14010222
Chicago/Turabian StyleShaheen, Marwa Y., Amani M. Basudan, Abdurahman A. Niazy, Jeroen J. J. P. van den Beucken, John A. Jansen, and Hamdan S. Alghamdi. 2021. "Histological and Histomorphometric Analyses of Bone Regeneration in Osteoporotic Rats Using a Xenograft Material" Materials 14, no. 1: 222. https://doi.org/10.3390/ma14010222
APA StyleShaheen, M. Y., Basudan, A. M., Niazy, A. A., van den Beucken, J. J. J. P., Jansen, J. A., & Alghamdi, H. S. (2021). Histological and Histomorphometric Analyses of Bone Regeneration in Osteoporotic Rats Using a Xenograft Material. Materials, 14(1), 222. https://doi.org/10.3390/ma14010222








