Preliminary Characterization of Glass/Alumina Composite Using Laser Powder Bed Fusion (L-PBF) Additive Manufacturing
Abstract
1. Introduction
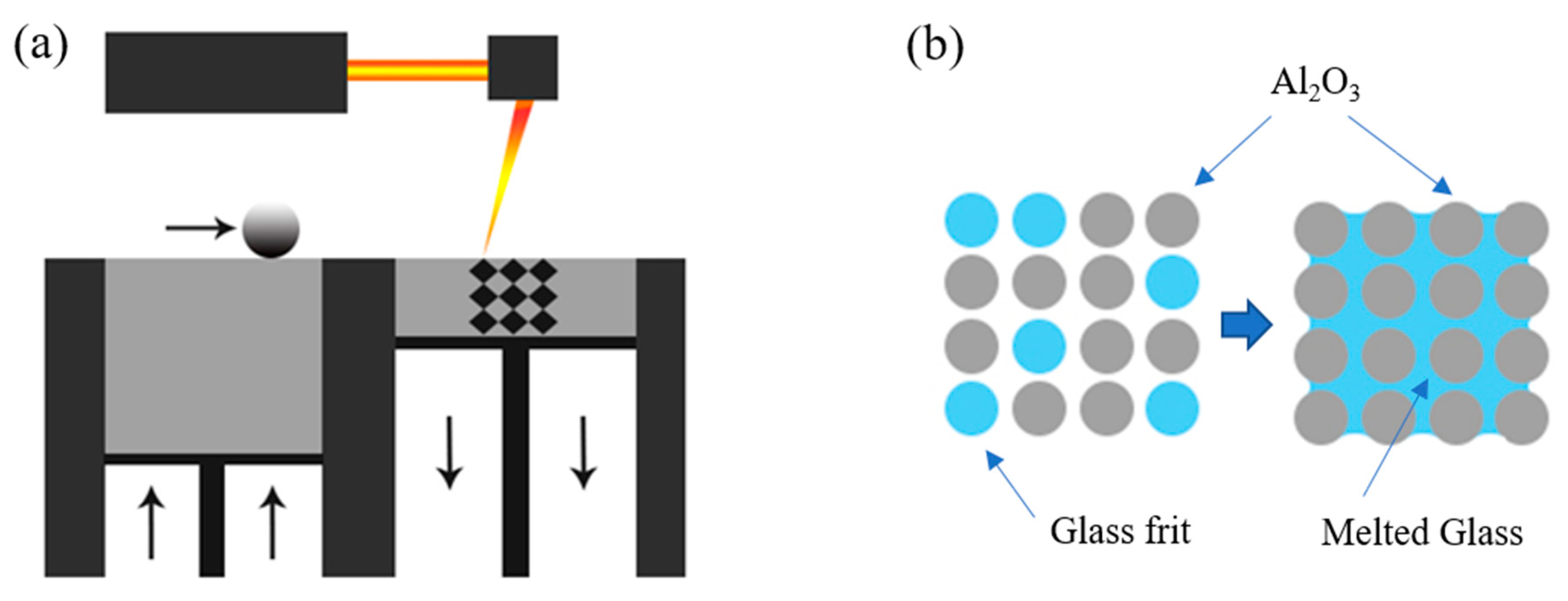
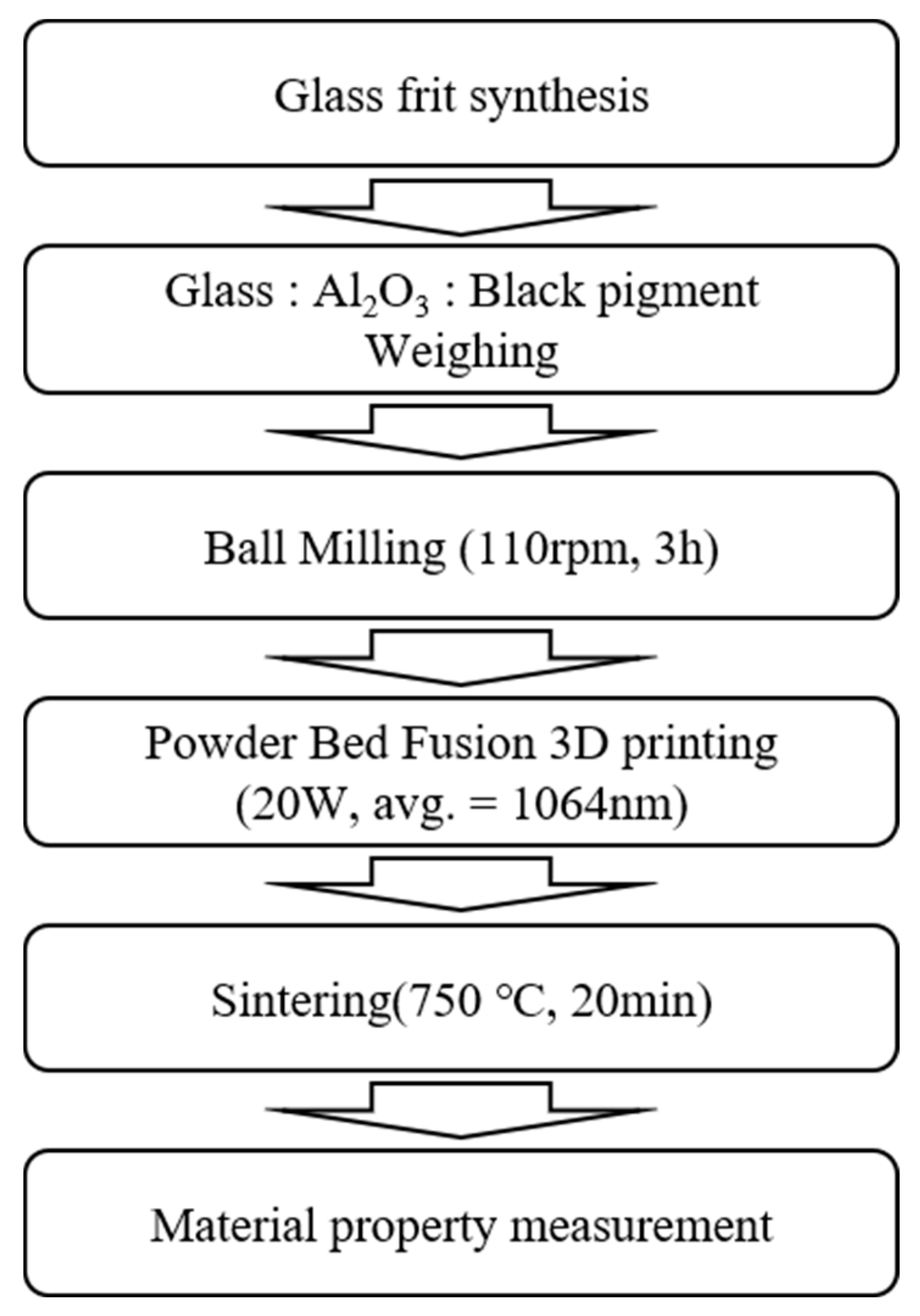
2. Materials and Methods
3. Results and Discussion
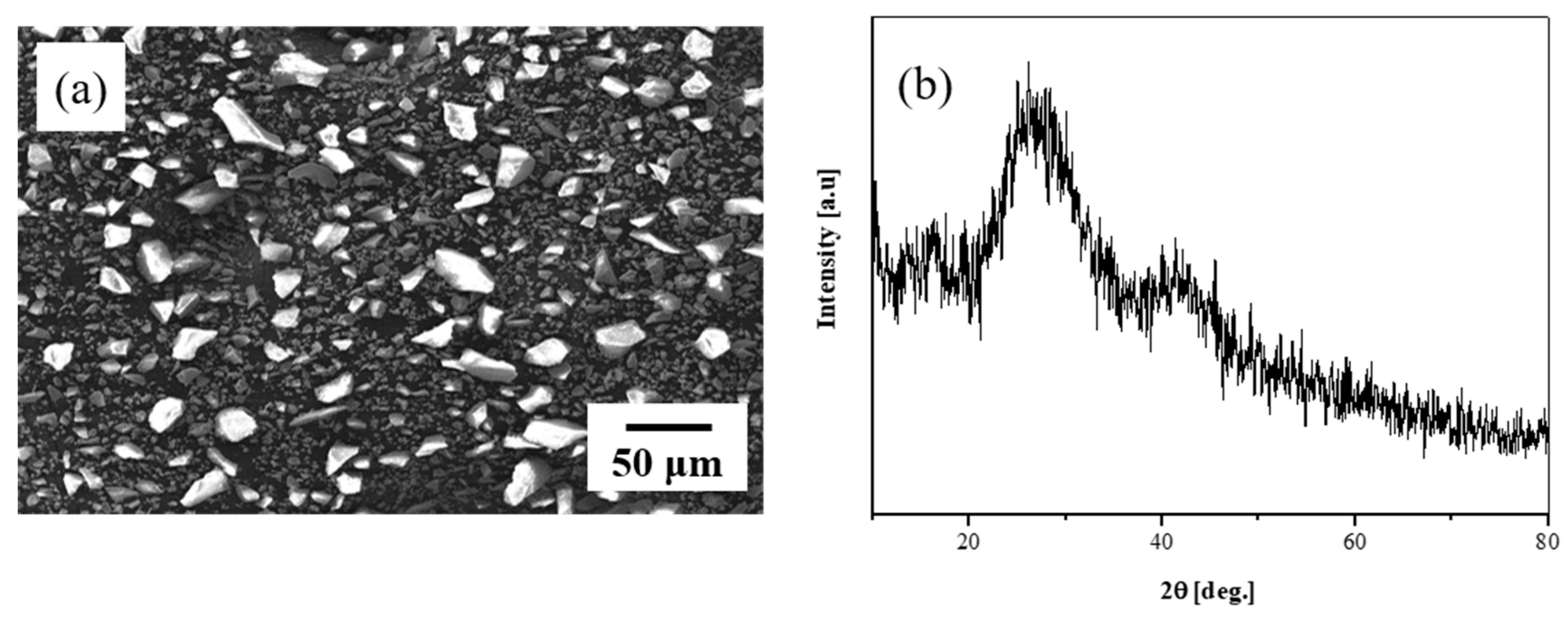
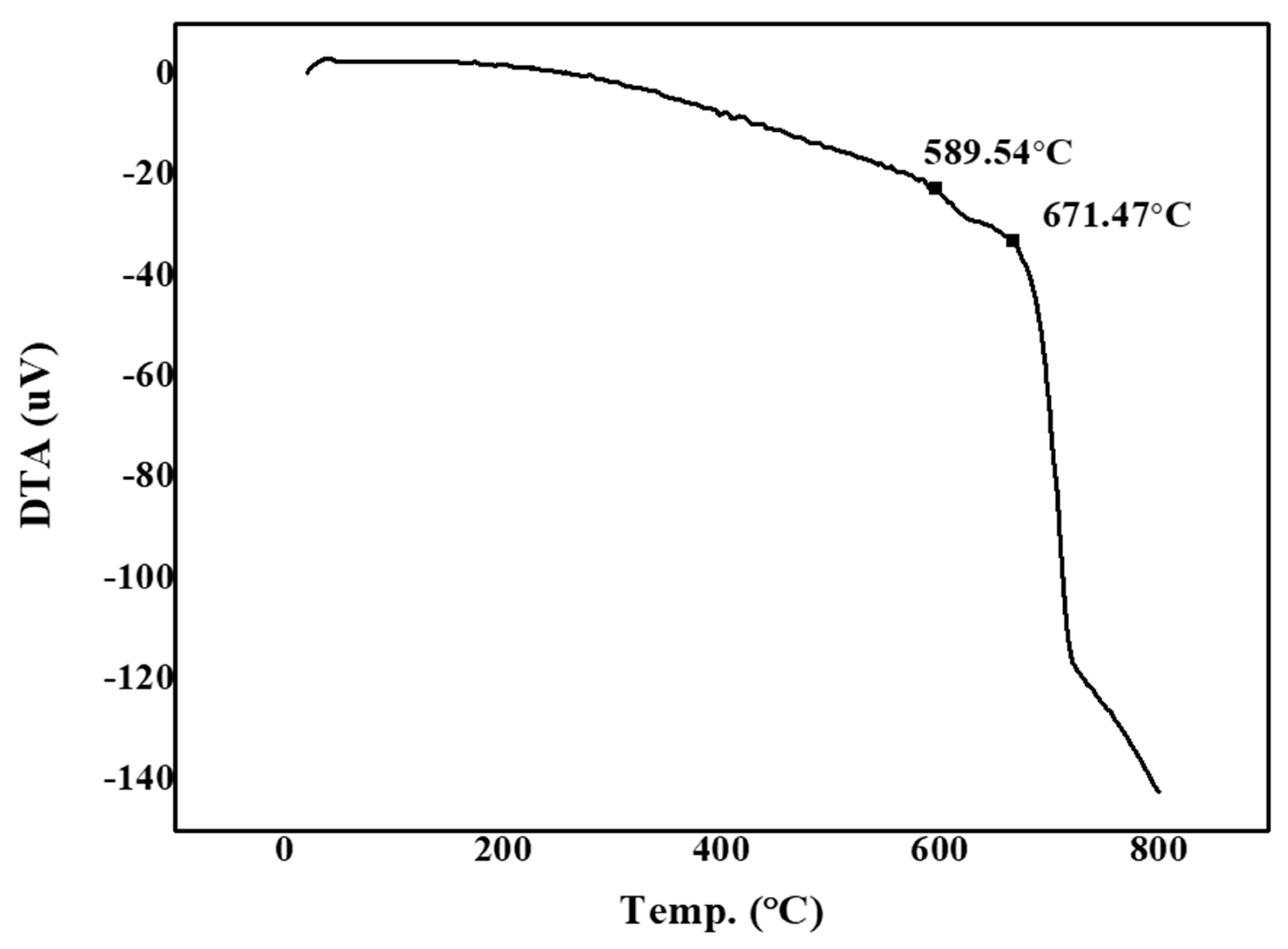
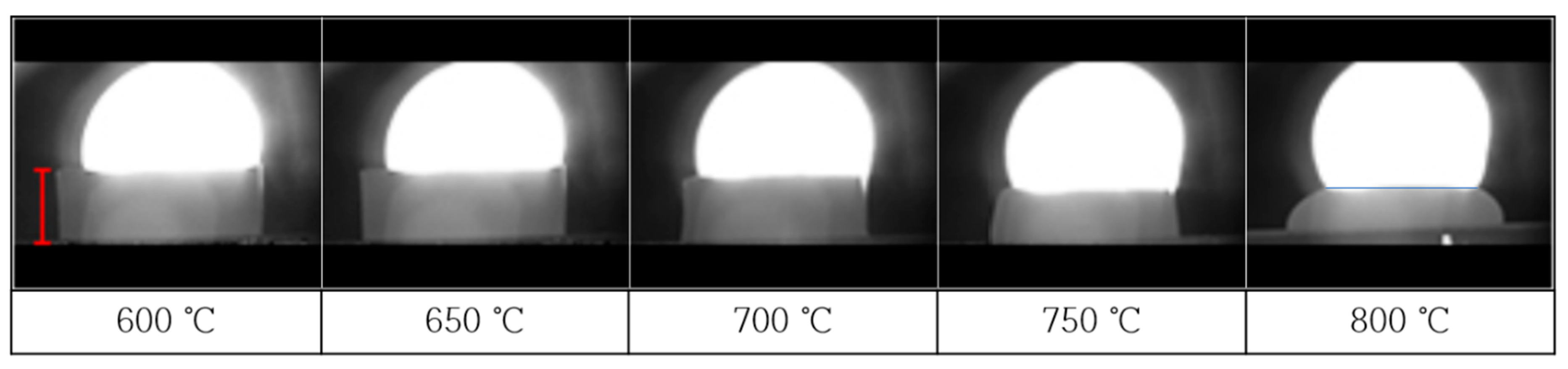
3.1. Results of Glass Sintering at 750 °C
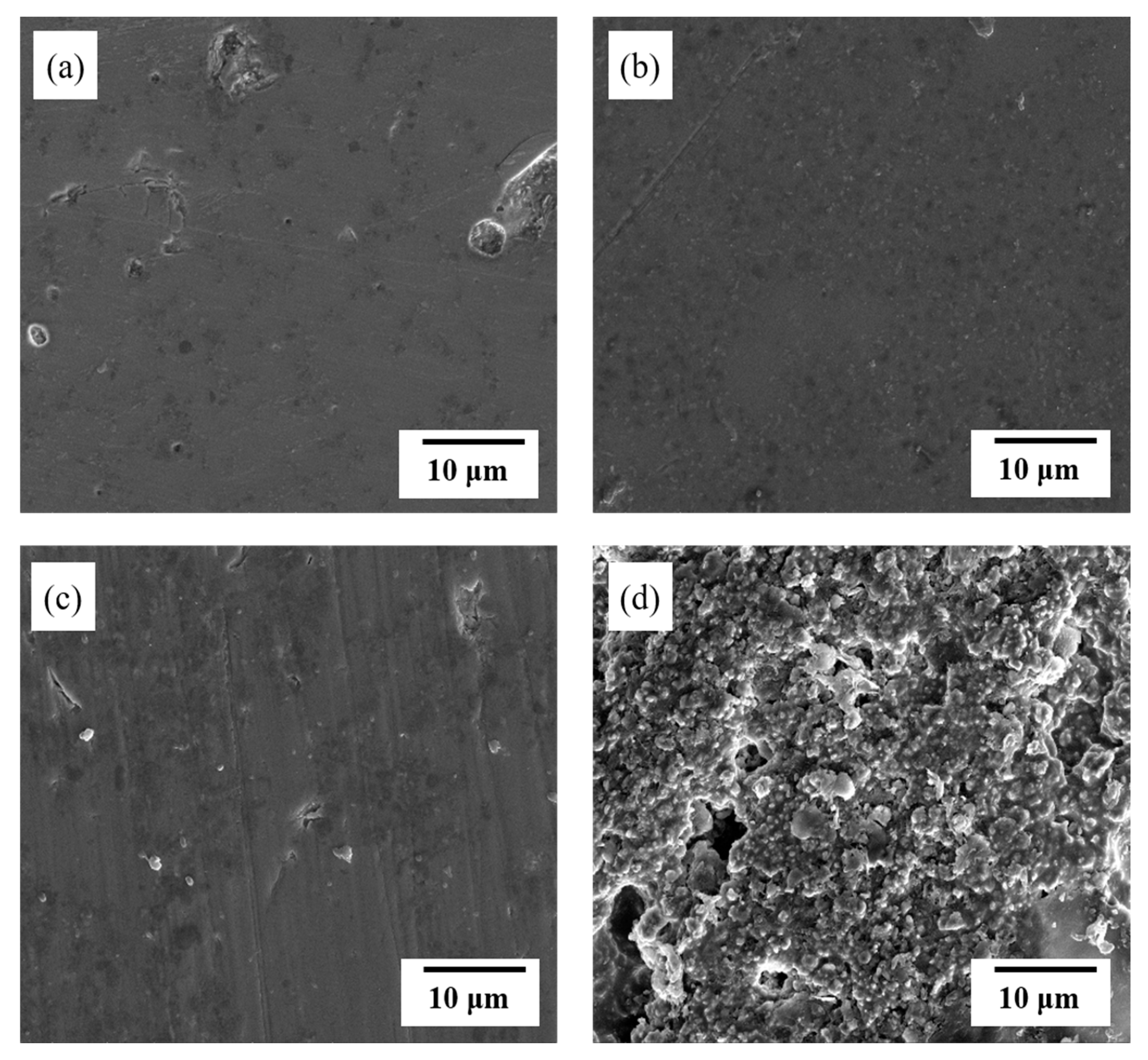
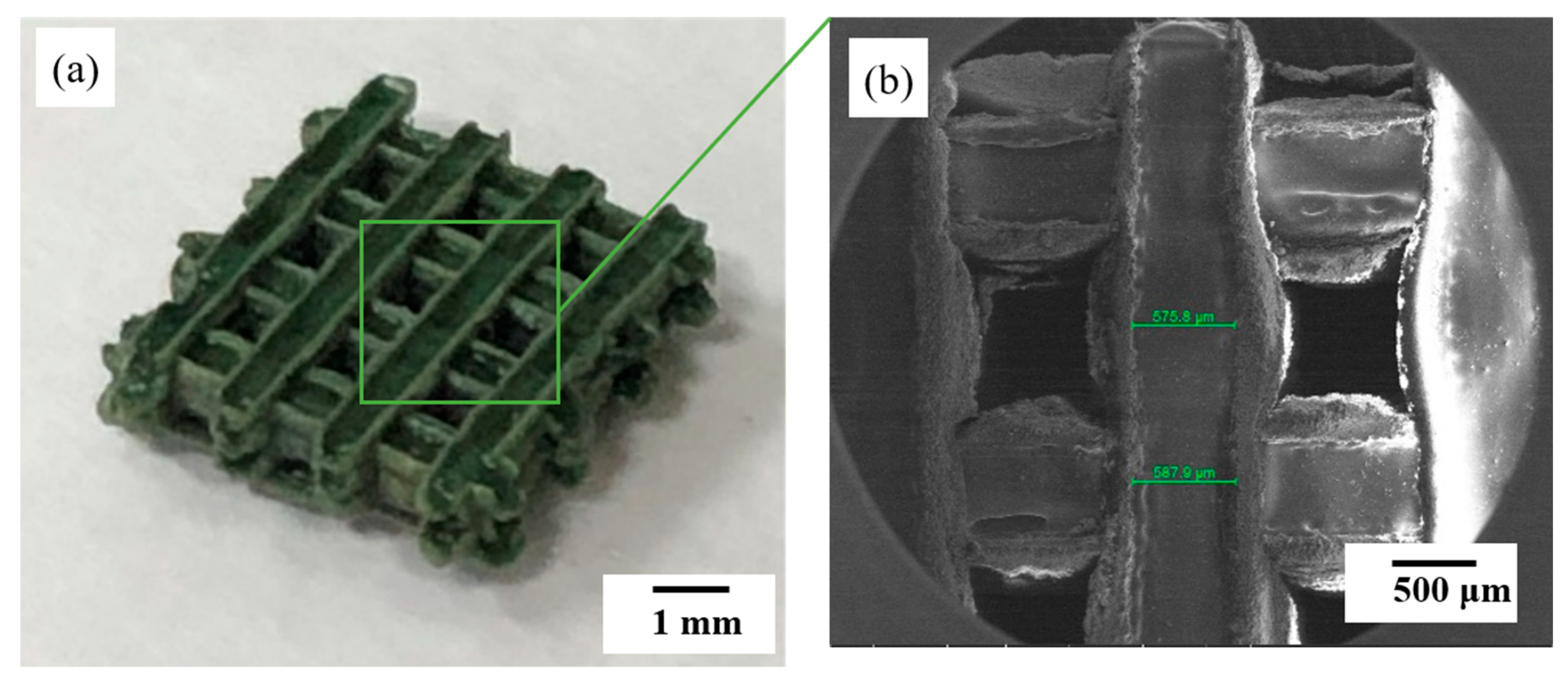
3.2. Mixing Ratio of Fabricated Glass/Al2O3 Composite and Mechanical-Property Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Graf, D.; Burchard, S.; Crespo, J.; Megnin, C.; Gutsch, S.; Zacharias, M.; Hanemann, T. Influence of Al2O3 nanoparticle addition on a uv cured polyacrylate for 3d inkjet printing. Polymers 2019, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.; Cheon, K.-H.; Park, C.; Jang, T.-S.; Kim, H.-E.; Jung, H.-D. Fabrication of poly (lactic acid)/Ti composite scaffolds with enhanced mechanical properties and biocompatibility via fused filament fabrication (fff)–based 3d printing. Addit. Manuf. 2019, 30, 100883. [Google Scholar] [CrossRef]
- Jung, H.-D.; Jang, T.-S.; Lee, J.E.; Park, S.J.; Son, Y.; Park, S.-H. Enhanced bioactivity of titanium-coated polyetheretherketone implants created by a high-temperature 3d printing process. Biofabrication 2019, 11, 045014. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, E.; Iuliano, L.; Minetola, P.; Salmi, A. Redesign and cost estimation of rapid manufactured plastic parts. Rapid Prototyp. J. 2010. [Google Scholar] [CrossRef]
- Atzeni, E.; Salmi, A. Economics of additive manufacturing for end-usable metal parts. Int. J. Adv. Manuf. Technol. 2012, 62, 1147–1155. [Google Scholar] [CrossRef]
- Kotz, F.; Arnold, K.; Bauer, W.; Schild, D.; Keller, N.; Sachsenheimer, K.; Nargang, T.M.; Richter, C.; Helmer, D.; Rapp, B.E. Three-dimensional printing of transparent fused silica glass. Nature 2017, 544, 337–339. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3d printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Okada, A. Automotive and industrial applications of structural ceramics in japan. J. Eur. Ceram. Soc. 2008, 28, 1097–1104. [Google Scholar] [CrossRef]
- Rödel, J.; Kounga, A.B.; Weissenberger-Eibl, M.; Koch, D.; Bierwisch, A.; Rossner, W.; Hoffmann, M.J.; Danzer, R.; Schneider, G. Development of a roadmap for advanced ceramics: 2010–2025. J. Eur. Ceram. Soc. 2009, 29, 1549–1560. [Google Scholar] [CrossRef]
- Baek, J.; Lee, H.; Jang, T.-S.; Song, J.; Kim, H.-E.; Jung, H.-D. Incorporation of calcium sulfate dihydrate into hydroxyapatite microspheres to improve the release of bone morphogenetic protein-2 and accelerate bone regeneration. ACS Biomater. Sci. Eng. 2018, 4, 846–856. [Google Scholar] [CrossRef]
- Klein, J.; Stern, M.; Franchin, G.; Kayser, M.; Inamura, C.; Dave, S.; Weaver, J.C.; Houk, P.; Colombo, P.; Yang, M. Additive manufacturing of optically transparent glass. 3D Print. Addit. Manuf. 2015, 2, 92–105. [Google Scholar] [CrossRef]
- Luo, J.; Pan, H.; Kinzel, E.C. Additive manufacturing of glass. J. Manuf. Sci. Eng. 2014, 136. [Google Scholar] [CrossRef]
- Luo, J.; Bender, T.; Bristow, D.A.; Landers, R.G.; Goldstein, J.T.; Urbas, A.M.; Kinzel, E.C. Bubble Formation in Additive Manufacturing of Borosilicate Glass, Proceedings of the 2016 Annual International Solid Freeform Fabrication Symposium, Austin, TX, USA, 8–10 August 2016; University of Texas: Austin, TX, USA, 2016; pp. 998–1003. [Google Scholar]
- Luo, J.; Gilbert, L.J.; Qu, C.; Landers, R.G.; Bristow, D.A.; Kinzel, E.C. Additive manufacturing of transparent soda-lime glass using a filament-fed process. J. Manuf. Sci. Eng. 2017, 139. [Google Scholar] [CrossRef]
- Luo, J.; Gilbert, L.J.; Bristow, D.A.; Landers, R.G.; Goldstein, J.T.; Urbas, A.M.; Kinzel, E.C. Additive manufacturing of glass for optical applications. In Laser 3D Manufacturing III; International Society for Optics and Photonics: Bellingham, WA, USA, 2016; p. 97380Y. [Google Scholar]
- Schmid, C. The Chemistry of Inkjet Inks; World Scientific Publishing: Hackensack, NJ, USA, 2008; p. 123. [Google Scholar]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3d printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Dorfinger, P.; Stampfl, J.; Liska, R. Toughening of photopolymers for stereolithography (sl). In Materials Science Forum; Trans Tech Publ: Schwyz, Switzerland, 2015; pp. 53–59. [Google Scholar]
- Nguyen, D.T.; Meyers, C.; Yee, T.D.; Dudukovic, N.A.; Destino, J.F.; Zhu, C.; Duoss, E.B.; Baumann, T.F.; Suratwala, T.; Smay, J.E. 3d-printed transparent glass. Adv. Mater. 2017, 29, 1701181. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, C.; Chou, T.-W. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Johnsen, B.; Kinloch, A.; Mohammed, R.; Taylor, A.; Sprenger, S. Toughening mechanisms of nanoparticle-modified epoxy polymers. Polymer 2007, 48, 530–541. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, W.; Wu, H.; Huang, R.; He, R.; Jiang, Q.; Chen, Y.; Ji, X.; Tian, Z.; Wu, S. Research into the mechanical properties, sintering mechanism and microstructure evolution of Al2O3-ZrO2 composites fabricated by a stereolithography-based 3d printing method. Mater. Chem. Phys. 2018, 207, 1–10. [Google Scholar] [CrossRef]
- Santoliquido, O.; Colombo, P.; Ortona, A. Additive manufacturing of ceramic components by digital light processing: A comparison between the “bottom-up” and the “top-down” approaches. J. Eur. Ceram. Soc. 2019, 39, 2140–2148. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, W.; Wu, H.; Song, X.; Chen, Y.; Cheng, L.; He, F.; Chen, S.; Wu, S. Preparation of a defect-free alumina cutting tool via additive manufacturing based on stereolithography–optimization of the drying and debinding processes. Ceram. Int. 2016, 42, 11598–11602. [Google Scholar] [CrossRef]
- Crosby, A.J.; Lee, J.Y. Polymer nanocomposites: The “nano” effect on mechanical properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Ash, B.J.; Siegel, R.W.; Schadler, L.S. Mechanical behavior of alumina/poly (methyl methacrylate) nanocomposites. Macromolecules 2004, 37, 1358–1369. [Google Scholar] [CrossRef]
- Khmyrov, R.; Grigoriev, S.; Okunkova, A.; Gusarov, A. On the possibility of selective laser melting of quartz glass. Phys. Procedia 2014, 56, 345–356. [Google Scholar] [CrossRef]
- Khmyrov, R.; Protasov, C.; Grigoriev, S.; Gusarov, A. Crack-free selective laser melting of silica glass: Single beads and monolayers on the substrate of the same material. Int. J. Adv. Manuf. Technol. 2016, 85, 1461–1469. [Google Scholar] [CrossRef]
- Datsiou, K.C.; Saleh, E.; Spirrett, F.; Goodridge, R.; Ashcroft, I.; Eustice, D. Additive manufacturing of glass with laser powder bed fusion. J. Am. Ceram. Soc. 2019, 102, 4410–4414. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef]
- Wilkes, J.; Hagedorn, Y.C.; Meiners, W.; Wissenbach, K. Additive manufacturing of ZrO2-Al2O3 ceramic components by selective laser melting. Rapid Prototyp. J. 2013. [Google Scholar] [CrossRef]
- Awasthi, S.; Maurya, R.; Pandey, C.P.; Balani, K. Interfacial mechanics of carbonaceous reinforcements in electrophoretically deposited nickel coatings. Surf. Coat. Technol. 2017, 310, 79–86. [Google Scholar] [CrossRef]
- Asano, K. Preparation of alumina fiber-reinforced aluminum by squeeze casting and their machinability. Mater. Manuf. Process. 2015, 30, 1312–1316. [Google Scholar] [CrossRef]


| Classification | Unit | Value |
|---|---|---|
| Transition Temperature | °C | 589 |
| Softening Temperature | °C | 671 |
| Particle Size (d50) | µm | 15 |
| Particle Size (dmax) | µm | 50 |
| Al2O3 Content (wt % (vol %)) | Theoretical Density of Composite (g/cm3) | Experimental Density (% of Theoretical Density) |
|---|---|---|
| 10 (08) | 3.28 | 94.8 |
| 20 (17) | 3.35 | 92.5 |
| 30 (25) | 3.42 | 95.3 |
| 40 (34) | 3.49 | 85.1 |
| 50 (44) | 3.56 | 60.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, B.H.; Lee, J.W.; Cha, J.M.; Kim, I.-W.; Jung, H.-D.; Yoon, C.-B. Preliminary Characterization of Glass/Alumina Composite Using Laser Powder Bed Fusion (L-PBF) Additive Manufacturing. Materials 2020, 13, 2156. https://doi.org/10.3390/ma13092156
Bae BH, Lee JW, Cha JM, Kim I-W, Jung H-D, Yoon C-B. Preliminary Characterization of Glass/Alumina Composite Using Laser Powder Bed Fusion (L-PBF) Additive Manufacturing. Materials. 2020; 13(9):2156. https://doi.org/10.3390/ma13092156
Chicago/Turabian StyleBae, Byeong Hoon, Jeong Woo Lee, Jae Min Cha, Il-Won Kim, Hyun-Do Jung, and Chang-Bun Yoon. 2020. "Preliminary Characterization of Glass/Alumina Composite Using Laser Powder Bed Fusion (L-PBF) Additive Manufacturing" Materials 13, no. 9: 2156. https://doi.org/10.3390/ma13092156
APA StyleBae, B. H., Lee, J. W., Cha, J. M., Kim, I.-W., Jung, H.-D., & Yoon, C.-B. (2020). Preliminary Characterization of Glass/Alumina Composite Using Laser Powder Bed Fusion (L-PBF) Additive Manufacturing. Materials, 13(9), 2156. https://doi.org/10.3390/ma13092156






