Predicting the Thermodynamic Ideal Glass Transition Temperature in Glass-Forming Liquids
Abstract
1. Introduction
2. Expressions of Predicting TK
3. Methods
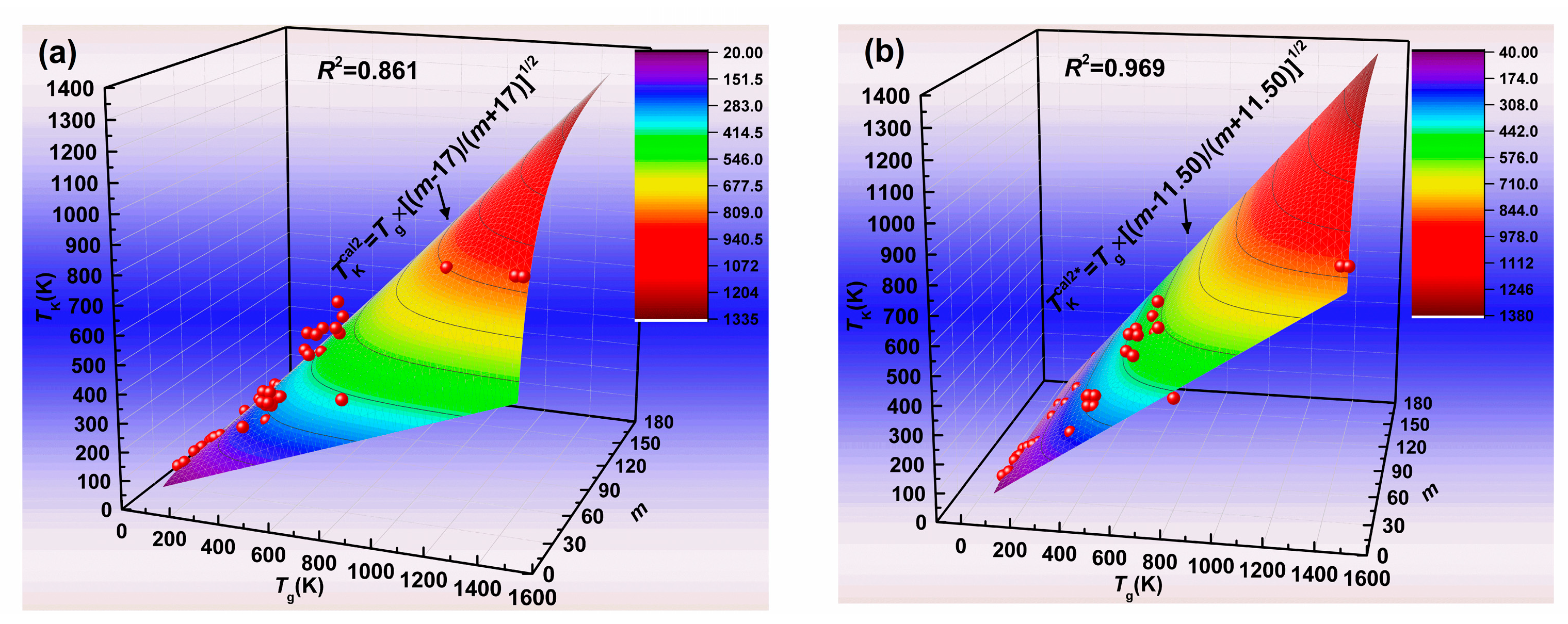
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fiore, G.; Ichim, I.; Battezzati, L. Thermal analysis, fragility and viscosity of Au-based metallic glasses. J. Non-Cryst. Solids 2010, 356, 2218–2222. [Google Scholar] [CrossRef]
- Glade, S.C.; Johnson, W.L. Viscous flow of the Cu47Ti34Zr11Ni8 glass forming alloy. J. Appl. Phys. 2000, 87, 7249–7251. [Google Scholar] [CrossRef]
- Kawamura, Y.; Inoue, A. Newtonian viscosity of supercooled liquid in a Pd40Ni40P20 metallic glass. Appl. Phys. Lett. 2000, 77, 1114–1116. [Google Scholar] [CrossRef]
- Busch, R.; Liu, W.; Johnson, W.L. Thermodynamics and kinetics of the Mg65Cu25Y10 bulk metallic glass forming liquid. J. Appl. Phys. 1998, 83, 4134–4141. [Google Scholar] [CrossRef]
- Debenedetti, P.G.; Stillinger, F.H. Supercooled liquids and the glass transition. Nature 2001, 410, 259–267. [Google Scholar] [CrossRef]
- Tanaka, H. Relation between thermodynamics and kinetics of glass-forming liquids. Phys. Rev. Lett. 2003, 90, 055701. [Google Scholar] [CrossRef] [PubMed]
- Kauzmann, W. The Nature of the Glassy State and the Behavior of Liquids at Low Temperatures. Chem. Rev. 1948, 43, 219–256. [Google Scholar] [CrossRef]
- Okamoto, P.R.; Lam, N.Q.; Rehn, L.E. Physics of Crystal-to-Glass Transformations. Solid State Phys. 1998, 52, 1–135. [Google Scholar] [CrossRef]
- Wilde, G.; Görler, G.P.; Willnecker, R.; Fecht, H.J. Calorimetric, thermomechanical, and rheological characterizations of bulk glass-forming Pd40Ni40P20. J. Appl. Phys. 2000, 87, 1141–1152. [Google Scholar] [CrossRef]
- Lu, Z.P.; Li, Y.; Liu, C.T. Glass-forming tendency of bulk La–Al–Ni–Cu–(Co) metallic glass-forming liquids. J. Appl. Phys. 2003, 93, 286–290. [Google Scholar] [CrossRef]
- Fan, G.J.; Löffler, J.F.; Wunderlich, R.K.; Fecht, H.J. Thermodynamics, enthalpy relaxation and fragility of the bulk metallic glass-forming liquid Pd43Ni10Cu27P20. Acta Mater. 2004, 52, 667–674. [Google Scholar] [CrossRef]
- Fan, G.J.; Choo, H.; Liaw, P.K. Fragility of metallic glass-forming liquids: A simple thermodynamic connection. J. Non-Cryst. Solids 2005, 351, 3879–3883. [Google Scholar] [CrossRef]
- Tanaka, H. Relationship among glass-forming ability, fragility, and short-range bond ordering of liquids. J. Non-Cryst. Solids 2005, 351, 678–690. [Google Scholar] [CrossRef]
- Jiang, Q.K.; Wang, X.D.; Nie, X.P.; Zhang, G.Q.; Ma, H.; Fecht, H.J.; Bendnarcik, J.; Franz, H.; Liu, Y.G.; Cao, Q.P.; et al. Zr–(Cu,Ag)–Al bulk metallic glasses. Acta Mater. 2008, 56, 1785–1796. [Google Scholar] [CrossRef]
- Gallino, I.; Schroers, J.; Busch, R. Kinetic and thermodynamic studies of the fragility of bulk metallic glass forming liquids. J. Appl. Phys. 2010, 108, 063501. [Google Scholar] [CrossRef]
- Fontana, G.D.; Battezzati, L. Thermodynamic and dynamic fragility in metallic glass-formers. Acta Mater. 2013, 61, 2260–2267. [Google Scholar] [CrossRef]
- Ruocco, G.; Sciortino, F.; Zamponi, F.; De Michele, C.; Scopigno, T. Landscapes and fragilities. J. Chem. Phys. 2004, 120, 10666–10680. [Google Scholar] [CrossRef]
- Battezzati, L.; Castellero, A.; Rizzi, P. On the glass transition in metallic melts. J. Non-Cryst. Solids 2007, 353, 3318–3326. [Google Scholar] [CrossRef]
- Li, P.; Wang, G.; Ding, D.; Shen, J. Glass forming ability and thermodynamics of new Ti-Cu-Ni-Zr bulk metallic glasses. J. Non-Cryst. Solids 2012, 358, 3200–3204. [Google Scholar] [CrossRef]
- Böhmer, R.; Ngai, K.L.; Angell, C.A.; Plazek, D.J. Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. 1993, 99, 4201–4209. [Google Scholar] [CrossRef]
- Venkataraman, S.; Biswas, K.; Wei, B.C.; Sordelet, D.J.; Eckert, J. On the fragility of Cu47Ti33Zr11Ni8Si1metallic glass. J. Phys. D Appl. Phys. 2006, 39, 2600–2608. [Google Scholar] [CrossRef]
- Meng, Q.G.; Zhang, S.G.; Li, J.G.; Bian, X.F. Strong liquid behavior of Pr55Ni25Al20 bulk metallic glass. J. Alloys Compd. 2007, 431, 191–196. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Zhang, Z.; Wen, P.; Pan, M.X.; Zhao, D.Q.; Wang, W.H.; Wang, W.L. A highly glass-forming alloy with low glass transition temperature. Appl. Phys. Lett. 2003, 82, 4699–4701. [Google Scholar] [CrossRef]
- Waniuk, T.A.; Busch, R.; Masuhr, A.; Johnson, W.L. Equilibrium viscosity of the Zr41.2Ti13.8Cu12.5Ni10Be22.5 bulk metallic glass-forming liquid and viscous flow during relaxation, phase separation, and primary crystallization. Acta Mater. 1998, 46, 5229–5236. [Google Scholar] [CrossRef]
- Fontana, G.D.; Castellero, A.; Battezzati, L. Thermodynamics and fragility of Fe-based glass forming melts. J. Non-Cryst. Solids 2016, 433, 103–108. [Google Scholar] [CrossRef]
- Busch, R.; Bakke, E.; Johnson, W.L. Viscosity of the supercooled liquid and relaxation at the glass transition of the Zr46.75Ti8.25Cu7.5Ni10Be27.5 bulk metallic glass forming alloy. Acta Mater. 1998, 46, 4725–4732. [Google Scholar] [CrossRef]
- Wang, L.M.; Angell, C.A.; Richert, R. Fragility and thermodynamics in nonpolymeric glass-forming liquids. J. Chem. Phys. 2006, 125, 074505. [Google Scholar] [CrossRef]
- Hunt, A. A simple connection between the melting temperature and the glass temperature in a kinetic theory of the glass transition. J. Phys. Condens. Matter 1992, 4, L429–L431. [Google Scholar] [CrossRef]
- Hunt, A. An explanation for the correlation between the glass transition temperature and the extrapolated divergence of the viscosity in Vogel-Fulcher phenomenology. Solid State Commun. 1993, 88, 377–379. [Google Scholar] [CrossRef]
- Lubchenko, V.; Wolynes, P.G. Barrier softening near the onset of nonactivated transport in supercooled liquids: Implications for establishing detailed connection between thermodynamic and kinetic anomalies in supercooled liquids. J.Chem. Phys. 2003, 119, 9088–9105. [Google Scholar] [CrossRef]
- Kato, H.; Wada, T.; Hasegawa, M.; Saida, J.; Inoue, A.; Chen, H.S. Fragility and thermal stability of Pt- and Pd-based bulk glass forming liquids and their correlation with deformability. Scripta Mater. 2006, 54, 2023–2027. [Google Scholar] [CrossRef]
- Gao, Q.; Jian, Z.Y. Measured and ideal glass transition temperatures of glass-forming liquids. J. Mol. Liq. 2019, 296. [Google Scholar] [CrossRef]
- Huang, D.; McKenna, G.B. New insights into the fragility dilemma in liquids. J. Chem. Phys. 2001, 114, 5621–5630. [Google Scholar] [CrossRef]
- Senkov, O.N. Correlation between fragility and glass-forming ability of metallic alloys. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef]
- Fiore, G.; Battezzati, L. Thermodynamic properties of the Pd77.5Cu6Si16.5 undercooled liquid. J. Alloys Compd. 2009, 483, 54–56. [Google Scholar] [CrossRef]
- Glade, S.C.; Busch, R.; Lee, D.S.; Johnson, W.L.; Wunderlich, R.K.; Fecht, H.J. Thermodynamics of Cu47Ti34Zr11Ni8, Zr52.5Cu17.9Ni14.6Al10Ti5 and Zr57Cu15.4Ni12.6Al10Nb5 bulk metallic glass forming alloys. J. Appl. Phys. 2000, 87, 7242–7248. [Google Scholar] [CrossRef]
- Kim, Y.J.; Busch, R.; Johnson, W.L.; Rulison, A.J.; Rhim, W.K. Metallic glass formation in highly undercooled Zr41.2Ti13.8Cu12.5Ni10.0Be22.5 during containerless electrostatic levitation processing. Appl. Phys. Lett. 1994, 65, 2136–2138. [Google Scholar] [CrossRef]
- Sipp, A.; Bottinga, Y.; Richet, P. New high viscosity data for 3D network liquids and new correlations between old parameters. J. Non-Cryst. Solids 2001, 288, 166–174. [Google Scholar] [CrossRef]
- Lu, Z.P.; Goh, T.T.; Li, Y.; Ng, S.C. Glass formation in La-based La–Al–Ni–Cu–(Co) alloys by Bridgman solidification and their glass forming ability. Acta Mater. 1999, 47, 2215–2224. [Google Scholar] [CrossRef]
- Evenson, Z.; Busch, R. Equilibrium viscosity, enthalpy recovery and free volume relaxation in a Zr44Ti11Ni10Cu10Be25 bulk metallic glass. Acta Mater. 2011, 59, 4404–4415. [Google Scholar] [CrossRef]
- Kuno, M.; Shadowspeaker, L.A.; Schroers, J.; Busch, R. Thermodynamics of The Pd43Ni10Cu27P20 Bulk Metallic Glass Forming Alloy. MRS Proc. 2004, 806. [Google Scholar] [CrossRef]



| Glass Formers | Tg (K) | m | TK (K) | TKcal1 (K) | TKcal1* (K) | TKcal2 (K) | TKcal2* (K) | TKnew (K) | TKnew* (K) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mg65Cu25Y10 | 404 [4] | 50 [34] | 320 [4] | 266.64 | 323.52 | 283.53 | 319.65 | 264.63 | 320.06 |
| 2 | Pd77.5Cu6Si16.5 | 637 [34] | 73 [34] | 560 [16,35] | 488.66 | 550.09 | 502.47 | 543.44 | 507.28 | 555.91 |
| 3 | Cu47Ti34Zr11Ni8 | 673 [34,36] | 59 [34] | 537 [36] | 479.08 | 559.39 | 500.30 | 552.42 | 482.27 | 558.08 |
| 4 | Zr41.2Ti13.8Cu12.5Ni10Be22.5 | 625 [34,37] | 46 [34] | 558 [6,13] | 394.02 | 489.67 | 424.04 | 484.12 | 390.27 | 482.86 |
| 5 | Zr46.75Ti8.25Cu7.5Ni10Be27.5 | 590 [13,34] | 46 [34] | 560 [13] | 371.96 | 462.25 | 400.30 | 457.01 | 368.42 | 455.82 |
| 6 | SiO2 | 1480 [38] | 25 [34] | 876 [38] | 473.60 | 890.37 | 645.92 | 900.08 | 620.11 | 907.07 |
| 6 | SiO2 | 1452 [34,38] | 25 [34] | 876 [38] | 464.64 | 873.52 | 633.70 | 883.05 | 608.38 | 889.91 |
| 7 | GeO2 | 816 [34,38] | 21 [34] | 418 [38] | 155.43 | 428.98 | 264.75 | 441.17 | 300.63 | 460.41 |
| 8 | Pd40Ni40P20 | 578 [3,13] | 46 [3,13] | 500 [9,13] | 364.39 | 452.85 | 392.15 | 447.72 | 360.92 | 446.55 |
| 9 | La55Al25Ni20 | 491 [13,34,39] | 42 [34] | 337 [10,13] | 292.26 | 374.56 | 319.61 | 370.73 | 290.45 | 368.45 |
| 9 | La55Al25Ni20 | 470.3 [10] | 42 [34] | 337 [10,13] | 279.94 | 358.77 | 306.14 | 355.10 | 278.21 | 352.91 |
| 10 | La55Al25Ni15Cu5 | 472 [13,34,39] | 37 [34] | 318 [10,13] | 255.14 | 344.94 | 287.25 | 342.25 | 258.09 | 339.07 |
| 10 | La55Al25Ni15Cu5 | 449.3 [10] | 37 [34] | 318 [10,13] | 242.86 | 328.35 | 273.44 | 325.79 | 245.68 | 322.76 |
| 11 | La55Al25Ni10Cu10 | 467 [13,34,39] | 35 [34] | 332 [10,13] | 240.17 | 334.11 | 274.76 | 331.99 | 246.41 | 328.74 |
| 11 | La55Al25Ni10Cu10 | 440.6 [10] | 35 [34] | 332 [10,13] | 226.59 | 315.22 | 259.23 | 313.22 | 232.48 | 310.16 |
| 12 | La55Al25Ni5Cu15 | 459 [13,34,39] | 42 [34] | 304 [10,13] | 273.21 | 350.15 | 298.78 | 346.57 | 271.52 | 344.44 |
| 12 | La55Al25Ni5Cu15 | 435 [10] | 42 [34] | 304 [10,13] | 258.93 | 331.84 | 283.16 | 328.44 | 257.32 | 326.43 |
| 13 | La55Al25Ni5Cu10Co5 | 466 [13,16,34,39] | 37 [16,34] | 363 [13,16] | 251.89 | 340.56 | 283.60 | 337.90 | 254.81 | 334.76 |
| 13 | La55Al25Ni5Cu10Co5 | 439.1 [10] | 37 [16,34] | 363 [13,16] | 237.35 | 320.90 | 267.23 | 318.39 | 240.10 | 315.44 |
| 14 | Zr46(Cu4.5/5.5Ag1/5.5)46Al8 | 703 [14] | 49 [14,16] | 671 [14,16] | 459.10 | 560.10 | 489.51 | 553.47 | 455.25 | 553.63 |
| 15 | Zr46Cu46Al8 | 715 [14,16] | 43 [14,16] | 596 [14,16] | 432.33 | 549.39 | 470.67 | 543.58 | 429.00 | 540.69 |
| 16 | Zr44Ti11Ni10Cu10Be25 | 620 [16,40] | 39 [16] | 504.5 [16] | 349.74 | 461.66 | 388.61 | 457.52 | 350.43 | 453.73 |
| 17 | Pd43Ni10Cu27P20 | 582 [15,16] | 65 [12,16] | 532 [15,16,41] | 429.78 | 492.82 | 445.28 | 486.71 | 438.19 | 494.47 |
| 17 | Pd43Ni10Cu27P20 | 576 [11] | 65 [12,16] | 447 [11,12] | 425.35 | 487.74 | 440.69 | 481.69 | 433.67 | 489.37 |
| 18 | Au77Ge13.6Si9.4 | 294 [16] | 85 [12,16] | 199 [16] | 235.20 | 259.55 | 240.05 | 256.58 | 250.74 | 264.83 |
| 19 | 2-metylpentane | 80.5 | 58 | 58 | 56.91 | 66.68 | 59.52 | 65.85 | 57.17 | 66.46 |
| 20 | Butyronitrile | 100 | 47 | 81.2 | 63.83 | 78.81 | 68.47 | 77.90 | 63.23 | 77.77 |
| 21 | Ethanol | 92.5 | 55 | 71 | 63.91 | 75.75 | 67.20 | 74.81 | 63.86 | 75.28 |
| 22 | n-propanol | 102.5 | 36.5 | 73 | 54.76 | 74.53 | 61.88 | 73.97 | 55.56 | 73.27 |
| 23 | Toluene | 126 | 59 | 96 | 89.69 | 104.73 | 93.67 | 103.42 | 90.29 | 104.49 |
| 24 | 1-2 propan diol | 172 | 52 | 127 | 115.77 | 139.06 | 122.50 | 137.36 | 115.16 | 137.81 |
| 25 | Glycerol | 190 | 53 | 135 | 129.06 | 154.29 | 136.26 | 152.40 | 128.55 | 153.06 |
| 26 | Triphenil phospate | 205 | 160 | 166 | 183.22 | 192.24 | 184.26 | 190.76 | 219.15 | 203.31 |
| 27 | Orthoterphenyl | 244 | 81 | 200 | 192.79 | 214.00 | 197.18 | 211.50 | 203.75 | 217.68 |
| 28 | m-toluidine | 187 | 79 | 154 | 146.76 | 163.42 | 150.28 | 161.50 | 154.42 | 165.98 |
| 29 | Propylene carbonate | 156 | 104 | 127 | 130.50 | 141.06 | 132.28 | 139.61 | 144.43 | 145.73 |
| 30 | Sorbitol | 266 | 93 | 226 | 217.38 | 237.51 | 221.10 | 234.91 | 235.60 | 243.71 |
| 31 | Selenium | 307 | 87 | 240 | 247.01 | 271.85 | 251.87 | 268.78 | 264.45 | 277.79 |
| 32 | ZnCl2 | 380 | 30 | 250 | 164.67 | 253.84 | 199.85 | 253.71 | 180.95 | 251.90 |
| 33 | As2S3 | 455 | 36 | 265 | 240.14 | 329.12 | 272.43 | 326.77 | 244.48 | 323.64 |
| 34 | CaAl2Si2O8 | 1118 | 53 | 815 | 759.40 | 907.90 | 801.76 | 896.78 | 756.43 | 900.63 |
| 35 | Propilen glycol | 167 | 52 | 127 | 112.40 | 135.01 | 118.94 | 133.37 | 111.81 | 133.81 |
| 36 | 3-Methyl pentane | 77 | 36 | 58.4 | 40.64 | 55.70 | 46.10 | 55.30 | 41.37 | 54.77 |
| 37 | 3-Bromopentane | 108 | 53 | 82.5 | 73.36 | 87.70 | 77.45 | 86.63 | 73.07 | 87.00 |
| 38 | 2-methyltetrahydrofuran | 91 | 65 | 69.3 | 67.20 | 77.06 | 69.62 | 76.10 | 68.51 | 77.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Jian, Z. Predicting the Thermodynamic Ideal Glass Transition Temperature in Glass-Forming Liquids. Materials 2020, 13, 2151. https://doi.org/10.3390/ma13092151
Gao Q, Jian Z. Predicting the Thermodynamic Ideal Glass Transition Temperature in Glass-Forming Liquids. Materials. 2020; 13(9):2151. https://doi.org/10.3390/ma13092151
Chicago/Turabian StyleGao, Qian, and Zengyun Jian. 2020. "Predicting the Thermodynamic Ideal Glass Transition Temperature in Glass-Forming Liquids" Materials 13, no. 9: 2151. https://doi.org/10.3390/ma13092151
APA StyleGao, Q., & Jian, Z. (2020). Predicting the Thermodynamic Ideal Glass Transition Temperature in Glass-Forming Liquids. Materials, 13(9), 2151. https://doi.org/10.3390/ma13092151





