Development of Perylene-Based Non-Fullerene Acceptors through Bay-Functionalization Strategy
Abstract
1. Introduction
1.1. Discovery of Perylene Dyes
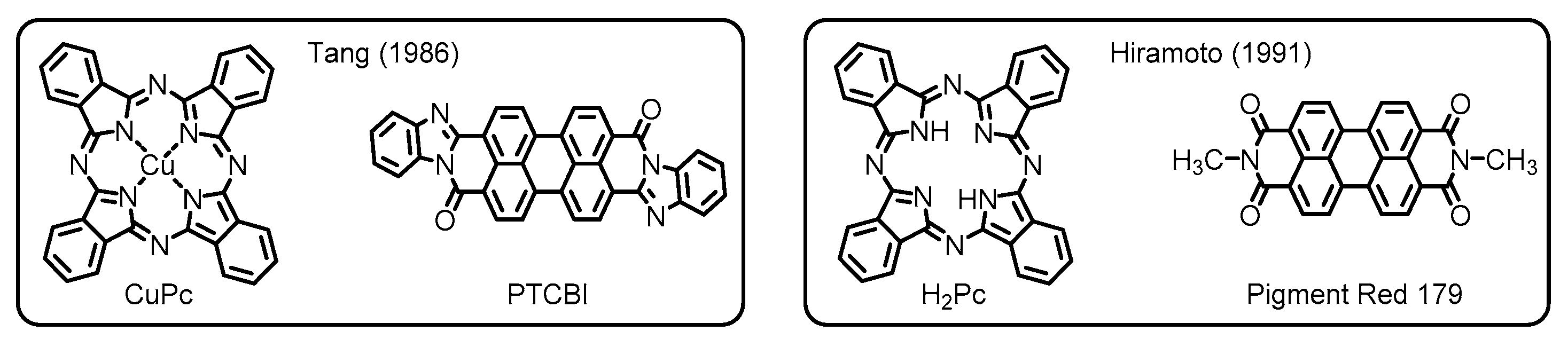
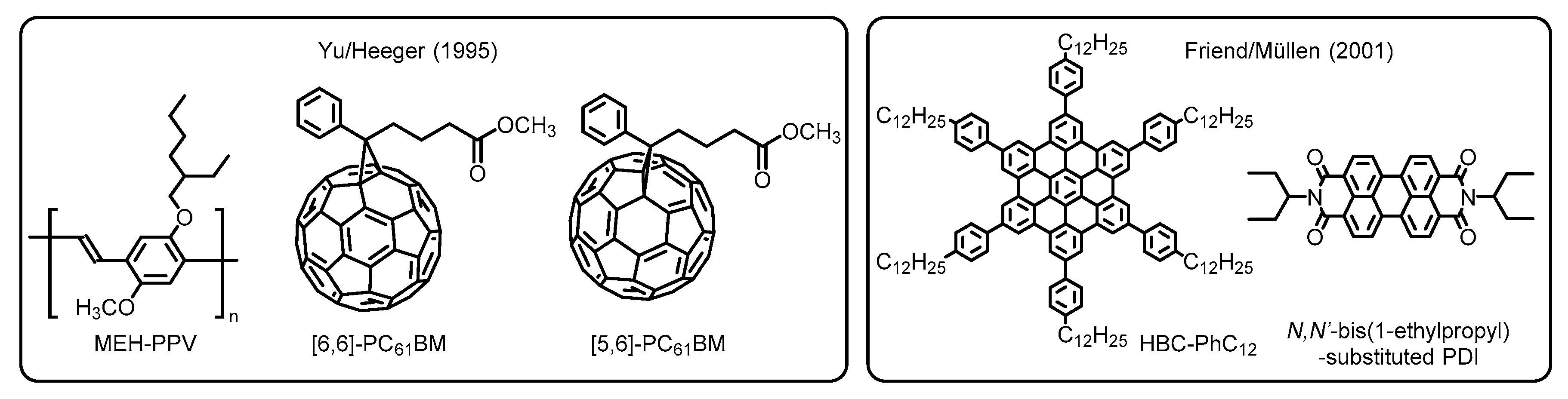
1.2. Application for Organic Photovoltaics
1.3. Development of Organic Photovoltaic Technology
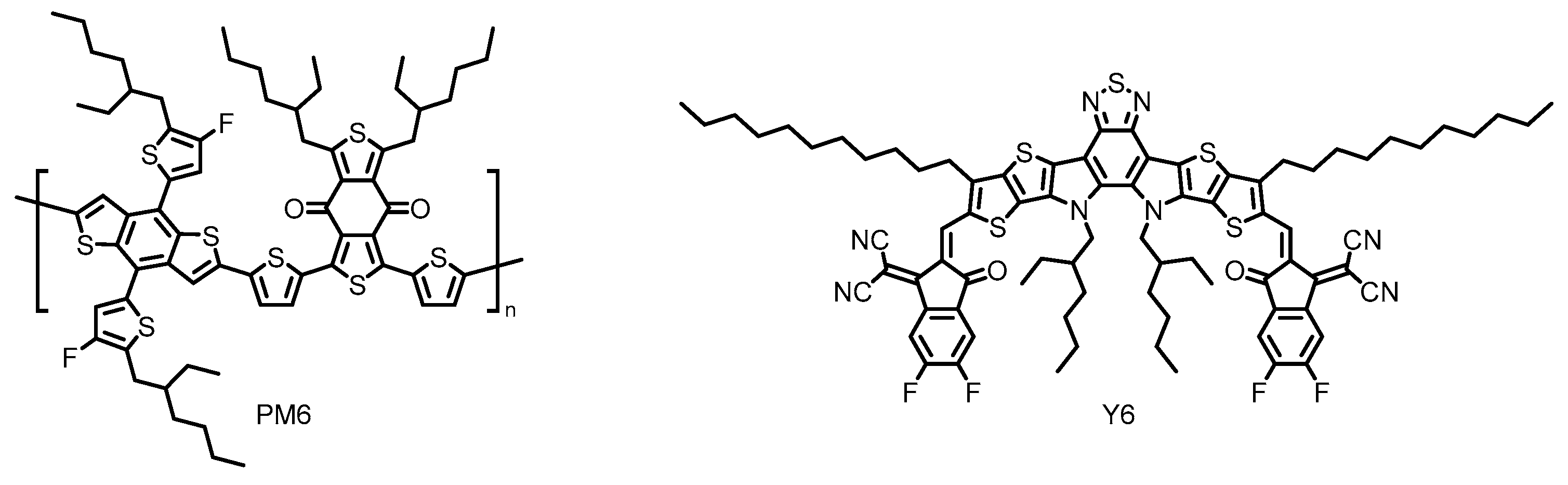
1.4. Non-Fullerene Acceptors for Organic Solar Cells
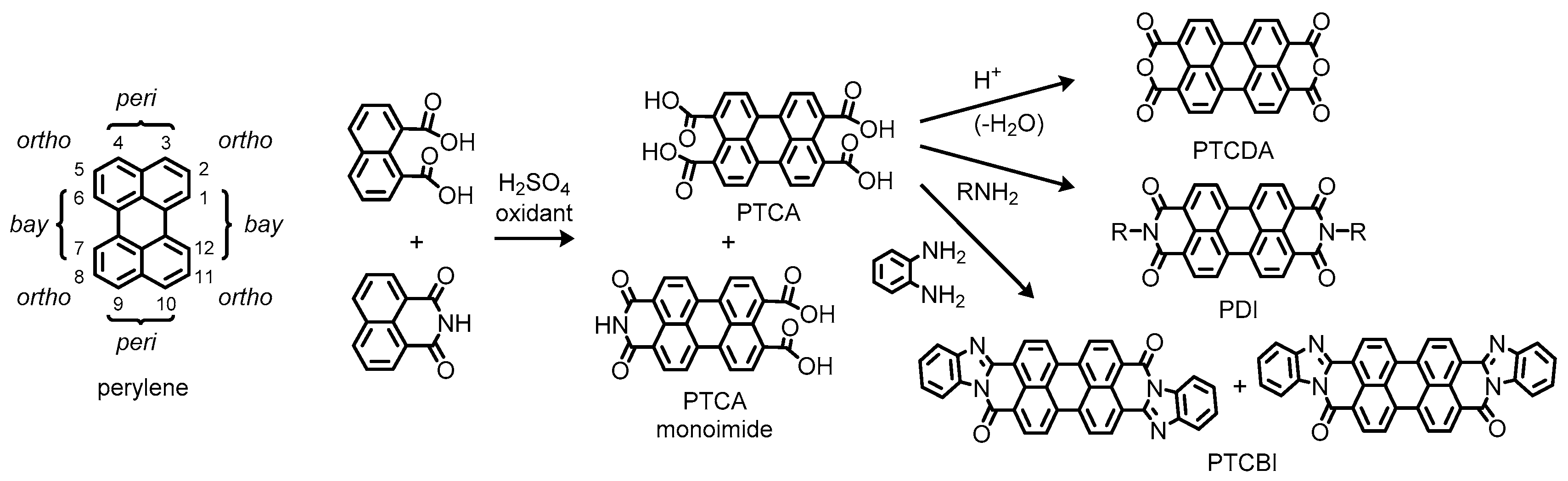
1.5. Chemical Modification of Perylene Systems
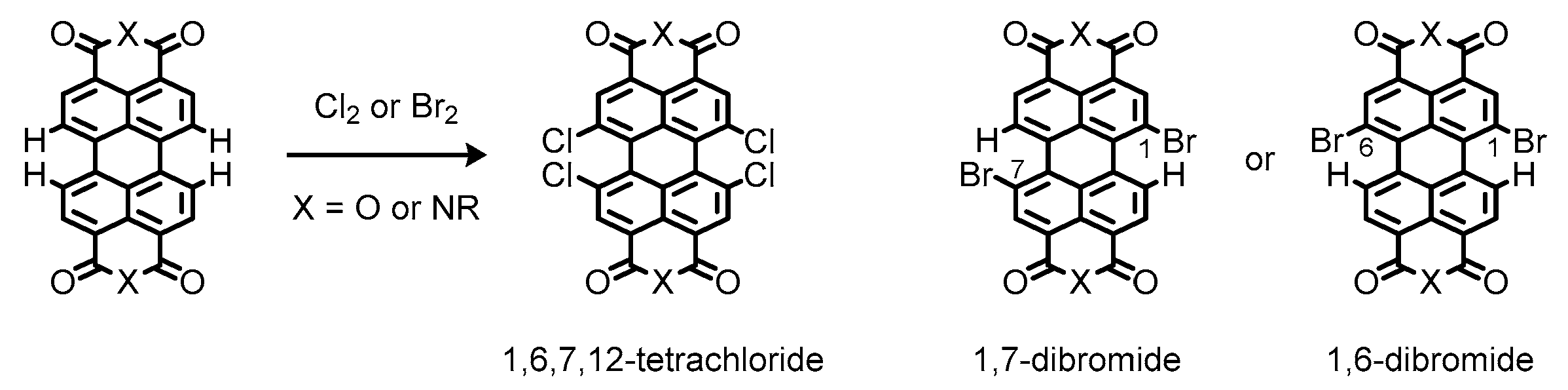
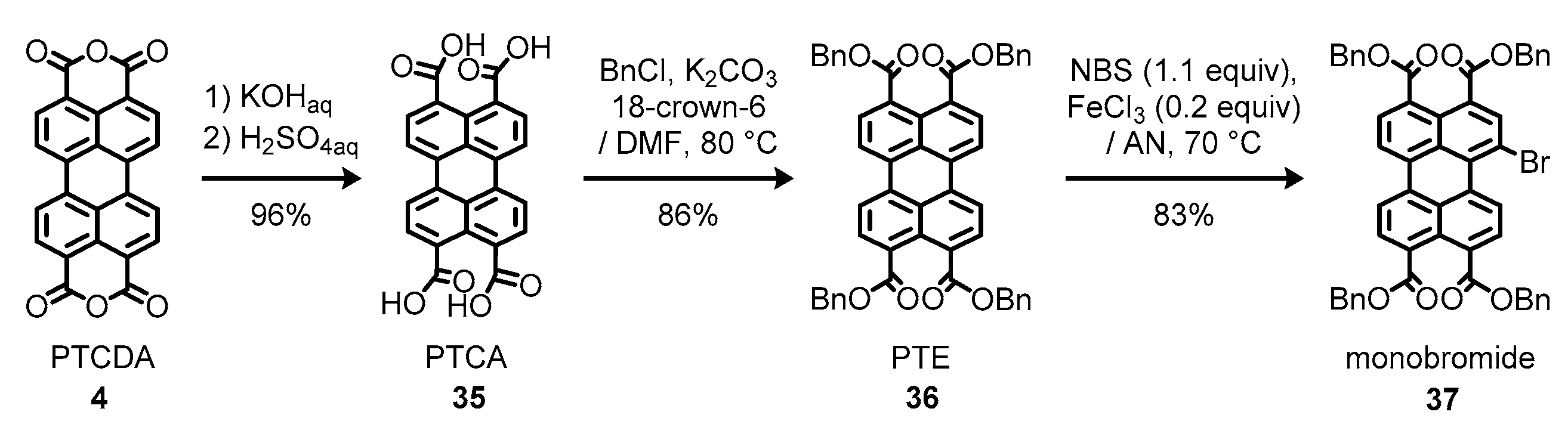
1.6. Synthetic Issues on Perylene Dyes
2. Monomeric Series of Perylene-Based Materials for Organic Photovoltaics
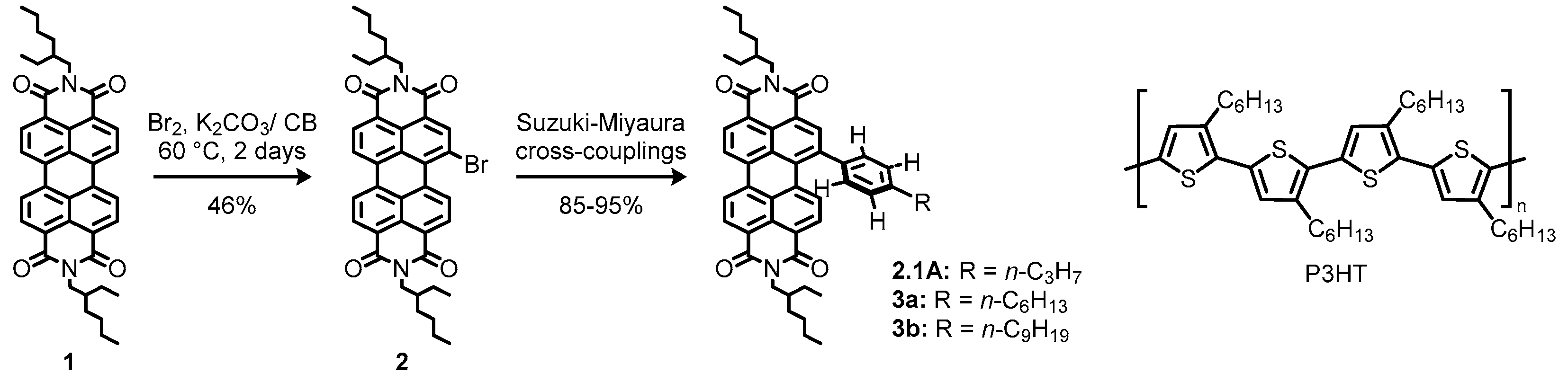
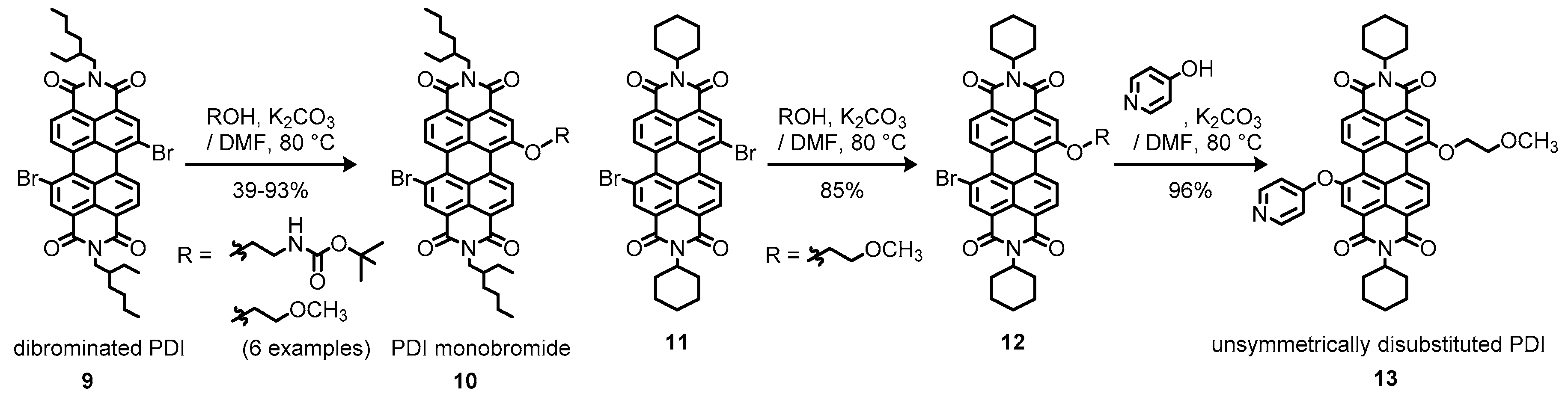
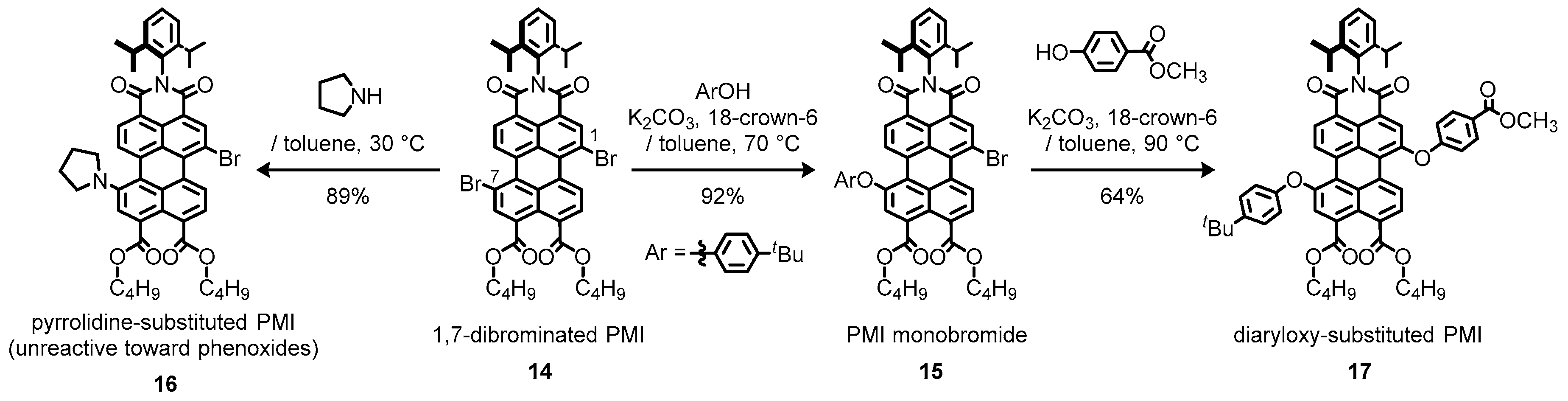
2.1. Bay-Monofunctionalized Perylene Diimides
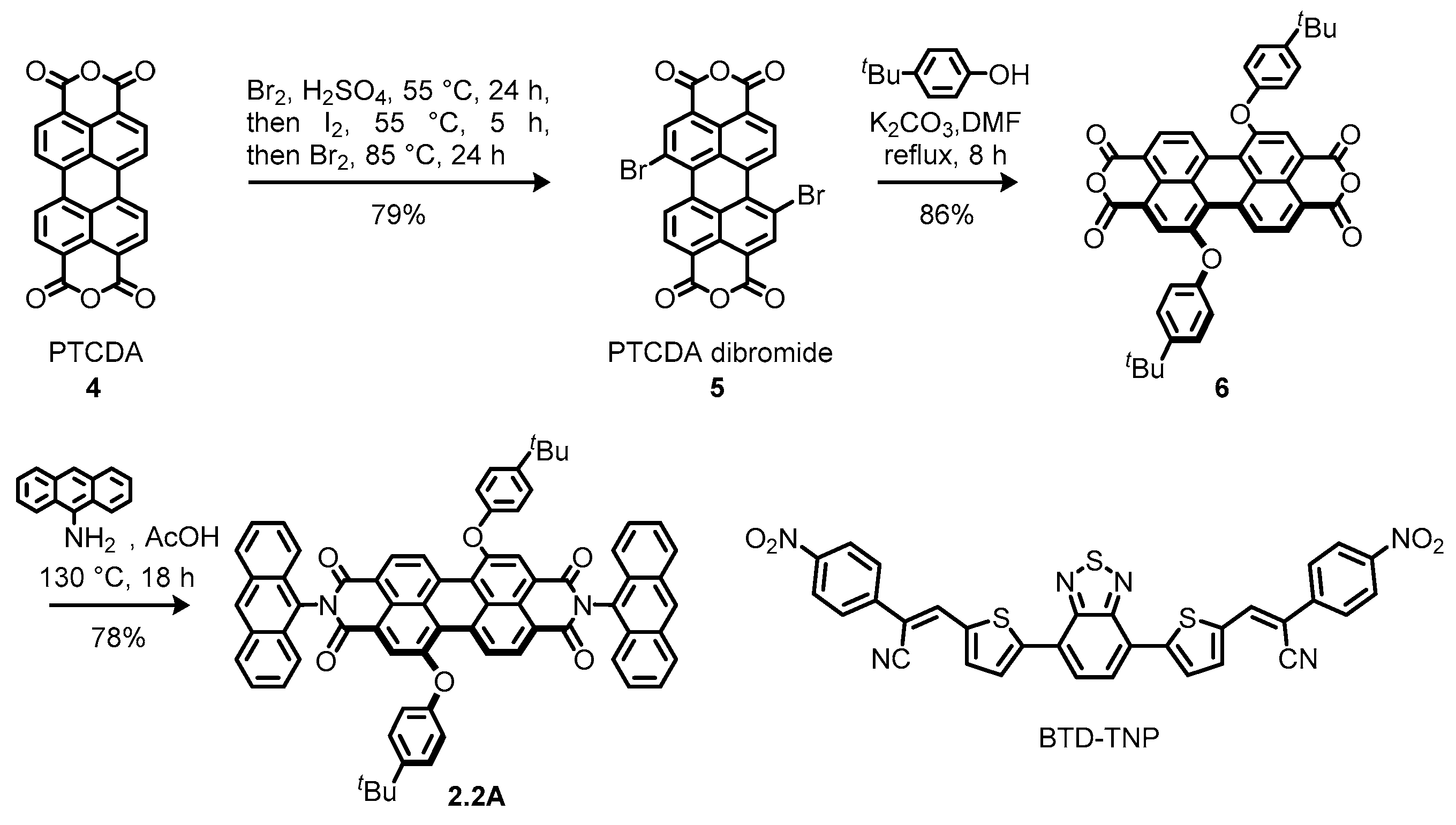
2.2. Bay-Difunctionalized Perylene Diimides
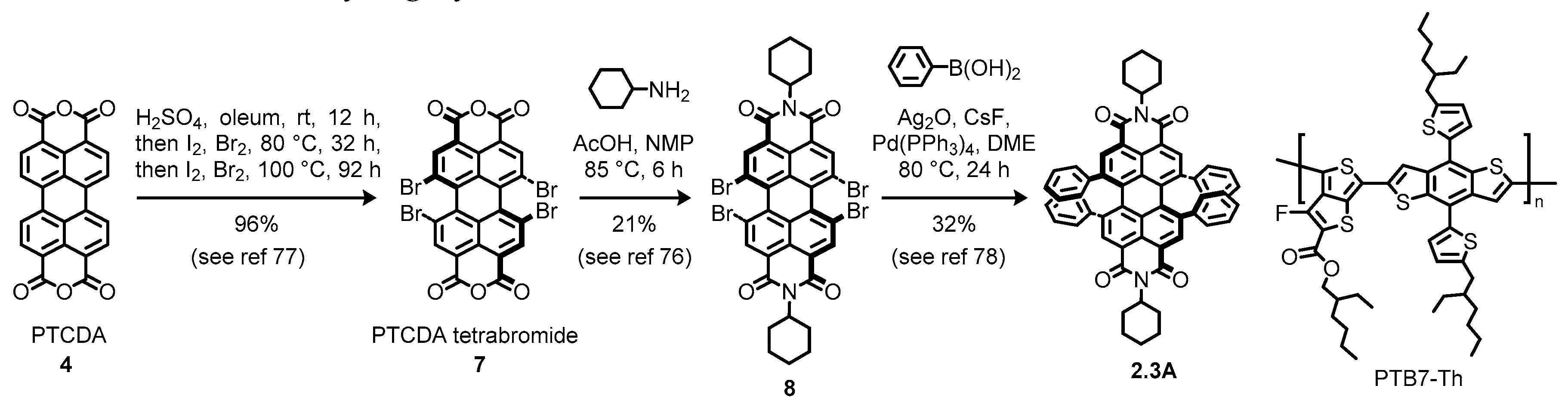
2.3. Fully Bay-Functionalized Perylene Diimides
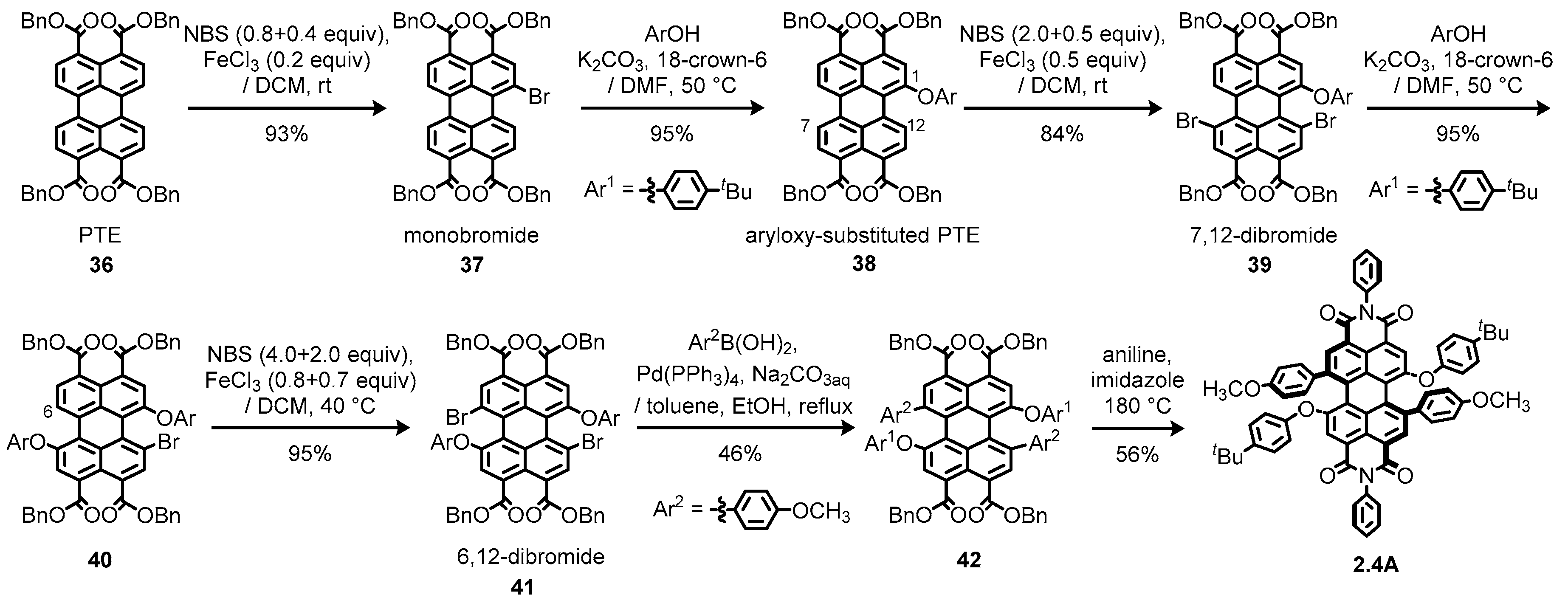
2.4. Multiply Bay-Functionalized Perylene Diimides
3. Integrated Series of Perylene-Based Materials for Organic Photovoltaics
3.1. Bay-Linked Dimeric Perylene Diimides
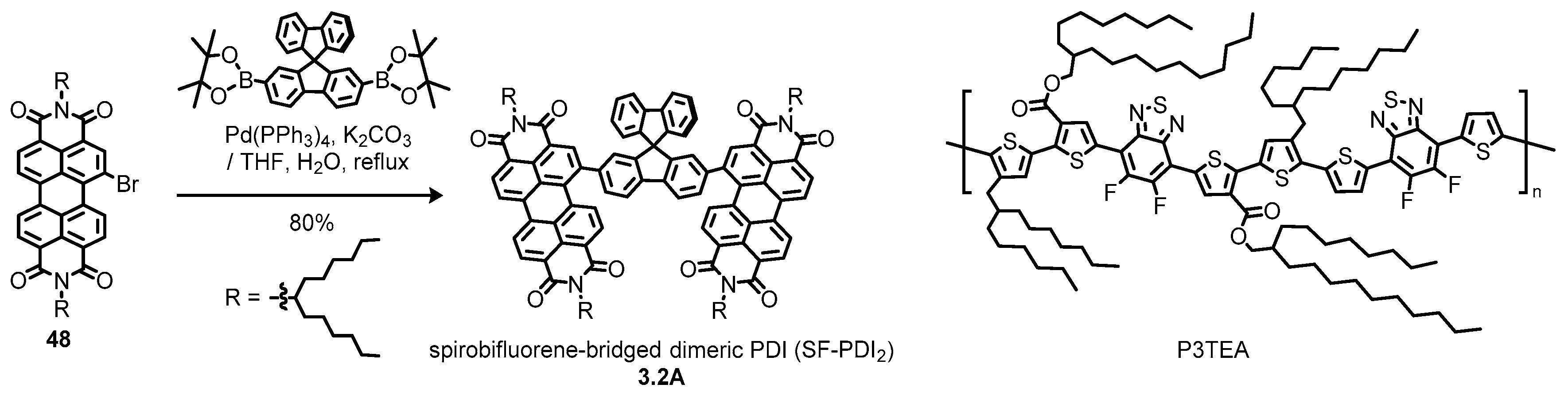
3.2. Bay-Bridged Dimeric Perylene Diimides
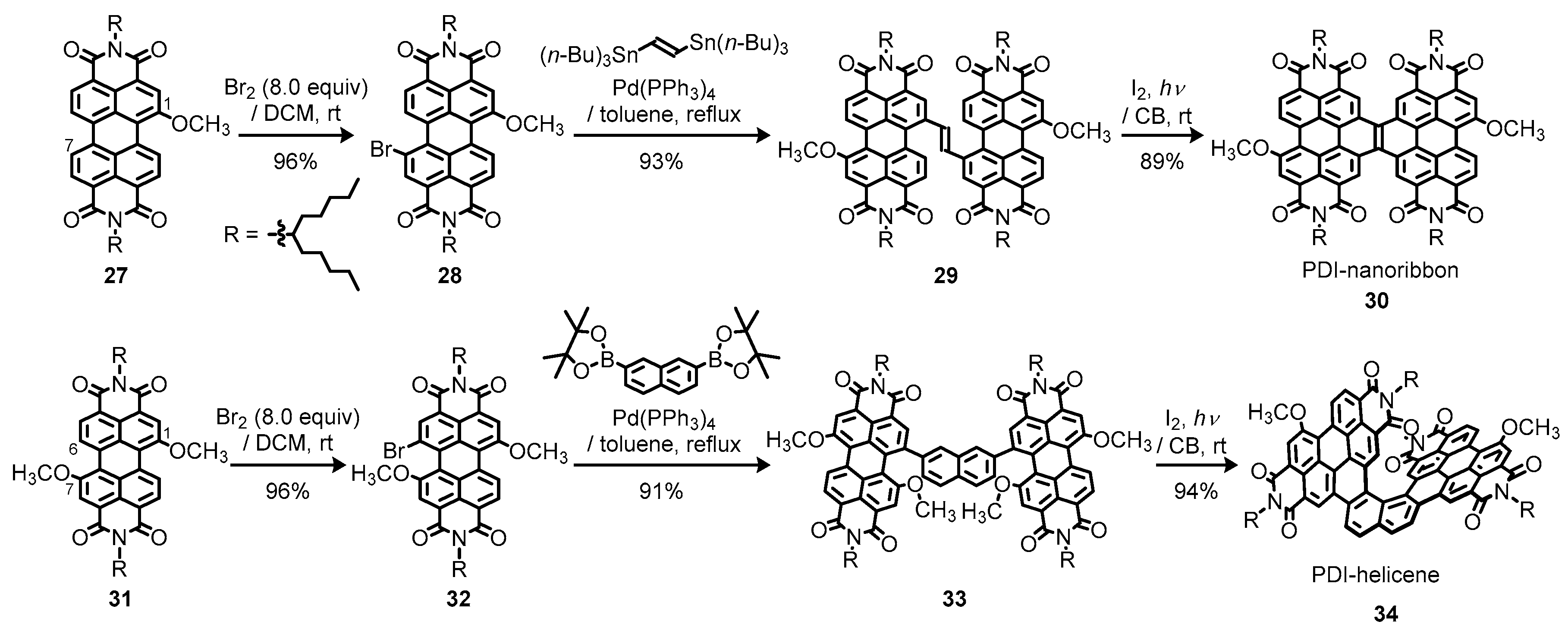
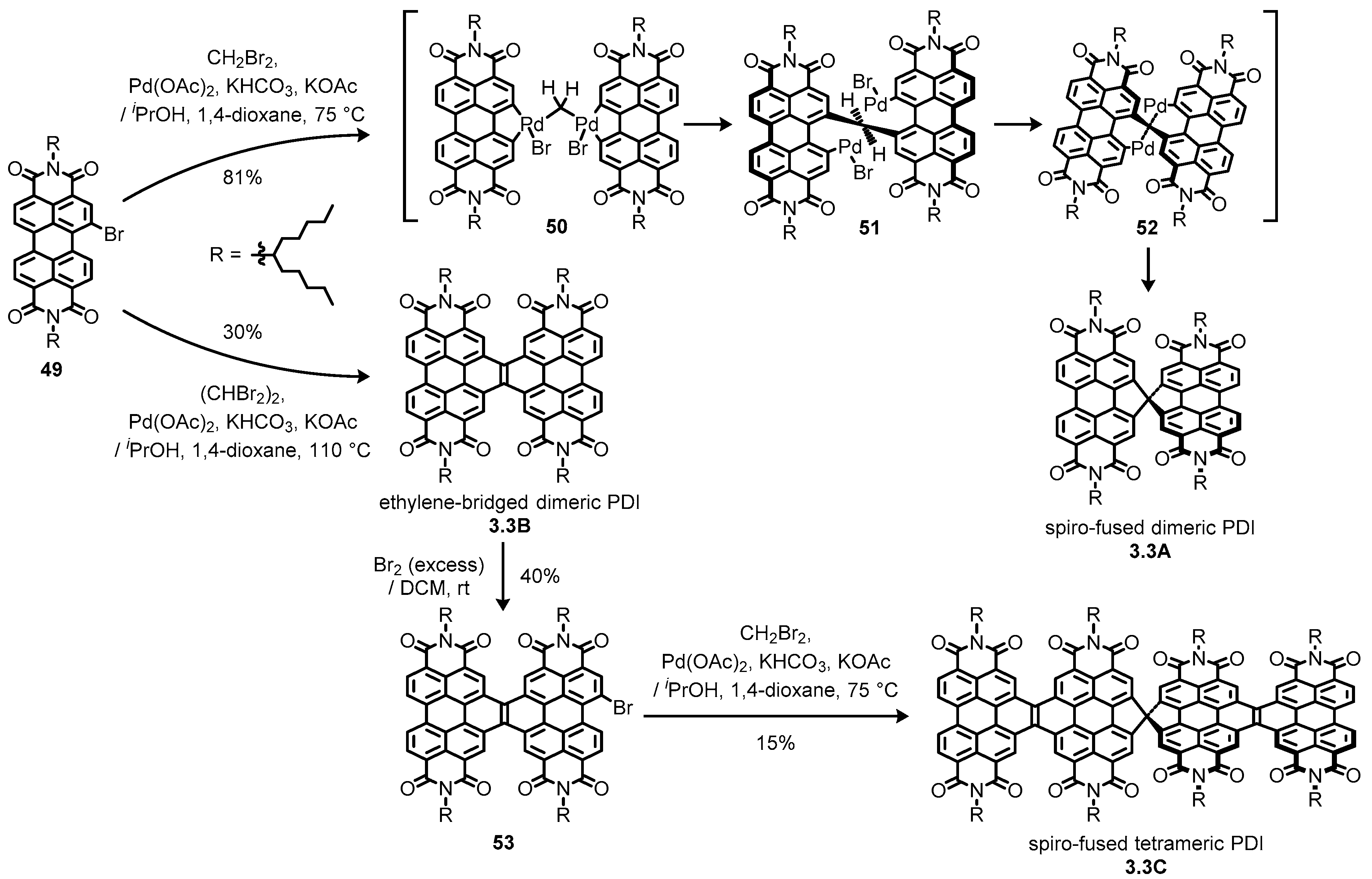
3.3. Bay-Fused Dimeric and Tetrameric Perylene Diimides
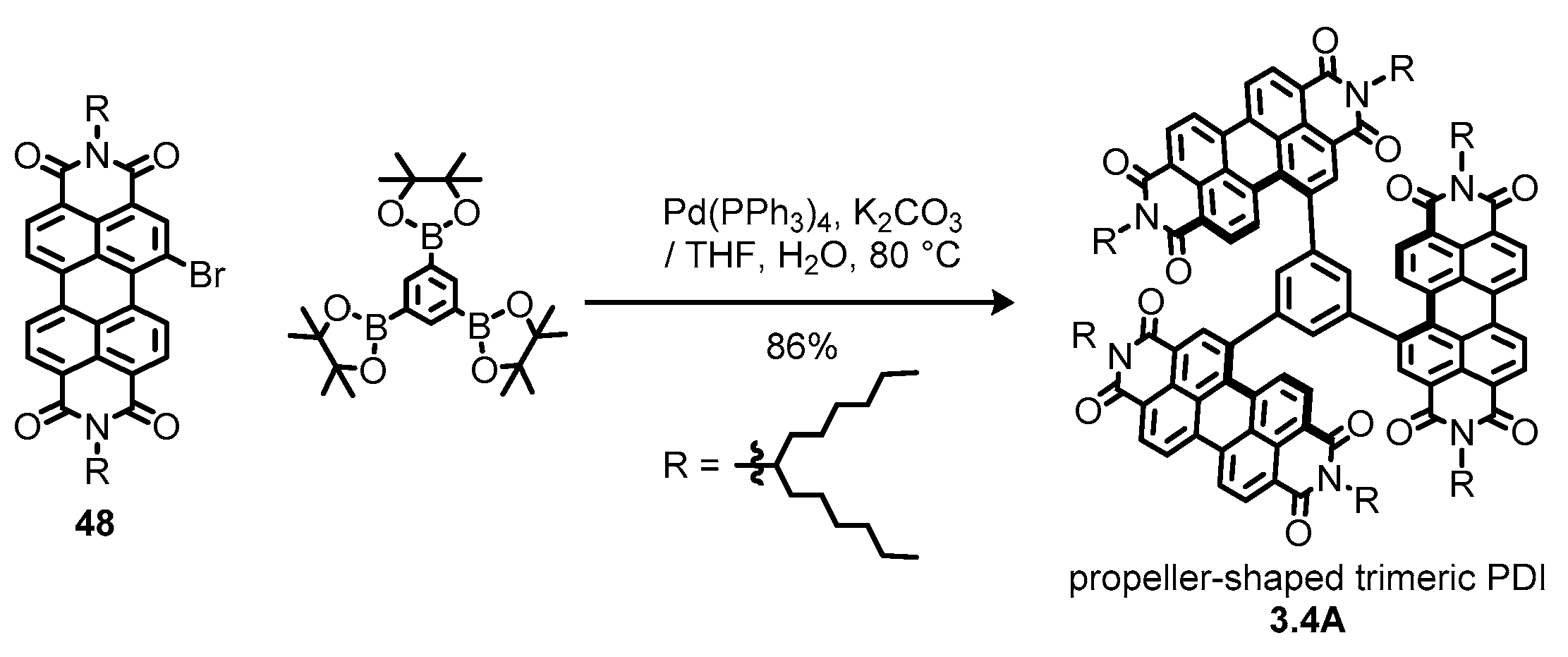
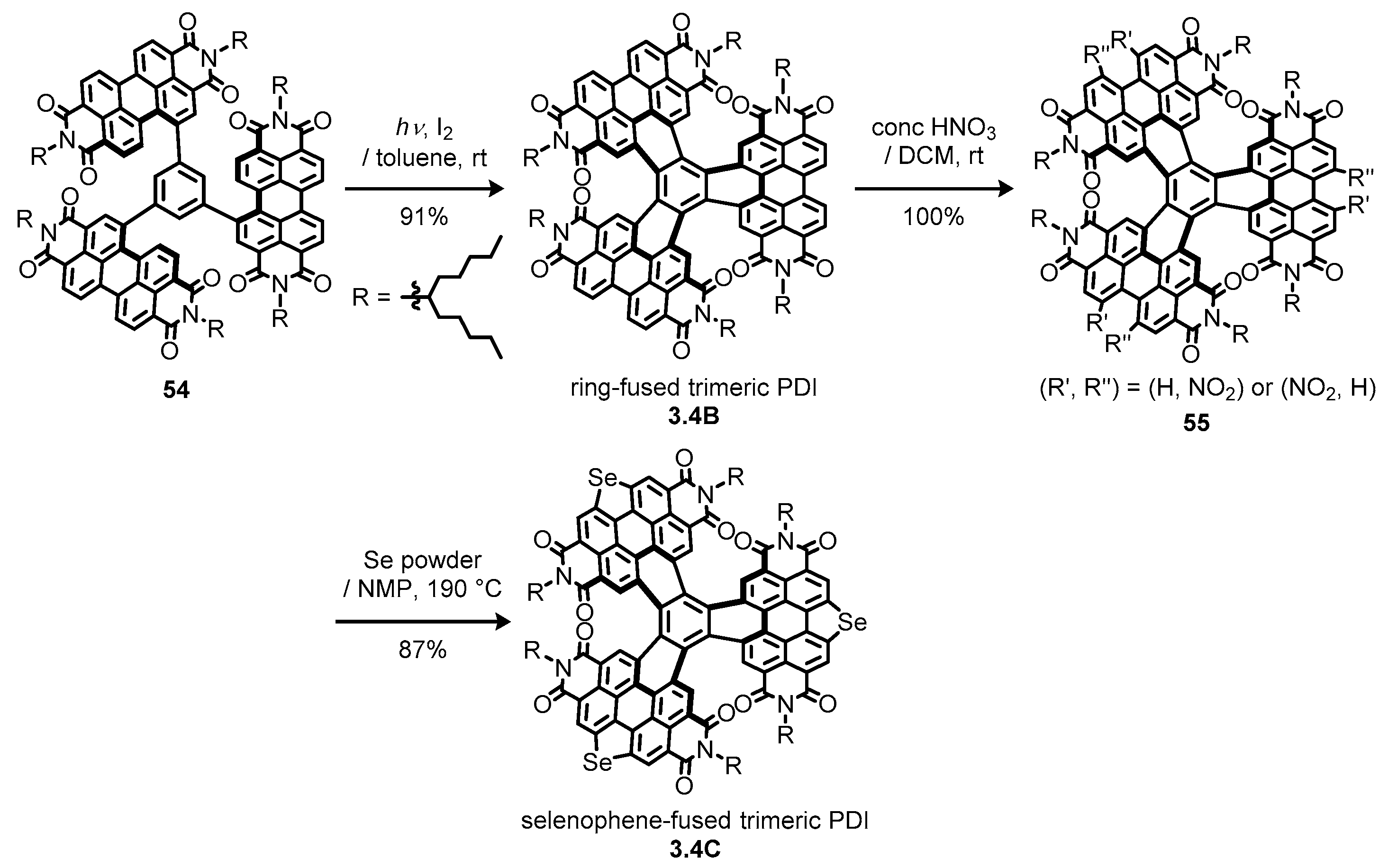
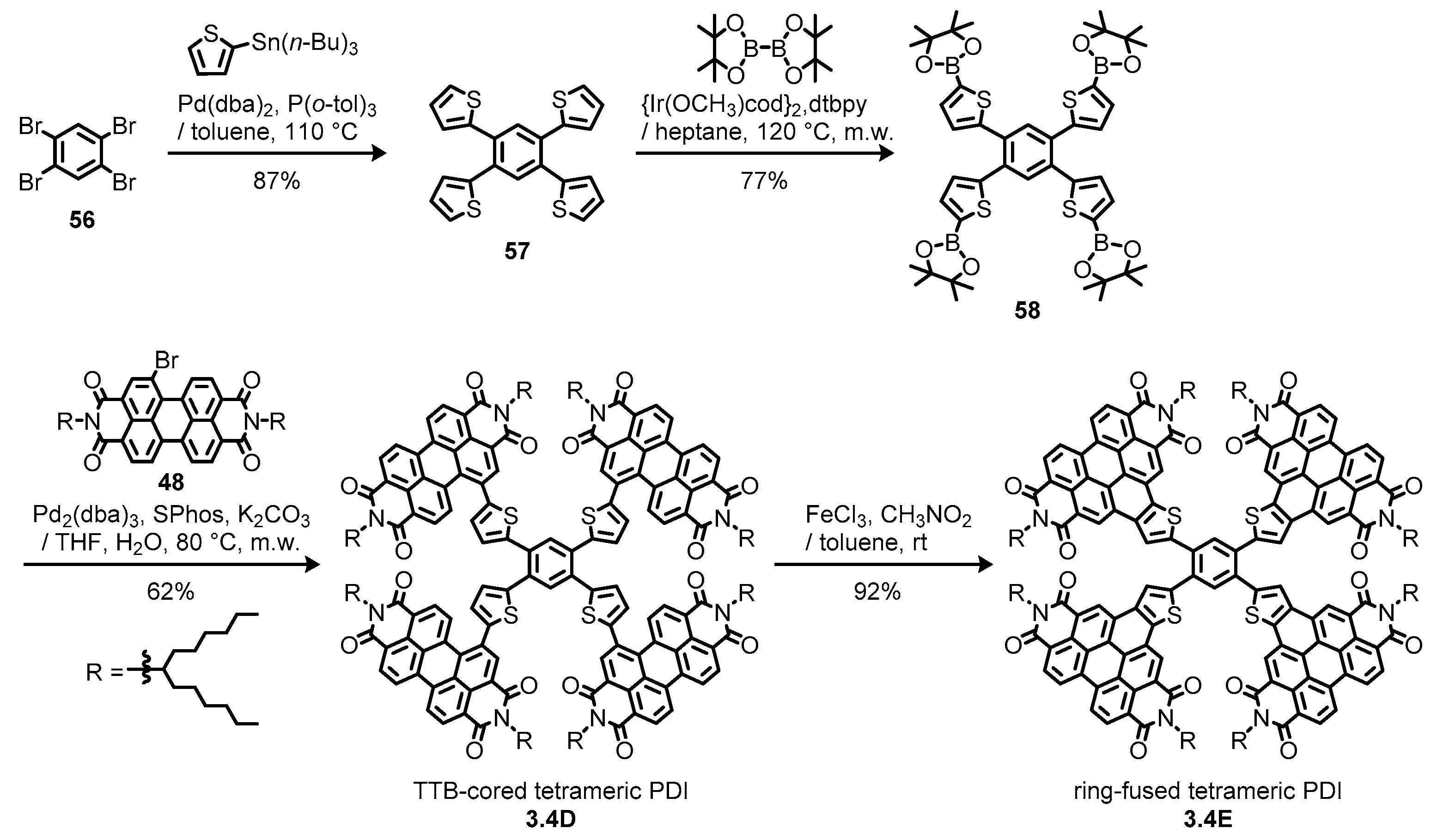
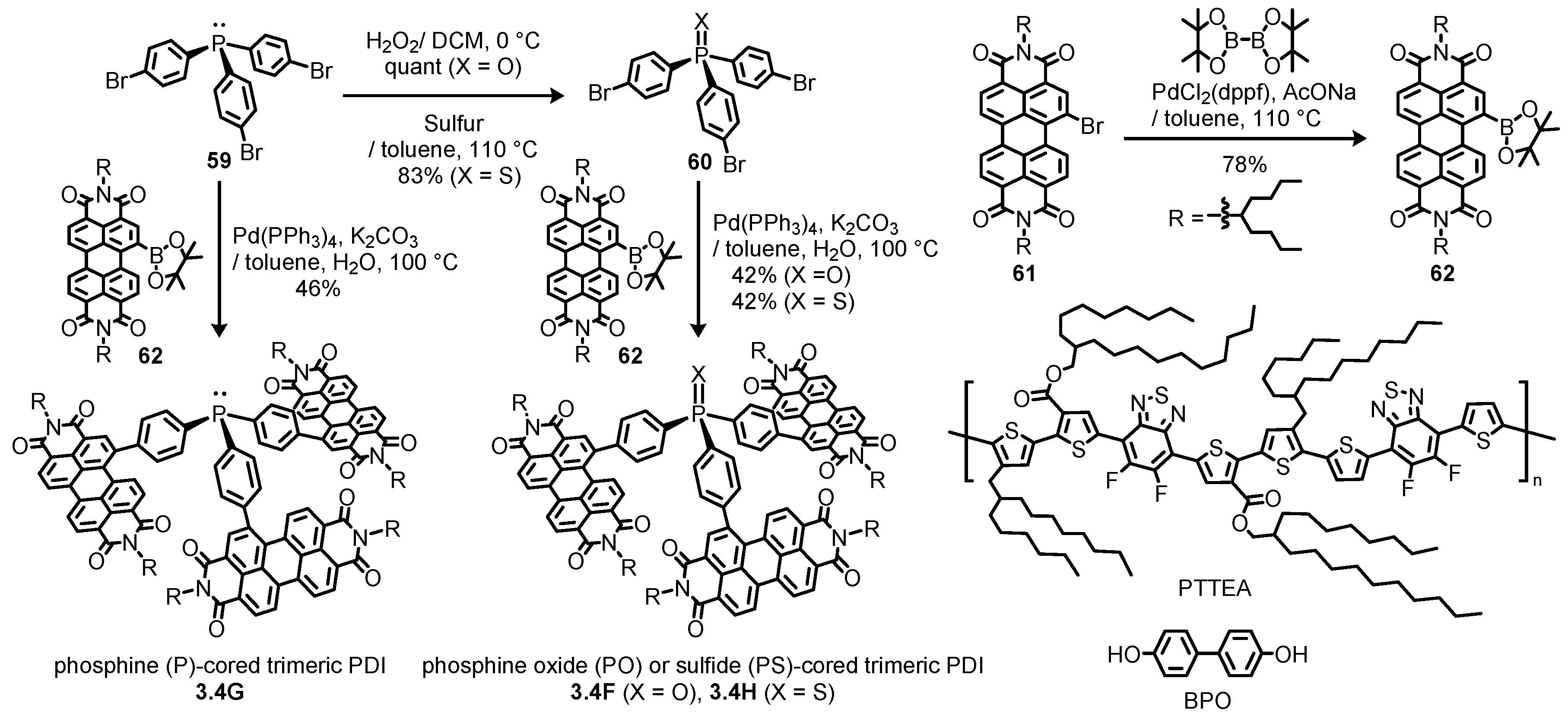
3.4. Propeller-Shaped Trimeric and Tetrameric Perylene Diimides
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akamatsu, H.; Inokuchi, H.; Matsunaga, Y. Electrical Conductivity of the Perylene-Bromine Complex. Nature 1954, 173, 168–169. [Google Scholar] [CrossRef]
- Wonneberger, H.; Reichelt, H.; Zagranyarski, Y.; Li, C.; Müllen, K.; Chen, L. Functionalisation of the Peri-Positions of Perylene and Naphthalene Monoimide via Versatile Building Blocks. US Patent US 9630973B2, 25 April 2017. [Google Scholar]
- Schmidt, M.P.; Neugebauer, W. Perylenetetracarboxylic Acids and Derivatives. US Patent US 1506545A, 26 August 1924. [Google Scholar]
- Pongratz, A. Process of Manufacturing Perylene Tetracarboxylic Acid Anhydride. US Patent US 1917153, 4 July 1933. [Google Scholar]
- Francis, E.M.; Simonsen, J.L. 111.Derivatives of Naphthalomethylimide. J. Chem. Soc. 1935, 496–499. [Google Scholar] [CrossRef]
- Kalle & Co. AG. Process of Producing Perylenetetracarboxylic Acids or Their Mono-Imides. Patent GB 221008, 1 September 1924. [Google Scholar]
- Kalle & Co. AG. Process for Producing Vat Colouring-Matters. Patent GB 201786, 9 August 1923. [Google Scholar]
- Kardos, M. Verfahren zur Darstellung eines Küpenfarbstoffes der Naphtalinreihe. Deutsches Reichspatent DE276357, 14 June 1913. [Google Scholar]
- Kardos, M. Verfahren zur Darstellung eines Küpenfarbstoffes der Naphtalinreihe. Deutsches Reichspatent DE276956, 10 October 1913. [Google Scholar]
- Cullinan, J.F.; Lyte, L.D. Acid Treatment of Heterocyclic Imide and Imidazole Vat Dyestuffs. US Patent US 2473015, 14 June 1949. [Google Scholar]
- Li, C.; Wonneberger, H. Perylene Imides for Organic Photovoltaics: Yesterday, Today, and Tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Spanggaard, H.; Krebs, F.C. A Brief History of the Development of Organic and Polymeric Photovoltaics. Sol. Energy Mater. Sol. Cells 2004, 83, 125–146. [Google Scholar] [CrossRef]
- Kearns, D.; Calvin, M. Photovoltaic Effect and Photoconductivity in Laminated Organic Systems. J. Chem. Phys. 1958, 29, 950. [Google Scholar] [CrossRef]
- Tang, C.W. Two-Layer Organic Photovoltaic Cell. App. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Hiramoto, M.; Fujiwara, H.; Yokoyama, M. Three-Layered Organic Solar Cell with a Photoactive Interlayer of Codeposited Pigments. App. Phys. Lett. 1991, 58, 1062–1064. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; Fechtenkötter, A.; Müllen, K.; Moons, E.; Friend, R.H.; MacKenzie, J.D. Self-Organized Discotic Liquid Crystals for High-Efficiency Organic Photovoltaics. Science 2001, 293, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Brus, V.V.; Lee, J.; Luginbuhl, B.; Ko, S.J.; Bazan, G.C.; Nguyen, T.Q. Solution-Processed Semitransparent Organic Photovoltaics: From Molecular Design to Device Performance. Adv. Mater. 2019, 31, 1900904. [Google Scholar] [CrossRef]
- Burke, D.J.; Lipomi, D.J. Green Chemistry for Organic Solar Cells. Energy Environ. Sci. 2013, 6, 2053–2066. [Google Scholar] [CrossRef]
- Deng, R.; Chang, N.L.; Ouyang, Z.; Chong, C.M. A Techno-Economic Review of Silicon Photovoltaic Module Recycling. Renew. Sustain. Energy Rev. 2019, 109, 532–550. [Google Scholar] [CrossRef]
- Lee, J.; Ko, S.-J.; Lee, H.; Huang, J.; Zhu, Z.; Seifrid, M.; Vollbrecht, J.; Brus, V.V.; Karki, A.; Wang, H.; et al. Side-Chain Engineering of Nonfullerene Acceptors for Near-Infrared Organic Photodetectors and Photovoltaics. ACS Energy Lett. 2019, 4, 1401–1409. [Google Scholar] [CrossRef]
- Mulligan, C.J.; Bilen, C.; Zhou, X.; Belcher, W.J.; Dastoor, P.C. Levelised Cost of Electricity for Organic Photovoltaics. Sol. Energy Mater. Sol. Cells 2015, 133, 26–31. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Ramakrishna, S.; Aberle, A. Recent Progress in Flexible-Wearable Solar Cells for Self-Powered Electronic Devices. Energy Environ. Sci. 2020, 13, 685–743. [Google Scholar] [CrossRef]
- Darling, S.B.; You, F. The Case for Organic Photovoltaics. RSC Adv. 2013, 3, 17633–17648. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Z.; Sun, Y. Polymer Donors for High-Performance Non-Fullerene Organic Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 1433–7851. [Google Scholar] [CrossRef]
- Sonar, P.; Fong Lim, J.P.; Chan, K.L. Organic Non-Fullerene Acceptors for Organic Photovoltaics. Energy Environ. Sci. 2011, 4, 1558–1574. [Google Scholar] [CrossRef]
- Mishra, A.; Bäuerle, P. Small Molecule Organic Semiconductors on the Move: Promises for Future Solar Energy Technology. Angew. Chem. Int. Ed. 2012, 51, 1433–7851. [Google Scholar] [CrossRef]
- Naveed, H.B.; Zhou, K.; Ma, W. Interfacial and Bulk Nanostructures Control Loss of Charges in Organic Solar Cells. Acc. Chem. Res. 2019, 52, 2904–2915. [Google Scholar] [CrossRef]
- Collavini, S.; Delgado, J.L. Fullerenes: The Stars of Photovoltaics. Sustain. Energ. Fuels 2018, 2, 2480–2493. [Google Scholar] [CrossRef]
- Duan, L.; Elumalai, N.K.; Zhang, Y.; Uddin, A. Progress in Non-Fullerene Acceptor Based Organic Solar Cells. Sol. Energy Mater. Sol. Cells 2019, 193, 22–65. [Google Scholar] [CrossRef]
- Wang, H.; Cao, J.; Yu, J.; Zhang, Z.; Geng, R.; Yang, L.; Tang, W. Molecular Engineering of Central Fused-Ring Cores of Non-Fullerene Acceptors for High-Efficiency Organic Solar Cells. J. Mater. Chem. A 2019, 7, 2050–7488. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Liu, L.; Kan, Y.; Gao, K.; Wang, J.; Zhao, M.; Chen, H.; Zhao, C.; Jiu, T.; Jen, A.-K.-Y.; Li, Y. Graphdiyne Derivative as Multifunctional Solid Additive in Binary Organic Solar Cells with 17.3% Efficiency and High Reproductivity. Adv. Mater. 2020, 32, 1907604. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Xian, K.; Zhang, T.; Hong, L.; Wang, Y.; Xu, Y.; Ma, K.; An, C.; et al. Single-Junction Organic Photovoltaic Cells with Approaching 18% Efficiency. Adv. Mater. 2020, 1908205. [Google Scholar] [CrossRef]
- Speller, E.M.; Clarke, A.J.; Luke, J.; Lee, H.K.H.; Durrant, J.R.; Li, N.; Wang, T.; Wong, H.C.; Kim, J.-S.; Tsoi, W.C.; et al. From Fullerene Acceptors to Non-Fullerene Acceptors: Prospects and Challenges in the Stability of Organic Solar Cells. J. Mater. Chem. A 2019, 7, 2050–7488. [Google Scholar] [CrossRef]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical Review of The Molecular Design Progress in Non-Fullerene Electron Acceptors Towards Commercially Viable Organic Solar Cells. Chem. Soc. Rev. 2019, 48, 1596–1625. [Google Scholar] [CrossRef]
- Jones, B.A.; Ahrens, M.J.; Yoon, M.-H.; Facchetti, A.; Marks, T.J.; Wasielewski, M.R. High-Mobility Air-Stable n-Type Semiconductors with Processing Versatility: Dicyanoperylene-3,4:9,10-Bis(dicarboximides). Angew. Chem. Int. Ed. 2004, 43, 6363–6366. [Google Scholar] [CrossRef]
- Minder, N.A.; Ono, S.; Chen, Z.; Facchetti, A.; Morpurgo, A.F. Band-Like Electron Transport in Organic Transistors and Implication of the Molecular Structure for Performance Optimization. Adv. Mater. 2012, 24, 503–508. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, L.; Yang, M.; Zhan, C.; Xie, Z.; Verpoort, F.; Xiao, S. Taking the Place of Perylene Diimide: Perylene Tetracarboxylic Tetraester as a Building Block for Polymeric Acceptors to Achieve Higher Open Circuit Voltage in All-Polymer Bulk Heterojunction Solar Cells. Polym. Chem. 2013, 4, 5612–5620. [Google Scholar] [CrossRef]
- Avlasevich, Y.; Li, C.; Müllen, K. Synthesis and Applications of Core-Enlarged Perylene Dyes. J. Mater. Chem. 2010, 20, 3814–3826. [Google Scholar] [CrossRef]
- Nakazono, S.; Easwaramoorthi, S.; Kim, D.; Shinokubo, H.; Osuka, A. Synthesis of Arylated Perylene Bisimides through C−H Bond Cleavage under Ruthenium Catalysis. Org. Lett. 2009, 11, 5426–5429. [Google Scholar] [CrossRef] [PubMed]
- Clikeman, T.T.; Bukovsky, E.V.; Wang, X.-B.; Chen, Y.-S.; Rumbles, G.; Strauss, S.H.; Boltalina, O.V. Core Perylene Diimide Designs via Direct Bay- and ortho-(Poly)trifluoromethylation: Synthesis, Isolation, X-ray Structures, Optical and Electronic Properties. Eur. J. Org. Chem. 2015, 6641–6654. [Google Scholar] [CrossRef]
- Dey, S.; Efimov, A.; Lemmetyinen, H. Diaryl-Substituted Perylene Bis(imides): Synthesis, Separation, Characterization and Comparison of Electrochemical and Optical Properties of 1,7- and 1,6-Regioisomer. Eur. J. Org. Chem. 2012, 2367–2374. [Google Scholar] [CrossRef]
- Weil, T.; Vosch, T.; Hofkens, J.; Peneva, K.; Müllen, K. The Rylene Colorant Family—Tailored Nanoemitters for Photonics Research and Applications. Angew. Chem. Int. Ed. 2010, 49, 9068–9093. [Google Scholar] [CrossRef]
- Nowak-Król, A.; Würthner, F. Progress in the Synthesis of Perylene Bisimide Dyes. Org. Chem. Front. 2019, 6, 1272–1318. [Google Scholar] [CrossRef]
- Würthner, F. Perylene Bisimide Dyes as Versatile Building Blocks for Functional Supramolecular Architectures. Chem. Commun. 2004, 14, 1564–1579. [Google Scholar] [CrossRef]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3,4,9,10-tetracarboxylic Acid Diimides: Synthesis, Physical Properties, and Use in Organic Electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef]
- Dubey, R.K.; Westerveld, N.; Grozema, F.C.; Sudhölter, E.J.R.; Jager, W.F. Facile Synthesis of Pure 1,6,7,12-Tetrachloroperylene-3,4,9,10-tetracarboxy Bisanhydride and Bisimide. Org. Lett. 2015, 17, 1882–1885. [Google Scholar] [CrossRef]
- Böhm, A.; Arms, H.; Henning, G.; Blaschka, P. (BASF AG) 1,7-Diaroxy-oder-Arylthiosubstituierte Perylen-3,4,9,10-Tetracarbonsäuren, deren Dianhydride und Diimide. Germain Patent DE 19547209A1, 19 June 1997. [Google Scholar]
- Würthner, F.; Stepanenko, V.; Chen, Z.; Saha-Möller, C.R.; Kocher, N.; Stalke, D. Preparation and Characterization of Regioisomerically Pure 1,7-Disubstituted Perylene Bisimide Dyes. J. Org. Chem. 2004, 69, 7933–7939. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Saha-Möller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Datar, A.; Naddo, T.; Huang, J.; Oitker, R.; Yen, M.; Zhao, J.; Zang, L. Effect of Side-Chain Substituents on Self-Assembly of Perylene Diimide Molecules: Morphology Control. J. Am. Chem. Soc. 2006, 128, 7390–7398. [Google Scholar] [CrossRef] [PubMed]
- Rajasingh, P.; Cohen, R.; Shirman, E.; Shimon, L.J.W.; Rybtchinski, B. Selective Bromination of Perylene Diimides under Mild Conditions. J. Org. Chem. 2007, 72, 5973–5979. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Zhang, Q.; Gao, X. Non-Fullerene Small Molecule Acceptors Based on Perylene Diimides. J. Mater. Chem. A 2016, 4, 17604–17622. [Google Scholar] [CrossRef]
- Izawa, S.; Shintaku, N.; Kikuchi, M.; Hiramoto, M. Importance of Interfacial Crystallinity to Reduce Open-Circuit Voltage Loss in Organic Solar Cells. Appl. Phys. Lett. 2019, 115, 153301. [Google Scholar] [CrossRef]
- Vollbrecht , J.; Bock, H.; Wiebeler, C.; Schumacher, S.; Kitzerow, H. Polycyclic Aromatic Hydrocarbons Obtained by Lateral Core Extension of Mesogenic Perylenes: Absorption and Optoelectronic Properties. Chem. Eur. J. 2014, 20, 12026–12031. [Google Scholar] [CrossRef] [PubMed]
- Lütke Eversloh, C.; Li, C.; Müllen, K. Core-Extended Perylene Tetracarboxdiimides: The Homologous Series of Coronene Tetracarboxdiimides. Org. Lett. 2011, 13, 4148–4150. [Google Scholar] [CrossRef]
- Hartnett, P.E.; Timalsina, A.; Matte, H.S.S.R.; Zhou, N.; Guo, X.; Zhao, W.; Facchetti, A.; Chang, R.P.H.; Hersam, M.C.; Wasielewski, M.R.; et al. Slip-Stacked Perylenediimides as an Alternative Strategy for High Efficiency Nonfullerene Acceptors in Organic Photovoltaics. J. Am. Chem. Soc. 2014, 136, 16345–16356. [Google Scholar] [CrossRef]
- Sharenko, A.; Gehrig, D.; Laquai, F.; Nguyen, T.-Q. The Effect of Solvent Additive on the Charge Generation and Photovoltaic Performance of a Solution-Processed Small Molecule: Perylene Diimide Bulk Heterojunction Solar Cell. Chem. Mater. 2014, 26, 4109–4118. [Google Scholar] [CrossRef]
- Hartnett, P.E.; Margulies, E.A.; Matte, H.S.S.R.; Hersam, M.C.; Marks, T.J.; Wasielewski, M.R. Effects of Crystalline Perylenediimide Acceptor Morphology on Optoelectronic Properties and Device Performance. Chem. Mater. 2016, 28, 3928–3936. [Google Scholar] [CrossRef]
- Liao, H.-C.; Ho, C.-C.; Chang, C.-Y.; Jao, M.-H.; Darling, S.B.; Su, W.-F. Additives for Morphology Control in High-Efficiency Organic Solar Cells. Mater. Today 2013, 16, 326–336. [Google Scholar] [CrossRef]
- Yi, J.; Ma, Y.; Dou, J.; Lin, Y.; Wang, Y.; Ma, C.-Q.; Wang, H. Influence of para-Alkyl Chain Length of the Bay-Phenyl Unit on Properties and Photovoltaic Performance of Asymmetrical Perylenediimide Derivatives. Dyes Pigments 2016, 126, 86–95. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Liu, T.; Yu, Y.; Liu, H.; Li, Y.; Zhu, D. Anthraceno-Perylene Bisimides: The Precursor of a New Acene. Org. Lett. 2011, 13, 5692–5695. [Google Scholar] [CrossRef]
- Yi, J.; Wang, J.; Lin, Y.; Gao, W.; Ma, Y.; Tan, H.; Wang, H.; Ma, C.-Q. Molecular Geometry Regulation of Bay-Phenyl Substituted Perylenediimide Derivatives with Bulky Alkyl Chain for Use in Organic Solar Cells as the Electron Acceptor. Dyes Pigments 2017, 136, 335–346. [Google Scholar] [CrossRef]
- Simón Marqués, P.; Tintori, F.; Andrés Castán, J.M.; Josse, P.; Dalinot, C.; Allain, M.; Welch, G.; Blanchard, P.; Cabanetos, C. Indeno[1,2-b]thiophene End-capped Perylene Diimide: Should the 1,6-Regioisomers be systematically considered as a byproduct? Sci. Rep. 2020, 10, 3262. [Google Scholar] [CrossRef]
- Sharma, G.D.; Balraju, P.; Mikroyannidis, J.A.; Stylianakis, M.M. Bulk Heterojunction Organic Photovoltaic Devices Based on Low Band Gap Small Molecule BTD-TNP and Perylene–Anthracene Diimide. Sol. Energy Mater. Sol. Cells 2009, 93, 2025–2028. [Google Scholar] [CrossRef]
- Koyuncu, S.; Kus, M.; Demic, S.; Kaya, İ.; Ozdemir, E.; Icli, S. Electrochemical and Optical Properties of Novel Donor-Acceptor Thiophene-Perylene-Thiophene Polymers. J. Polym. Sci. A Polym. Chem. 2008, 46, 1974–1989. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Stylianakis, M.M.; Roy, M.S.; Suresh, P.; Sharma, G.D. Synthesis, Photophysics of Two New Perylene Bisimides and Their Photovoltaic Performances in Quasi Solid State Dye Sensitized Solar Cells. J. Power Sources 2009, 194, 1171–1179. [Google Scholar] [CrossRef]
- Kelber, J.; Achard, M.-F.; Durola, F.; Bock, H. Distorted Arene Core Allows Room-Temperature Columnar Liquid-Crystal Glass with Minimal Side Chains. Angew. Chem. Int. Ed. 2012, 51, 5200–5203. [Google Scholar] [CrossRef]
- Eccher, J.; Zajaczkowski, W.; Faria, G.C.; Bock, H.; von Seggern, H.; Pisula, W.; Bechtold, I.H. Thermal Evaporation versus Spin-Coating: Electrical Performance in Columnar Liquid Crystal OLEDs. ACS Appl. Mater. Interfaces 2015, 7, 16374–16381. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, J.; Wiebeler, C.; Schumacher, S.; Bock, H.; Kitzerow, H. Enhanced Columnar Mesophase Range through Distortions in Arene Cores. Mol. Cryst. Liq. Cryst. 2017, 646, 66–73. [Google Scholar] [CrossRef]
- Würthner, F. Bay-Substituted Perylene Bisimides: Twisted Fluorophores for Supramolecular Chemistry. Pure Appl. Chem. 2009, 78, 2341–2349. [Google Scholar] [CrossRef]
- Nagarajan, K.; Mallia, A.R.; Muraleedharan, K.; Hariharan, M. Enhanced Intersystem Crossing in Core-Twisted Aromatics. Chem. Sci. 2017, 8, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gu, W.; Lv, L.; Chen, Y.; Yang, Y.; Ye, P.; Wu, J.; Hong, L.; Peng, A.; Huang, H. Triplet Tellurophene-Based Acceptors for Organic Solar Cells. Angew. Chem. Int. Ed. 2018, 57, 1096–1102. [Google Scholar] [CrossRef]
- Würthner, F.; Sautter, A.; Schilling, J. Synthesis of Diazadibenzoperylenes and Characterization of Their Structural, Optical, Redox, and Coordination Properties. J. Org. Chem. 2002, 67, 3037–3044. [Google Scholar] [CrossRef]
- Fan, L.; Xu, Y.; Tian, H. 1,6-Disubstituted Perylene Bisimides: Concise Synthesis and Characterization as Near-Infrared Fluorescent Dyes. Tetrahedron Lett. 2005, 46, 4443–4447. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, S.; Sun, X.; Liu, Y.; Zhu, D. Suzuki Coupling Reaction of 1,6,7,12-Tetrabromoperylene Bisimide. Org. Lett. 2006, 8, 867–870. [Google Scholar] [CrossRef]
- Cai, Y.; Huo, L.; Sun, X.; Wei, D.; Tang, M.; Sun, Y. High Performance Organic Solar Cells Based on a Twisted Bay-Substituted Tetraphenyl Functionalized Perylenediimide Electron Acceptor. Adv. Energy Mater. 2015, 5, 1500032. [Google Scholar] [CrossRef]
- Yin, Z.; Wei, J.; Zheng, Q. Interfacial Materials for Organic Solar Cells: Recent Advances and Perspectives. Adv. Sci. 2016, 3, 1500362. [Google Scholar] [CrossRef]
- Zhang, X.; Pang, S.; Zhang, Z.; Ding, X.; Zhang, S.; He, S.; Zhan, C. Facile Synthesis of 1-Bromo-7-Alkoxyl Perylene Diimide Dyes: Toward Unsymmetrical Functionalizations at the 1,7-Positions. Tetrahedron Lett. 2012, 53, 1094–1097. [Google Scholar] [CrossRef]
- Dubey, R.K.; Westerveld, N.; Eustace, S.J.; Sudhölter, E.J.R.; Grozema, F.C.; Jager, W.F. Synthesis of Perylene-3,4,9,10-Tetracarboxylic Acid Derivatives Bearing Four Different Substituents at the Perylene Core. Org. Lett. 2016, 18, 5648–5651. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Eustace, S.J.; van Mullem, J.S.; Sudhölter, E.J.R.; Grozema, F.C.; Jager, W.F. Perylene Bisimide Dyes with up to Five Independently Introduced Substituents: Controlling the Functionalization Pattern and Photophysical Properties Using Regiospecific Bay Substitution. J. Org. Chem. 2019, 84, 9532–9547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhan, C.; Zhang, X.; Yao, J. Orientation of Bromination in Bay-Region of Perylene Diimides. Tetrahedron 2013, 69, 8155–8160. [Google Scholar] [CrossRef]
- Khokhlov, K.; Schuster, N.J.; Ng, F.; Nuckolls, C. Functionalized Helical Building Blocks for Nanoelectronics. Org. Lett. 2018, 20, 1991–1994. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.B. Photochemical Oxidative Cyclisation of Stilbenes and Stilbenoids—The Mallory-Reaction. Molecules 2010, 15, 4334–4358. [Google Scholar] [CrossRef]
- Takahashi, M.; Suzuki, Y.; Ichihashi, Y.; Yamashita, M.; Kawai, H. 1,3,8,10-Tetrahydro-2,9-Diazadibenzo[cd,lm]Perylenes: Synthesis of Reduced Perylene Bisimide Analogues. Tetrahedron Lett. 2007, 48, 357–359. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. Halogenation of Aromatic Compounds by N-Chloro-, N-Bromo-, and N-Iodosuccinimide. Chem. Lett. 2003, 32, 932–933. [Google Scholar] [CrossRef]
- Takahashi, M.; Asaba, K.; Lua, T.T.; Inuzuka, T.; Uemura, N.; Sakamoto, M.; Sengoku, T.; Yoda, H. Controllable Monobromination of Perylene Ring System: Synthesis of Bay-Functionalized Perylene Dyes. J. Org. Chem. 2018, 83, 624–631. [Google Scholar] [CrossRef]
- Fujimoto, K.; Izawa, S.; Arikai, Y.; Sugimoto, S.; Oue, H.; Inuzuka, T.; Uemura, N.; Sakamoto, M.; Hiramoto, M.; Takahashi, M. Regioselective Bay-Functionalization of Perylenes Toward Tailor-Made Synthesis of Acceptor Materials for Organic Photovoltaics. ChemPlusChem 2020, 85, 285–293. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, J.; Chow, P.C.Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Zhang, J.; Huang, F.; Yan, H. Nonfullerene Acceptor Molecules for Bulk Heterojunction Organic Solar Cells. Chem. Rev. 2018, 118, 3447–3507. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Meng, D.; Cai, Y.; Fan, B.; Li, Y.; Jiang, W.; Huo, L.; Sun, Y.; Wang, Z. Non-Fullerene-Acceptor-Based Bulk-Heterojunction Organic Solar Cells with Efficiency over 7%. J. Am. Chem. Soc. 2015, 137, 11156–11162. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Qian, H.; Xiang, J.; Qu, J.; Wang, Z. Highly Regiospecific Synthetic Approach to Monobay-Functionalized Perylene Bisimide and Di(Perylene Bisimide). Org. Lett. 2009, 11, 3084–3087. [Google Scholar] [CrossRef]
- Pagoaga, B.; Giraudet, L.; Hoffmann, N. Synthesis and Characterisation of 1,7-Di- and Inherently Chiral 1,12-Di- and 1,6,7,12-Tetraarylperylenetetracarbox-3,4:9,10-diimides. Eur. J. Org. Chem. 2014, 5178–5195. [Google Scholar] [CrossRef]
- Jiang, W.; Ye, L.; Li, X.; Xiao, C.; Tan, F.; Zhao, W.; Hou, J.; Wang, Z. Bay-Linked Perylene Bisimides as Promising Non-Fullerene Acceptors for Organic Solar Cells. Chem. Commun. 2014, 50, 1024–1026. [Google Scholar] [CrossRef]
- Meng, D.; Sun, D.; Zhong, C.; Liu, T.; Fan, B.; Huo, L.; Li, Y.; Jiang, W.; Choi, H.; Kim, T.; et al. High-Performance Solution-Processed Non-Fullerene Organic Solar Cells Based on Selenophene-Containing Perylene Bisimide Acceptor. J. Am. Chem. Soc. 2016, 138, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Hendsbee, A.D.; Sun, J.-P.; Law, W.K.; Yan, H.; Hill, I.G.; Spasyuk, D.M.; Welch, G.C. Synthesis, Self-Assembly, and Solar Cell Performance of N-Annulated Perylene Diimide Non-Fullerene Acceptors. Chem. Mater. 2016, 28, 7098–7109. [Google Scholar] [CrossRef]
- Liu, T.; Meng, D.; Cai, Y.; Sun, X.; Li, Y.; Huo, L.; Liu, F.; Wang, Z.; Russell, T.P.; Sun, Y. High-Performance Non-Fullerene Organic Solar Cells Based on a Selenium-Containing Polymer Donor and a Twisted Perylene Bisimide Acceptor. Adv. Sci. 2016, 3, 1600117. [Google Scholar] [CrossRef]
- Fan, Y.; Ziabrev, K.; Zhang, S.; Lin, B.; Barlow, S.; Marder, S.R. Comparison of the Optical and Electrochemical Properties of Bi(perylene diimide)s Linked through Ortho and Bay Positions. ACS Omega 2017, 2, 377–385. [Google Scholar] [CrossRef]
- Vespa, M.; Cann, J.R.; Dayneko, S.V.; Melville, O.A.; Hendsbee, A.D.; Zou, Y.; Lessard, B.H.; Welch, G.C. Synthesis of a Perylene Diimide Dimer with Pyrrolic N–H Bonds and N-Functionalized Derivatives for Organic Field-Effect Transistors and Organic Solar Cells. Eur. J. Org. Chem. 2018, 4592–4599. [Google Scholar] [CrossRef]
- Nazari, M.; Martell, M.; Welsh, T.A.; Melville, O.; Li, Z.; Cann, J.; Cieplechowicz, E.; Zou, Y.; Lessard, B.H.; Welch, G.C. Benzyl and Fluorinated Benzyl Side Chains for Perylene Diimide Non-Fullerene Acceptors. Mater. Chem. Front. 2018, 2, 2272–2276. [Google Scholar] [CrossRef]
- Yan, Q.; Zhou, Y.; Zheng, Y.-Q.; Pei, J.; Zhao, D. Towards Rational Design of Organic Electron Acceptors for Photovoltaics: A Study Based on Perylenediimide Derivatives. Chem. Sci. 2013, 4, 4389–4394. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Qian, D.; Gautam, B.; Yang, G.; Zhao, J.; Bergqvist, J.; Zhang, F.; Ma, W.; Ade, H.; et al. Fast Charge Separation in a Non-Fullerene Organic Solar Cell with a Small Driving Force. Nat. Energy 2016, 1, 16089. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Q.; Cai, Z.; Zheng, T.; Chen, W.; Lu, J.; Yu, L. Electron Acceptors Based on α-Substituted Perylene Diimide (PDI) for Organic Solar Cells. Chem. Mater. 2016, 28, 1139–1146. [Google Scholar] [CrossRef]
- Hartnett, P.E.; Matte, H.S.S.R.; Eastham, N.D.; Jackson, N.E.; Wu, Y.; Chen, L.X.; Ratner, M.A.; Chang, R.P.H.; Hersam, M.C.; Wasielewski, M.R.; et al. Ring-Fusion as a Perylenediimide Dimer Design Concept for High-Performance Non-Fullerene Organic Photovoltaic Acceptors. Chem. Sci. 2016, 7, 3543–3555. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, S.; Zhang, J.; Oswald, V.F.; Amassian, A.; Marder, S.R.; Blakey, S.B. KOtBu-Initiated Aryl C–H Iodination: A Powerful Tool for the Synthesis of High Electron Affinity Compounds. J. Am. Chem. Soc. 2016, 138, 3946–3949. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, X.; Zheng, Y.; Yu, G.; Yao, J.; Zhan, C. A Comparative Study of Photovoltaic Performance Between Non-Fullerene and Fullerene Based Organic Solar Cells. RSC Adv. 2016, 6, 43715–43718. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, T.; Zeng, W.; Xie, D.; Luo, Z.; Sun, Y.; Yang, C. Thienobenzene-Fused Perylene Bisimide as a Non-Fullerene Acceptor for Organic Solar Cells with a High Open-Circuit Voltage and Power Conversion Efficiency. Mater. Chem. Front. 2017, 1, 749–756. [Google Scholar] [CrossRef]
- McAfee, S.M.; Dayneko, S.V.; Josse, P.; Blanchard, P.; Cabanetos, C.; Welch, G.C. Simply Complex: The Efficient Synthesis of an Intricate Molecular Acceptor for High-Performance Air-Processed and Air-Tested Fullerene-Free Organic Solar Cells. Chem. Mater. 2017, 29, 1309–1314. [Google Scholar] [CrossRef]
- Park, M.; Jung, J.W. Anthracene-Based Perylene Diimide Electron-Acceptor for Fullerene-Free Organic Solar Cells. Dyes Pigments 2017, 143, 301–307. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, Q.; Li, W.; Zhao, Y.; Luo, Z.; Zhang, F.; Yang, C. Isomeric Small Molecule Acceptors Based on Perylene Diimide and Spirobifluorene for Non-Fullerene Organic Solar Cells. Dyes Pigments 2017, 146, 151–158. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, H.; Liu, X.; Liu, C.; Shan, H.; Xia, J.; Xu, Z.; Chen, F.; Chen, Z.-K.; Huang, W. Impact of Fluorine Atoms on Perylene Diimide Derivative for Fullerene-Free Organic Photovoltaics. Chem. Asian J. 2017, 12, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.-F.; Cai, Y.; Liu, K.-K.; Song, X.-X.; Liu, J.-J.; Liu, X.; Sun, Y.; Zhang, R.; Wang, J.-L. Rational Design of Two-Dimensional PDI-Based Small Molecular Acceptor from Extended Indacenodithiazole Core for Organic Solar Cells. Dyes Pigments 2017, 147, 31–39. [Google Scholar] [CrossRef]
- Eom, S.H.; Kim, H.S.; Do, H.J.; Lee, U.-H.; Wibowo, F.T.A.; Hwang, D.-H.; Yoon, S.C.; Jung, I.H. n-Type Core Effect on Perylene Diimide Based Acceptors for Panchromatic Fullerene-Free Organic Solar Cells. Dyes Pigments 2018, 156, 318–325. [Google Scholar] [CrossRef]
- Welsh, T.A.; Laventure, A.; Welch, G.C. Direct (Hetero)Arylation for the Synthesis of Molecular Materials: Coupling Thieno[3,4-c]pyrrole-4,6-dione with Perylene Diimide to Yield Novel Non-Fullerene Acceptors for Organic Solar Cells. Molecules 2018, 23, 931. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, G.; Xie, R.; Wang, Z.; Zhong, W.; Li, Y.; Huang, F.; Cao, Y. A Rational Design and Synthesis of Cross-Conjugated Small Molecule Acceptors Approaching High-Performance Fullerene-Free Polymer Solar Cells. Chem. Mater. 2018, 30, 4331–4342. [Google Scholar] [CrossRef]
- Carlotti, B.; Cai, Z.; Kim, H.; Sharapov, V.; Madu, I.K.; Zhao, D.; Chen, W.; Zimmerman, P.M.; Yu, L.; Goodson, T. Charge Transfer and Aggregation Effects on the Performance of Planar vs Twisted Nonfullerene Acceptor Isomers for Organic Solar Cells. Chem. Mater. 2018, 30, 4263–4276. [Google Scholar] [CrossRef]
- Farinhas, J.; Molina, D.; Olcina, A.; Costa, C.; Alcácer, L.; Fernández-Lázaro, F.; Sastre-Santos, Á.; Charas, A. Oligo(Ethylene Oxide) Chains in Fluorene Bridge Units of Perylenediimide Dimers as an Efficient Strategy for Improving the Photovoltaic Performance in Organic Solar Cells. Dyes Pigments 2019, 161, 188–196. [Google Scholar] [CrossRef]
- Welsh, T.A.; Laventure, A.; Alahmadi, A.F.; Zhang, G.; Baumgartner, T.; Zou, Y.; Jäkle, F.; Welch, G.C. Borane Incorporation in a Non-Fullerene Acceptor to Tune Steric and Electronic Properties and Improve Organic Solar Cell Performance. ACS Appl. Energy Mater. 2019, 2, 1229–1240. [Google Scholar] [CrossRef]
- Laventure, A.; Stanzel, S.; Payne, A.-J.; Lessard, B.H.; Welch, G.C. A Ring Fused N-Annulated PDI Non-Fullerene Acceptor for High Open Circuit Voltage Solar Cells Processed from Non-Halogenated Solvents. Synth. Met. 2019, 250, 55–62. [Google Scholar] [CrossRef]
- Gao, G.; Liang, N.; Geng, H.; Jiang, W.; Fu, H.; Feng, J.; Hou, J.; Feng, X.; Wang, Z. Spiro-Fused Perylene Diimide Arrays. J. Am. Chem. Soc. 2017, 139, 15914–15920. [Google Scholar] [CrossRef]
- Shi, G.; Chen, D.; Jiang, H.; Zhang, Y.; Zhang, Y. Synthesis of Fluorenes Starting from 2-Iodobiphenyls and CH2Br2 through Palladium-Catalyzed Dual C–C Bond Formation. Org. Lett. 2016, 18, 2958–2961. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Trinh, M.T.; Chen, R.; Wang, W.; Khlyabich, P.P.; Kumar, B.; Xu, Q.; Nam, C.-Y.; Sfeir, M.Y.; Black, C.; et al. Efficient Organic Solar Cells with Helical Perylene Diimide Electron Acceptors. J. Am. Chem. Soc. 2014, 136, 15215–15221. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, W.; Li, C.-Z.; Liu, F.; Zhang, Y.; Shi, M.; Chen, H.; Russell, T.P. A Simple Perylene Diimide Derivative with a Highly Twisted Geometry as an Electron Acceptor for Efficient Organic Solar Cells. J. Mater. Chem. A 2016, 4, 10659–10665. [Google Scholar] [CrossRef]
- Meng, D.; Fu, H.; Xiao, C.; Meng, X.; Winands, T.; Ma, W.; Wei, W.; Fan, B.; Huo, L.; Doltsinis, N.L.; et al. Three-Bladed Rylene Propellers with Three-Dimensional Network Assembly for Organic Electronics. J. Am. Chem. Soc. 2016, 138, 10184–10190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Huang, J.; Hu, H.; Zhang, G.; Ma, T.; Chow, P.C.Y.; Ade, H.; Pan, D.; Yan, H. Ring-Fusion of Perylene Diimide Acceptor Enabling Efficient Nonfullerene Organic Solar Cells with a Small Voltage Loss. J. Am. Chem. Soc. 2017, 139, 16092–16095. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.E.M.; Cho, K.T.; Zhang, Y.; Urbani, M.; Tabet, N.; de la Torre, G.; Nazeeruddin, M.K.; Torres, T. Tetrathienoanthracene and Tetrathienylbenzene Derivatives as Hole-Transporting Materials for Perovskite Solar Cell. Adv. Energy Mater. 2018, 8, 1800681. [Google Scholar] [CrossRef]
- Dössel, L.; Gherghel, L.; Feng, X.; Müllen, K. Graphene Nanoribbons by Chemists: Nanometer-Sized, Soluble, and Defect-Free. Angew. Chem. Int. Ed. 2011, 50, 2540–2543. [Google Scholar] [CrossRef] [PubMed]
- Ran, N.A.; Love, J.A.; Heiber, M.C.; Jiao, X.; Hughes, M.P.; Karki, A.; Wang, M.; Brus, V.V.; Wang, H.; Neher, D.; et al. Charge Generation and Recombination in an Organic Solar Cell with Low Energetic Offsets. Adv. Energy Mater. 2018, 8, 1701073. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, Y.; Alotaibi, A.; Yuan, J.; Jiang, C.; Xin, J.; Liu, X.; Collins, B.A.; Zhang, F.; Ma, W. Molecular and Energetic Order Dominate the Photocurrent Generation Process in Organic Solar Cells with Small Energetic Offsets. ACS Energy Lett. 2020, 5, 589–596. [Google Scholar] [CrossRef]
- Zhang, G.; Feng, J.; Xu, X.; Ma, W.; Li, Y.; Peng, Q. Perylene Diimide-Based Nonfullerene Polymer Solar Cells with over 11% Efficiency Fabricated by Smart Molecular Design and Supramolecular Morphology Optimization. Adv. Funct. Mater. 2019, 29, 1906587. [Google Scholar] [CrossRef]
- Smith, J.N.; Hook, J.M.; Lucas, N.T. Superphenylphosphines: Nanographene-Based Ligands That Control Coordination Geometry and Drive Supramolecular Assembly. J. Am. Chem. Soc. 2018, 140, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium(0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Wang, J.; Hou, J.; Li, Y.; Zhu, D.; Zhan, X. A Star-Shaped Perylene Diimide Electron Acceptor for High-Performance Organic Solar Cells. Adv. Mater. 2014, 26, 5137–5142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Zhang, K.; Wang, H.; Xiao, Y. A Soluble Ladder-Conjugated Star-Shaped Oligomer Composed of Four Perylene Diimide Branches and a Fluorene Core: Synthesis and Properties. Chem. Eur. J. 2014, 20, 10170–10178. [Google Scholar] [CrossRef]
- Liu, Y.; Mu, C.; Jiang, K.; Zhao, J.; Li, Y.; Zhang, L.; Li, Z.; Lin, J.Y.L.; Hu, H.; Ma, T.; et al. A Tetraphenylethylene Core-Based 3D Structure Small Molecular Acceptor Enabling Efficient Non-Fullerene Organic Solar Cells. Adv. Mater. 2015, 27, 1015–1020. [Google Scholar] [CrossRef]
- Lee, J.; Singh, R.; Sin, D.H.; Kim, H.G.; Song, K.C.; Cho, K. A Nonfullerene Small Molecule Acceptor with 3D Interlocking Geometry Enabling Efficient Organic Solar Cells. Adv. Mater. 2016, 28, 69–76. [Google Scholar] [CrossRef]
- Lin, H.; Chen, S.; Hu, H.; Zhang, L.; Ma, T.; Lai, J.Y.L.; Li, Z.; Qin, A.; Huang, X.; Tang, B.; et al. Reduced Intramolecular Twisting Improves the Performance of 3D Molecular Acceptors in Non-Fullerene Organic Solar Cells. Adv. Mater. 2016, 28, 8546–8551. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xiong, W.; Liu, T.; Cheng, W.; Wu, K.; Sun, Y.; Yang, C. Triphenylamine-Cored Star-Shape Compounds as Non-Fullerene Acceptor for High-Efficiency Organic Solar Cells: Tuning the Optoelectronic Properties by S/Se-Annulated Perylene Diimide. Org. Electron. 2017, 41, 166–172. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, X.; Yan, H.; Wu, W.; Li, Z.; Peng, Q. Pronounced Effects of a Triazine Core on Photovoltaic Performance–Efficient Organic Solar Cells Enabled by a PDI Trimer-Based Small Molecular Acceptor. Adv. Mater. 2017, 29, 1605115. [Google Scholar] [CrossRef]
- Yi, M.; Yi, J.; Wang, J.; Wang, L.; Gao, W.; Lin, Y.; Luo, Q.; Tan, H.; Ma, C.-Q.; Wang, H. Perylenediimide Derivatives Based on a Dendritic Oligothiophene Core as Electron Acceptor for Use in Polymer Solar Cells. Dyes Pigments 2017, 139, 498–508. [Google Scholar] [CrossRef]
- Zhang, A.; Li, C.; Yang, F.; Zhang, J.; Wang, Z.; Wei, Z.; Li, W. An Electron Acceptor with Porphyrin and Perylene Bisimides for Efficient Non-Fullerene Solar Cells. Angew. Chem. Int. Ed. 2017, 56, 2694–2698. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Huang, M.; Zhao, B.; Zhang, J.; Tan, S. A Trilobal Non-Fullerene Electron Acceptor Based on Benzo[1,2-b:3,4-b′:5,6-b″] Trithiophene and Perylenediimide for Polymer Solar Cells. Synth. Met. 2017, 227, 122–130. [Google Scholar] [CrossRef]
- Meng, D.; Fu, H.; Fan, B.; Zhang, J.; Li, Y.; Sun, Y.; Wang, Z. Rigid Nonfullerene Acceptors Based on Triptycene–Perylene Dye for Organic Solar Cells. Chem. Asian J. 2017, 12, 1286–1290. [Google Scholar] [CrossRef]
- Zhang, Y.; Kan, B.; Ke, X.; Wang, Y.; Feng, H.; Zhang, H.; Li, C.; Wan, X.; Chen, Y. 3-Dimensional Non-Fullerene Acceptors Based on Triptycene and Perylene Diimide for Organic Solar Cells. Org. Electron. 2017, 50, 458–465. [Google Scholar] [CrossRef]
- Sun, H.; Song, X.; Xie, J.; Sun, P.; Gu, P.; Liu, C.; Chen, F.; Zhang, Q.; Chen, Z.-K.; Huang, W. PDI Derivative through Fine-Tuning the Molecular Structure for Fullerene-Free Organic Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 29924–29931. [Google Scholar] [CrossRef] [PubMed]
- Jeanbourquin, X.A.; Rahmanudin, A.; Yu, X.; Johnson, M.; Guijarro, N.; Yao, L.; Sivula, K. Amorphous Ternary Charge-Cascade Molecules for Bulk Heterojunction Photovoltaics. ACS Appl. Mater. Interfaces 2017, 9, 27825–27831. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, L.; Shao, M.; Wu, Y.; Zeng, D.; Cai, X.; Duan, J.; Zhang, X.; Gao, X. Fine-Tuning the Quasi-3D Geometry: Enabling Efficient Nonfullerene Organic Solar Cells Based on Perylene Diimides. ACS Appl. Mater. Interfaces 2018, 10, 762–768. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Xia, S.; Zhou, F.; Luo, Z.; Luo, J.; He, F.; Yang, C. 9,9′-Bifluorenylidene-Core Perylene Diimide Acceptors for As-Cast Non-Fullerene Organic Solar Cells: The Isomeric Effect on Optoelectronic Properties. Chem. Eur. J. 2018, 24, 4149–4156. [Google Scholar] [CrossRef]
- Tang, F.; Wu, K.; Zhou, Z.; Wang, G.; Pei, Y.; Zhao, B.; Tan, S. Rational Design of Truxene-Bridged PDI Trimers as Acceptors for Efficient Organic Solar Cells. Dyes Pigments 2018, 156, 276–284. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Y.; Wu, J.; Xia, D.; Hong, S.; Wu, X.; Yu, J.; Zhang, S.; Peng, A.; Huang, H. Iris-Like Acceptor with Most PDI Units for Organic Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 28812–28818. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Q.; Tao, Y.; Guo, Y.; Yang, J.; Liu, Y.; Zhao, L.; Xie, Z.; Huang, W. Fully Conjugated Block Copolymers for Single-Component Solar Cells: Synthesis, Purification, and Characterization. New J. Chem. 2016, 40, 1825–1833. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Awartani, O.; Zhao, J.; Han, H.; Ade, H.; Zhao, D.; Yan, H. A Vinylene-Bridged Perylenediimide-Based Polymeric Acceptor Enabling Efficient All-Polymer Solar Cells Processed under Ambient Conditions. Adv. Mater. 2016, 28, 8483–8489. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Awartani, O.; Han, H.; Zhao, J.; Ade, H.; Yan, H.; Zhao, D. Improved Performance of All-Polymer Solar Cells Enabled by Naphthodiperylenetetraimide-Based Polymer Acceptor. Adv. Mater. 2017, 29, 1700309. [Google Scholar] [CrossRef]
- Lai, W.; Li, C.; Zhang, J.; Yang, F.; Colberts, F.J.M.; Guo, B.; Wang, Q.M.; Li, M.; Zhang, A.; Janssen, R.A.J.; et al. Diketopyrrolopyrrole-Based Conjugated Polymers with Perylene Bisimide Side Chains for Single-Component Organic Solar Cells. Chem. Mater. 2017, 29, 7073–7077. [Google Scholar] [CrossRef]
- Feng, G.; Li, J.; Colberts, F.J.M.; Li, M.; Zhang, J.; Yang, F.; Jin, Y.; Zhang, F.; Janssen, R.A.J.; Li, C.; et al. “Double-Cable” Conjugated Polymers with Linear Backbone toward High Quantum Efficiencies in Single-Component Polymer Solar Cells. J. Am. Chem. Soc. 2017, 139, 18647–18656. [Google Scholar] [CrossRef]
- Cheng, J.; Li, B.; Ren, X.; Liu, F.; Zhao, H.; Wang, H.; Wu, Y.; Chen, W.; Ba, X. Highly Twisted Ladder-Type Backbone Bearing Perylene Diimides for Non-Fullerene Acceptors in Organic Solar Cells. Dyes Pigments 2019, 161, 221–226. [Google Scholar] [CrossRef]



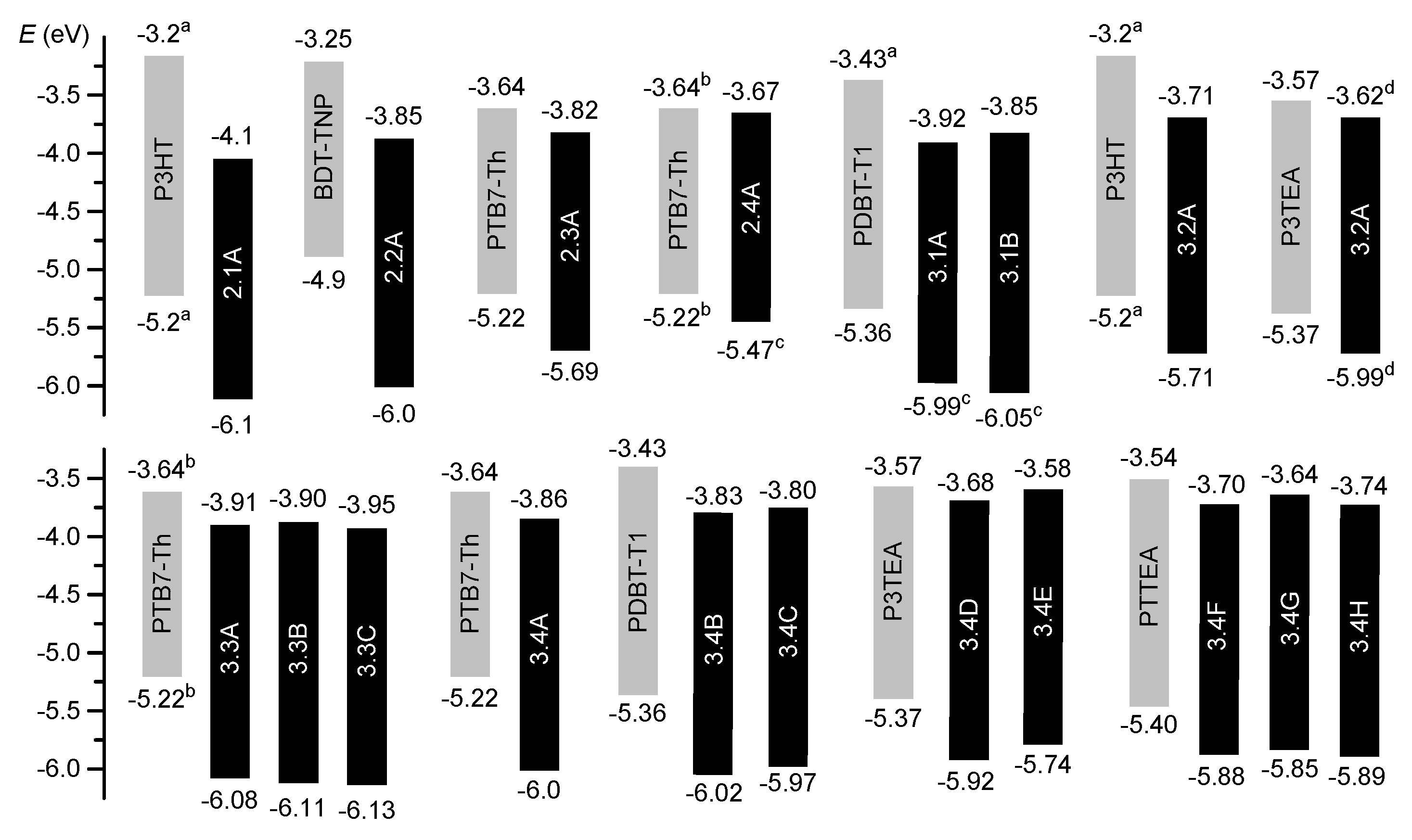
| Comp 1 | Num of PDI 2 | Donor 3 | PCE 4 | Voc (V) 4 | JSC (mA cm−2) 4 | FF 4 | Ref 5 |
|---|---|---|---|---|---|---|---|
| 2.1A | 1 | P3HT | 0.77% | 0.63 | 1.93 | 0.66 | 62 |
| 2.2A | 1 | BTD-TNP | 2.85% | 0.92 | 6.6 | 0.47 | 66 |
| 2.3A | 1 | PTB7-Th | 4.1% | 0.87 | 10.1 | 0.41 | 78 |
| 2.4A | 1 | PTB7-Th | 1.74% | 1.00 | 4.94 | 0.352 | 89 |
| 3.1A | 2 | PDBT-T1 | 5.40% | 0.87 | 10.16 | 0.61 | 91 |
| 3.1B | 2 | PDBT-T1 | 7.16% | 0.90 | 11.98 | 0.66 | 91 |
| 3.2A | 2 | P3HT | 2.35% | 0.61 | 5.92 | 0.65 | 101 |
| 3.2A | 2 | P3TEA | 9.47% | 1.11 | 13.27 | 0.643 | 102 |
| 3.3A | 2 | PTB7-Th | 5.18% | 0.83 | 12.67 | 0.47 | 120 |
| 3.3B | 2 | PTB7-Th | 6.43% | 0.80 | 13.50 | 0.56 | 120 |
| 3.3C | 4 | PTB7-Th | 7.18% | 0.77 | 14.58 | 0.60 | 120 |
| 3.4A | 3 | PTB7-Th | 5.65% | 0.83 | 13.12 | 0.52 | 123 |
| 3.4B | 3 | PDBT-T1 | 8.28% | 0.97 | 12.01 | 0.701 | 124 |
| 3.4C | 3 | PDBT-T1 | 9.28% | 1.00 | 12.53 | 0.717 | 124 |
| 3.4D | 4 | P3TEA | 7.11% | 1.05 | 12.06 | 0.529 | 125 |
| 3.4E | 4 | P3TEA | 10.58% | 1.13 | 13.89 | 0.659 | 125 |
| 3.4F | 3 | PTTEA | 11.01% | 0.99 | 14.89 | 0.747 | 130 |
| 3.4G | 3 | PTTEA | 6.19% | 1.02 | 12.25 | 0.495 | 130 |
| 3.4H | 3 | PTTEA | 9.67% | 0.97 | 14.20 | 0.702 | 130 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujimoto, K.; Takahashi, M.; Izawa, S.; Hiramoto, M. Development of Perylene-Based Non-Fullerene Acceptors through Bay-Functionalization Strategy. Materials 2020, 13, 2148. https://doi.org/10.3390/ma13092148
Fujimoto K, Takahashi M, Izawa S, Hiramoto M. Development of Perylene-Based Non-Fullerene Acceptors through Bay-Functionalization Strategy. Materials. 2020; 13(9):2148. https://doi.org/10.3390/ma13092148
Chicago/Turabian StyleFujimoto, Keisuke, Masaki Takahashi, Seiichiro Izawa, and Masahiro Hiramoto. 2020. "Development of Perylene-Based Non-Fullerene Acceptors through Bay-Functionalization Strategy" Materials 13, no. 9: 2148. https://doi.org/10.3390/ma13092148
APA StyleFujimoto, K., Takahashi, M., Izawa, S., & Hiramoto, M. (2020). Development of Perylene-Based Non-Fullerene Acceptors through Bay-Functionalization Strategy. Materials, 13(9), 2148. https://doi.org/10.3390/ma13092148






