Improving the Performance of Zn-Air Batteries with N-Doped Electroexfoliated Graphene
Abstract
1. Introduction
2. Materials and Methods
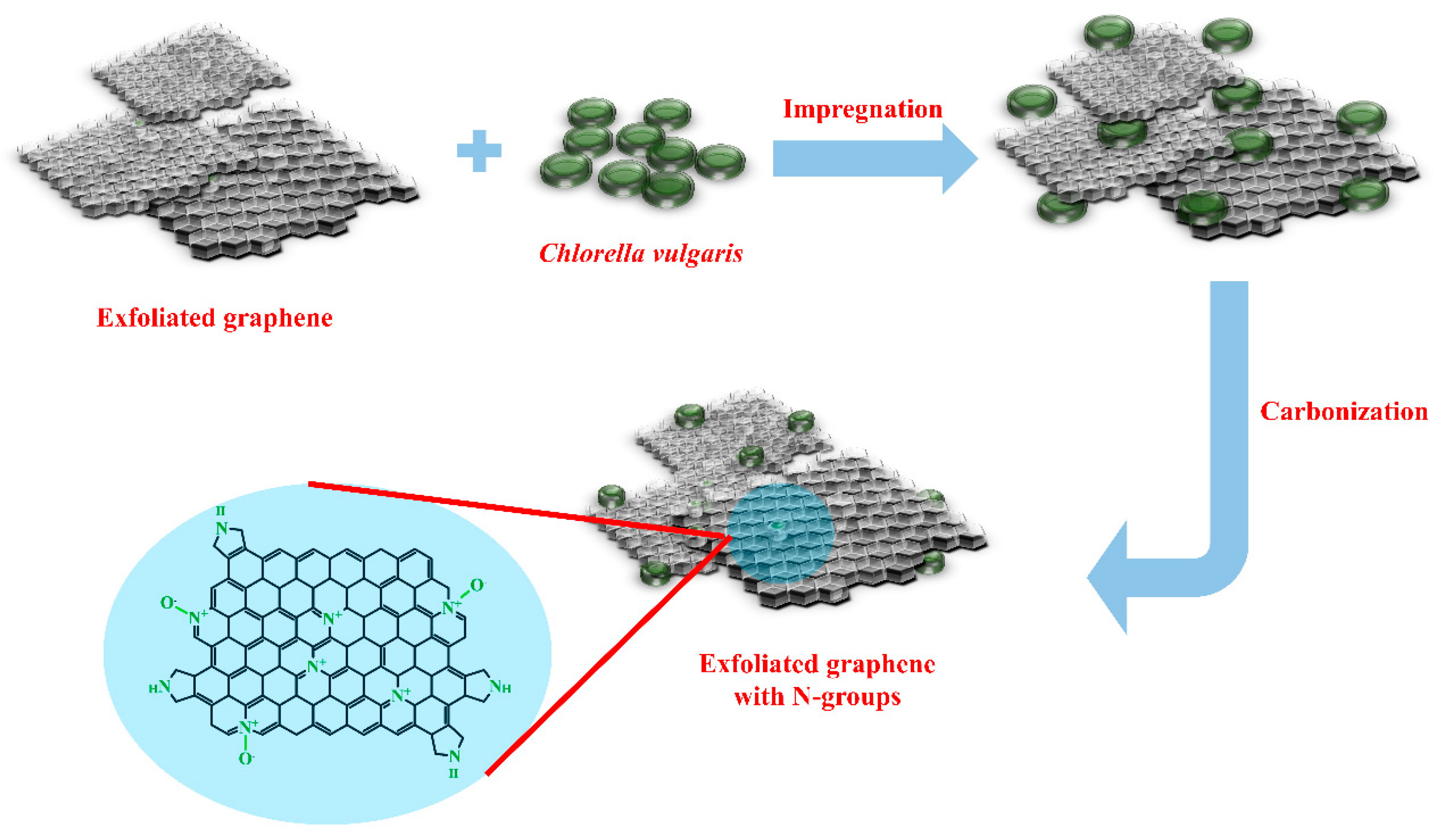
2.1. Preparation of N-Doped Graphene
2.2. Physicochemical Characterization
2.3. Electrochemical Measurements
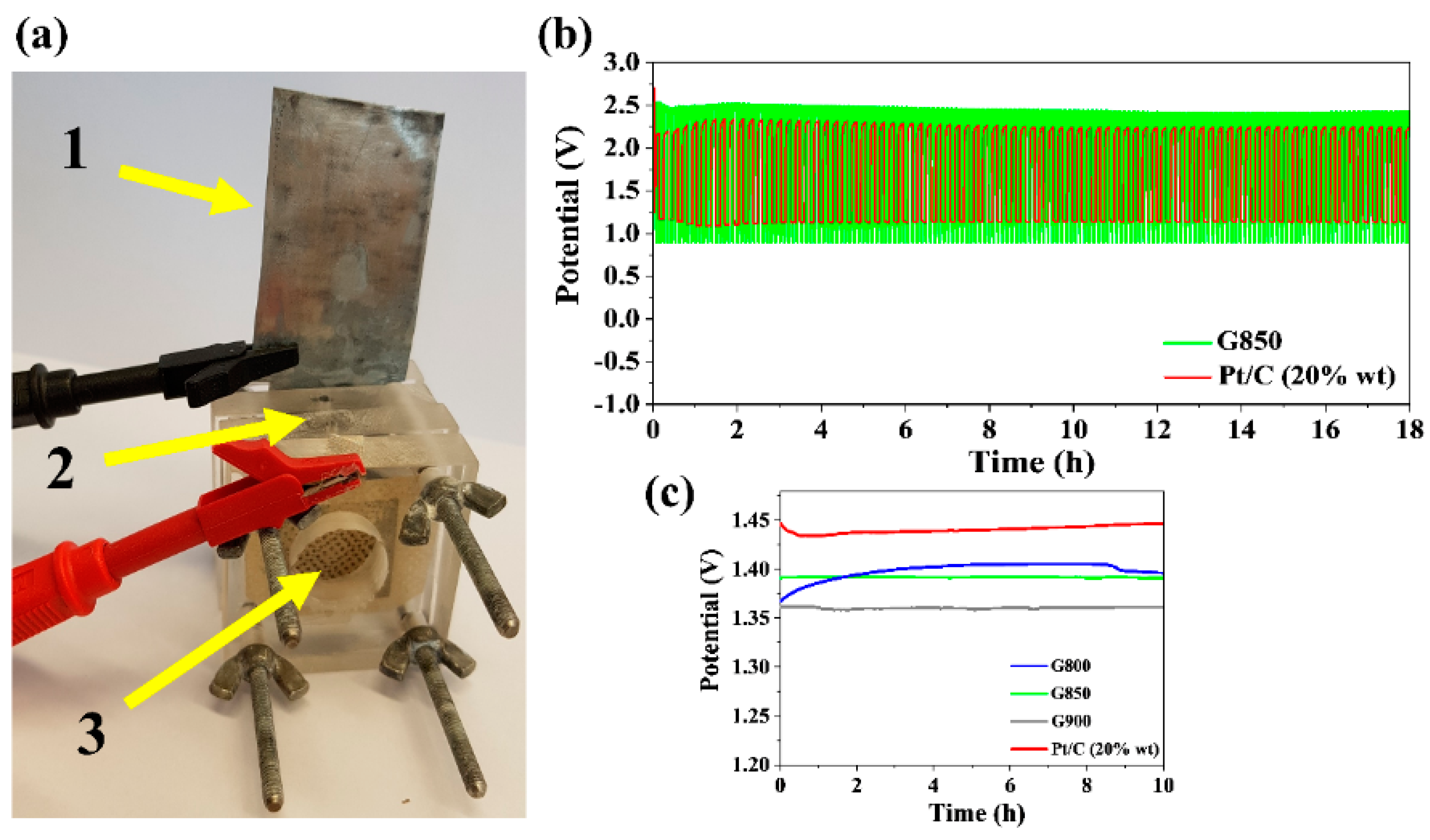
2.4. Manufacturing a Zn-Air Battery
3. Results and Discussion
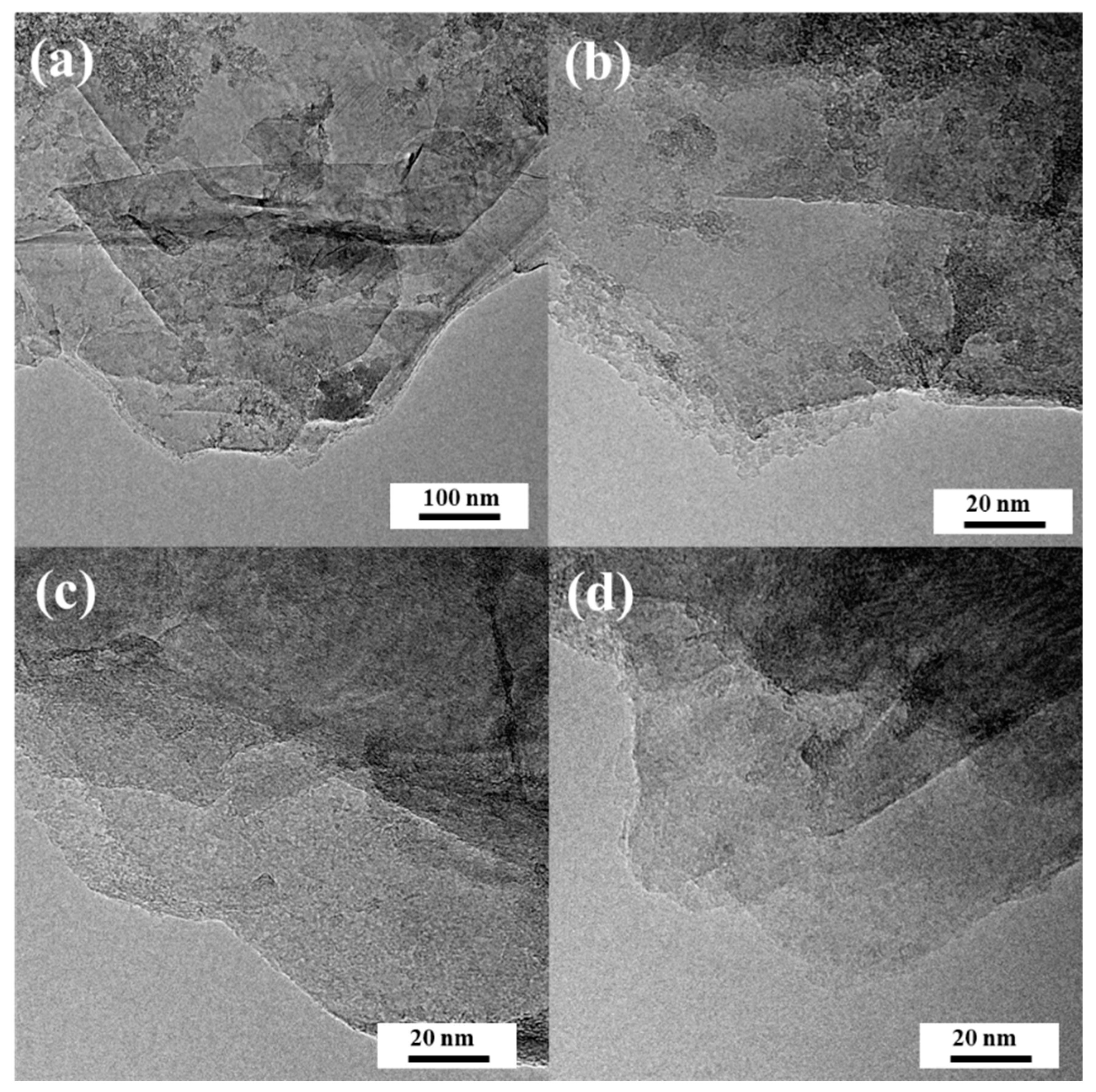
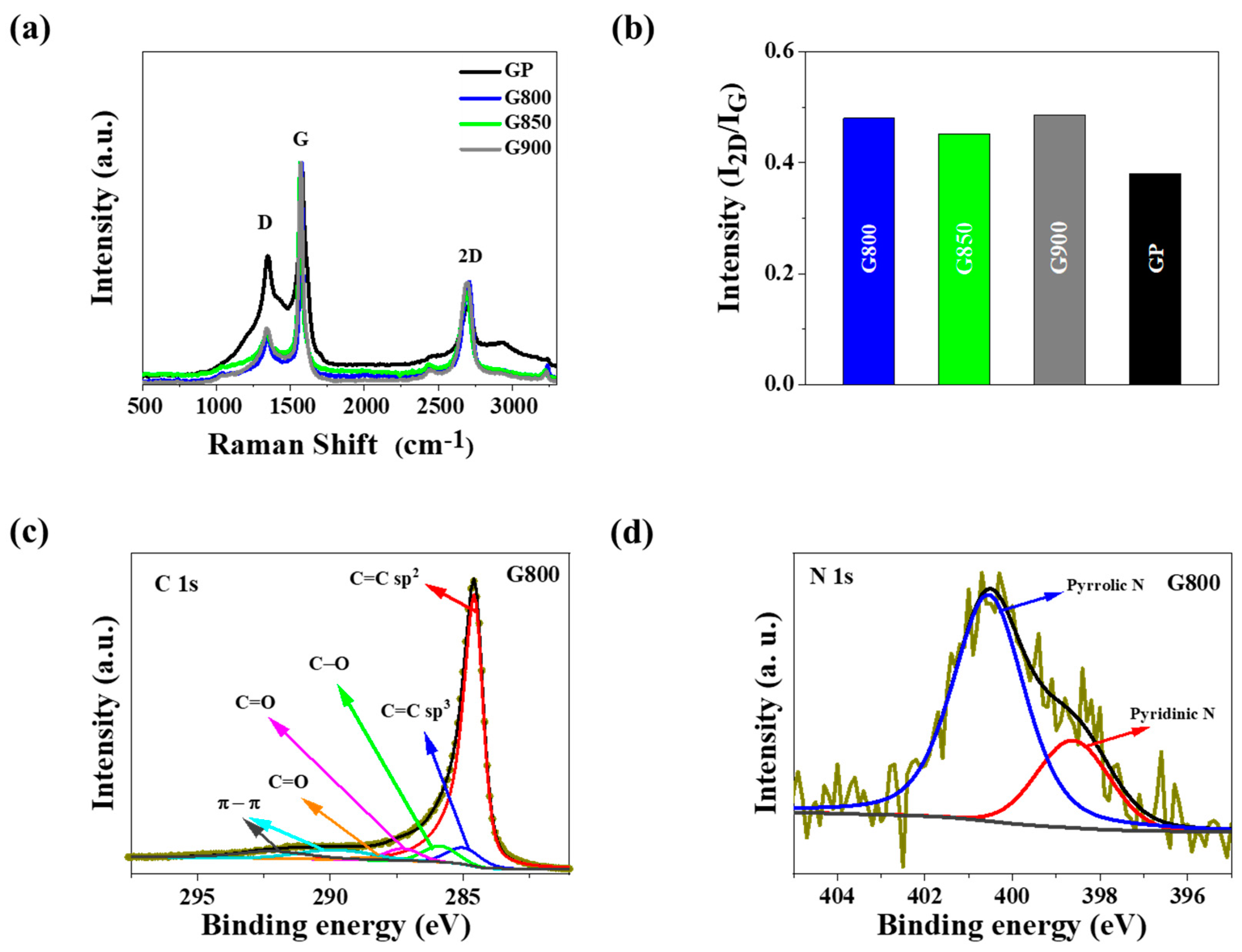
3.1. Material Characterization
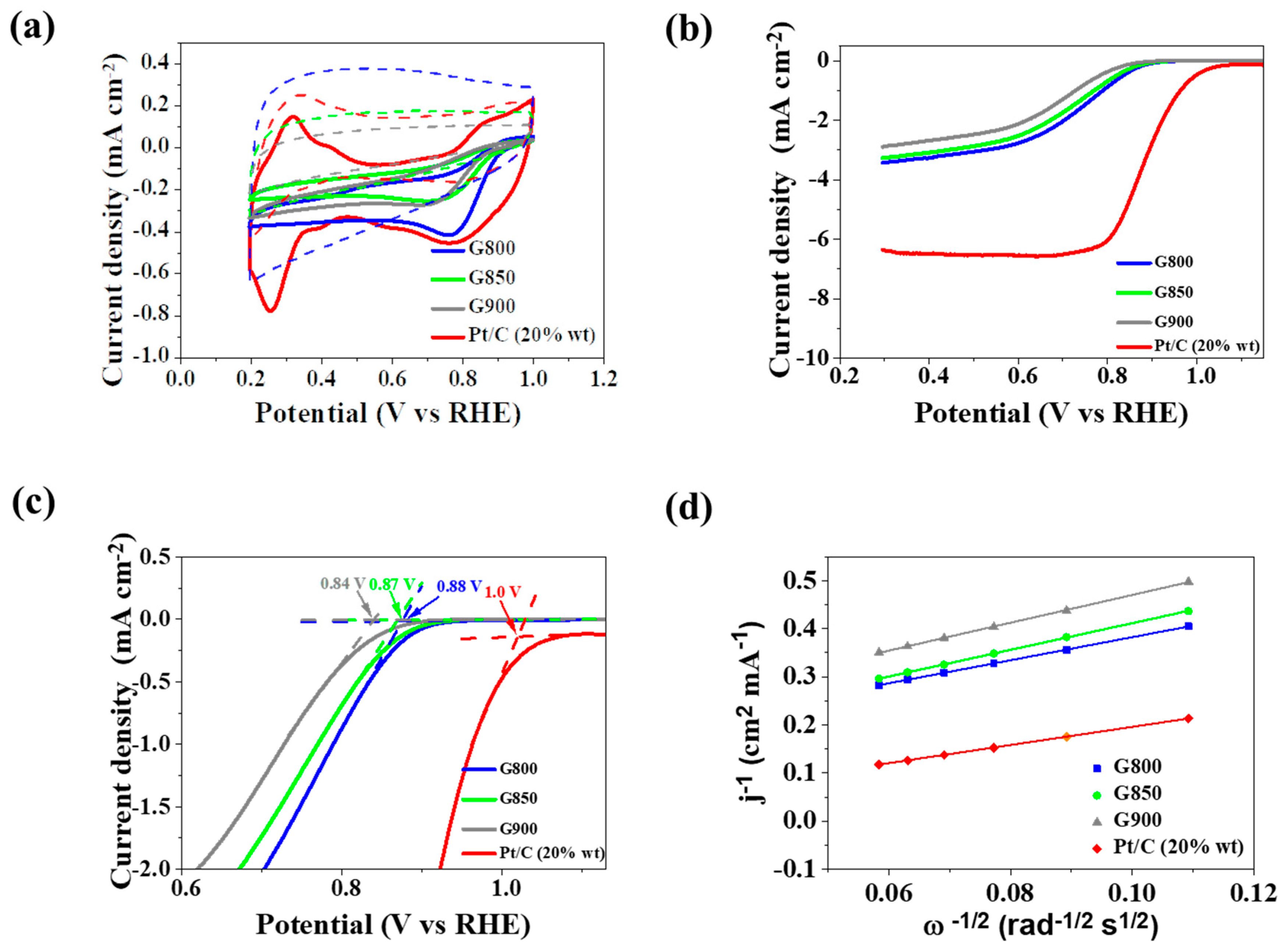
3.2. Electroctrochemical Performance
3.3. Rechargeable Zn-Air Battery Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Tai Kim, S.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal–air batteries with high energy density: Li–air versus Zn–air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Christensen, J.; Albertus, P.; Sanchez-Carrera, R.S.; Lohmann, T.; Kozinsky, B.; Liedtke, R.; Ahmed, J.; Kojic, A. A critical review of Li/air batteries. J. Electrochem. Soc. 2011, 159, R1. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Tang, Y.; Zhang, L.; Li, Y. Zinc–air batteries: Are they ready for prime time? Chem. Sci. 2019, 10, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-F.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Khan, Z.; Vagin, M.; Crispin, X. Can Hybrid Na–Air Batteries Outperform Nonaqueous Na–O2 Batteries? Adv. Sci. 2020, 7, 1902866. [Google Scholar] [CrossRef]
- Bruce, P.G.; Hardwick, L.J.; Abraham, K. Lithium-air and lithium-sulfur batteries. MRS Bull. 2011, 36, 506–512. [Google Scholar] [CrossRef]
- Aurbach, D.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 2016, 1, 1–11. [Google Scholar] [CrossRef]
- Fan, Y.; Ida, S.; Staykov, A.; Akbay, T.; Hagiwara, H.; Matsuda, J.; Kaneko, K.; Ishihara, T. Ni-Fe Nitride Nanoplates on Nitrogen-Doped Graphene as a Synergistic Catalyst for Reversible Oxygen Evolution Reaction and Rechargeable Zn-Air Battery. Small 2017, 13, 1700099. [Google Scholar] [CrossRef]
- Cao, R.; Lee, J.S.; Liu, M.; Cho, J. Recent progress in non-precious catalysts for metal-air batteries. Adv. Energy Mater. 2012, 2, 816–829. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, D.U.; Nazar, L.F.; Chen, Z. Oxygen reduction reaction using MnO2 nanotubes/nitrogen-doped exfoliated graphene hybrid catalyst for Li-O2 battery applications. J. Electrochem. Soc. 2013, 160, A344–A350. [Google Scholar] [CrossRef]
- Jiang, K.; Zhao, D.; Guo, S.; Zhang, X.; Zhu, X.; Guo, J.; Lu, G.; Huang, X. Efficient oxygen reduction catalysis by subnanometer Pt alloy nanowires. Sci. Adv. 2017, 3, e1601705. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.-Y.; Su, D. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, C.; Li, W.; Guo, H.; Su, X.; He, P.; Wang, Y.; Xia, Y. Ruthenium oxide coated ordered mesoporous carbon nanofiber arrays: A highly bifunctional oxygen electrocatalyst for rechargeable Zn–air batteries. J. Mater. Chem. A 2016, 4, 6282–6289. [Google Scholar] [CrossRef]
- Cai, P.; Ci, S.; Zhang, E.; Shao, P.; Cao, C.; Wen, Z. FeCo alloy nanoparticles confined in carbon layers as high-activity and robust cathode catalyst for Zn-air battery. Electrochim. Acta 2016, 220, 354–362. [Google Scholar] [CrossRef]
- Nam, G.; Park, J.; Choi, M.; Oh, P.; Park, S.; Kim, M.G.; Park, N.; Cho, J.; Lee, J.-S. Carbon-coated core–shell Fe–Cu nanoparticles as highly active and durable electrocatalysts for a Zn–air battery. ACS Nano 2015, 9, 6493–6501. [Google Scholar] [CrossRef]
- Fu, G.; Cui, Z.; Chen, Y.; Xu, L.; Tang, Y.; Goodenough, J.B. Hierarchically mesoporous nickel-iron nitride as a cost-efficient and highly durable electrocatalyst for Zn-air battery. Nano Energy 2017, 39, 77–85. [Google Scholar] [CrossRef]
- Yang, Z.K.; Lin, L.; Xu, A.W. 2D Nanoporous Fe−N/C Nanosheets as Highly Efficient Non-Platinum Electrocatalysts for Oxygen Reduction Reaction in Zn-Air Battery. Small 2016, 12, 5710–5719. [Google Scholar] [CrossRef]
- Wang, X.; Sebastian, P.; Smit, M.A.; Yang, H.; Gamboa, S. Studies on the oxygen reduction catalyst for zinc–air battery electrode. J. Power Sources 2003, 124, 278–284. [Google Scholar] [CrossRef]
- Yin, J.; Shen, L.; Li, Y.; Lu, M.; Sun, K.; Xi, P. CoFe2O4 nanoparticles as efficient bifunctional catalysts applied in Zn–air battery. J. Mater. Res. 2018, 33, 590–600. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, T.; Jiang, H.; Wei, L.; Zhang, Z. Facile preparation of high-performance MnO2/KB air cathode for Zn-air batteries. Electrochim. Acta 2016, 222, 1438–1444. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Xue, D.; Guan, C.; Xi, P.; Gao, D.; Huang, W. Energy-level engineered hollow N-doped NiS1.03 for Zn–Air batteries. Energy Storage Mater. 2020, 25, 202–209. [Google Scholar] [CrossRef]
- Liu, P.; Ran, J.; Xia, B.; Xi, S.; Gao, D.; Wang, J. Bifunctional Oxygen Electrocatalyst of Mesoporous Ni/NiO Nanosheets for Flexible Rechargeable Zn–Air Batteries. Nano Micro Lett. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Chen, Z. Nitrogen doped carbon nanotubes and their impact on the oxygen reduction reaction in fuel cells. Carbon 2010, 48, 3057–3065. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.; Dou, S.; Wang, S.; Zhang, J.; Gao, X.; Ma, J.; Yu, Y. Nitrogen-doped hierarchically porous carbon networks: Synthesis and applications in lithium-ion battery, sodium-ion battery and zinc-air battery. Electrochim. Acta 2016, 219, 592–603. [Google Scholar] [CrossRef]
- Liang, H.-W.; Wu, Z.-Y.; Chen, L.-F.; Li, C.; Yu, S.-H. Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: An efficient metal-free oxygen reduction electrocatalyst for zinc-air battery. Nano Energy 2015, 11, 366–376. [Google Scholar] [CrossRef]
- Lin, H.; Chen, D.; Lu, C.; Zhang, C.; Qiu, F.; Han, S.; Zhuang, X. Rational synthesis of N/S-doped porous carbons as high efficient electrocatalysts for oxygen reduction reaction and Zn-Air batteries. Electrochim. Acta 2018, 266, 17–26. [Google Scholar] [CrossRef]
- Tan, A.-D.; Wang, Y.-F.; Fu, Z.-Y.; Tsiakaras, P.; Liang, Z.-X. Highly effective oxygen reduction reaction electrocatalysis: Nitrogen-doped hierarchically mesoporous carbon derived from interpenetrated nonporous metal-organic frameworks. Appl. Catal. B Environ. 2017, 218, 260–266. [Google Scholar] [CrossRef]
- Wu, Y.; Nagata, S.; Nabae, Y. Genuine four-electron oxygen reduction over precious-metal-free catalyst in alkaline media. Electrochim. Acta 2019, 319, 382–389. [Google Scholar] [CrossRef]
- Geng, D.; Ding, N.; Hor, T.A.; Liu, Z.; Sun, X.; Zong, Y. Potential of metal-free “graphene alloy” as electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 1795–1810. [Google Scholar] [CrossRef]
- Vikkisk, M.; Kruusenberg, I.; Joost, U.; Shulga, E.; Kink, I.; Tammeveski, K. Electrocatalytic oxygen reduction on nitrogen-doped graphene in alkaline media. Appl. Catal. B Environ. 2014, 147, 369–376. [Google Scholar] [CrossRef]
- Hu, J.; Wang, L.; Shi, L.; Huang, H. Oxygen reduction reaction activity of LaMn1-xCoxO3-graphene nanocomposite for zinc-air battery. Electrochim. Acta 2015, 161, 115–123. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yim, W.-L.; Suryanto, B.H.; Zhao, C. Electrocatalytic oxygen evolution at surface-oxidized multiwall carbon nanotubes. J. Am. Chem. Soc. 2015, 137, 2901–2907. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Yue, X.; Jia, J.; Guo, S. Bamboo-like carbon nanotube/Fe3C nanoparticle hybrids and their highly efficient catalysis for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 1436–1439. [Google Scholar] [CrossRef]
- Wang, T.; Kaempgen, M.; Nopphawan, P.; Wee, G.; Mhaisalkar, S.; Srinivasan, M. Silver nanoparticle-decorated carbon nanotubes as bifunctional gas-diffusion electrodes for zinc–air batteries. J. Power Sources 2010, 195, 4350–4355. [Google Scholar] [CrossRef]
- Liu, X.; Park, M.; Kim, M.G.; Gupta, S.; Wang, X.; Wu, G.; Cho, J. High-performance non-spinel cobalt–manganese mixed oxide-based bifunctional electrocatalysts for rechargeable zinc–air batteries. Nano Energy 2016, 20, 315–325. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, C.; Xu, J.; Zhao, Y.; Zhang, J.; Guan, L. Nest-like assembly of the doped single-walled carbon nanotubes with unique mesopores as ultrastable catalysts for high power density Zn-air battery. Carbon 2018, 128, 46–53. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, L.; Shi, R.; Zhang, X.; Waterhouse, G.I.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. 3D carbon nanoframe scaffold-immobilized Ni3FeN nanoparticle electrocatalysts for rechargeable zinc-air batteries’ cathodes. Nano Energy 2017, 40, 382–389. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.-F.; Yan, Y.-M.; Wang, F.; Deng, C.; Rooney, D.; Sun, K.-N. CoO nanoparticles embedded in three-dimensional nitrogen/sulfur co-doped carbon nanofiber networks as a bifunctional catalyst for oxygen reduction/evolution reactions. Carbon 2016, 106, 84–92. [Google Scholar] [CrossRef]
- Mulyadi, A.; Zhang, Z.; Dutzer, M.; Liu, W.; Deng, Y. Facile approach for synthesis of doped carbon electrocatalyst from cellulose nanofibrils toward high-performance metal-free oxygen reduction and hydrogen evolution. Nano Energy 2017, 32, 336–346. [Google Scholar] [CrossRef]
- Park, G.S.; Lee, J.-S.; Kim, S.T.; Park, S.; Cho, J. Porous nitrogen doped carbon fiber with churros morphology derived from electrospun bicomponent polymer as highly efficient electrocatalyst for Zn–air batteries. J. Power Sources 2013, 243, 267–273. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, Z.; Ge, X.; Yang, S.; Peng, Y.; Kang, Z.; Liu, Z.; Lee, J.Y.; Zhao, D. A metal-free ORR/OER bifunctional electrocatalyst derived from metal-organic frameworks for rechargeable Zn-Air batteries. Carbon 2017, 111, 641–650. [Google Scholar] [CrossRef]
- Song, L.-T.; Wu, Z.-Y.; Liang, H.-W.; Zhou, F.; Yu, Z.-Y.; Xu, L.; Pan, Z.; Yu, S.-H. Macroscopic-scale synthesis of nitrogen-doped carbon nanofiber aerogels by template-directed hydrothermal carbonization of nitrogen-containing carbohydrates. Nano Energy 2016, 19, 117–127. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.B.; Dai, L. BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2012, 51, 4209–4212. [Google Scholar] [CrossRef]
- Kim, D.-W.; Li, O.L.; Saito, N. Enhancement of ORR catalytic activity by multiple heteroatom-doped carbon materials. Phys. Chem. Chem. Phys. 2015, 17, 407–413. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. N, P-Codoped Carbon Networks as Efficient Metal-free Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Angew. Chem. Int. Ed. 2016, 55, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Ilnicka, A.; Skorupska, M.; Kamedulski, P.; Lukaszewicz, J.P. Electro-Exfoliation of Graphite to Graphene in an Aqueous Solution of Inorganic Salt and the Stabilization of Its Sponge Structure with Poly (Furfuryl Alcohol). Nanomaterials 2019, 9, 971. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]
- Ilnicka, A.; Lukaszewicz, J.P.; Shimanoe, K.; Yuasa, M. Urea treatment of nitrogen-doped carbon leads to enhanced performance for the oxygen reduction reaction. J. Mater. Res. 2018, 33, 1612–1624. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Liu, Y.; Geng, X.; Yang, H.; Sun, C.; An, B. Synthesis and ORR performance of nitrogen-doped ordered microporous carbon by CVD of acetonitrile vapor using silanized zeolite as template. Appl. Surf. Sci. 2020, 504, 144438. [Google Scholar] [CrossRef]
- Mao, X.; Cao, Z.; Chen, S.; Jia, J.; Li, X.; Yin, Y.; Yang, S. Facile synthesis of N, P-doped hierarchical porous carbon framework catalysts based on gelatin/phytic acid supermolecules for electrocatalytic oxygen reduction. Int. J. Hydrog. Energy 2019, 44, 5890–5898. [Google Scholar] [CrossRef]
- Borghei, M.; Laocharoen, N.; Kibena-Põldsepp, E.; Johansson, L.-S.; Campbell, J.; Kauppinen, E.; Tammeveski, K.; Rojas, O.J. Porous N, P-doped carbon from coconut shells with high electrocatalytic activity for oxygen reduction: Alternative to Pt-C for alkaline fuel cells. Appl. Catal. B Environ. 2017, 204, 394–402. [Google Scholar] [CrossRef]
- Ilnicka, A.; Kamedulski, P.; Lukaszewicz, J.P. Pyrolysis of Chlorella vulgaris as a green chemistry method for manufacturing of nitrogen doped porous carbon materials of high application potential. Mater. Express 2017, 7, 25–34. [Google Scholar] [CrossRef]
- Malard, L.; Pimenta, M.; Dresselhaus, G.; Dresselhaus, M. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Wang, L.; Ni, Z.; Wang, Z.; Wang, R.; Koo, C.K.; Shen, Z.; Thong, J.T. Probing layer number and stacking order of few-layer graphene by Raman spectroscopy. Small 2010, 6, 195–200. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Zhang, X.; Han, W.-P.; Lu, Y.; Shi, W.; Wu, J.-B.; Tan, P.-H. Raman spectroscopy at the edges of multilayer graphene. Carbon 2015, 85, 221–224. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Genet, M.J. XPS analysis of bio-organic systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.T.; Hor, T.A.; Zong, Y.; Liu, Z. Oxygen reduction in alkaline media: From mechanisms to recent advances of catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Wu, J.; Nabae, Y.; Muthukrishnan, A.; Ohsaka, T. Electrochemical deposition and dissolution of Fe species for N-doped carbon to understand the degradation mechanism of Pt-free oxygen reduction catalysts. Electrochim. Acta 2016, 214, 307–312. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, D.; Niwa, H.; Harada, Y.; Oshima, M.; Ofuchi, H.; Nabae, Y.; Okajima, T.; Ohsaka, T. Enhancement in Kinetics of the Oxygen Reduction Reaction on a Nitrogen-Doped Carbon Catalyst by Introduction of Iron via Electrochemical Methods. Langmuir 2015, 31, 5529–5536. [Google Scholar] [CrossRef]
- Genet, M.J.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS Analysis of Biosystems and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2008; pp. 177–307. [Google Scholar]
- Ilnicka, A.; Gauden, P.A.; Terzyk, A.P.; Lukaszewicz, J.P. Nano-structured carbon matrixes obtained from chitin and chitosan by a novel method. J. Nanosci. Nanotechnol. 2016, 16, 2623–2631. [Google Scholar] [CrossRef]
- Ilnicka, A.; Lukaszewicz, J.P. Synthesis of N-rich microporous carbon materials from chitosan by alkali activation using Na2CO3. Mater. Sci. Eng. B 2015, 201, 66–71. [Google Scholar] [CrossRef]
- Yasuda, S.; Yu, L.; Kim, J.; Murakoshi, K. Selective nitrogen doping in graphene for oxygen reduction reactions. Chem. Commun. 2013, 49, 9627–9629. [Google Scholar] [CrossRef]
- Kim, E.-A.; Neto, A.C. Graphene as an electronic membrane. EPL Europhys. Lett. 2008, 84, 57007. [Google Scholar] [CrossRef]
- Asefa, T. Metal-free and noble metal-free heteroatom-doped nanostructured carbons as prospective sustainable electrocatalysts. Acc. Chem. Res. 2016, 49, 1873–1883. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444. [Google Scholar] [CrossRef]
- Khan, Z.; Park, S.O.; Yang, J.; Park, S.; Shanker, R.; Song, H.-K.; Kim, Y.; Kwak, S.K.; Ko, H. Binary N, S-doped carbon nanospheres from bio-inspired artificial melanosomes: A route to efficient air electrodes for seawater batteries. J. Mater. Chem. A 2018, 6, 24459–24467. [Google Scholar] [CrossRef]
- Wiggins-Camacho, J.D.; Stevenson, K.J. Effect of nitrogen concentration on capacitance, density of states, electronic conductivity, and morphology of N-doped carbon nanotube electrodes. J. Phys. Chem. C 2009, 113, 19082–19090. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, R.; Liao, H.; Hou, Y. Synthesis of amino-functionalized graphene as metal-free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2013, 2, 88–97. [Google Scholar] [CrossRef]
- Tsyganov, D.; Bundaleska, N.; Dias, A.; Henriques, J.; Felizardo, E.; Abrashev, M.; Kissovski, Z.; do Rego, A.M.; Ferraria, A.; Tatarova, E. Microwave plasma-based direct synthesis of free-standing N-graphene. Phys. Chem. Chem. Phys. 2020, 22, 4772–4787. [Google Scholar] [CrossRef]
- Legrand, U.; Boudreault, R.; Meunier, J.L. Decoration of N-functionalized graphene nanoflakes with copper-based nanoparticles for high selectivity CO2 electroreduction towards formate. Electrochim. Acta 2019, 318, 142–150. [Google Scholar] [CrossRef]
| Sample | Binding Energy (eV) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 284.6 | 285 | 286.3 | 287.7 | 288.6 | 289.6 | 292.1 | 532 | 533.3 | 398.7 | 400.5 | |
| Elemental Content (at.%) | |||||||||||
| C | O | N | |||||||||
| G800 | 64.5 | 4.9 | 4.7 | 3.6 | 0.8 | 6.1 | 5.6 | 0.8 | 6.1 | 0.4 | 1.2 |
| G850 | 63.1 | 7.8 | 4.9 | 3.5 | 1 | 5.6 | 5.5 | 1.1 | 4.3 | 0.8 | 1.4 |
| G900 | 62.6 | 6.8 | 5.3 | 3.7 | 0.8 | 6.5 | 4.6 | 0.9 | 5.7 | 0.3 | 1.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilnicka, A.; Skorupska, M.; Romanowski, P.; Kamedulski, P.; Lukaszewicz, J.P. Improving the Performance of Zn-Air Batteries with N-Doped Electroexfoliated Graphene. Materials 2020, 13, 2115. https://doi.org/10.3390/ma13092115
Ilnicka A, Skorupska M, Romanowski P, Kamedulski P, Lukaszewicz JP. Improving the Performance of Zn-Air Batteries with N-Doped Electroexfoliated Graphene. Materials. 2020; 13(9):2115. https://doi.org/10.3390/ma13092115
Chicago/Turabian StyleIlnicka, Anna, Malgorzata Skorupska, Piotr Romanowski, Piotr Kamedulski, and Jerzy P. Lukaszewicz. 2020. "Improving the Performance of Zn-Air Batteries with N-Doped Electroexfoliated Graphene" Materials 13, no. 9: 2115. https://doi.org/10.3390/ma13092115
APA StyleIlnicka, A., Skorupska, M., Romanowski, P., Kamedulski, P., & Lukaszewicz, J. P. (2020). Improving the Performance of Zn-Air Batteries with N-Doped Electroexfoliated Graphene. Materials, 13(9), 2115. https://doi.org/10.3390/ma13092115








