Influence of Bio-Based Plasticizers on the Properties of NBR Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Processing of Plasticized Compounds
2.2. Tests
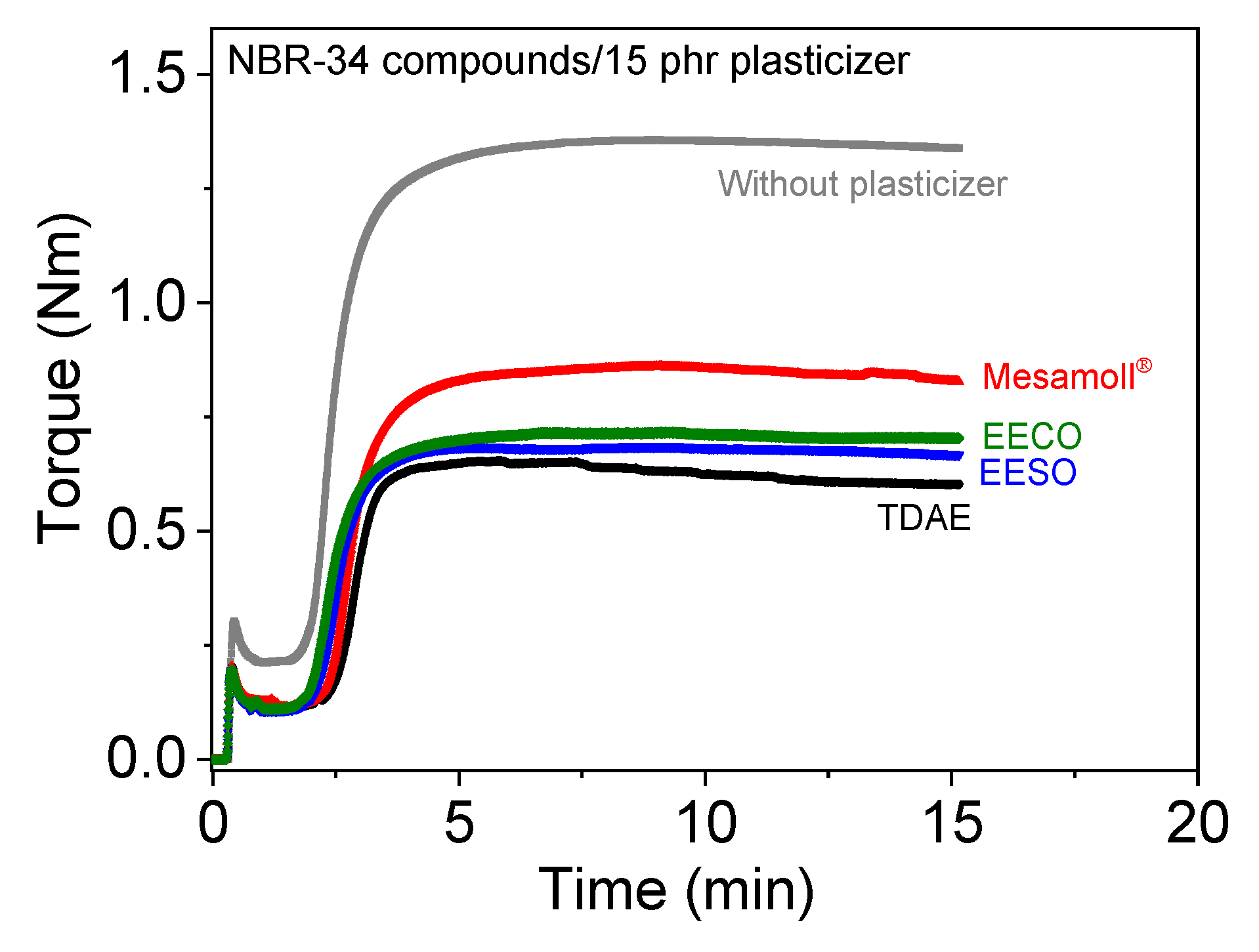
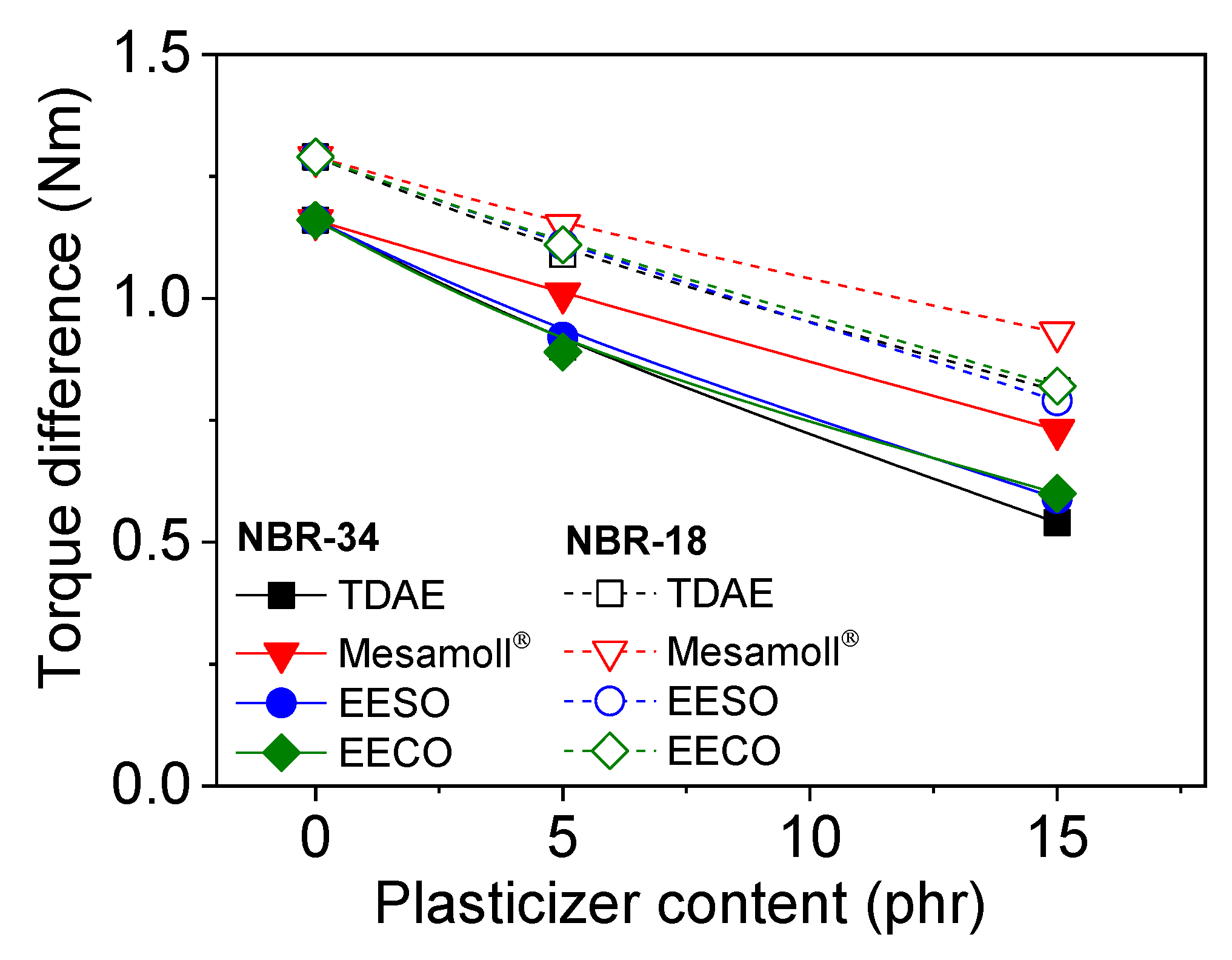
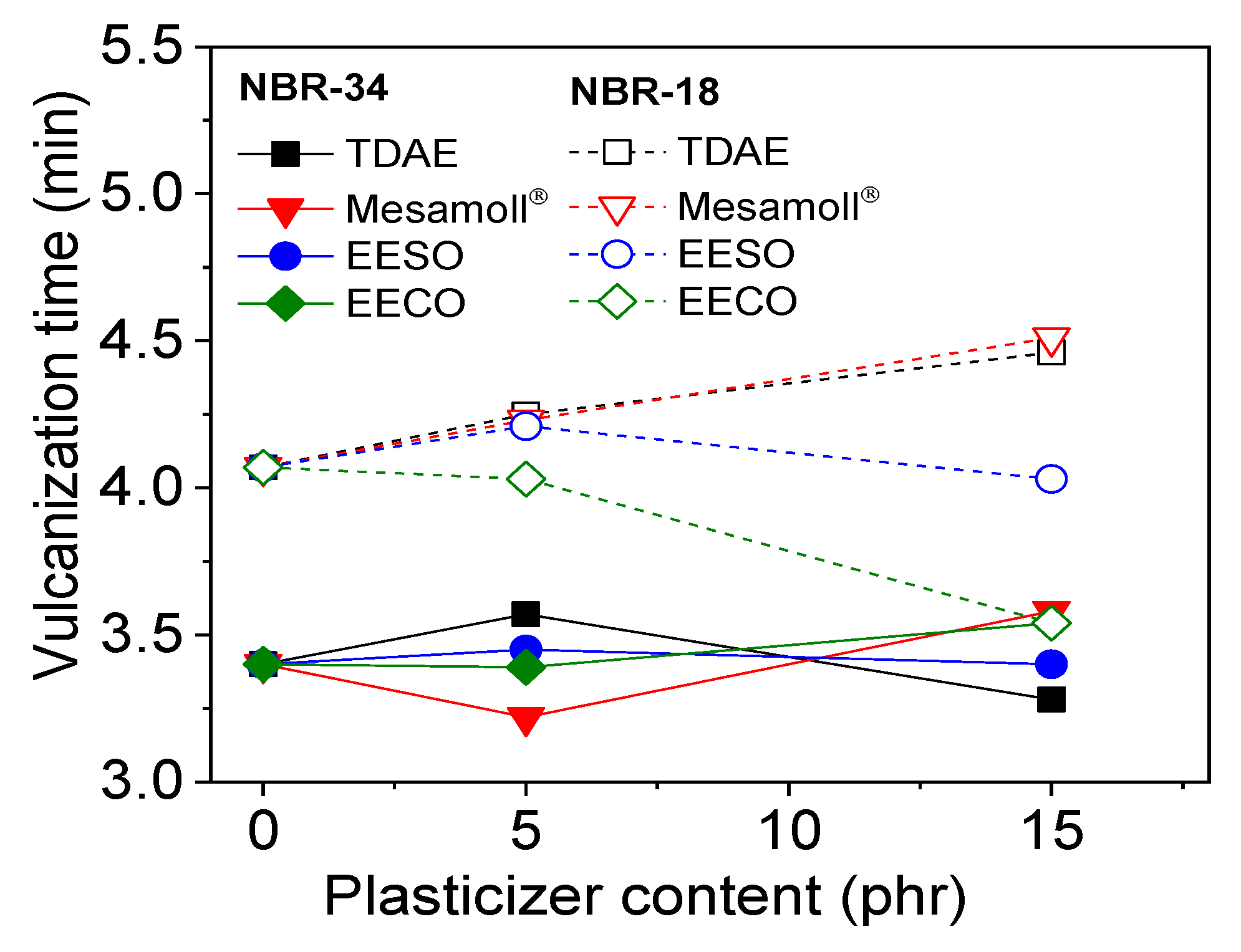
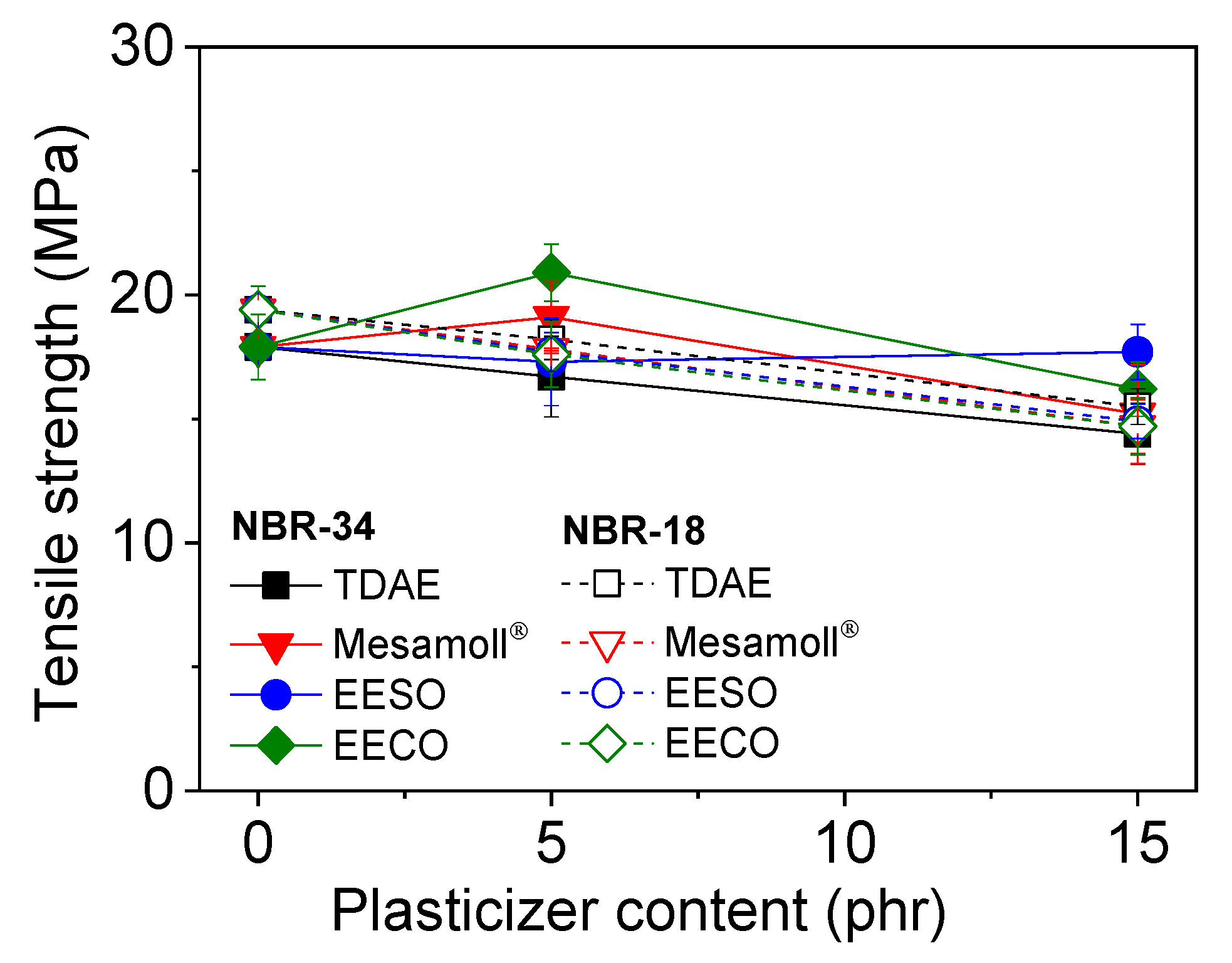
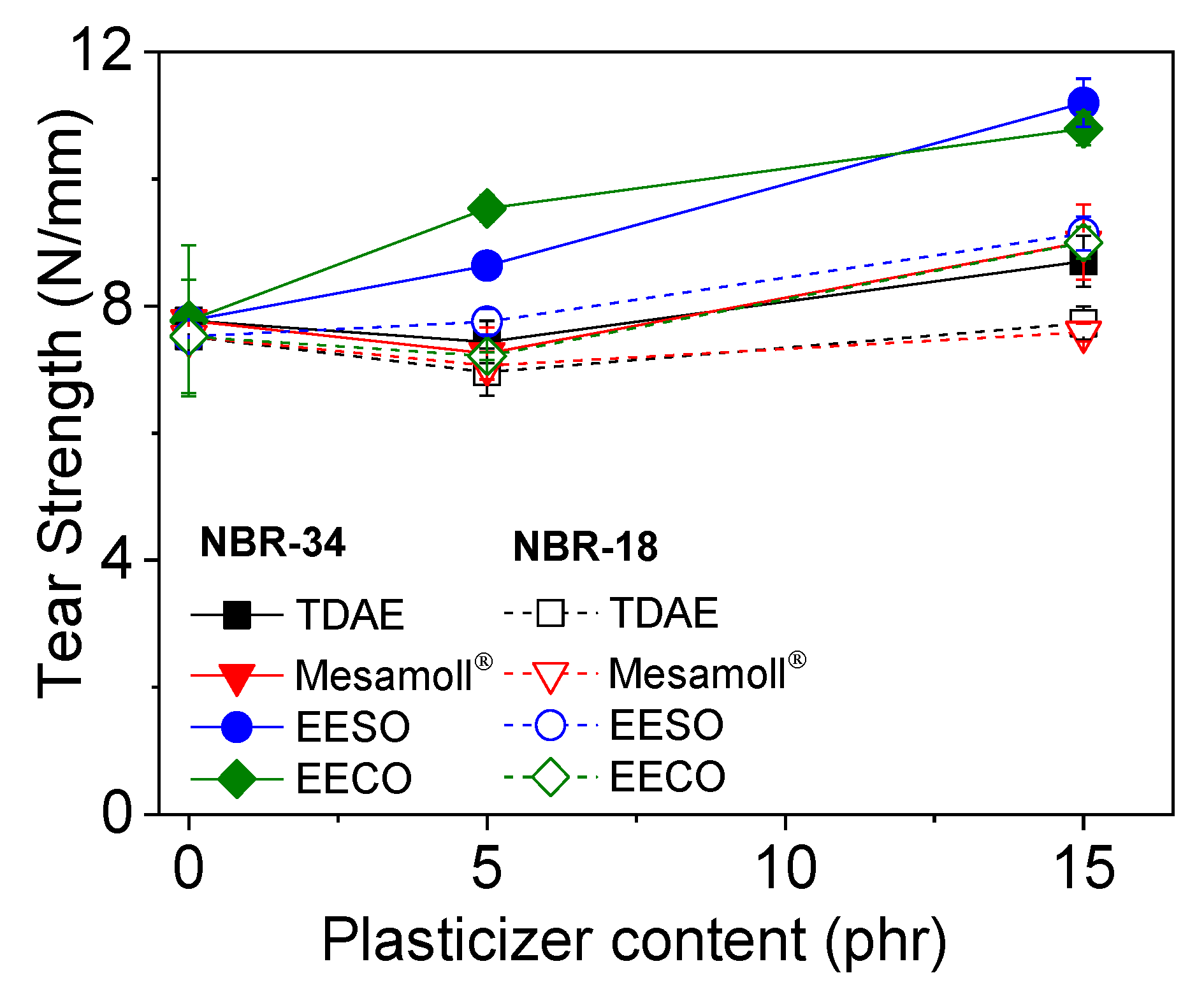
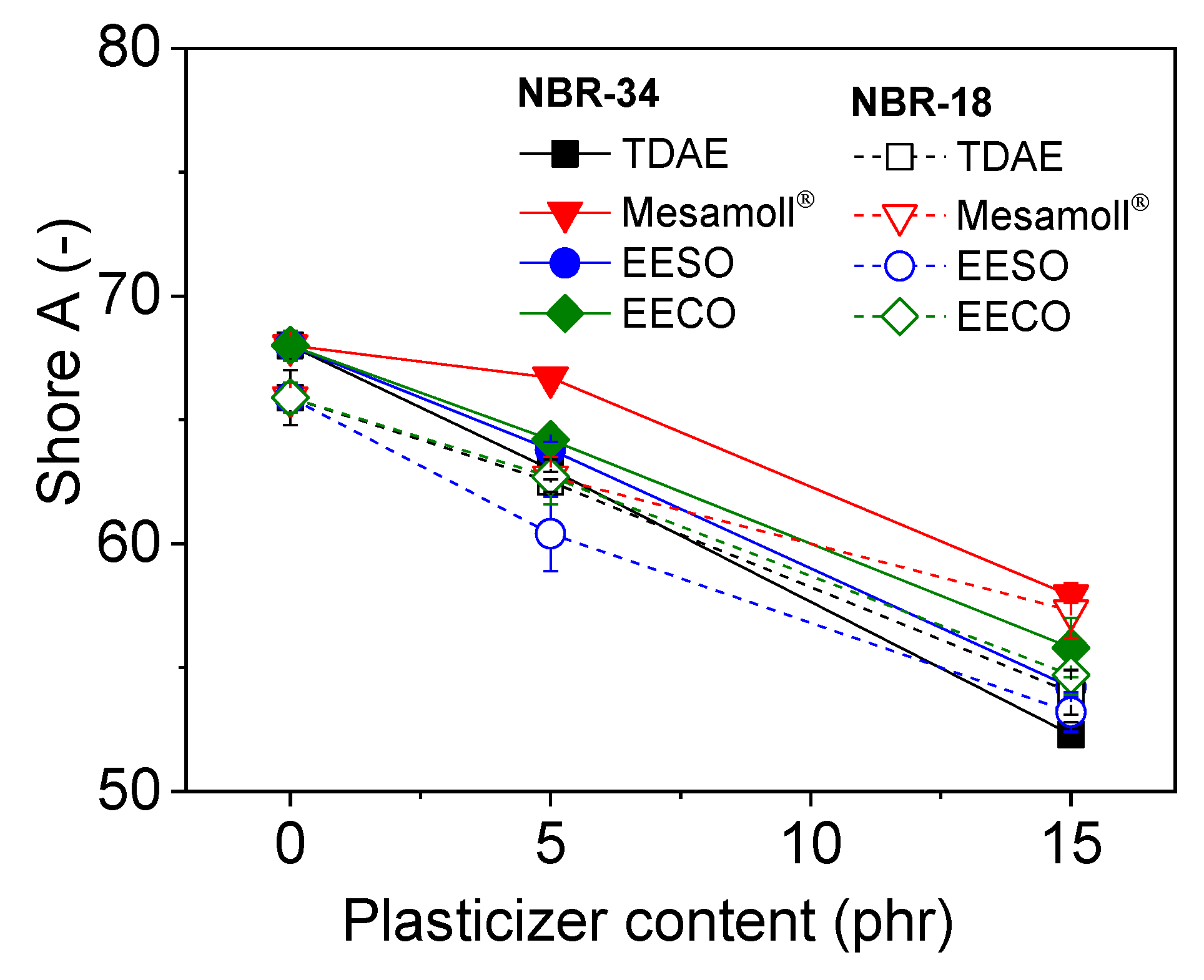
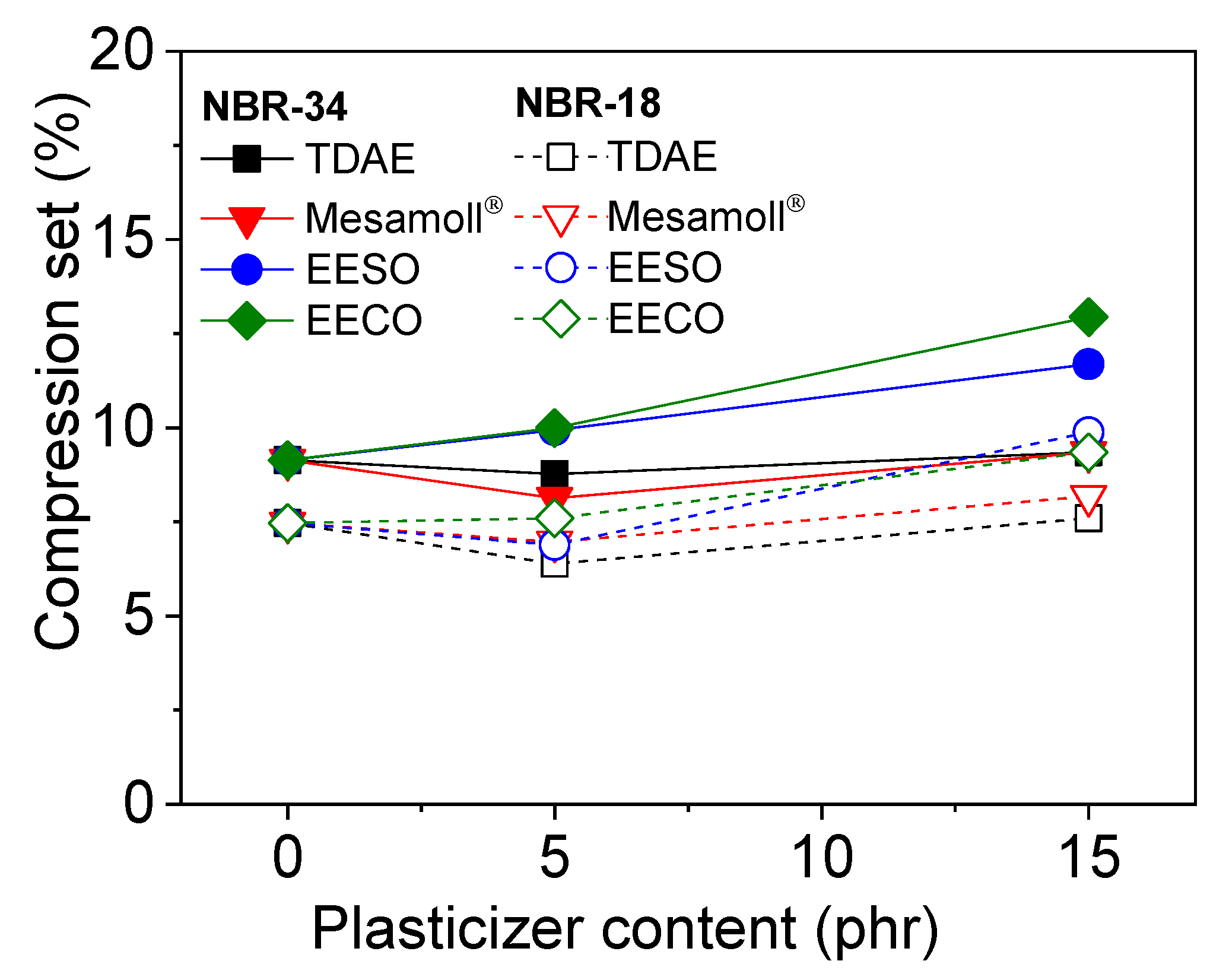
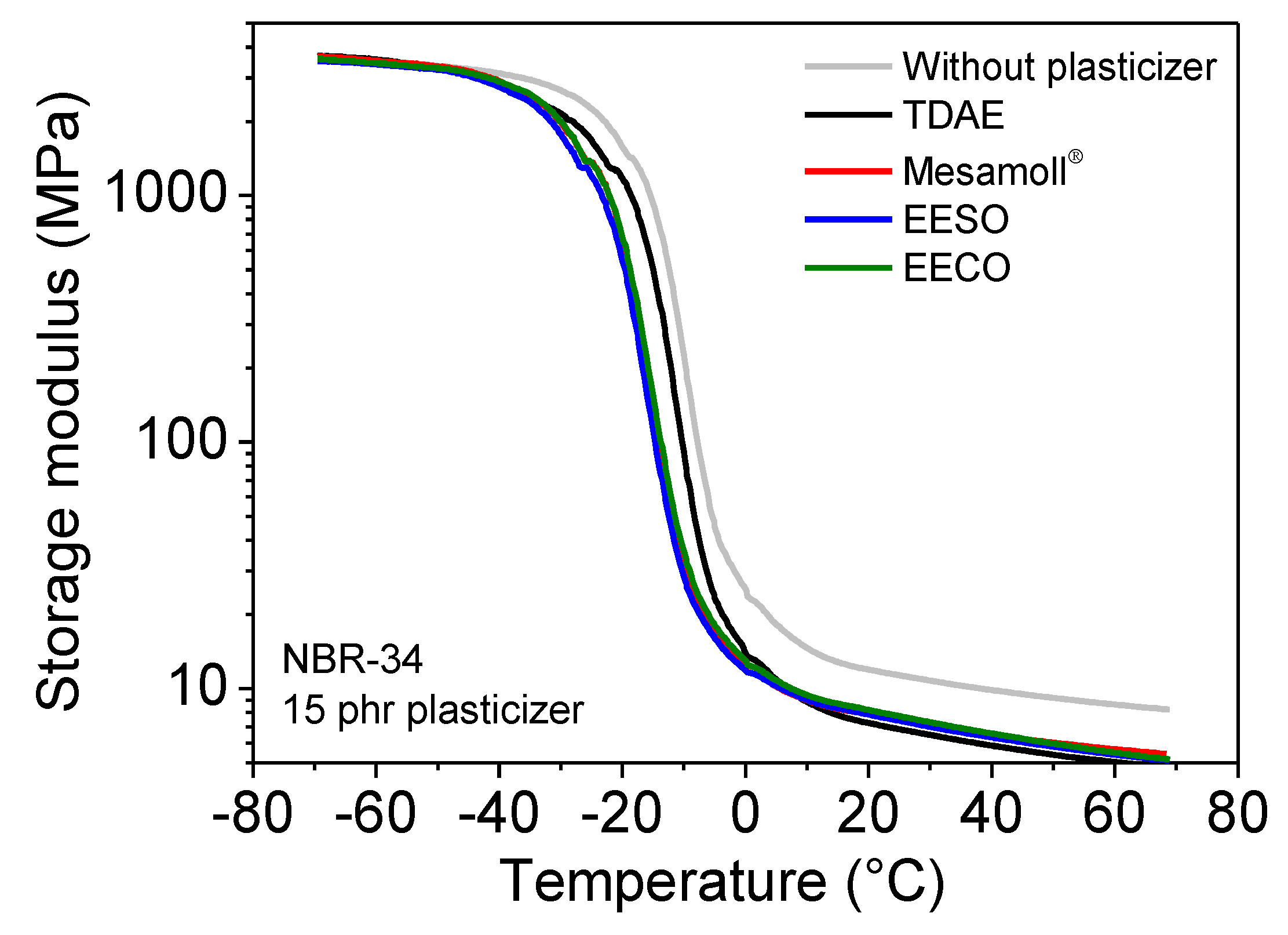
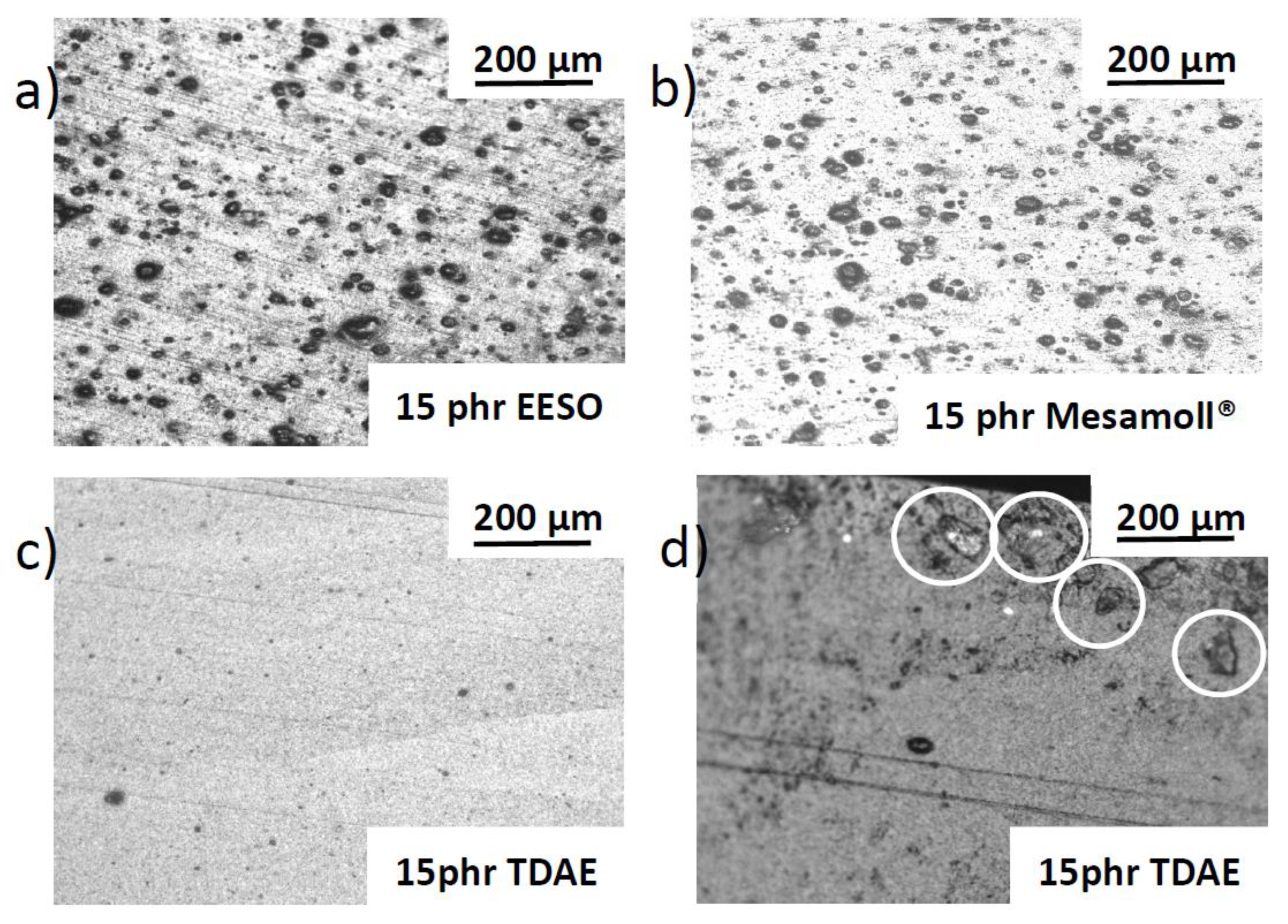
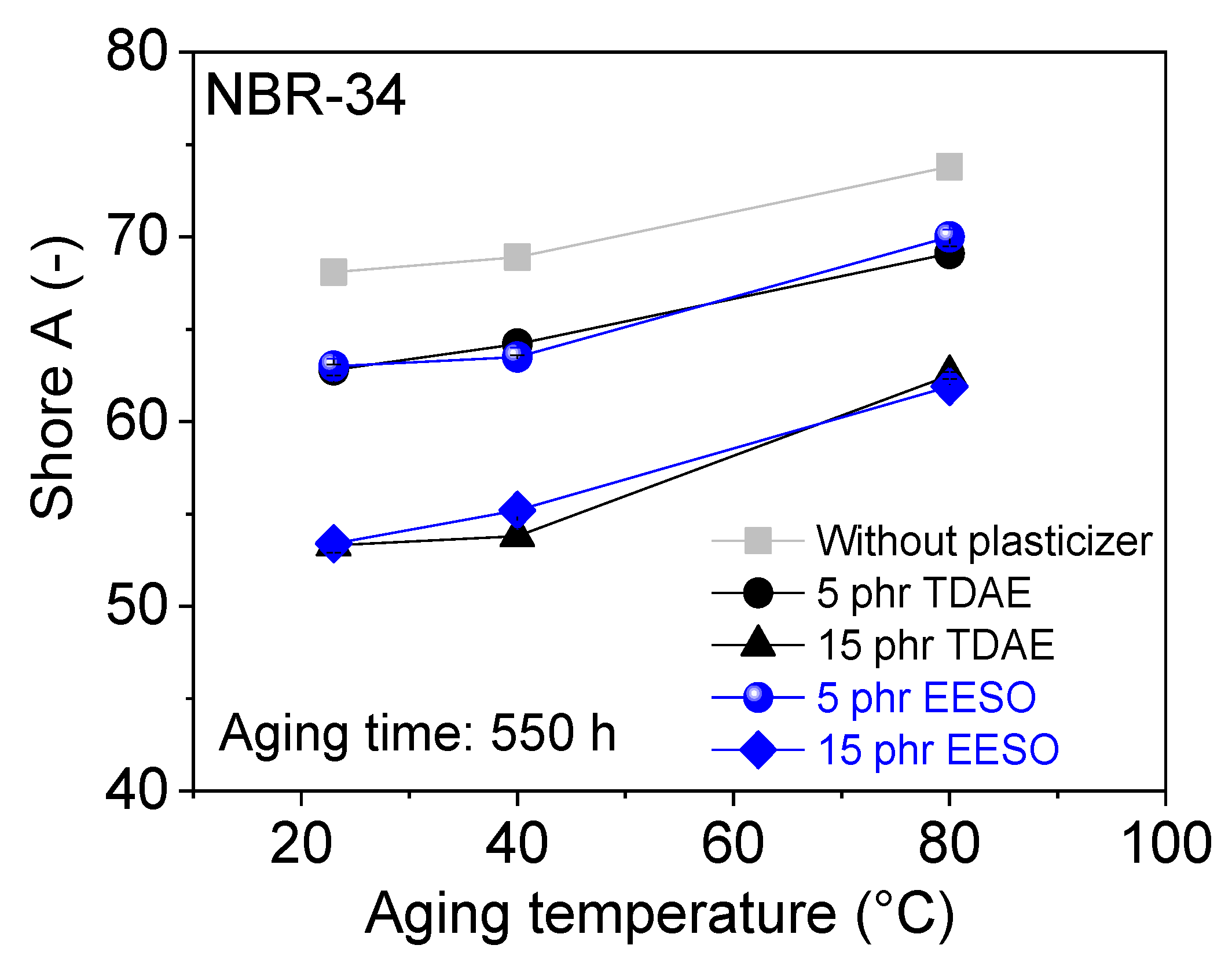
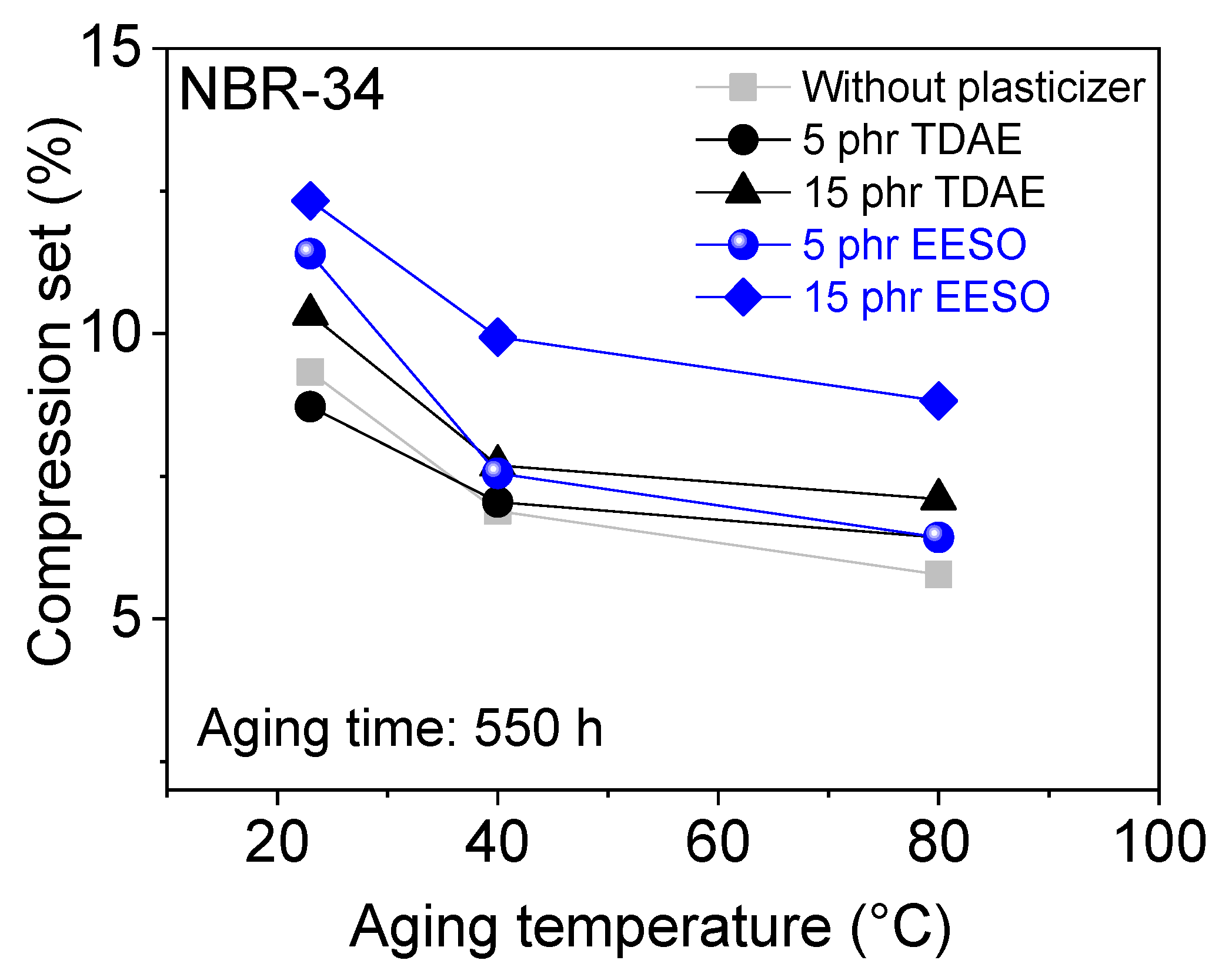
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khalaf, A.; Yehia, A.; Ismail, M.N.; El-Sabbagh, S. High Performance Oil Resistant Rubber. Open J. Org. Polym. Mater. 2012, 2, 89–94. [Google Scholar] [CrossRef][Green Version]
- Hofmann, W. Nitrile Rubber, Rubber Chemistry and Technology, 1st ed.; Lancaster, P., Ed.; American Chemical Society: Lancaster, UK, 1964. [Google Scholar]
- Waters, T.F. Fundamentals of Manufacturing for Engineers; CRC Press: London, UK, 1996; p. 96. [Google Scholar]
- Raju, P.; Nandanan, V.; Kutty, S.K.N. A Study on the Use of Coconut Oil as Plasticiser in Natural Rubber Compounds. J. Rubber Res. 2007, 10, 1–16. [Google Scholar]
- Petrović, Z.S.; Ionescu, M.; Milic, J.; Halladay, J.R. Soybean oil plasticizers as replacement of petroleum oil in rubber. Rubber Chem. Technol. 2013, 86, 233–249. [Google Scholar] [CrossRef]
- Wilkes, C.D.C.; Summers, J. PVC Handbook; Hanser Publications: Munich, Germany; Cincinnati, OH, USA, 2005; pp. 172–193. [Google Scholar]
- Dasgupta, S.; Agrawal, S.; Bandyopadhyay, S.; Chakraborty, S.; Mukhopadhyay, R.; Malkani, R.; Ameta, S. Characterization of eco-friendly processing aids for rubber compound. Polym. Test. 2007, 26, 489–500. [Google Scholar] [CrossRef]
- Aisbl, E. Replacement of Highly Aromatic Oils in Tyres; European Tyre Rubber Manufacturers’ Association: Brussels, Belgium, 2010; pp. 1–7. [Google Scholar]
- Null, V. Safe process oils for tires with low environmental impact. KGK-Kautsch. Gummi Kunstst. 1999, 52, 799–805. [Google Scholar]
- Fernandez, S.S.; Kunchandy, S.; Ghosh, S. Linseed Oil Plasticizer Based Natural Rubber/Expandable Graphite Vulcanizates: Synthesis and Characterizations. J. Polym. Environ. 2015, 23, 526–533. [Google Scholar] [CrossRef]
- Raju, P.; Nandanan, V.; Kutty, S.K.N. A Study on the Use of Castor Oil as Plasticizer in Natural Rubber Compounds. Prog. Rubber Plast. Recycl. Technol. 2007, 23, 169–180. [Google Scholar] [CrossRef]
- Kuriakose, A.P.; Rajendran, G. Use of rice-bran oil in the compounding of styrene butadiene rubber. J. Mater. Sci. 1995, 30, 2257–2262. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Shou, J.-Q.; Zhang, Z.-Y.; Zhao, G.-Z.; Liu, Y. Effect of curing temperature on properties of semi-efficient vulcanized natural rubber. J. Elastomers Plast. 2015, 48, 331–339. [Google Scholar] [CrossRef]
- Wypych, G. Effect of plasticizers on properties of plasticized materials. In Handbook of Plasticizers, 2nd ed.; William Andrew Publications: Toronto, ON, Canada, 2012; pp. 209–306. [Google Scholar]
- Kirkpatrick, A. Some Relations between Molecular Structure and Plasticizing Effect. J. Appl. Phys. 1940, 11, 255–261. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Second-Order Transition Temperatures and Related Properties of Polystyrene. I. Influence of Molecular Weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Clark, F.W. Industrial and engineering chemistry. Chem. Ind. 1941, 60, 1089–1090. [Google Scholar]
- Khalaf, A.; Ward, A.; El-Kader, A.E.A.; El-Sabbagh, S. Effect of selected vegetable oils on the properties of acrylonitrile-butadiene rubber vulcanizates. Polimery 2015, 60, 43–56. [Google Scholar] [CrossRef]
- Zhu, L.; Cheung, C.S.; Zhang, W.; Huang, Z. Compatibility of different biodiesel composition with acrylonitrile butadiene rubber (NBR). Fuel 2015, 158, 288–292. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Zhang, L.; Zhao, Y.; Vyzhimov, R.; Tan, T.; Fong, H. Investigation of Palm Oil as Green Plasticizer on the Processing and Mechanical Properties of Ethylene Propylene Diene Monomer Rubber. Ind. Eng. Chem. Res. 2016, 55, 2784–2789. [Google Scholar] [CrossRef]
- Pechurai, W.; Chiangta, W.; Tharuen, P. Effect of Vegetable Oils as Processing Aids in SBR Compounds. Macromol. Symp. 2015, 354, 191–196. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, J.; Yang, R.; Iervolino, R.; Barbera, S. Effect of lubricating oil on thermal aging of nitrile rubber. Polym. Degrad. Stab. 2018, 151, 136–143. [Google Scholar] [CrossRef]
- Zong, Z.; Soucek, M.D.; Liu, Y.; Hu, J. Cationic photopolymerization of epoxynorbornane linseed oils: The effect of diluents. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3440–3456. [Google Scholar] [CrossRef]
- ISO 7619. Rubber, Vulcanized or Thermoplastic—Determination Indentation Hardness Part 1 Durometer Method (Shore Hardness); International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- DIN ISO 37:2011(E). Rubber Vulcanized or Thermoplastic—Determiknation of Tensile Stress-Strain Properties; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- ISO 6721. (Plastics—Determination of Dynamic Mechanical Properties); International Organization for Standardization: Geneva, Switzerland, 2008; pp. 1–8. [Google Scholar]
- ASTM D 2663. Standard Test Methods for Carbon Black—Dispersion in Rubber 1; ASTM International: West Conshohocken, PA, USA, 2000; pp. 1–6. [Google Scholar]
- Le, H.; Ilisch, S.; Radusch, H.J. Characterization of the effect of the filler dispersion on the stress relaxation behavior of carbon black filled rubber composites. Polymer 2009, 50, 2294–2303. [Google Scholar] [CrossRef]
- Kukreja, T.R.; Chauhan, R.; Choe, S.; Kundu, P.P. Effect of the doses and nature of vegetable oil on carbon black/rubber interactions: Studies on castor oil and other vegetable oils. J. Appl. Polym. Sci. 2003, 87, 1574–1578. [Google Scholar] [CrossRef]
- Coran, A.Y. Vulcanization: Conventional and Dynamic. Rubber Chem. Technol. 1995, 68, 351–375. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zlatanić, A.; Lava, C.C.; Sinadinović-Fišer, S. Epoxidation of soybean oil in toluene with peroxoacetic and peroxoformic acids—Kinetics and side reactions. Eur. J. Lipid Sci. Technol. 2002, 104, 293–299. [Google Scholar] [CrossRef]
- Krejsa, M.R.; Koenig, J.L. A Review of Sulfur Crosslinking Fundamentals for Accelerated and Unaccelerated Vulcanization. Rubber Chem. Technol. 1993, 66, 376–410. [Google Scholar] [CrossRef]
- Gong, L.; Nguyen, M.H.T.; Oh, E.-S. High polar polyacrylonitrile as a potential binder for negative electrodes in lithium ion batteries. Electrochem. Commun. 2013, 29, 45–47. [Google Scholar] [CrossRef]
- Hassan, A.; Wahit, M.U.; Chee, C.Y. Mechanical and Morphological Properties of PP/LLPDE/NR Blends—Effects of Polyoctenamer. Polym. Technol. Eng. 2005, 44, 1245–1256. [Google Scholar] [CrossRef]
- Kern Sears, J.; Darby, J., Jr. The Technology of Plasticizers; John Wiley Sons: New York, NY, USA, 1982; Volume 20, p. 459. [Google Scholar]
- Kukreja, T.; Kumar, D.; Prasad, K.; Chauhan, R.; Choe, S.; Kundu, P.P. Optimisation of physical and mechanical properties of rubber compounds by response surface methodology—Two component modelling using vegetable oil and carbon black. Eur. Polym. J. 2002, 38, 1417–1422. [Google Scholar] [CrossRef]
- McKeen, L.W. 1—Introduction to Creep, Polymers, Plastics and Elastomers. In The Effect of Creep and Other Time Related Factors on Plastics and Elastomers, 3rd ed.; McKeen, L.W., Ed.; William Andrew Publishing: Boston, MA, USA, 2015; pp. 1–41. [Google Scholar]
- Schuring, D.J.; Futamura, S. Rolling Loss of Pneumatic Highway Tires in the Eighties. Rubber Chem. Technol. 1990, 63, 315–367. [Google Scholar] [CrossRef]
- Flanigan, C.; Beyer, L.; Klekamp, D.; Rohweder, D.; Haakenson, D. Using bio-based plasticizers, alternative rubber. Br. J. Psychiatry 2013, 2, 15–19. [Google Scholar]
- Wang, Z.; Zhang, X.; Wang, R.; Kang, H.; Qiao, B.; Ma, J.; Zhao, X.; Wang, H. Synthesis and Characterization of Novel Soybean-Oil-Based Elastomers with Favorable Processability and Tunable Properties. Macromolecules 2012, 45, 9010–9019. [Google Scholar] [CrossRef]
- Alneamah, M.; Almaamori, M. Study of Thermal Stability of Nitrile Rubber/Polyimide Compounds. Int. J. Mater. Chem. 2015, 5, 1–3. [Google Scholar]
- Doma, A.S.; Kamoun, E.A.; Abboudy, S.; Belal, M.A.; Khattab, S.N.; A El-Bardan, A. Compatibilization of Vulcanized SBR/NBR Blends using Cis-Polybutadiene Rubber: Influence of Blend Ratio on Elastomer Properties. Eur. J. Eng. Res. Sci. 2019, 3, 135–143. [Google Scholar] [CrossRef]
- David, F. Cadogan, Phthalic acid and Derivates. In Ullmann’s Encyclopedia of Industrial Chemistry; ICI Chemicals and Polymers: Cheshire, UK, 2012; pp. 35–154. [Google Scholar]
- Brown, R.; Soulagnet, G. Microhardness profiles on aged rubber compounds. Polym. Test. 2001, 20, 295–303. [Google Scholar] [CrossRef]
- Kundu, P.P.; Kukreja, T.R. Surface modification of carbon black by vegetable oil? Its effect on the rheometric, hardness, abrasion, rebound resilience, tensile, tear, and adhesion properties. J. Appl. Polym. Sci. 2002, 84, 256–260. [Google Scholar] [CrossRef]
- Budrugeac, P. Accelerated thermal ageing of nitrile-butadiene rubber under air pressure. Polym. Degrad. Stab. 1995, 47, 129–132. [Google Scholar] [CrossRef]



| Name | Comments | Content (phr) | Time of Addition to the Kneader (min) |
|---|---|---|---|
| NBR | 34% ACN, 18% ACN | 100 | 0 |
| Carbon black | N 550 | 40 | 1 |
| Oil | TDAE, Mesamoll®, EESO, EECO | 0, 5, 15 | 1 |
| Stearic acid | Processing aids | 1 | 1 |
| ZnO | Activator | 3 | 1 |
| 6PPD1 | Antioxidant | 1.5 | 1 |
| Sulfur | Crosslinking agent | 1.75 | 5 |
| CBS2 | Accelerator | 1.05 | 5 |
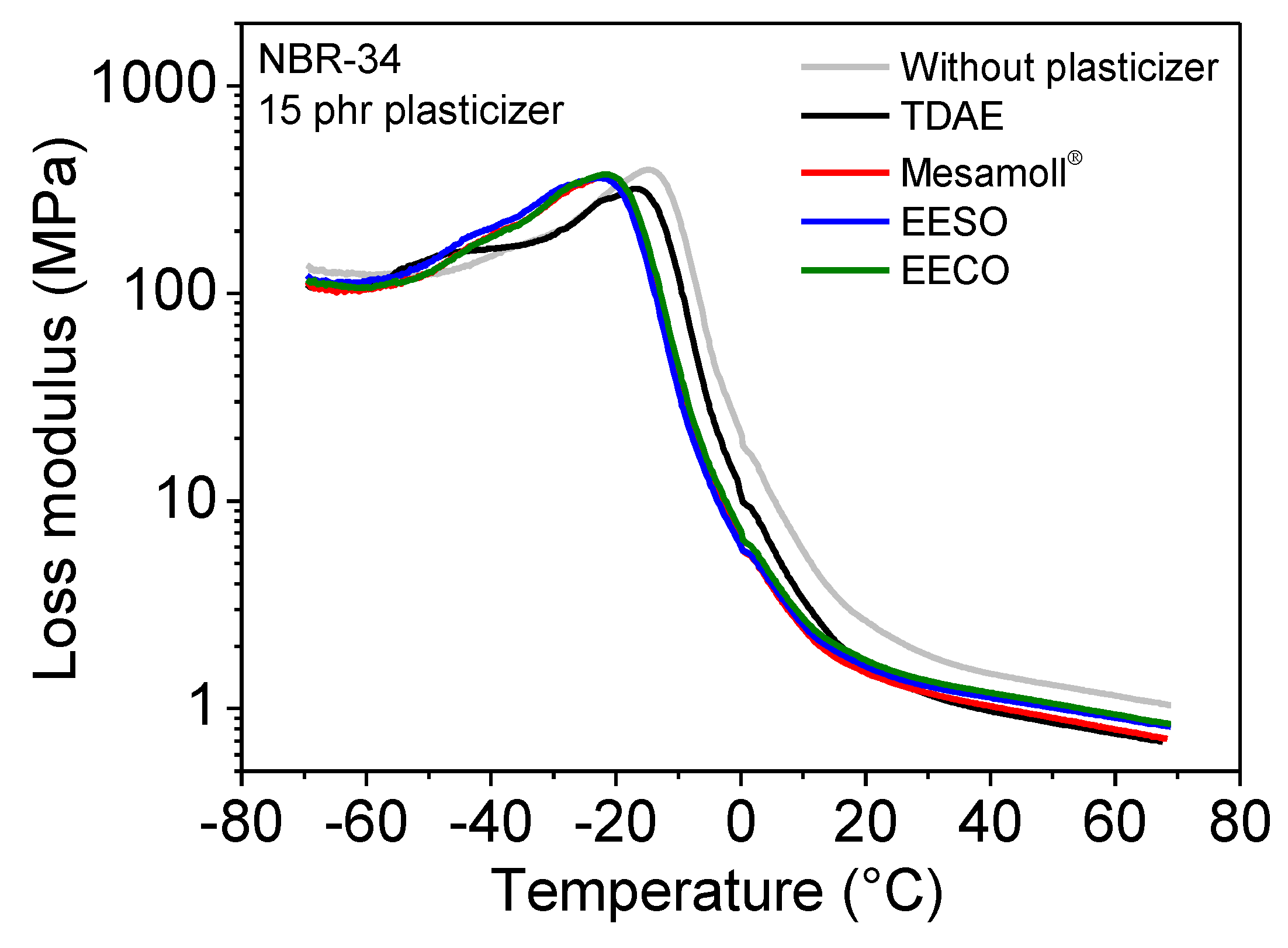
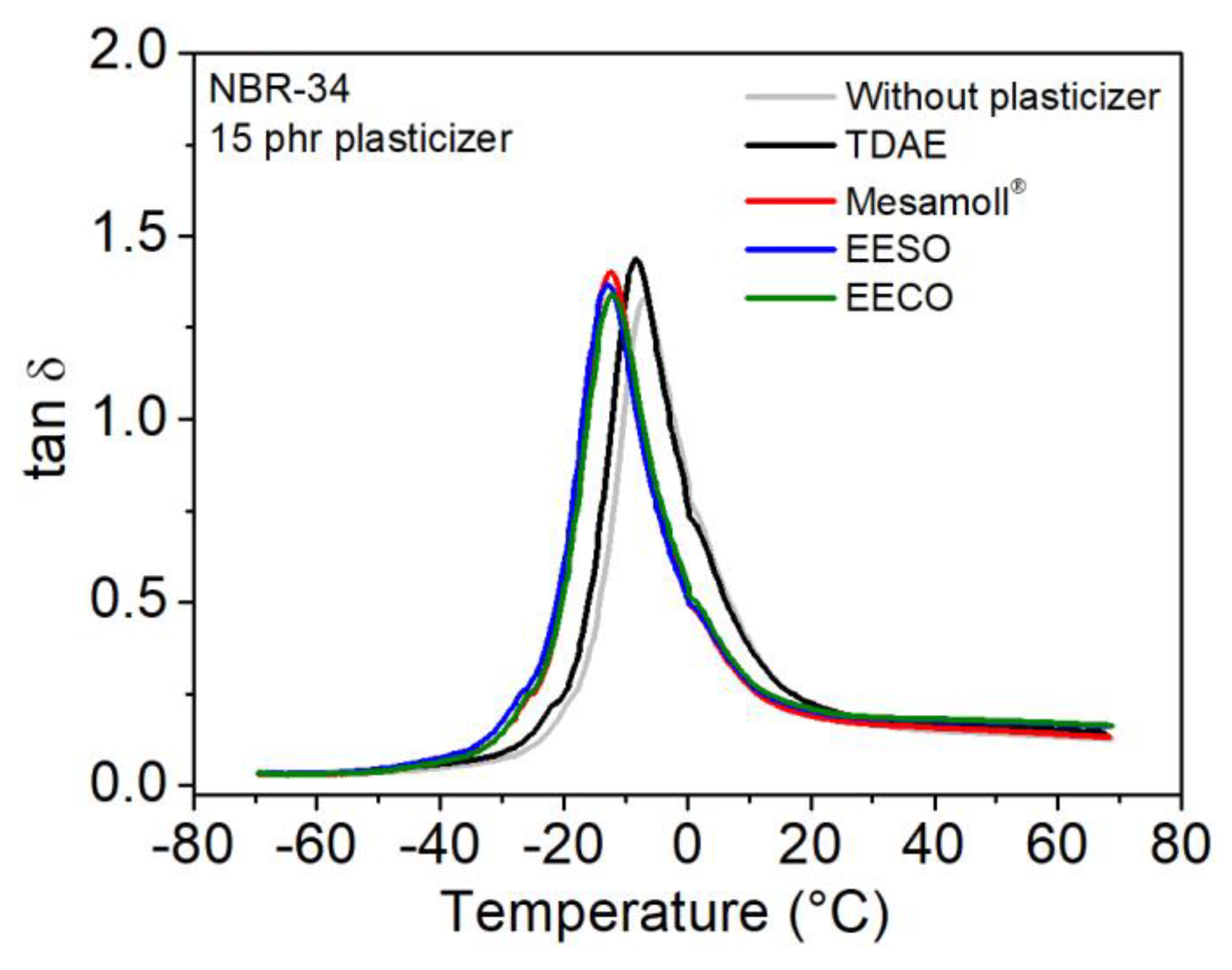
| NBR-34 | tan δ | Tg(°C) | E’ (MPa) at 23 °C | E” (MPa) at 23 °C |
|---|---|---|---|---|
| Without plasticizer | 1.32 | −6.88 | 11.53 | 2.31 |
| 15 phr TDAE | 1.43 | −7.34 | 6.98 | 1.44 |
| 15 phr Mesamoll® | 1.40 | −12.11 | 7.66 | 1.37 |
| 15 phr EESO | 1.36 | −14.11 | 7.55 | 1.47 |
| 15 phr EECO | 1.34 | −12.56 | 7.91 | 1.57 |
| NBR-34 | Decomposition % (250 °C) | Decomposition % (350 °C) | Decomposition % (450 °C) | Decomposition % (550 °C) |
|---|---|---|---|---|
| Without plasticizer | 0.96 | 2.92 | 27.55 | 66.89 |
| 15 phr TDAE | 1.28 | 7.4182 | 33.23 | 69.74 |
| 15 phr Mesamoll® | 1.73 | 9.44 | 33.08 | 68.66 |
| 15 phr EESO | 1.17 | 7.60 | 33.6 | 68.72 |
| 15 phr EECO | 1.28 | 7.36 | 33.74 | 69.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Oßwald, K.; Reincke, K.; Langer, B. Influence of Bio-Based Plasticizers on the Properties of NBR Materials. Materials 2020, 13, 2095. https://doi.org/10.3390/ma13092095
Rahman MM, Oßwald K, Reincke K, Langer B. Influence of Bio-Based Plasticizers on the Properties of NBR Materials. Materials. 2020; 13(9):2095. https://doi.org/10.3390/ma13092095
Chicago/Turabian StyleRahman, Md Mahbubur, Katja Oßwald, Katrin Reincke, and Beate Langer. 2020. "Influence of Bio-Based Plasticizers on the Properties of NBR Materials" Materials 13, no. 9: 2095. https://doi.org/10.3390/ma13092095
APA StyleRahman, M. M., Oßwald, K., Reincke, K., & Langer, B. (2020). Influence of Bio-Based Plasticizers on the Properties of NBR Materials. Materials, 13(9), 2095. https://doi.org/10.3390/ma13092095






