In Situ Ternary Boride: Effects on Densification Process and Mechanical Properties of WC-Co Composite Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Composite Powder
2.2. Preparation of Coatings
2.3. Analysis
3. Results and Discussion
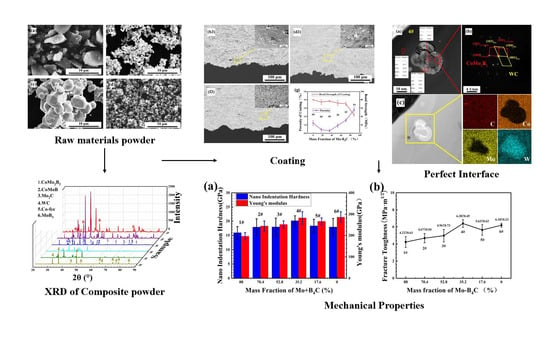
3.1. Characterization of the Raw Materials
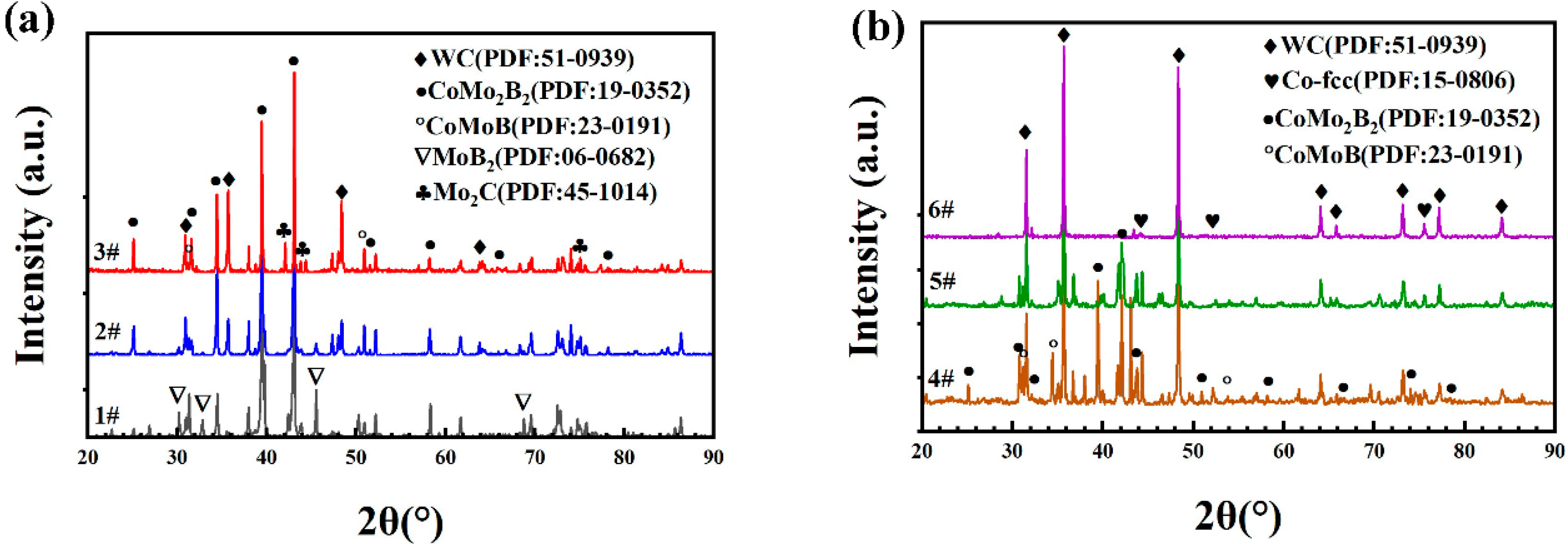
3.2. Characterization of the Prepared Composite Powders
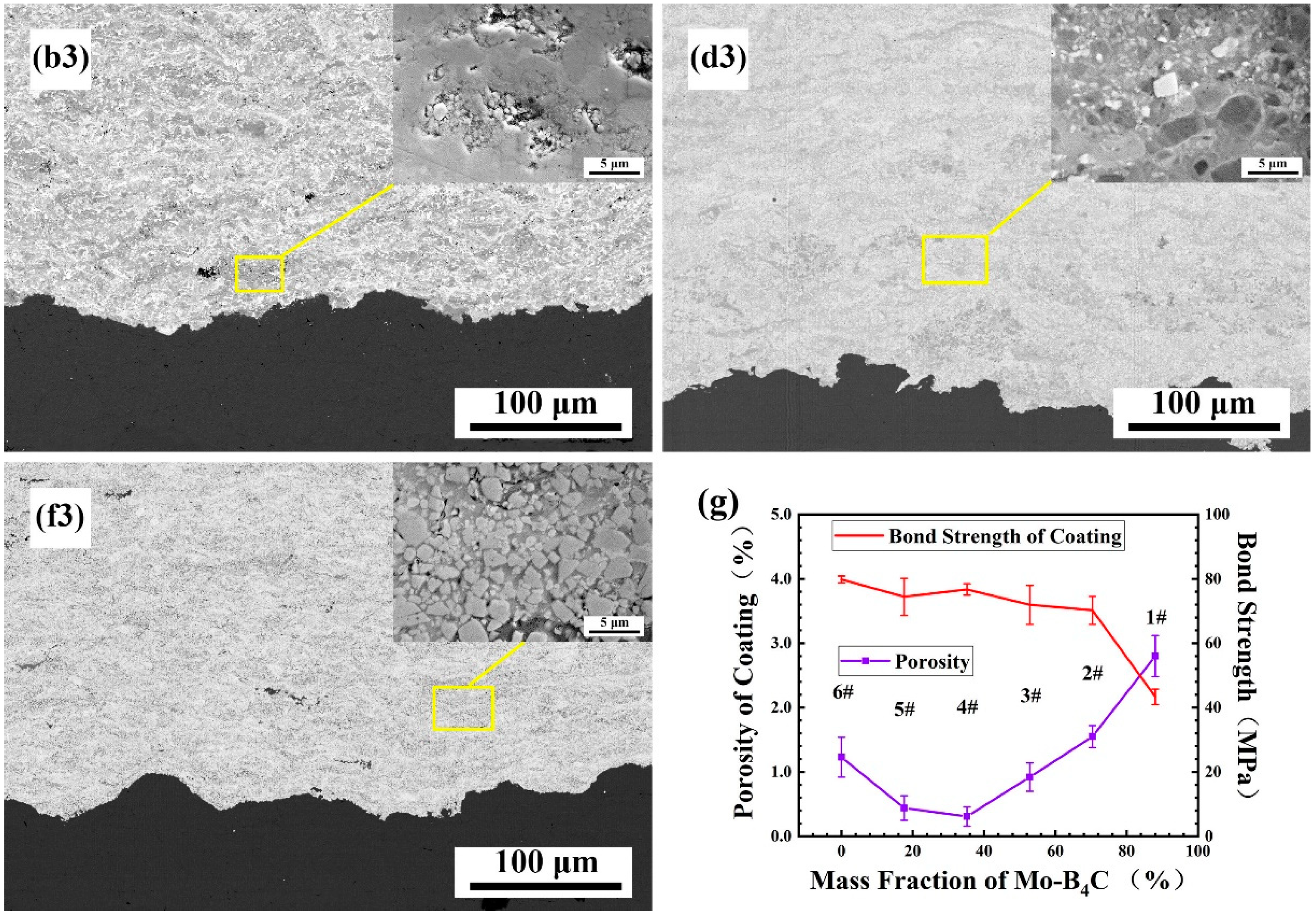
3.3. Effect of Mo–B4C on the Morphology and Densification Process of the Coating
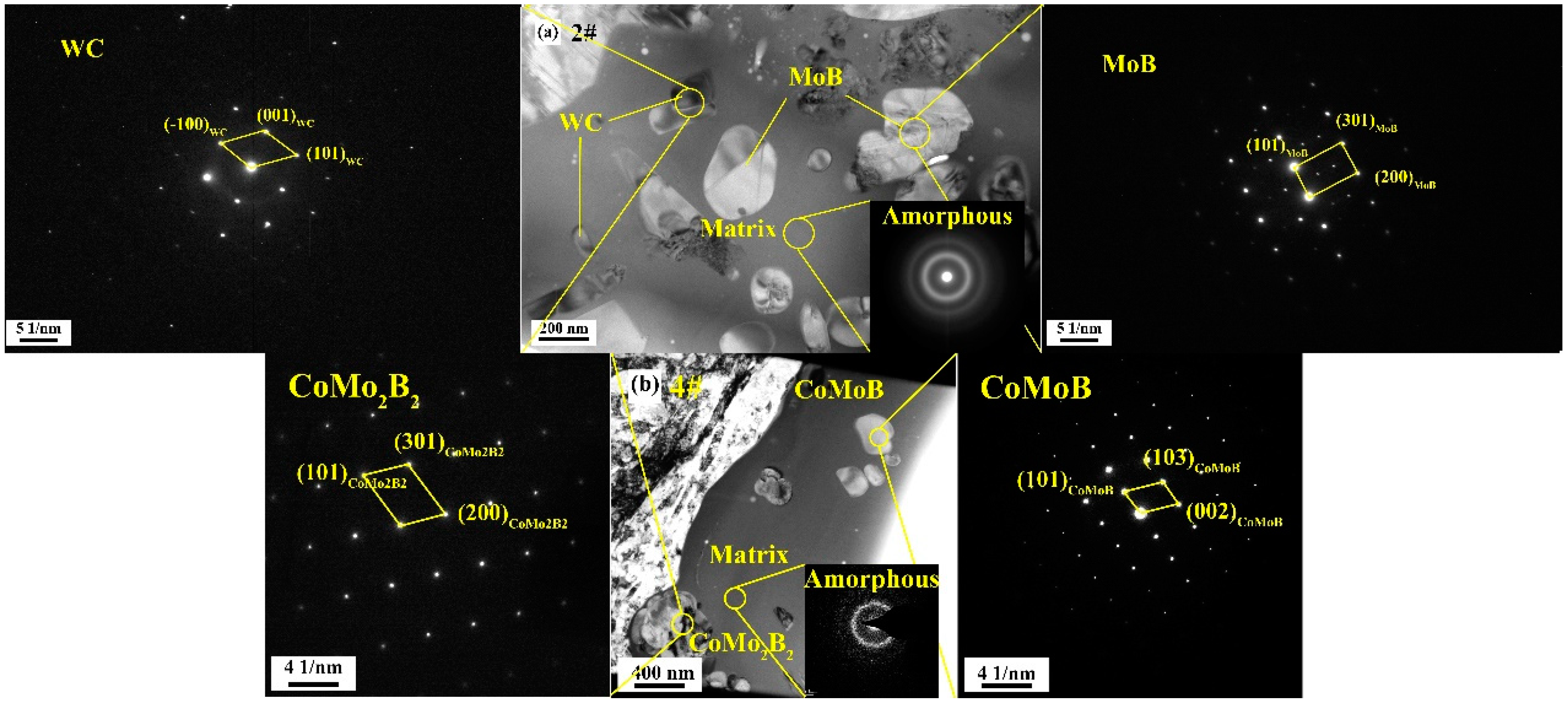
3.4. Microstructural Evolution of Coating
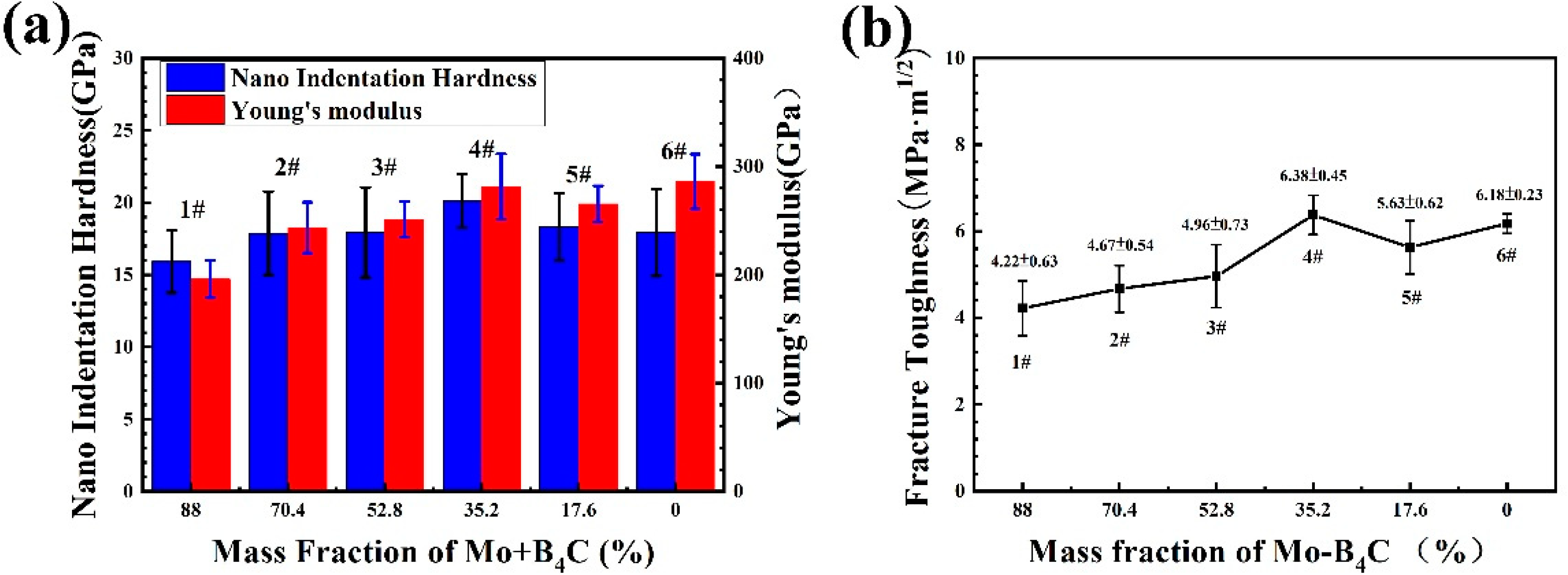
3.5. Mechanical Property Evolution of Coating by Mo–B4C Addition
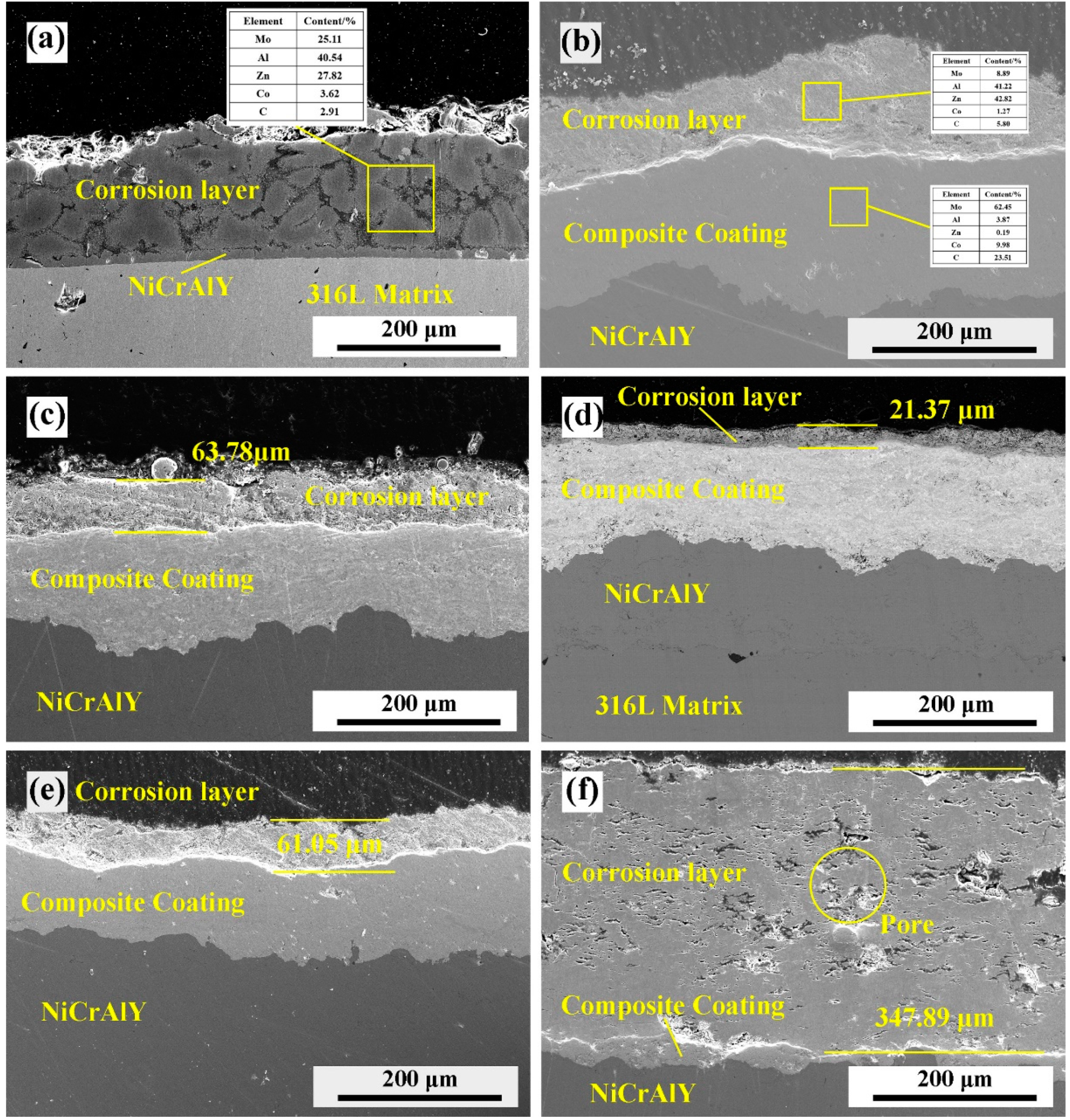
3.6. Corrosion Resistance Property Evolution of Coating by Mo–B4C Addition
4. Conclusions
- Composite powders of different proportions of Mo–B4C, WC, and Co were prepared by mixing, granulation, and heat treatment. When a small amount of Mo–B4C was added, it reacted with Co to produce ternary borides CoMo2B2 and CoMoB. When Mo–B4C was added to excess, Mo and B4C further reacted to generate MoB and MoB2.
- Boride ceramics were formed in situ to replace part of the Co bonding phase to improve corrosion resistance. When the amount of Mo–B4C added was 35.2%, some pores were present in the composite powder, but the combination between components was good. When Mo–B4C was added to excess, then numerous pores were visible in the composite powder, and the combination of particles was poor.
- When the amount of Mo–B4C was 35.2%, the mechanical properties of the prepared coating reached optimal values: minimum porosity of 0.31 ± 0.15%, bonding strength of 77.81 ± 1.77 Mpa, nanoindentation hardness of 20.12 ± 1.85 GPa, Young’s modulus of 281.52 ± 30.22 GPa, and fracture toughness of 6.38 ± 0.45 MPa·m1/2.
- The strengthening mechanism of Mo–B4C in WC–Co composite coatings was as follows: the addition of Mo–B4C reacted with Co to form ternary borides CoMo2B2 and CoMoB. Owing to the in-situ reaction, a perfect interface formed between Co and ternary boride. Simultaneously, ternary boride also formed a perfect interface with Mo and WC of a particular crystallographic orientation. Therefore, the addition of Mo–B4C improved the interface with WC–Co, forming a dense microstructure. This change improved the mechanical properties and corrosion resistance property of the coating.
Author Contributions
Funding
Conflicts of Interest
References
- Tsipas, D.N.; Triantafyllidis, G.K.; Kiplagat, J.K.; Psillaki, P. Degradation behavior of boronized carbon and high alloy steels in molten aluminum and zinc. Mater. Lett. 1998, 37, 128–131. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Y.; Zhang, Y.; Feng, S.; Du, J.; Liu, D.; Ouyang, T.; Suo, J.; Cai, S. Improving the corrosion resistance of Fe-21Cr-9Mn alloy in liquid zinc by heat treatment. Corros. Sci. 2016, 111, 362–369. [Google Scholar] [CrossRef]
- Ren, X.J.; Mei, X.Z.; She, J.; Jiang-hong, M.A. Materials Resistance to Liquid Zinc Corrosion on Surface of Sink Roll. J. Iron Steel Res. Int. 2007, 14, 130–136. [Google Scholar] [CrossRef]
- Wang, W.J.; Lin, J.P.; Wang, Y.L.; Chen, G.L. The corrosion of intermetallic alloys in liquid zinc. J. Alloy. Compd. 2007, 428, 243. [Google Scholar] [CrossRef]
- Cao, X.M.; Yi, L.; Run, W. Mechanism of liquid zinc corrosion on metals. Int. J. Miner. Metall. Mater. 1997, 3, 22–26. [Google Scholar]
- Armstrong, R.W. The Hardness and Strength Properties of WC-Co Composites. Materials 2011, 4, 1287–1308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Jia, J.H.; Wang, B.X.; Wang, Y. Two-Step Spark Plasma Sintering Process of Ultrafine Grained WC-12Co-0.2VC Cemented Carbide. Materials 2019, 12, 2443. [Google Scholar] [CrossRef] [PubMed]
- Konyashin, I.; Zaitsev, A.; Meledin, A.; Mayer, J.; Loginov, P.; Levashov, E.; Ries, B. Interfaces between Model Co-W-C Alloys with Various Carbon Contents and Tungsten Carbide. Materials 2018, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Bjorndal, M.; Bardal, E.; Rogne, T.; Eggen, T.G. Erosion and corrosion properties of WC coatings and duplex stainless steel in sand-containing synthetic sea water. Wear 1995, 186–187, 508–514. [Google Scholar] [CrossRef]
- Souza, V.A.; Neville, A. Corrosion and erosion damage mechanisms during erosion-corrosion of WC−Co−Cr cermet coatings. Wear 2008, 255, 146–156. [Google Scholar] [CrossRef]
- Wang, W.J.; Lin, J.P.; Wang, Y.L. Corrosion of 316 stainless steel/WC-Co coating in liquid zinc. J. Aeronaut. Mater. 2006, 26, 56–60. [Google Scholar]
- Ying, K.L.; Dian, R.Y.; Dong, L.Z. Study on the Material of Inside Heating Casing for Galvanizing by Dipping. Shandong Ceram. 2006, 29, 16–19. [Google Scholar]
- Shimizu, J.; Ohmura, E.; Kobayashi, Y.; Kiyoshima, S.; Eda, H. Molecular Dynamics Simulation of Flattening Process of a High-Temperature, High-Speed Droplet-Influence of Impact Parameters. J. Therm. Spray Technol. 2007, 16, 722–728. [Google Scholar] [CrossRef]
- Lv, H.; Nie, P.; Yan, Y.; Sun, B. Characterization and adhesion strength study of detonation-sprayed MoB-CoCr alloy coatings on 2Cr13 stainless steel substrate. J. Coat. Technol. Res. 2010, 7, 801–807. [Google Scholar] [CrossRef]
- Khan, F.F.; Bae, G.; Kang, K.; Na, H.; Kim, J.; Jeong, T.; Lee, C. Evaluation of Die-Soldering and Erosion Resistance of High Velocity Oxy-Fuel Sprayed MoB-Based Cermet Coatings. J. Therm. Spray Technol. 2011, 20, 1022–1034. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R. Corrosion Behavior of Low-Pressure Plasma Sprayed MoB/CoCr Coatings Exposed to Molten Al-12.07wt.%Si Alloy. Procedia Eng. 2012, 27, 1766–1773. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.F.; Deng, C.M.; Song, J.B.; Deng, C.; Liu, M.; Zhou, K. MoB-CoCr as alternatives to WC-12Co for stainless steel protective coating and its corrosion behavior in molten zinc. Surf. Coat. Technol. 2013, 235, 811–818. [Google Scholar] [CrossRef]
- Sugiyama, S.; Taimatsu, H. Mechanical properties of WC-WB-W2B composites prepared by reaction sintering of B4C-W-WC powders. J. Eur. Ceram. Soc. 2004, 24, 871–876. [Google Scholar] [CrossRef]
- Wang, H.; Hou, C.; Liu, X.; Liu, X.; Song, X. Phase evolution in synthesis of nanocrystalline WC-η composite powder by solid-state in situ reactions. Int. J. Refract. Met. Hard Mater. 2018, 71, 21–27. [Google Scholar] [CrossRef]
- Song, H.P. Preparation and Study of a New Ternary Borides Based Ceramic Coating. Ph.D. Thesis, Shanghai Jiao Tong University, Shanghai, China, 2010. [Google Scholar]
- Sun, Y.H.; Wu, J.H.; He, L.K.; Liu, B.; Zhang, C.; Meng, Q.; Zhang, X. Influence of B4C coating on graphitization for diamond/WC-Fe-Ni composite. Int. J. Refract. Met. Hard Mater. 2020, 88, 105208. [Google Scholar] [CrossRef]



| Sample | WC | Mo, B4C | Co | ||
|---|---|---|---|---|---|
| Mo + B4C | Mo | B4C | |||
| 1# | 0 | 88.0 | 78.8 | 9.2 | 12.0 |
| 2# | 17.6 | 70.4 | 63.1 | 7.3 | 12.0 |
| 3# | 35.2 | 52.8 | 47.3 | 5.5 | 12.0 |
| 4# | 52.8 | 35.2 | 31.5 | 3.7 | 12.0 |
| 5# | 70.4 | 17.6 | 15.8 | 1.8 | 12.0 |
| 6# | 88.0 | 0 | 0 | 0 | 12.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, J.; Yu, Y.; Liu, B.; Jia, C.; Wu, C. In Situ Ternary Boride: Effects on Densification Process and Mechanical Properties of WC-Co Composite Coating. Materials 2020, 13, 1995. https://doi.org/10.3390/ma13081995
Bao J, Yu Y, Liu B, Jia C, Wu C. In Situ Ternary Boride: Effects on Densification Process and Mechanical Properties of WC-Co Composite Coating. Materials. 2020; 13(8):1995. https://doi.org/10.3390/ma13081995
Chicago/Turabian StyleBao, Junfeng, Yueguang Yu, Bowen Liu, Chengchang Jia, and Chao Wu. 2020. "In Situ Ternary Boride: Effects on Densification Process and Mechanical Properties of WC-Co Composite Coating" Materials 13, no. 8: 1995. https://doi.org/10.3390/ma13081995
APA StyleBao, J., Yu, Y., Liu, B., Jia, C., & Wu, C. (2020). In Situ Ternary Boride: Effects on Densification Process and Mechanical Properties of WC-Co Composite Coating. Materials, 13(8), 1995. https://doi.org/10.3390/ma13081995