Development of an Adsorbing System Made of DMS-1 Mesh Modified by Amino Groups to Remove Pb(II) Ions from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the DMS-1 Mesoporous Silica
2.2. Synthesis of Amine-Functionalized DMS-1
2.3. Adsorption Experiments
2.4. Physico-Chemical Characterization of the Supporting Materials
3. Results
3.1. Characterization of Mesoporous Silica Materials
3.2. FTIR Analysis
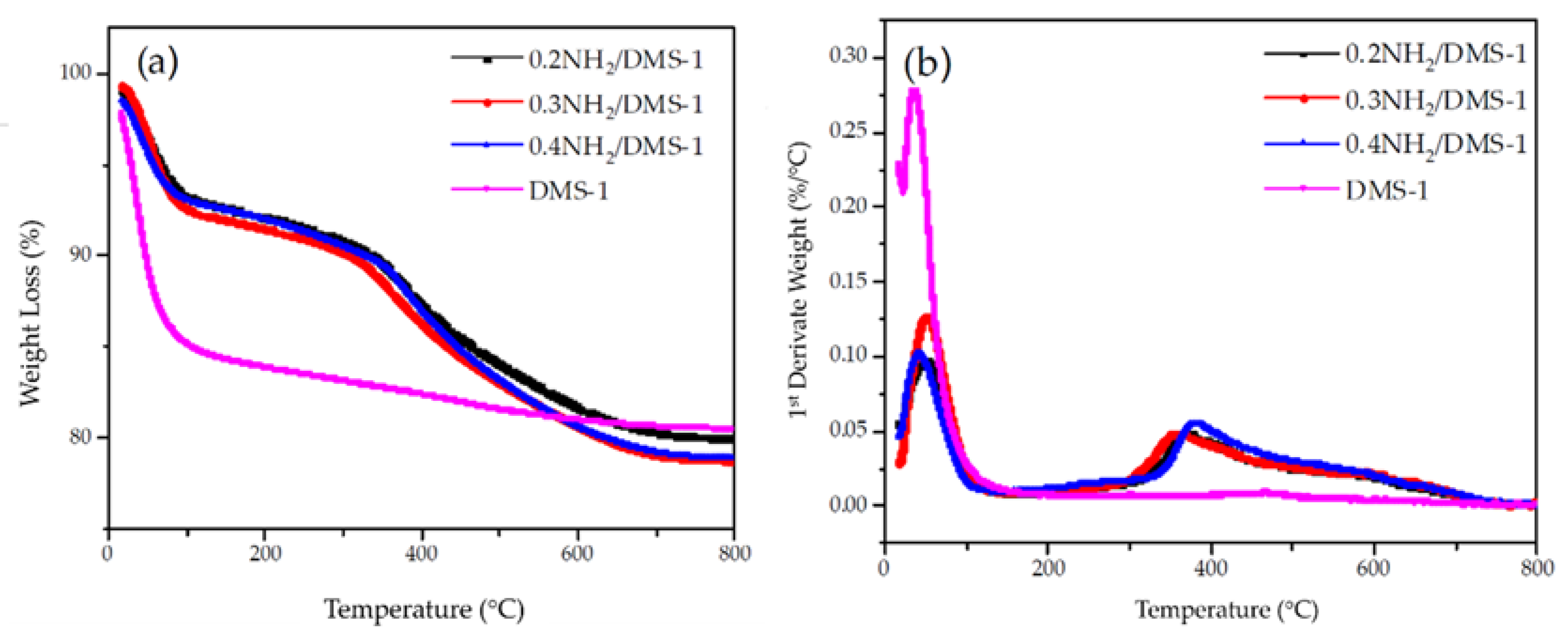
3.3. Thermogravimetric Analysis (TGA-DTG)
3.4. Determination of Amino Concentration in DMS-1 Adsorbent
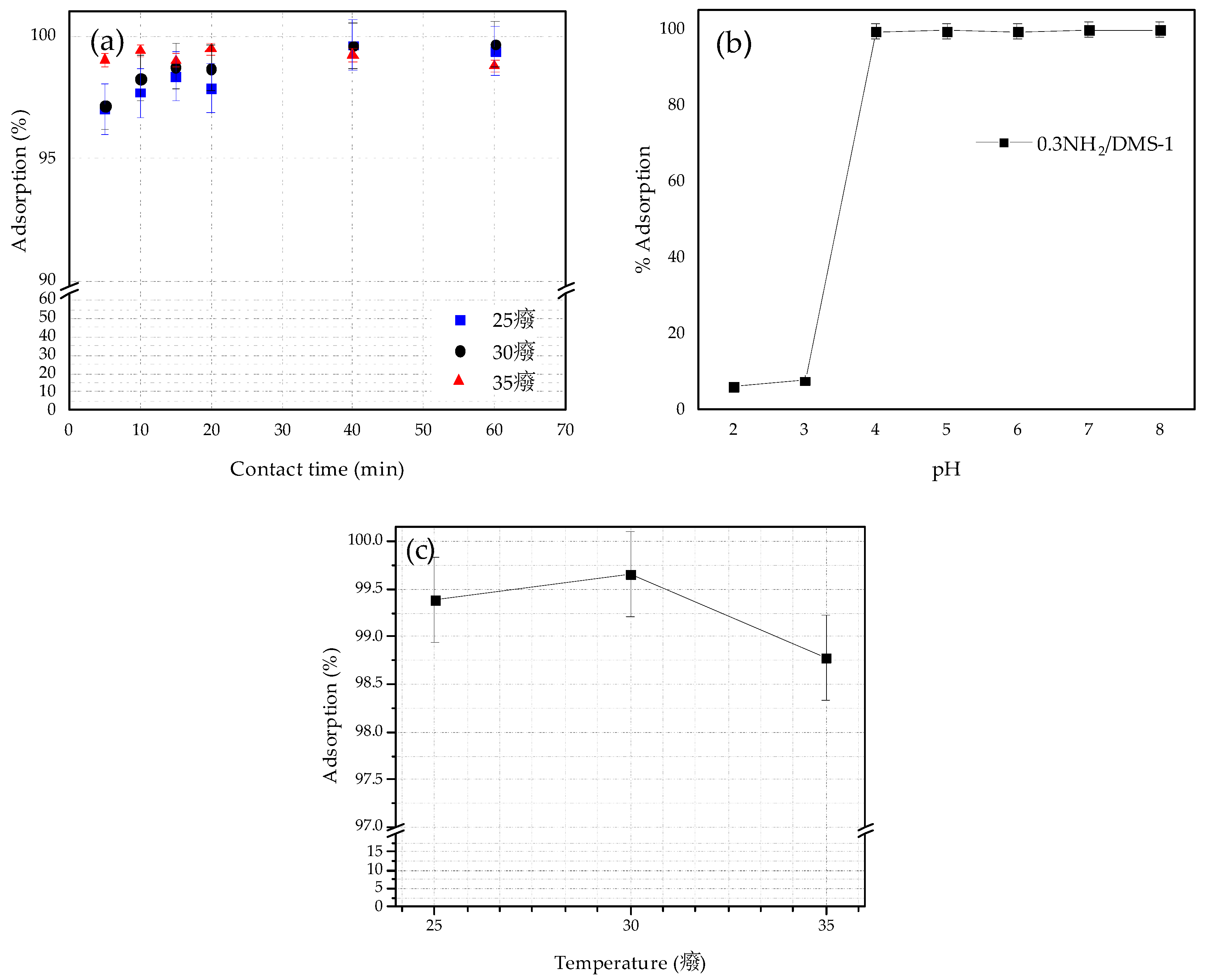
3.5. Adsorption Tests
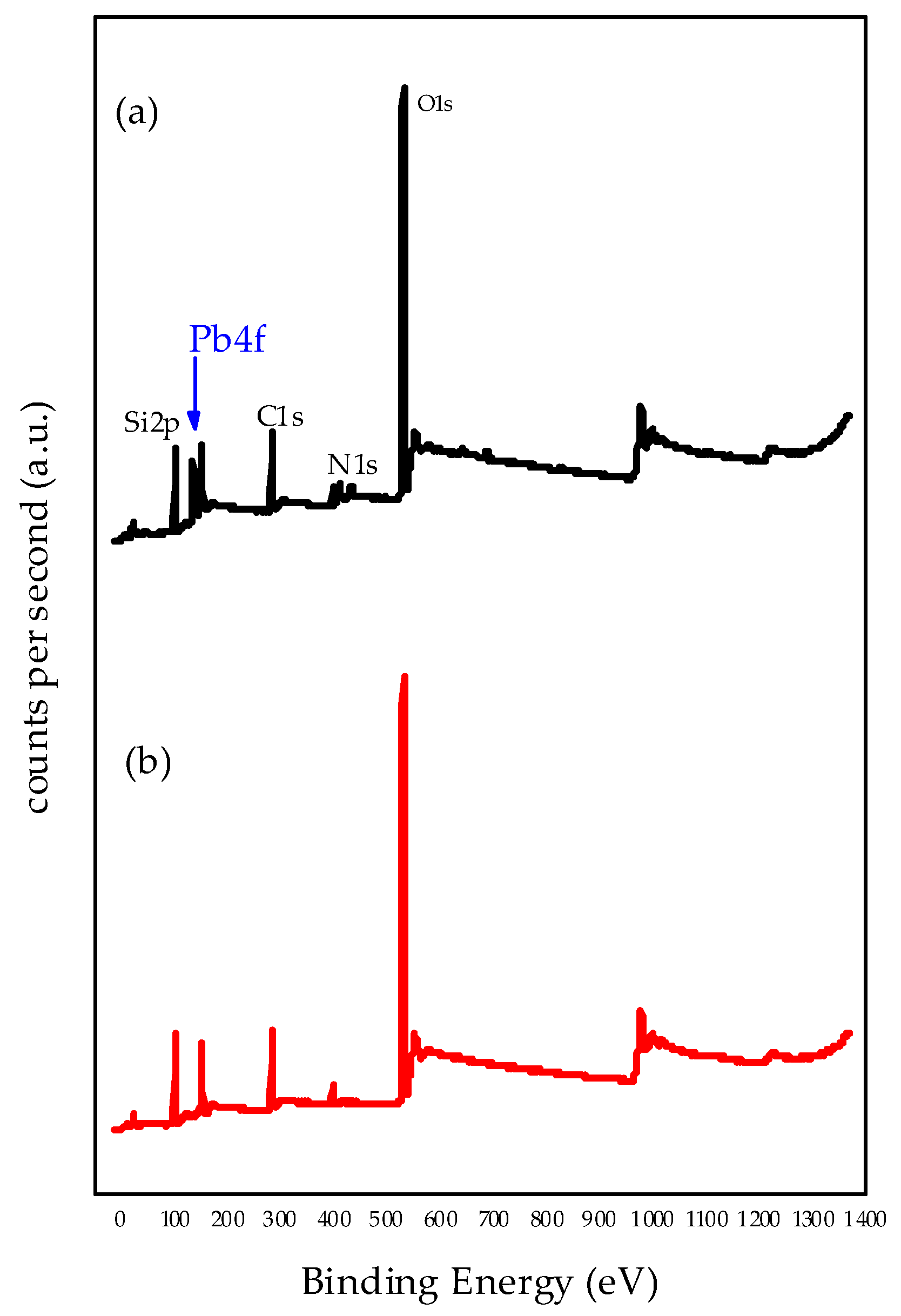
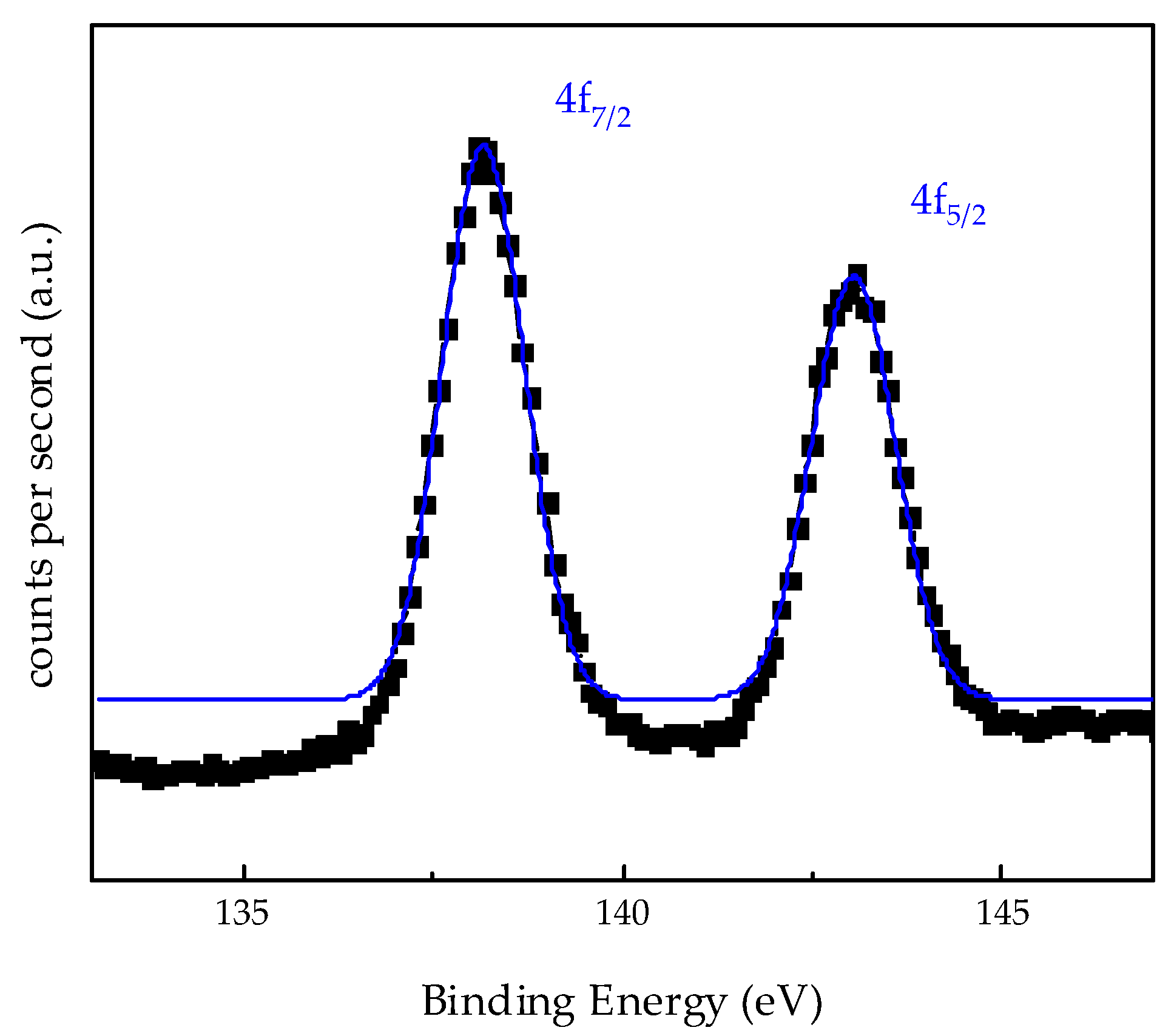
3.6. X-ray Photoelectron Spectroscopy (XPS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, C.-Y.; Wong, C.-S.; Chung, C.-J.; Wu, M.-Y.; Huang, Y.-L.; Ao, P.-L.; Lin, Y.-F.; Lin, Y.-C.; Shiue, H.-S.; Su, C.-T.; et al. The association between plasma selenium and chronic kidney disease related to lead, cadmium and arsenic exposure in a Taiwanese population. J. Hazard. Mater. 2019, 375, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R.; Hasan, M.M.; Islam, A.; Rahman, M.M.; Asiri, A.M.; Khaleque, M.A.; Sheikh, M.C. Offering an innovative composited material for effective lead(II) monitoring and removal from polluted water. J. Clean. Prod. 2019, 231, 214–223. [Google Scholar] [CrossRef]
- Mohammadyan, M.; Moosazadeh, M.; Borji, A.; Khanjani, N.; Moghadam, S.R. Exposure to lead and its effect on sleep quality and digestive problems in soldering workers. Environ. Monit. Assess. 2019, 191, 184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peterson, K.E.; Montgomery, K.; Sánchez, B.N.; Zhang, Z.; Afeiche, M.C.; Cantonwine, D.E.; Ettinger, A.S.; Cantoral, A.; Schnaas, L.; et al. Early lead exposure and childhood adiposity in Mexico city. Int. J. Hyg. Environ. Health 2019, 222, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Njati, S.Y.; Maguta, M.M. Lead-based paints and children’s PVC toys are potential sources of domestic lead poisoning—A review. Environ. Pollut. 2019, 249, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Digman, T.; Kaufmann, R.B.; LeStourgeon, L.; Brown, M.J. Control of Lead Sources in the United States, 1970–2017: Public Health Progress and Current Challenges to Eliminating Lead Exposure. J. Public Health Manag. Pract. 2019, 25, S13–S22. [Google Scholar]
- Cassleman, K.L.; Dorrance, K.A.; Todd, A.C. Neuropsychiatric Implications of Chronic Lead Exposure. Mil. Med. 2019, 1–5. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Djordjevic, A.B.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Shahat, A. Functionalized novel mesoporous adsorbent for selective lead(II) ions monitoring and removal from wastewater. Sens. Actuators B Chem. 2014, 203, 854–863. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M. A novel fine-tuning mesoporous adsorbent for simultaneous lead(II) detection and removal from wastewater. Sens. Actuators B Chem. 2014, 202, 395–403. [Google Scholar] [CrossRef]
- Ma, T.; Wu, X.; Cai, Q.; Wang, Y.; Xiao, L.; Tian, Y.; Li, H. Lead poisoning disturbs oligodendrocytes differentiation involved in decreased expression of NCX3 inducing intracellular calcium overload. Int. J. Mol. Sci. 2015, 16, 19096–19110. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, J.; Jia, P.; Liu, Y.; An, J.; Cao, B.; Pan, K. Hierarchically structured polyacrylonitrile nanofiber mat as highly efficient lead adsorbent for water treatment. Chem. Eng. J. 2015, 262, 775–784. [Google Scholar] [CrossRef]
- Hsueh, Y.M.; Lee, C.Y.; Chien, S.N.; Chen, W.J.; Shiue, H.S.; Huang, S.R.; Lin, M.I.; Mu, S.C.; Hsieh, R.L. Association of blood heavy metals with developmental delays and health status in children. Sci. Rep. 2017, 7, 43608. [Google Scholar] [CrossRef] [PubMed]
- Olympio, K.P.K.; Gonçalves, C.; Günther, W.M.R.; Bechara, E.J.H. Neurotoxicity and aggressiveness triggered by low-level lead in children: A review. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2009, 26, 266–275. [Google Scholar] [CrossRef]
- Secretaría de Salud, Norma Oficial Mexicana NOM-127-SSA1-1994. In Salud Ambiental, Agua Para uso y Consumo Humano-Límites Permisibles de Calidad y Tratamientos a Que Debe Someterse el Agua Para su Potabilización; Diario Oficial de la Federación: Ciudad de México, México, 2000.
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; rad, A.J.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef]
- Dong, Q.; Guo, X.; Huang, X.; Liu, L.; Tallon, R.; Taylor, B.; Chen, J. Selective removal of lead ions through capacitive deionization: Role of ion-exchange membrane. Chem. Eng. J. 2019, 361, 1535–1542. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Cecilia, J.A.; Moya, E.M.O.; Cavalcante, C.L.; Azevedo, D.C.S.; Rodríguez-Castellón, E. “Low cost” pore expanded SBA-15 functionalized with amine groups applied to CO2 adsorption. Materials 2015, 8, 2495–2513. [Google Scholar] [CrossRef]
- Bensacia, N.; Moulay, S.; Hulea, O.; Boos, A.; Garin, F. Kinetic and equilibrium studies of lead(II) adsorption from aqueous media by KIT-6 mesoporous silica functionalized with –COOH. Comptes Rendus Chim. 2014, 17, 869–880. [Google Scholar] [CrossRef]
- Luka, M.; Polarz, S. Wiring functional groups in mesoporous organosilica materials. J. Mater. Chem. C 2015, 3, 2195–2203. [Google Scholar] [CrossRef][Green Version]
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Malgras, V.; Ataee-Esfahani, H.; Wang, H.; Jiang, B.; Li, C.; Wu, K.C.W.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for Mesoporous Metals. Adv. Mater. 2016, 28, 993–1010. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal–organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
- Mansor, A.F.; Mohidem, N.A.; Wan, W.N.I.; Zawawi, M.; Othman, N.S.; Endud, S.; Mat, H. The optimization of synthesis conditions for laccase entrapment in mesoporous silica microparticles by response surface methodology. Microporous Mesoporous Mater. 2016, 220, 308–314. [Google Scholar] [CrossRef]
- Huang, M.; Li, L.; Zheng, S. Mesoporous silica with block copolymer templates: Modulation of porosity via block copolymer reaction with silica. Microporous Mesoporous Mater. 2016, 225, 9–25. [Google Scholar] [CrossRef]
- Dey, S.; Anderson, S.T.; Mayanovic, R.A.; Sakidja, R.; Landskron, K.; Kokoszka, B.; Mandal, M.; Wang, Z. Experimental and theoretical investigation of a mesoporous KxWO3 material having superior mechanical strength. Nanoscale 2016, 8, 2937–2943. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Li, Y.; Guragain, S.; Pramanik, M.; Alshehri, S.M.; Ahamad, T.; Liu, Z.; Yamauchi, Y. Synthesis of Mesoporous Transition-Metal Phosphates by Polymeric Micelle Assembly. Chem. A Eur. J. 2016, 22, 7463–7467. [Google Scholar] [CrossRef]
- Wen, X.; Zeng, Z.; Du, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Xu, P.; Zhang, C.; Wan, J.; et al. Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere 2019, 222, 865–871. [Google Scholar] [CrossRef]
- Jian, J.; Kuang, D.; Wang, X.; Zhou, H.; Gao, H.; Sun, W.; Yuan, Z.; Zeng, J.; You, K.; Luo, H. Highly dispersed Co/SBA-15 mesoporous materials as efficient and stable catalyst for partial oxidation of cyclohexane with molecular oxygen. Mater. Chem. Phys. 2020, 246, 122814. [Google Scholar] [CrossRef]
- Doustkhah, E.; Lin, J.; Rostamnia, S.; Len, C.; Luque, R.; Luo, X.; Bando, Y.; Wu, K.C.W.; Kim, J.; Yamauchi, Y.; et al. Development of Sulfonic-Acid-Functionalized Mesoporous Materials: Synthesis and Catalytic Applications. Chem. A Eur. J. 2019, 25, 1614–1635. [Google Scholar] [CrossRef]
- Acosta-Silva, Y.J.; Nava, R.; Hernández-Morales, V.; Macías-Sánchez, S.A.; Pawelec, B. TiO2/DMS-1 disordered mesoporous silica system: Structural characteristics and methylene blue photodegradation activity. Microporous Mesoporous Mater. 2013, 170, 181–188. [Google Scholar] [CrossRef]
- Hernández-Morales, V.; Nava, R.; Acosta-Silva, Y.J.; MacÍas-Sánchez, S.A.; Pérez-Bueno, J.J.; Pawelec, B. Adsorption of lead (II) on SBA-15 mesoporous molecular sieve functionalized with -NH2groups. Microporous Mesoporous Mater. 2012, 160, 133–142. [Google Scholar] [CrossRef]
- Moreno-Martell, A.; Pawelec, B.; Nava, R.; Mota, N.; Escamilla-Perea, L.; Navarro, R.M.; Fierro, J.L.G. CO oxidation at 20 °C on Au catalysts supported on mesoporous silica: Effects of support structural properties and modifiers. Materials 2018, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chen, C.H.; Cheng, S.; Li, H.Y. Adsorption of Pb(II) and Cu(II) metal ions on functionalized large-pore mesoporous silica. Int. J. Environ. Sci. Technol. 2016, 13, 65–76. [Google Scholar] [CrossRef]
- Ultreras, N.T.A.; Castillo, R.R.V.; Mendoza, R.N.; Ledesma, C.P.; Castro, G.N.; Torres, M.G.O.; Gonzales, M.V.P. Development of functionalized mesoporous meshes DMS-1 applicable for the adsorption and removal of mercury in water. In Proceedings of the 2017 XIII International Engineering Congress (CONIIN), Santiago de Queretaro, Mexico, 15–19 May 2017. [Google Scholar] [CrossRef]
- Palos-Barba, V.; Moreno-Martell, A.; Hernández-Morales, V.; Peza-Ledesma, C.L.; Rivera-Muñoz, E.M.; Nava, R.; Pawelec, B. SBA-16 Cage-Like Porous Material Modified with with APTES as an Adsorbent for Pb2+ Ions Removal from Aqueous Solution. Materials 2020, 13, 927. [Google Scholar] [CrossRef] [PubMed]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A.; Lindo, M.; Gascón, V. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J. Hazard. Mater. 2009, 163, 213–221. [Google Scholar] [CrossRef]
- Nonkumwong, J.; Ananta, S.; Srisombat, L. Effective removal of lead(II) from wastewater by amine-functionalized magnesium ferrite nanoparticles. RSC Adv. 2016, 6, 47382–47393. [Google Scholar] [CrossRef]
- Mehdinia, A.; Shegefti, S.; Shemirani, F. Removal of lead(II), copper(II) and zinc(II) ions from aqueous solutions using magnetic amine-functionalized mesoporous silica nanocomposites. J. Braz. Chem. Soc. 2015, 26, 2249–2257. [Google Scholar] [CrossRef]
- Showkat, A.M.; Zhang, Y.P.; Min, S.K.; Gopalan, A.I.; Reddy, K.R.; Lee, K.P. Analysis of heavy metal toxic ions by adsorption onto amino-functionalized ordered mesoporous silica. Bull. Korean Chem. Soc. 2007, 28, 1985–1992. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric sufactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Fuertes, A.B. Synthesis of mesostructured silica with tailorable textural porosity and particle size. Mater. Lett. 2004, 58, 1494–1497. [Google Scholar] [CrossRef]
- Navarro, A.; Maldonado, H.; Campos, K.; Ramos, K. Elucidación del efecto del pH en la adsorción de metales pesados mediante biopolímeros naturales: Cationes divalentes y superficies activas. Rev. Iberoam. Polím. 2006, 7, 113–126. [Google Scholar]
- Shahbazi, A.; Younesi, H.; Badiei, A. Functionalized SBA-15 mesoporous silica by melamine-based dendrimer amines for adsorptive characteristics of Pb(II), Cu(II) and Cd(II) heavy metal ions in batch and fixed bed column. Chem. Eng. J. 2011, 168, 505–518. [Google Scholar] [CrossRef]
- Boss, C.B.; Fredeen, K.J. Concepts, Instrumentation and Techniques in Inductively Coupled plasma Optical Emission Spectrometry; Perkin Elmer Inc.: Shelton, CT, USA, 1999. [Google Scholar]
- Zuo, S.; Liu, W.; Yao, C.; Li, X.; Luo, S.; Wu, F.; Kong, Y.; Liu, X. One-pot template-free fabrication of hollow mesoporous sodalite nanospheres for drug release. Appl. Clay Sci. 2016, 119, 170–174. [Google Scholar] [CrossRef]
- Schneider, P. Adsorption isotherms of microporous-mesoporous solids revisited. Appl. Catal. A Gen. 1995, 129, 157–165. [Google Scholar] [CrossRef]
- Boevski, I.; Daskalova, N.; Havezov, I. Determination of barium, chromium, cadmium, manganese, lead and zinc in atmospheric particulate matter by inductively coupled plasma atomic emission spectrometry (ICP-AES). Spectrochim. Acta Part B At. Spectrosc. 2000, 55, 1643–1657. [Google Scholar] [CrossRef]
- Karandashev, V.K.; Turanov, A.N.; Orlova, T.A.; Lezhnev, A.E.; Nosenko, S.V.; Zolotareva, N.I.; Moskvitina, I.R. Use of the inductively coupled plasma mass spectrometry for element analysis of environmental objects. Inorg. Mater. 2008, 44, 1491–1500. [Google Scholar] [CrossRef]
- Yamasaki, S.I. Inductively Coupled Plasma Mass Spectrometry in Environmental Analysis. Encycl. Anal. Chem. Appl. Theory Instrum. 2006. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- L’vov, B.V. Thermal Decomposition of Solids and Melts: New Thermochemical Approach to the Mechanism, Kinetics and Methodology; Springer: Dordrecht, Neherlands, 2007. [Google Scholar]
- Buckle, E.R. Thermogravimetric analysis: The method of isobaric dehydration. J. Phys. Chem. 1959, 63, 1231–1235. [Google Scholar] [CrossRef]
- Möllmann, K.P.; Vollmer, M. Fourier transform infrared spectroscopy in physics laboratory courses. Eur. J. Phys. 2013, 34, S123. [Google Scholar] [CrossRef]
- Lerner, L. Corrigendum: Fourier transform infrared spectroscopy: An undergraduate experiment (2016 Eur. J. Phys. 37 065303). Eur. J. Phys. 2017, 38, 019501. [Google Scholar] [CrossRef]
- Biggs, P.; Holdsworth, F.J.; Wayne, R.P. A low-cost Fourier transform spectrometer for the visible and near-infrared regions. J. Phys. E 1987, 20, 1005. [Google Scholar] [CrossRef]
- Laghaei, M.; Sadeghi, M.; Ghalei, B.; Dinari, M. The effect of various types of post-synthetic modifications on the structure and properties of MCM-41 mesoporous silica. Prog. Org. Coat. 2016, 90, 163–170. [Google Scholar] [CrossRef]
- Solovyov, L.A. Diffraction analysis of mesostructured mesoporous materials. Chem. Soc. Rev. 2013, 42, 3708–3720. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Romig, A.D., Jr.; Lyman, C.E.; Fiori, C.; Lifshin, E. Scanning Electron Microscopy and X-Ray Microanalysis; Springer: Jersey, NJ, USA, 1992. [Google Scholar]
- Joy, D.C. The theory and practice of high-resolution scanning electron microscopy. Ultramicroscopy 1991, 37, 216–233. [Google Scholar] [CrossRef]
- Hollander, J.M.; Jolly, W.L. X-Ray Photoelectron Spectroscopy. Acc. Chem. Res. 1970, 3, 193–200. [Google Scholar] [CrossRef]
- Martínez-Klimov, M.E.; Hernandez-Hipólito, P.; Klimova, T.E.; Solís-Casados, D.A.; Martínez-García, M. Development of reusable palladium catalysts supported on hydrogen titanate nanotubes for the Heck reaction. J. Catal. 2016, 342, 138–150. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tomita, M.; Kato, K. Synthesis of amino-functionalized mesoporous silica sheets and their application for metal ion capture. J. Asian Ceram. Soc. 2015, 3, 70–76. [Google Scholar] [CrossRef]
- Sahu, D.R.; Hong, L.Y.; Wang, S.-C.; Huang, J.-L. Synthesis, analysis and characterization of ordered mesoporous TiO2/SBA-15 matrix: Effect of calcination temperature. Microporous Mesoporous Mater. 2009, 117, 640–649. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Wang, L.; Shi, J.; Yu, J.; Yan, D. Synthesis of nanostructured mesoporous silica materials containing manganese. Nanostruct. Mater. 1998, 10, 1289–1299. [Google Scholar] [CrossRef]
- Rahiala, H.; Beurroies, I.; Eklund, T.; Hakala, K.; Gougeon, R.; Trens, P.; Rosenholm, J.B. Preparation and Characterization of MCM-41 Supported Metallocene Catalysts for Olefin Polymerization. J. Catal. 1999, 188, 14–23. [Google Scholar] [CrossRef]
- Dutoit, D.C.M.; Schneider, M.; Fabrizioli, P.; Baiker, A. Vanadia−Silica Low-Temperature Aerogels: Influence of Aging and Vanadia Loading on Structural and Chemical Properties. Chem. Mater. 1996, 8, 734–743. [Google Scholar] [CrossRef]
- Almeida, R.M.; Guiton, T.A.; Pantano, C.G. Characterization of silica gels by infrared reflection spectroscopy. J. Non-Cryst. Solids 1990, 121, 193–197. [Google Scholar] [CrossRef]
- Khojastehnezhad, A.; Moeinpour, F.; Vafaei, M. Molybdenum oxide supported on silica (MoO3/SiO2): An efficient and reusable catalyst for the synthesis of 1,8-dioxodecahydroacridines under solvent-free conditions. J. Mex. Chem. Soc. 2015, 59, 29–35. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Wei, K.; Shu, L.; Guo, W.; Wu, Y.; Zeng, X. Synthesis of Amino-Functionalized Hexagonal Mesoporous Silica for Adsorption of Pb2+. Chin. J. Chem. 2011, 29, 143–149. [Google Scholar] [CrossRef]
- Kantam, M.L.; Sreekanth, P. One-pot synthesis of conjugated nitroalkenes by diamino-functionalised mesoporous material. Catal. Lett. 1999, 57, 227–231. [Google Scholar] [CrossRef]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, T.-J.; Gao, H.; Jin, Y. High density silanization of nano-silica particles using γ-aminopropyltriethoxysilane (APTES). Appl. Surf. Sci. 2015, 351, 646–654. [Google Scholar] [CrossRef]
- Blázquez, G.; Martín-Lara, M.A.; Tenorio, G.; Calero, M. Batch biosorption of lead(II) from aqueous solutions by olive tree pruning waste: Equilibrium, kinetics and thermodynamic study. Chem. Eng. J. 2011, 168, 170–177. [Google Scholar] [CrossRef]
- Altin, O.; Ozbelge, O.H.; Dogu, T. Effect of pH, flow rate and concentration on the sorption of Pb and Cd on montmorillonite: I. Experimental. J. Chem. Technol. Biotechnol. 1999, 74, 1131–1138. [Google Scholar] [CrossRef]
- Hao, S.; Zhong, Y.; Pepe, F.; Zhu, W. Adsorption of Pb2+ and Cu2+ on anionic surfactant-templated amino-functionalized mesoporous silicas. Chem. Eng. J. 2012, 189–190, 160–167. [Google Scholar] [CrossRef]
- Penilla, R.P.; Bustos, A.G.; Elizalde, S.G. Immobilization of Cs, Cd, Pb and Cr by synthetic zeolites from Spanish low-calcium coal fly ash. Fuel 2006, 85, 823–832. [Google Scholar] [CrossRef]
- Etienne, M.; Walcarius, A. Analytical investigation of the chemical reactivity and stability of aminopropyl-grafted silica in aqueous medium. Talanta 2003, 59, 1173–1188. [Google Scholar] [CrossRef]
- Huang, J.; Ye, M.; Qu, Y.; Chu, L.; Chen, R.; He, Q.; Xu, D. Pb (II) removal from aqueous media by EDTA-modified mesoporous silica SBA-15. J. Colloid Interface Sci. 2012, 385, 137–146. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Alwi, S.R.W.; Webb, C.; Ghasemi, N.; Muhamad, I.I. Treatment of lead-contaminated water using activated carbon adsorbent from locally available papaya peel biowaste. J. Clean. Prod. 2016, 118, 210–222. [Google Scholar] [CrossRef]
- Raji, F.; Saraeian, A.; Pakizeh, M.; Attarzadeh, F. Removal of Pb(ii) from aqueous solution by mesoporous silica MCM-41 modified by ZnCl2: Kinetics, thermodynamics, and isotherms. RSC Adv. 2015, 5, 37066–37077. [Google Scholar] [CrossRef]
- Shimoda, K.; Park, J.-S.; Hinoki, T.; Kohyama, A. Influence of surface structure of SiC nano-sized powder analyzed by X-ray photoelectron spectroscopy on basic powder characteristics. Appl. Surf. Sci. 2007, 253, 9450–9456. [Google Scholar] [CrossRef]
- Hijikata, Y.; Yaguchi, H.; Yoshikawa, M.; Yoshida, S. Composition analysis of SiO2/SiC interfaces by electron spectroscopic measurements using slope-shaped oxide films. Appl. Surf. Sci. 2001, 184, 161–166. [Google Scholar] [CrossRef]
- Wagner, C.D.; Joshi, A. The auger parameter, its utility and advantages: A review. J. Electron Spectros. Relat. Phenom. 1988, 47, 283–313. [Google Scholar] [CrossRef]
- Van Ooij, W.J.; Surman, D.; Yasuda, H.K. Plasma-polymerized coatings of trimethylsilane deposited on cold-rolled steel substrates Part 2. Effect of deposition conditions on corrosion performance. Prog. Org. Coat. 1995, 25, 319–337. [Google Scholar] [CrossRef]
- Alfonsetti, R.; Lozzi, L.; Passacantando, M.; Picozzi, P.; Santucci, S. XPS studies on SiOx thin films. Appl. Surf. Sci. 1993, 70–71, 222–225. [Google Scholar] [CrossRef]
- Mariscal, R.; Soria, J.; Peña, M.A.; Fierro, J.L.G. Structure and reactivity of undoped and sodium- doped PbO/α-Al2O3 catalysts for oxidative coupling of methane. Appl. Catal. A Gen. 1994, 111, 79–97. [Google Scholar] [CrossRef]
- Thomas, J.M.; Tricker, M.J. Electronic structure of the oxides of lead. Part 2—An XPS study of bulk rhombic PbO, tetragonal PbO, β-PbO2 and Pb3O4. J. Chem. Soc. 1975, 71, 329–336. [Google Scholar] [CrossRef]
- Pederson, L.R. Two-dimensional chemical-state plot for lead using XPS. J. Electron Spectros. Relat. Phenom. 1982, 28, 203–209. [Google Scholar] [CrossRef]
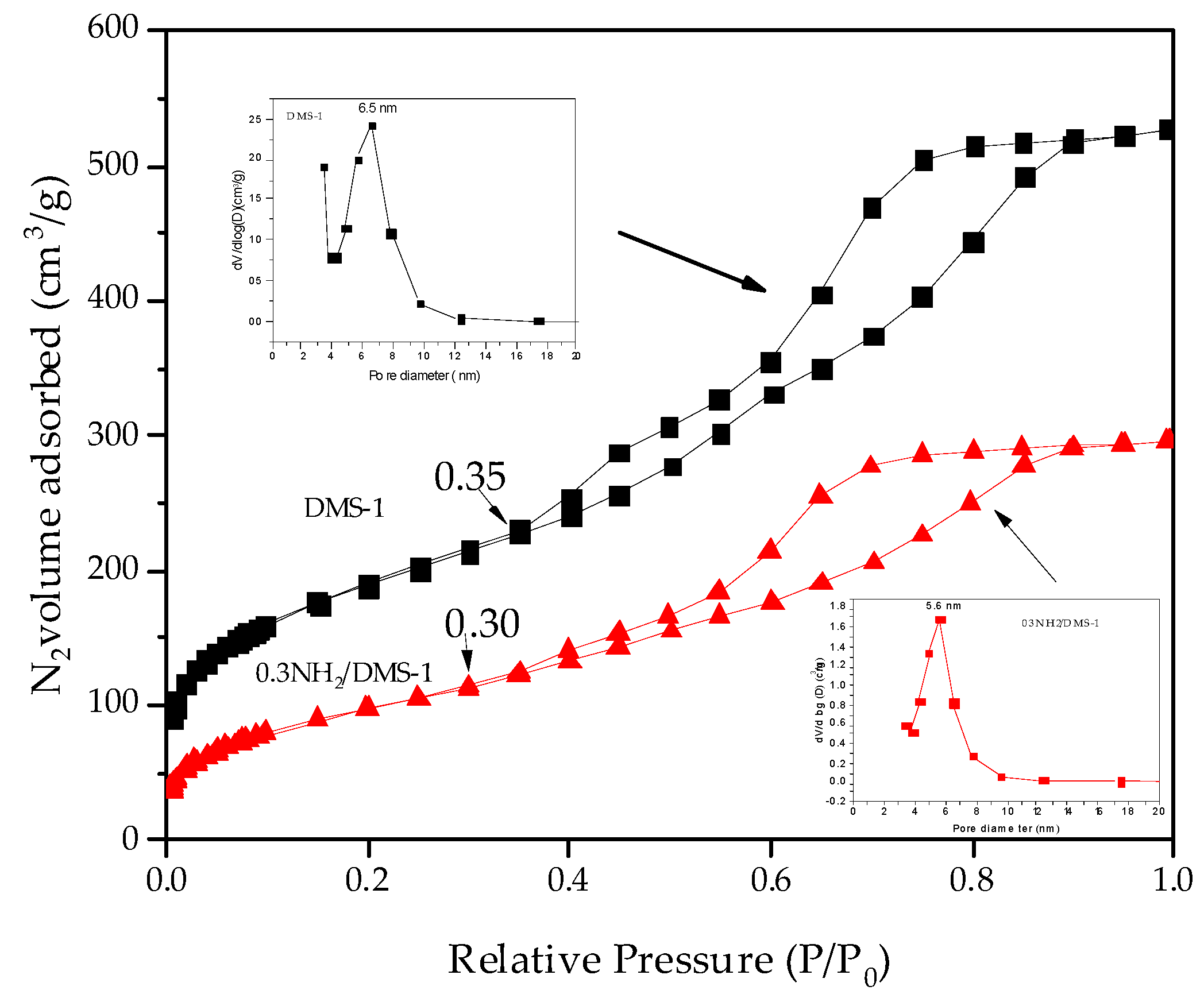
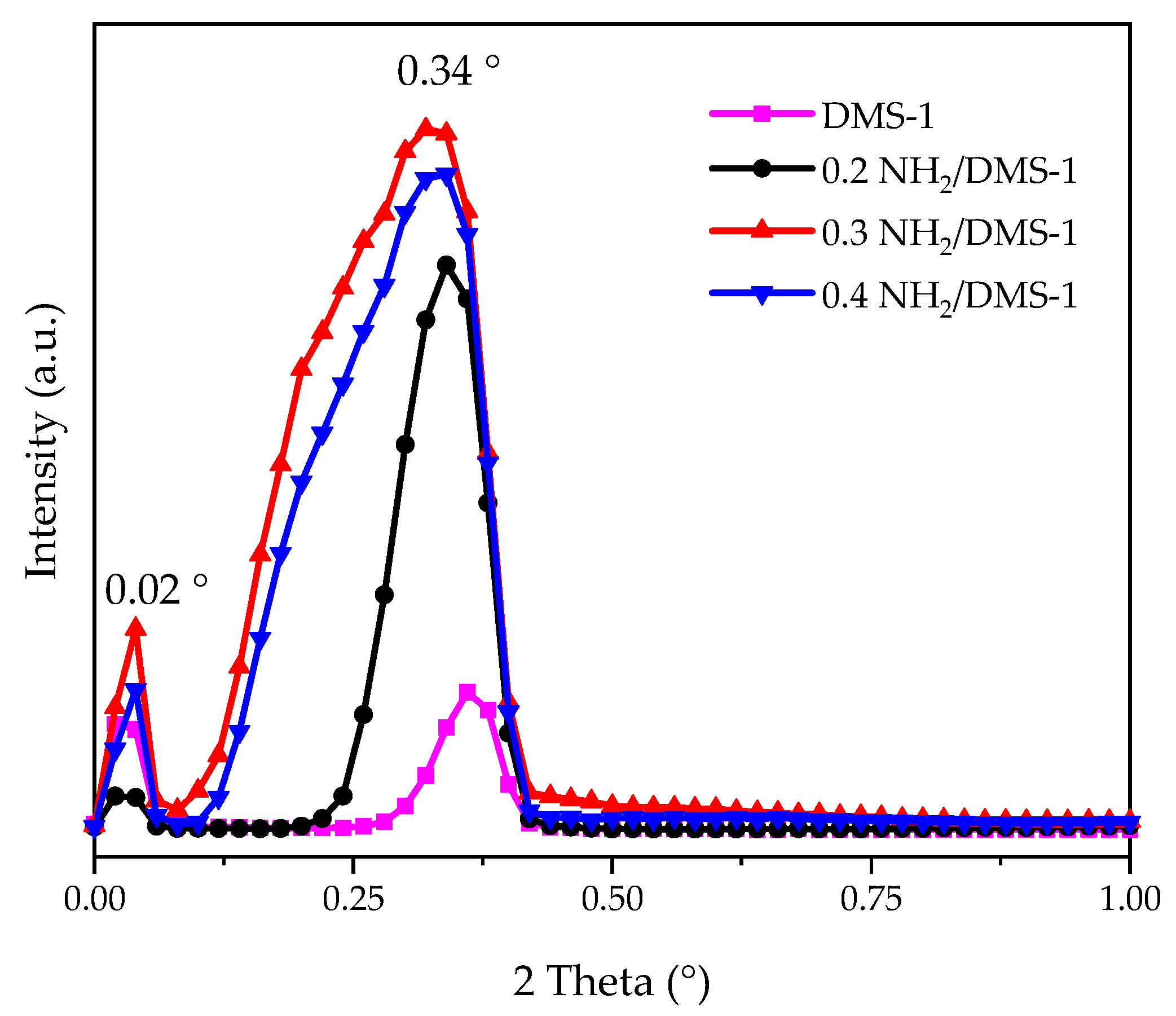
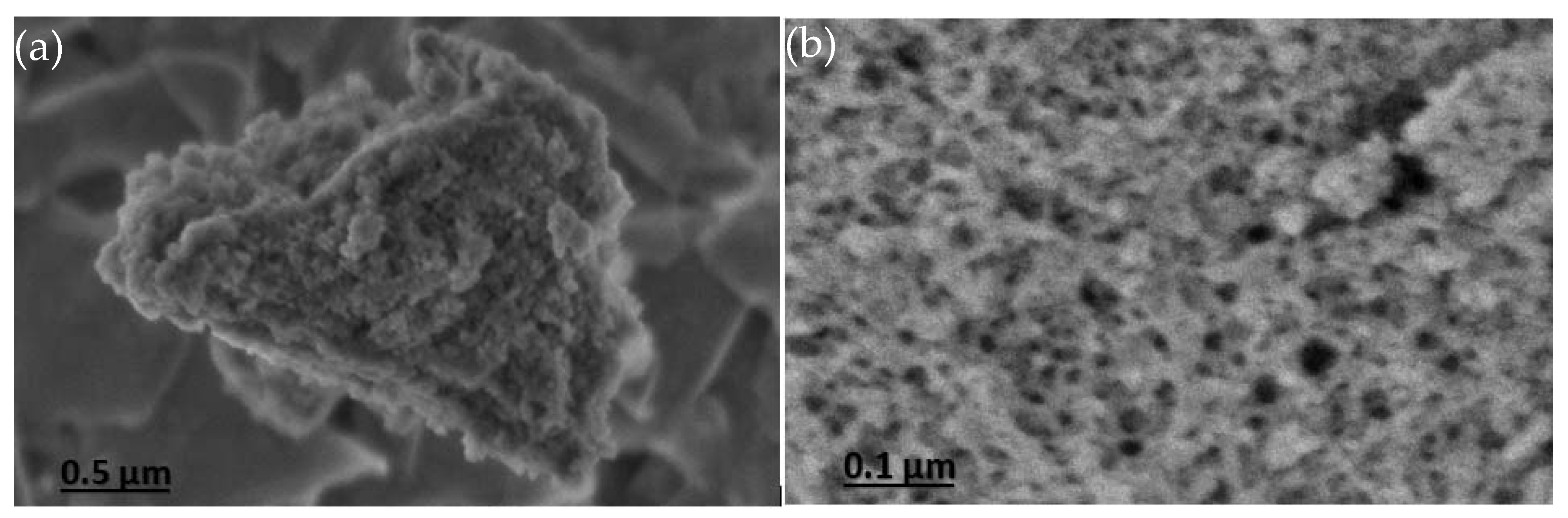
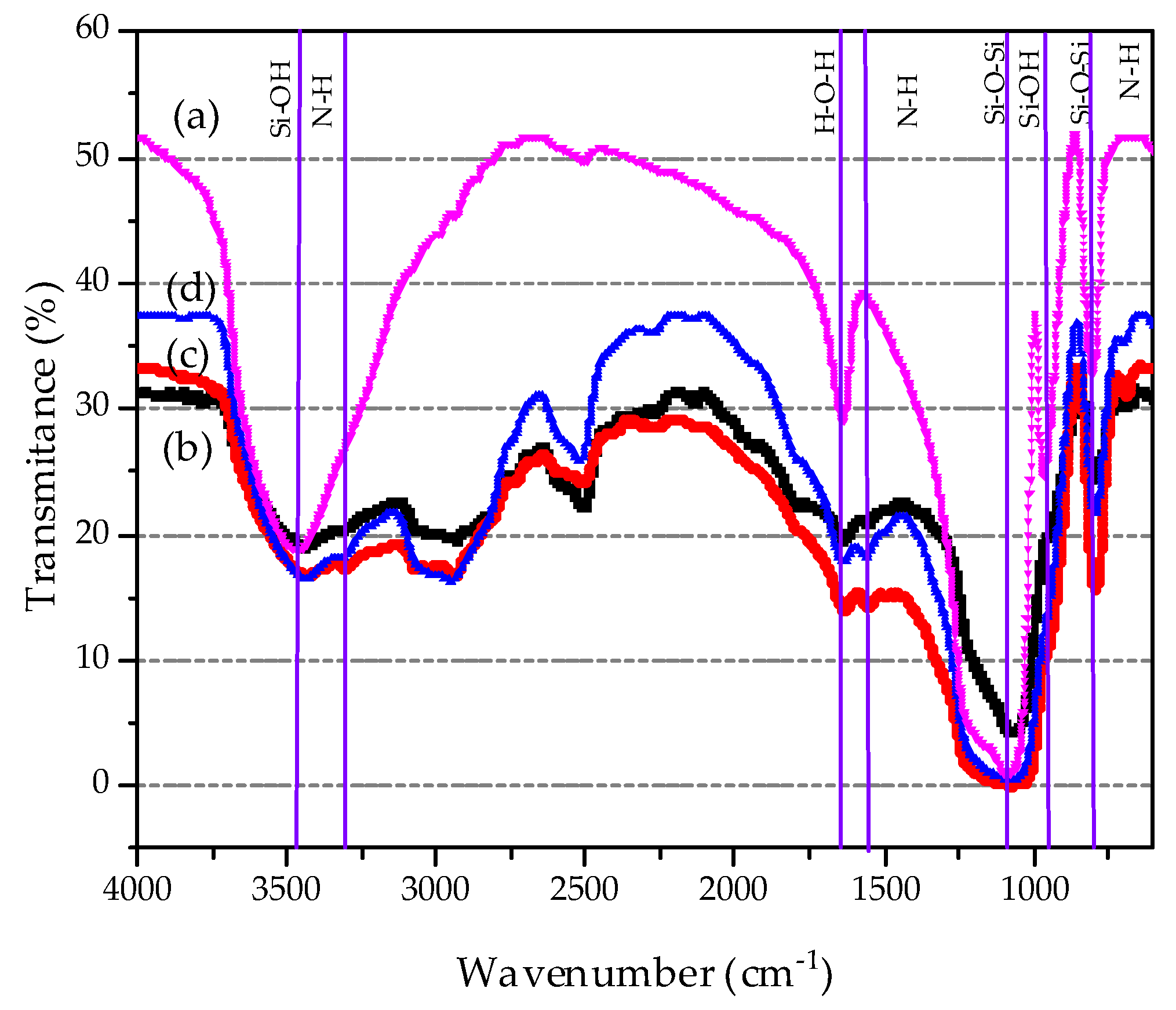


| Sample | SBET (m2/g) | Vpmeso (cm3/g) | Dp (nm) |
|---|---|---|---|
| DMS-1 | 668 | 0.67 | 6.5 |
| 0.3 NH2/DMS-1 | 354 | 0.39 | 5.6 |
| XPS Data | Before Adsorption (eV) | After Adsorption (eV) |
|---|---|---|
| Si 2p | 102.88 | 102.92 |
| O 1s | 530.64 | 530.60 |
| C 1s | 283.17 | 283.17 |
| N 1s | 399.27 400.96 | 399.30 401.07 |
| Pb4f7/2 | - | 138.17 |
| - | 143.04 | |
| N/Si | 0.15 | 0.13 |
| Pb/N | - | 1.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palos-Barba, V.; Lugo-Nabor, C.; Velázquez-Castillo, R.R.; Solís-Casados, D.A.; Peza-Ledesma, C.L.; Rivera-Muñoz, E.M.; Nava, R.; Pawelec, B. Development of an Adsorbing System Made of DMS-1 Mesh Modified by Amino Groups to Remove Pb(II) Ions from Water. Materials 2020, 13, 1914. https://doi.org/10.3390/ma13081914
Palos-Barba V, Lugo-Nabor C, Velázquez-Castillo RR, Solís-Casados DA, Peza-Ledesma CL, Rivera-Muñoz EM, Nava R, Pawelec B. Development of an Adsorbing System Made of DMS-1 Mesh Modified by Amino Groups to Remove Pb(II) Ions from Water. Materials. 2020; 13(8):1914. https://doi.org/10.3390/ma13081914
Chicago/Turabian StylePalos-Barba, Viviana, Cecilia Lugo-Nabor, Rodrigo R. Velázquez-Castillo, Dora Alicia Solís-Casados, Carmen L. Peza-Ledesma, Eric M. Rivera-Muñoz, Rufino Nava, and Barbara Pawelec. 2020. "Development of an Adsorbing System Made of DMS-1 Mesh Modified by Amino Groups to Remove Pb(II) Ions from Water" Materials 13, no. 8: 1914. https://doi.org/10.3390/ma13081914
APA StylePalos-Barba, V., Lugo-Nabor, C., Velázquez-Castillo, R. R., Solís-Casados, D. A., Peza-Ledesma, C. L., Rivera-Muñoz, E. M., Nava, R., & Pawelec, B. (2020). Development of an Adsorbing System Made of DMS-1 Mesh Modified by Amino Groups to Remove Pb(II) Ions from Water. Materials, 13(8), 1914. https://doi.org/10.3390/ma13081914






